Health systems are facing the most serious global pandemic crisis in a century. Containing and mitigating the spread and infection rate of the coronavirus SARS‑CoV‑2 is the first priority of public health authorities to distribute the number of infections over time and, if possible, reduce the incidence of the disease it causes (COVID‑19). However, beyond containment, additional measures – operational, financial, and R&D – are needed to provide effective patient care and reduce the pressure on health systems to manageable levels. The main focus of this brief is on the policies aimed at providing effective care and managing the pressure on health systems. Four key measures health systems are putting in place in response to the epidemic are considered: 1) ensuring access of the vulnerable to diagnostics and treatment; 2) strengthening and optimising health system capacity to respond to the rapid increase in caseloads; 3) how to leverage digital solutions and data to improve surveillance and care; and 4) how to improve R&D for accelerated development of diagnostics, treatments and vaccines. Access our COVID‑19 OECD Health System Response Tracker to learn more about the latest in OECD countries' responses to the COVID‑19 pandemic.
Beyond containment: Health systems responses to COVID-19 in the OECD

Abstract
Containment and mitigation policies are essential for health care systems to lower the peak in demand for care and, hopefully, reverse the flow of the COVID‑19 epidemics. In the absence of prophylaxis through a vaccine and more effective treatments, non-medical countermeasures have been an important priority of health systems (see Figure 1). At this stage, data on the cost-effectiveness of containment and mitigation policies is still limited. The available evidence is discussed in more detail in another OECD brief. Nevertheless, health systems are already being overburdened by the surge in demand for diagnostics and treatment, and health providers are being faced with unprecedented levels of demand. This brief presents the main responses of health systems across OECD countries and the innovations and that have been adopted to address the existing constraints.
Figure 1. Main public health policy responses to epidemics, including COVID‑19 pandemic
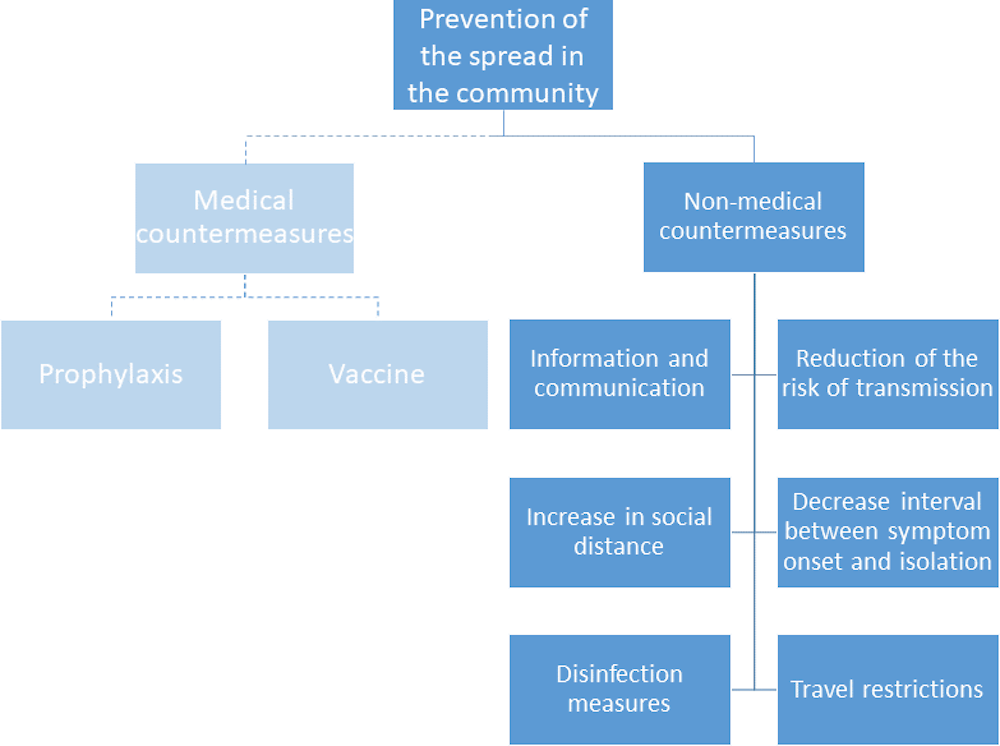
Note: as effective medical countermeasures are not currently available for COVID‑19, non-medical containment and mitigation measures are the main public health tool in the fight against COVID‑19.
Source: Adapted from OECD (2020[1]), “Containment and mitigation policy actions are key to fight the COVID‑19 pandemic”.
The size of the epidemics
Figure 2 shows the number of new cases of COVID‑19 for a set of countries over time, as compiled by the European Centre for Disease Prevention and Control (European Centre for Disease Prevention and Control (ECDC) Public Health Emergency Team et al., 2020[2]). The lines are adjusted for the time lag across countries depending on when the outbreak started. The figure shows that most countries in the European Union/European Economic Area and the United Kingdom follow a very similar pattern of growth observed in the Hubei Province in China.
Figure 2. Time distribution of cumulative incidence of COVID‑19 per country.
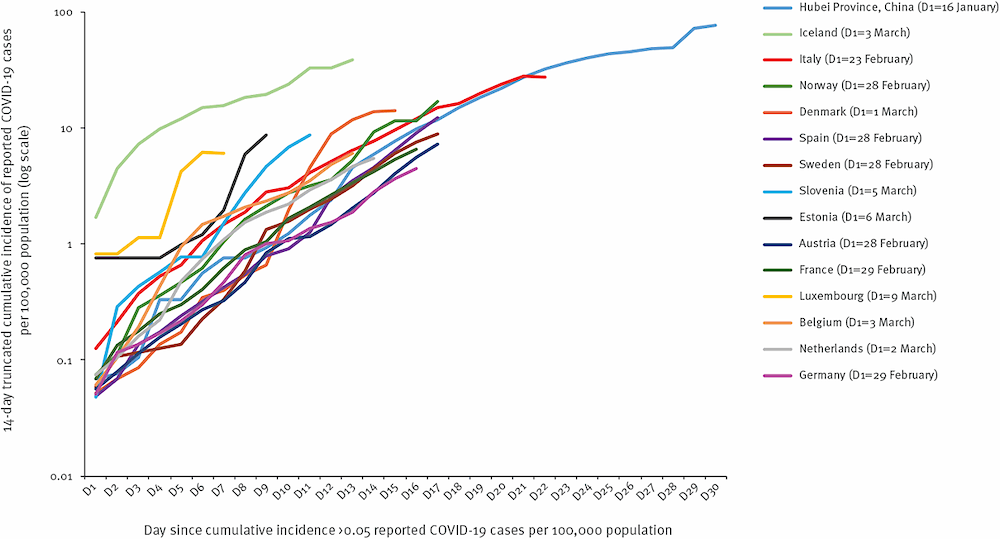
Note: Time distribution of the 14-day truncated cumulative incidence of COVID‑19 for 14-day truncated cumulative incidence ≥ 4.0 cases per 100 000 population and > 30 notified cases. The 14-day truncated cumulative incidence of COVID‑19 cases distribution in each country is compared with that of Hubei Province, China.
Source: European Centre for Disease Prevention and Control (ECDC) Public Health Emergency Team et al. (2020[2]), “Rapidly increasing cumulative incidence of coronavirus disease (COVID‑19) in the European Union/European Economic Area and the United Kingdom. 1 January to 15 March 2020”, https://doi.org/10.2807/1560-7917.ES.2020.25.11.2000285, reproduced here under a Creative Commons Attribution 4.0 International License.
Mortality follows similar patterns, with fragile populations at highest risk. The fatality rate – i.e. the number of deaths on the number of people with a known diagnosis of the disease – follows an exponential curve by age, with relatively low case fatality rates for those aged below 50 and growing fatality rates for the elderly. Individuals with other chronic conditions such as cardiovascular diseases and diabetes are also at higher risk.
Health systems response to the epidemic
Ensuring affordability of diagnostics and treatment to all
A critical task for health systems confronted with the spread of the coronavirus is to protect the health of all citizens, so this requires that both diagnosis/testing and appropriate care should be readily available, affordable and provided in a safe environment. The spread of the contagion does not respect borders nor discriminate between poor and rich people, even if the severity of resulting symptoms or the ability of health systems to cope differ. Governments across the OECD are therefore trying to mitigate the impact that containment and treatment have on the more vulnerable sections of the population.
The spread of the contagion does not respect borders nor discriminate between poor and rich people, even if the severity of resulting symptoms or the ability of health systems to cope differ
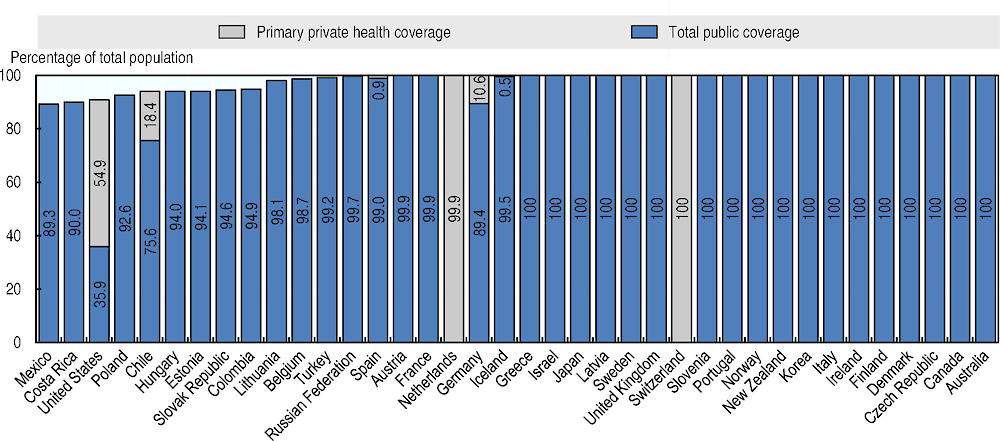
Although nearly all OECD countries provide universal health coverage to their populations, gaps persist in some countries. Population coverage for core services remains below 95% in seven OECD countries, and is lowest in Mexico, the United States and Poland (Figure 3). Mexico has expanded coverage since 2004, but gaps remain. In the United States, the uninsured tend to be working-age adults with lower education or income levels. In Ireland, though coverage is universal, less than half of the population are covered for the cost of GP visits.
Figure 3. Population coverage for a core set of services, 2017 (or nearest year)
Even among countries with universal coverage, inequalities in health status and unmet needs for care persist. For example, according to data from before the COVID-19 crisis nearly 30% of people in the lowest income quintile forgo care because of affordability, three times higher than those in the richest quintile, on average across countries. More generally, just over a fifth of all spending on health care comes directly from patients through out-of-pocket payments, on average across OECD countries. Whilst in terms of treatment, the cost-sharing requirements are generally low (households cover only 6% of inpatient and 8% of outpatient costs on average) households cover on average almost a quarter of spending on regular diagnostic laboratory tests.
Across the OECD, governments have implemented measures to ensure affordability of both diagnostic testing and subsequent treatment of COVID‑19 (Box 1). It should be noted that while affordability is a prerequisite to access to diagnostics and treatment, organisational barriers and health system capacity issues play a crucial role. For example, access to testing depends on a test being both physical available, and it being affordable, as well as laboratory capacity. In addition to affordability, countries have adopted different approaches for organising availability of testing, and this are discussed in more detail on Section 2.2.
Box 1. Measures implemented to improve affordability to diagnostics and treatment in selected OECD countries.
In the United States, steps have been taken to increase access to both diagnostic testing and treatment by the federal government, state governments, and private insurers. Legislation passed into law on 18 March 2020 requires private health plans to provide coverage for COVID‑19 diagnostic testing with no out-of-pocket costs. This legislation also provides COVID‑19 testing with no cost sharing under Medicare and Medicaid. Laboratory costs for COVID‑19 testing for uninsured individuals will be funded by the National Disaster Medical System.
In Japan, RT-polymerase chain reaction (RT-PCR) testing and subsequent treatment are fully funded by the central government. COVID‑19 is categorised as a designated disease and therefore the legislation applied is different from those applies to other diseases (covered by health insurance). Tests are now included in the health insurance coverage without any cost-sharing component, to increase testing.
France has a very regulated insurance coverage with limited out-of-pocket payments incurred by the population. The price of a coronavirus test has been regulated at EUR 54 with 60% covered through the Social Security and the rest through complementary private insurance.
Korea provides tests and subsequent treatment free of charge to patients and the cost is covered by central and local governments and the health insurance public corporation. South Korea also provides a subsidy to individuals who need to be isolated (both self-isolation and hospitalisation) to support their living costs and penalises those who are suspected to be infected if they refuse to receive diagnostic test or subsequent treatment or go through self-isolation.
In Germany, the costs of the COVID‑19 tests are covered by the Statutory Health Insurance (SHI) when recommended by a doctor. The SHI pay EUR 59 per test as agreed between SHI funds and provider representative in the context of the self-governing structures. Patients who are not deemed in need for testing may still purchase tests privately at a higher price.
Mexico is currently providing full coverage to the test only in public hospitals for patients that comply with the case definition set by the Ministry of Health. People can take the test in private providers but they have to pay the full price in most of cases. Media reports that the test price can range between EUR 227 and EUR 378, while the Ministry of Health has stated that the production cost of the test is EUR 87.
Chile covers the cost of tests taken for public health purposes and for all the beneficiaries of the public insurer (FONASA) that comply with the clinical criteria defined by the Ministry of Health. For private providers having agreements with FONASA, the co-payment is capped at around EUR 15. Those covered by a private insurer may have different co-payment according to their insurance plan, but the full price ranges between EUR 40 and EUR 67.
Turkey is currently providing tests to those displaying symptoms or who have contact with positive cases in 24 designated hospitals across the country. The costs of testing and any subsequent treatment are covered by the Social Security Institution (SGK - Sosyal Guvenlik Kurumu).
Chile covers the cost of tests taken for public health purposes and for all the beneficiaries of the public insurer (FONASA) that comply with the clinical criteria defined by the Ministry of Health. For private providers having agreements with FONASA, the co-payment is capped at around EUR 15. Those covered by a private insurer may have different co-payment according to their insurance plan, but the full price ranges between EUR 40 and EUR 67.
Turkey is currently providing tests to those displaying symptoms or who have contact with positive cases in 24 designated hospitals across the country. The costs of testing and any subsequent treatment are covered by the Social Security Institution (SGK - Sosyal Guvenlik Kurumu).
The current crisis demonstrates the importance of universal health coverage as a key element for the resilience of health systems. High levels of out-of-pocket payments may deter people from seeking early diagnosis and treatment, and thus contribute to an acceleration in the rate of transmission. However, even in health systems that have already achieved universal coverage, an epidemic caused by newly discovered pathogens requires an early response to clarify coverage for new diagnostic tests and treatments that were not previously included in the health benefit package.
Boosting and optimising health system capacity
Boosting and optimising the capacity of health systems to respond to the surge in the demand for care associated with COVID‑19 cases has been one of the major challenges faced by countries. Surge demand has put particularly pressure on access to diagnostics, hospitalisations, and critical care treatment of the most complex cases. Policy responses of health systems can be organised along three key “S” priorities: mobilising staff (to diagnose and treat patients), supplies (of required equipment to diagnose people safely, and provide them with acute treatment when needed), and space (to diagnose people quickly and safely, to isolate suspected and confirmed cases, and to treat patients in hospital or in their home).
Policy responses of health systems can be organised along three key “S” priorities: mobilising staff, boosting supplies and optimising space
Staff: Mobilising inactive health professionals, adapting the roles and responsibilities of providers and protecting the health of health workers
As doctors, nurses and other health professionals were mobilised to play the role of first responders, health systems have sought ways to increase the number of staff available and to make the best use of their work. Some countries that spend a lot on health, such as Norway, Switzerland and Germany, have both relatively high numbers of doctors and nurses (Figure 4). Other things being equal, this provides them with a greater capacity to respond to the COVID‑19 epidemic, assuming that the activities of some of these doctors and nurses can be reallocated to deal with the crisis. Countries that spend relatively less on health generally have fewer doctors and nurses per population (such as Mexico, Turkey and Poland). The existing workforce in these countries will therefore be even more stretched in their attempts to address the additional demand for care arising from the epidemic.
Figure 4. Number of doctors and nurses in OECD countries, 2017 (or nearest year)
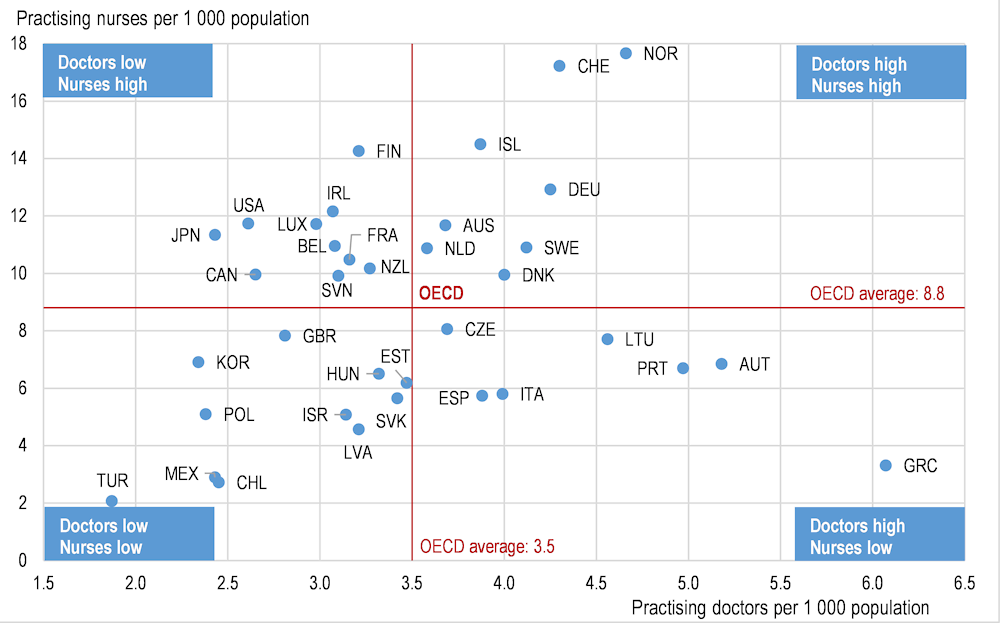
Note: In Portugal and Greece, data refer to all doctors licensed to practice, resulting in a large overestimation of the number of practising doctors (e.g. of around 30% in Portugal). In Austria and Greece, the number of nurses is underestimated as it only includes those working in hospital.
Regardless of their starting point, most countries that have already been hard-hit by the COVID‑19 have tried to increase the supply of staff to respond to the surge in demand – in both testing large numbers of people and providing acute treatments for those who need it (see Box 2). Several countries have tried to mobilise inactive and retired health professionals, although this has raised a concern that retired health professionals may be at greater risk of severe consequences and mortality from the coronavirus if they catch it, as it affects older people more severely. Some countries are mobilising military health professionals, to assist both in treatment and in relocation of patients or suspected cases. Countries have also mobilised students in medical, nursing and other health education programmes nearing the end of their studies to provide services to patients or to help in responding to public concerns through telephone hotlines.
Box 2. Measures implemented to boost and optimise staff capacity in selected OECD countries
The government of Italy announced on 9 March 2020, with subsequent updates, that retired doctors and nurses as well as medical students in their last year of training could be hired by the national health service for six months to boost the health workforce during the emergency – the aim was to recruit about 20 000 additional staff1.
France has also decided to mobilise its “sanitary reserve” (“réserve sanitaire”) to increase temporarily the supply of health workers. The reserve includes health professionals (doctors, nurses, care assistants), non-care hospital workers, psychologists, professionals from regional health agencies, and others – about 3 800 people were on this reserve as of early March 2020. They can be public sector employees, private sector employees, freelance workers, retirees or paramedical and medical students.
In Korea, additional health care professionals were recruited to dispatch to Taegu where a cluster of infected cases was found, providing an early, targeted response to mitigate the crisis.
The United Kingdom is also trying to call “back to duty” retired doctors and nurses, though the number of volunteers at least initially appeared to be fairly low.
In the Netherlands; former and retired medical support staff, as well as medical students are volunteering for work in the hospitals. Also parts of the medical military personnel provide specialised assistance. In civil society food banks and the like, find new volunteers to distribute food packages to the elderly or do their grocery shopping.
Health workers are at major risk of COVID‑19 infection if they are not properly protected. Therefore United Kingdom changed its guidelines in early March to require less stringent protective equipment than previously in some circumstances, freeing more stringent protection to be reserved for cases where health workers are most at risk.
Italy, Spain, Netherlands, and parts of Canada have put in place measures to ensure that health care workers have priority access to child care centres that would still remain open under certain conditions. This is to ensure that health professionals continue to work, even as schools and child care centres have closed.
Following the closure of early childhood care facilities and schools in early March to contain the spread of COVID‑19, France organised a system exclusively for health care staff. Early childhood care facilities in hospitals benefit from an exception so as to remain open and accommodate children of health professionals, applying appropriate health safety measures. Exceptions to the national closure of early childhood facilities and schools are also provided for children of hospital staff. Local school and health authorities work together to identify and provide care for the children of parents who are mobilised to work in the health sector. In some countries like Canada and the United States, pharmacists have been allowed to extend prescriptions beyond what they were previously allowed to do and to prescribe certain medications to allow doctors to focus on the more important cases and minimise the number of medical consultations.
1. DECRETO-LEGGE 17 Marzo 2020, n. 18
Some of the lessons already learned from the current coronavirus crisis about how to prepare better for these shocks in the future include:
In those countries where there is a chronic shortage of doctors, nurses or other skilled health workers, any additional pressure on health care systems arising from an epidemic or any other public health emergency becomes almost unmanageable as people already overstretched are asked to do even more. Hence, health workforce planning also needs to consider the probabilities and feasibility of preparing for scenarios beyond current or expected annual peak demand. For example, specifications for defences against natural disasters such as floods and earthquakes consider less frequent, high impact events such as 10-year or 100-year highs.
Planning for a “reserve army” of health workers, which was introduced in several countries after previous epidemics, have proven to be very useful to provide additional support to the regular workforce and allows for a more flexible management of human resources across regions. The European Medical Corps could be a reference for this practice1.
Crisis situations like the coronavirus epidemic can provide opportunities to change the traditional roles of different health care providers and expand the roles of some providers like nurses and pharmacists, so that they can take on some of the tasks from doctors and thereby allow them to spend their time more effectively on the most complex cases.
One major concern in all countries affected has been to protect the health of health workers to avoid spreading the virus from health care providers to patients in hospital and elsewhere and also to ensure that doctors, nurses and other health workers will be able to continue to provide care. Strategic reserves of masks and other protective equipment may be considered to avoid exposing doctors and other health workers to high risk of infections.
Supplies: Boosting supplies of required equipment to diagnose and treat patients safely
Ensuring a sustained availability of the needed equipment to diagnose and treat patients is a major concern for health policy deciders during the ongoing outbreak. For the immediate response to the current wave of the pandemic, policy should focus on the availability of sufficient diagnostic tests and emergency supplies. This can be done through international cooperation in purchasing, to avoid excessive purchases and stockpiling in one place creating shortages in others. It also requires demand planning, monitoring supply chains and ensuring that sufficient funds are allocated procuring basic goods that are simple to produce but for which margins and manufacturing capacity may be low. Initiatives such as the EU joint procurement scheme for medical counter measures could be developed further and broader international cooperation is required.
Diagnostic testing continues to be a bottle neck in some countries. Tests currently available are based on the real-time RT-polymerase chain reaction (rRT-PCR) principle and require trained staff, testing kits with reagents that allow for identification of the SARS‑CoV‑2 and machines to process the tests. Following publication of relevant RNA sequences of the virus, test kits are now being developed by research labs, public health authorities and private firms2. New testing kits have allowed to decrease the cycle times for processing tests and producing results, increasing lab throughput. Policy needs to ensure that, as new and reliable testing methods receive regulatory approval, sufficient capacity is available to scale up testing quickly. This requires demand planning, ensuring that sufficient physical and human resources are available locally to conduct the tests, monitoring of supply chains and international coordination in procurement, to ensure that tests are available where needed most.
The total number of tests performed varies substantially from country to country: as of 20 March 2020, Korea had conducted more than 6 100 tests per million inhabitants3, over 45 times more than the testing rate in the United States4. This pattern can be explained by a mix of strategic, logistic, capacity, regulatory, and even cultural considerations. Korea, for instance, has decided from the very beginning of the outbreak to very strictly track most possible cases. Innovative solutions were developed, such as drive-through COVID‑19 testing centres, where samples are taken while people stay in their car (see Section on Spaces: Boosting spaces to diagnose people safely and efficiently, to isolate suspected and confirmed cases, and to treat patients in hospital or their home). However, the drive-through testing was one component of a broader logistical strategy that included a strong infrastructure for test kit production, distribution and laboratory analysis, building on the lessons of previous SARS‑CoV‑1 and MERS outbreaks5. The United States experienced early problems with faulty tests and regulatory approvals, which delayed large availability of supplies for testing6. In Italy and France, on the other hand, authorities decided to limit testing to patients in serious conditions as the virus was spreading in those countries, to ensure they were provided with the right care while staying within health systems capacity. In Japan, tests were limited in the beginning given that health care resources to cope with the increased number of patients and the capacity to test were limited and tests were given mainly to diagnose those with serious conditions, but the test has become available more these days as the health system is organised better to deal with the increasing health care needs.
Other goods needed to tackle the epidemic include protective equipment such as masks, face shields, and hand sanitisers. The President of the European Commission, Ursula von der Leyen, announced on 10 March that “The European Commission is now taking stock of the available protective equipment and respiratory devices as well as their production and distribution capacity.” Countries have taken legal action to prevent smuggling of needed equipment so stocks can remain available for health professionals. The government of France set controls on the prices of hand sanitisers and has requisitioned all stocks of face masks and production in the coming months. Japan banned reselling of masks to assure their availability in health and long-term care facilities and provides subsidy to companies in order to produce more masks.
Availability of key devices such as ventilators for treating patients with serious respiratory symptoms has acquired great relevance. The Italian government will purchase 1 800 high intensity ventilators and 3 200 turbine-based ventilators to double the capacity of intensive and sub-intensive care units. In the United Kingdom, industrial consortia, including firms not traditionally involved in manufacturing of medical devices such as aerospace and automobile producers, have announced an intensive effort to produce new medical ventilator to meet the surge demand7.
Finally, availability of essential medicines needs to be closely monitored by each government. Even if the treatment of infected patients does not really require medications (even for most severe patients as these actually only need respiratory assistance and nursing care), the barriers to movements and trade may impact the availability of medicines in the medium term. Indeed, the production of Active Pharmaceutical Ingredients (APIs, i.e. the chemical raw materials required to produce effective medicines) is heavily concentrated in certain regions, e.g. China and, to a lesser extent, in India. The lock-down of the Wuhan province may have some impact on the global supply of medicines at some point (even if national regulatory authorities have not reported specific signals of this so far). Even closer monitoring of shortage notifications is needed so as to ensure that health professionals and policy makers are aware of the situation and can adjust their decisions and strategy accordingly. This crisis may also be an opportunity for governments to reconsider their dependence on certain countries for their supply in medicines and decide to make national/regional production of medicines more sustainable. Some governments have also taken measures to avoid shortages resulting from panic purchasing. In France, purchase of paracetamol has been limited to 1 pack per person (2 packs in case of symptoms) to avoid irrational stockpiling.
Spaces: Boosting spaces to diagnose people safely and efficiently, to isolate suspected and confirmed cases, and to treat patients in hospital or their home
The experience in China and Italy has highlighted the critical need to ensure adequate capacity of hospital beds in general and intensive care beds more specifically to address a surge of seriously ill patients from an infectious disease. The number of acute care beds in hospital provides a general indication of the capacity of hospitals to deliver acute care to patients. However, acute care is a broad category that typically encompasses units that provide not only intensive care, but also surgical and medical specialties, gynaecological and obstetrical services, and some psychiatric care. While some of the beds and other resources in these other hospital units may be temporarily converted into flexible intensive care units, a key point, especially for COVID‑19 treatment, is that intensive care beds need to be equipped with respiratory equipment.
Japan and Korea have the highest number of acute care hospital beds per capita, with over 7 beds per 1 000 people in 2017 (see Figure 5). Germany comes third with 6 beds per 1 000 people. Most OECD countries have between 2.5 and 5 acute care hospital beds per 1 000 people, but the numbers are lower in Mexico, Canada, Chile, Sweden, Israel, Spain and the United States, with less than 2.5 hospital beds per 1 000 people in 2017.
Figure 5. Acute care hospital beds in OECD countries, 2017 (or nearest year)
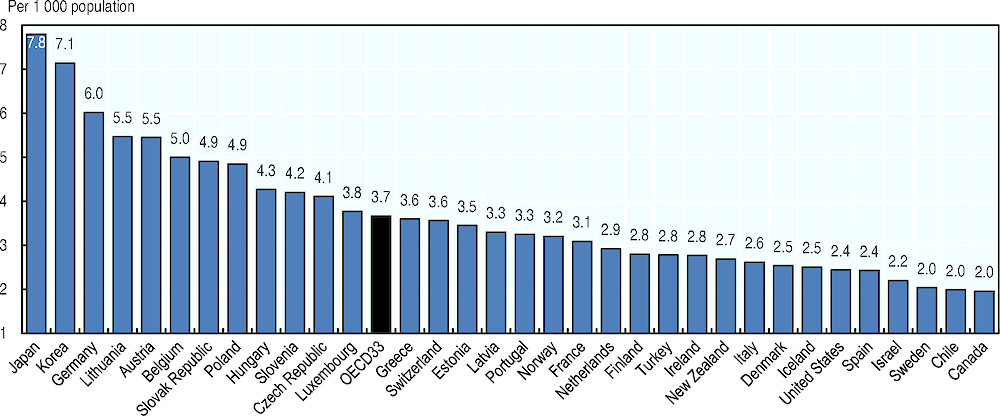
Note: Acute care beds include not only beds in intensive care units, but also beds in acute care units (e.g. all surgical units, all gynaecological and obstetric services, as well as acute psychiatric care beds in about half the countries). France, Japan and Latvia exclude psychiatric care beds.
Source: OECD Health Statistics 2019, https://doi.org/10.1787/health-data-en.
In addition to the number of hospital beds, occupancy rates give an indication of the normal activities of hospitals and the degree of spare capacity to deal with public health emergencies. High occupancy rates of acute care beds are symptomatic of a health system under pressure that has very limited capacity to handle an unexpected surge of patients requiring immediate hospitalisation. In many countries, a low supply of acute care hospital beds has been associated with a high occupancy rate during normal times, and this is the case in countries like Ireland, Israel, Canada and the United Kingdom (Figure 6). But some countries that have a relatively low number of acute care hospital beds also have relatively low occupancy rates, reflecting that there are some spare capacity given the level of usual activities. The United States is an example with an occupancy rate of only about 65% in 2017 (compared with an OECD average of 75%) despite the fact that the number of acute care hospital beds per population is substantially lower than the OECD average. However, there may be wide variations in bed occupancy rates across hospitals in each country and also over the year, so the occupancy rates can come to 100% in some hospitals during some peak seasons, with very little capacity to respond to crisis situations.
Figure 6. Occupancy rate of acute care beds in OECD countries, 2000 and 2017 (or nearest year)
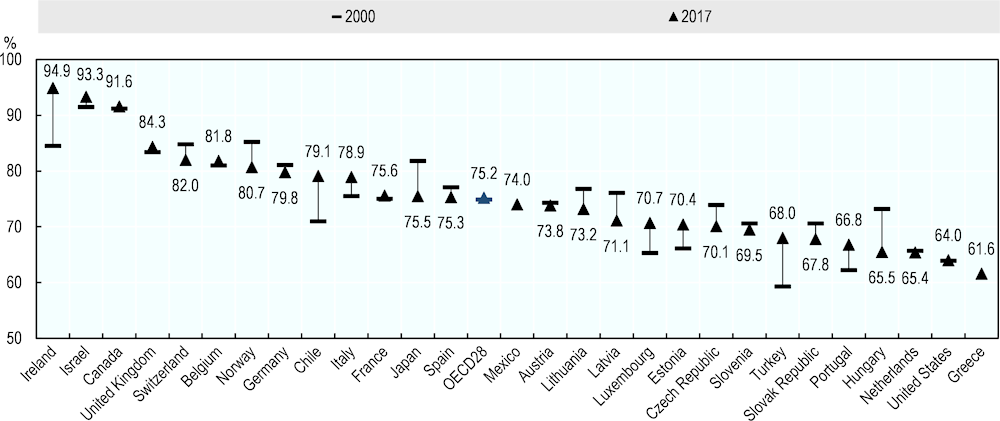
Note: The occupancy rate is calculated as the number of beds effectively occupied (bed-days) for acute care (HC.1 in SHA classification) divided by the number of beds available for acute care multiplied by 365 days, with the ratio multiplied by 100. France, Japan and Latvia exclude psychiatric care beds.
Source: OECD Health Statistics 2019, https://doi.org/10.1787/health-data-en.
Given the characteristics of the treatment required for the most severe COVID‑19 patients, the most important bottlenecks in hospital capacity are taking place in intensive care beds. While the availability of internationally comparable data is more limited, a preliminary analysis of most recent publicly available data suggests that, across ten OECD countries, the variation in capacity is ten-fold, ranging from a high of 33.9 critical beds per 100 000 population in Germany to a low of 3.3 beds per 100 000 in Mexico (Figure 7). These figures are somewhat similar to a previous analysis that used 2009 data (Rhodes et al., 2012[3]).
Given the characteristics of the treatment required for the most severe COVID 19 patients, the most important bottlenecks in hospital capacity are taking place in intensive care beds
Figure 7. Capacity of intensive care beds in selected OECD countries, 2020 (or nearest year)
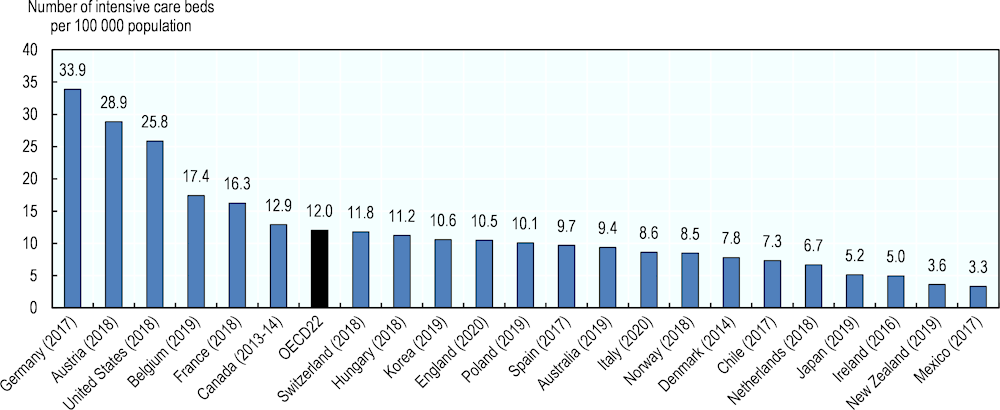
Note: There may be differences in the notion of intensive care affecting the comparability of the data. Data refers to adults only in Belgium, Ireland and Canada, to all ages in Germany, England and Spain. Data in France includes "lits de réanimation adulte" (except severe burns) and "lits de soins intensifs" (except neonatology) but excludes "lits de surveillance continue adulte et enfants" and "lits de réanimation enfants".
Source: German Federal Statistical Office, Austrian Ministry of Health, USA: Tsai, Jacobsen and Jha (2020), Belgian Ministry of Health, French Ministry of Health, Canadian Institute for Health Information, Hungarian National Health Insurance Fund, Korea: Phua, Faruq, Kulkarni et al. (2020), NHS England, Polish Ministry of Health, Spanish Ministry of Health, Australia: Edward Litton et al. (2020), Italy: Remuzzi and Remuzzi (2020), Norwegian Health Ministry, Danish Society of Anesthesiology and Intensive Medicine, Chilean Society of Internal Medicine (2020), Dutch Intensive Care Society, Japanese Society of Intensive Care Medicine, Irish Department of Health, New Zealand Ministry of Health, Mexican Ministry of Health.
In the current crisis, some responses taken by governments are around how to rapidly increase or optimise the use of existing capacity, and how to reduce the need for using of emergency rooms or hospital facilities), while other measures have been taken to minimise the need for using emergency rooms or hospitals in general (see Box 3). Also, the ECDC has put together a checklist to help hospitals prepare to receive and care for coronavirus patients (Box 4).
Box 3. Measures implemented to boost and optimise space in healthcare facilities in selected OECD countries
Korea pioneered the approach of putting in place drive-through centres together with a network of 96 public and private laboratories. More than 50 drive-through centres were put in place to boost the capacity to quickly identify cases with around 20 000 tests conducted every day. Drive-through testing has since been emulated elsewhere, including in several provinces in Canada (e.g. British Columbia, Alberta and Ontario), in several States in the United States (e.g. Connecticut, Colorado and Washington state), Australia, the United Kingdom, Belgium, and Germany.
In France, the intensive care capacity in the Eastern part of the country is overstretched and the authorities decided to set-up a military camp ICU centre to provide more bed capacity. The army will also be asked to transfer patients from regions not able to treat patients to those having less activity, so to spread to burden more evenly across the entire country. In Japan, some hospitals which were scheduled to open later in the year and hospitals which closed recently, are used to treat patients.
Italy and other countries are actively reorganising the supply of hospital beds, dedicating entire “aseptic” wards and creating new flexible intensive care units for patients infected with COVID‑19, while delaying non-urgent (elective) care. France has repurposed army camp hospitals for the same reason.
In Germany, the government has promised financial bonuses to hospitals that are able to increase and maintain intensive care beds. Furthermore, hospitals with capacity constraints need to know to which hospitals they can transfer patients. To this aim the Robert Koch Institute (RKI), the German Hospital Association (DKG) and the German Association of Intensive and Emergency Care (DIVI) have set up a website on March 17, where each hospital is asked to update dally their available capacity for intensive care with respiratory support. On a regional level this platform is expected to assist doctors in quickly identifying alternative places for treatment.
In the United States, measures have been adopted to encourage home hospitalisation with distance monitoring for patients who are medically stable and can receive care at home, or patients who received a hospital discharge following a hospitalisation with confirmed COVID‑19.
Box 4. ECDC checklist for hospitals preparing to care for coronavirus patients
The European Centre for Disease Control and Prevention (ECDC) has prepared in February 2020 a checklist to assess hospitals preparedness for the management of COVID‑19 patients, with a plan to update this checklist should new relevant information become available.
Elements to be assessed include:
the establishment of a core team to manage the event, including a member of the hospital management, the hospital infection control team, an infectious disease expert, and representatives from the intensive care unit (ICU) and emergency department, as well as key internal and external contact points
human, material and facility capacity (what is described here as staff, supplies and space capacity, including an assessment of the surge capacity of healthcare workers for triage, emergency department, ICU, laboratory, and the units where patients will be placed, and a calculation of the maximum facility capacity in terms of the maximum number of ICU beds and tors along with the required staff and supply capacity)
hand hygiene, personal protective equipment, and waste management, including adequate supplies of alcohol-based hand sanitisers for staff and patients and masks and other equipment to protect against contact and droplet
triage, first contact and prioritisation, including the establishment of procedures to separate suspected cases from other patients and isolation, and patient prioritisation (e.g. discharge criteria, criteria to postpone elective hospitalisations or interventions)
patient placement and visitor access, including an assessment of the capacity of isolation beds and ICU beds, and putting in place rules for access of visitors to the facility.
Lessons from the current crisis shows that the ability to create surge capacity in the three fronts – staff, supplies, and space – is a key characteristic of resilient health systems. In the long run, having excess idle capacity would be a diversion of much needed health systems, which were already experiencing constraints given the growing burden of non-communicable diseases, population ageing, increased citizen expectations, and costs associated with technological development. But the COVID‑19 crisis demonstrates the need for flexibility and adaptability in the use of existing resources, as well as planning for responding to surge in demand.
Leveraging digital data and tools to improve surveillance and care
The significance of reliable up-to-date information in dealing with disease outbreaks cannot be overstated. Digital technologies and interconnection are creating new opportunities to collect, combine, curate, analyse, present and use data to inform decisions before, during and after an outbreak. The digital transformation is giving countries new avenues to better detect, prevent, respond to, and recover from COVID‑19. At the same time, countries should manage the risks of rapid digitalisation, including diversion of resources to potentially ineffective digital tools, exacerbation of inequalities, and violation of privacy, both during and after the outbreak.
The digital transformation is giving countries new avenues to better detect, prevent, respond to, and recover from COVID‑19, but risks such as the violation of privacy should also be addressed
Detect: Use routine and big data for early warning and surveillance as well as digital diagnosis
Beyond notifying laboratory-confirmed cases to early warning and response systems, countries with standardised national electronic health records (EHRs), that produce high quality data, can extract routine data from those systems for real-time surveillance. Only eight OECD countries8 and Singapore have high technical and operational readiness to generate information from EHRs 9, but restrictions on using these data for surveillance or clinical trials might need to be lifted (Oderkirk, 2017[4]).
Some countries have leveraged national data to support controlling the outbreak (Wang, Ng and Brook, 2020[5]). This works by using real-time health data from existing insurance coverage databases, linked to other data such as customs and immigration data. During clinical visits, when health care providers scan patients’ health insurance cards, an alert can be issued based on patients’ travel history and clinical symptoms. This data can be analysed to identify and test patients for COVID‑19 who had severe respiratory symptoms.
Big data outside of the health system – from social media and web searches, to environment and satellites – can also help. The Canadian firm BlueDot, which uses machine learning to search global media for information on various infectious diseases, was one of the first to spot cases of COVID‑1910. It was not the only one though, with Boston service HealthMap and American firm Metabiota also catching early signs11. This type of big data insights could be especially useful when linked to traditional health system data; for example, the United States’ Centers for Disease Control and Prevention (CDC) are working with academic researchers to feed machine learning algorithms with administrative data from CDC plus Google searches and Twitter activity to predict the number of infections in real-time12. At least another eight OECD countries13 regularly link key national health datasets from across the health and health care sectors for monitoring and research.14
Smartphones and mobile data have been leveraged for detection and control in Korea and Israel. In Korea, when a person tests positive for the virus and the CDC cannot identify all of the people they may have been in contact with, then detailed information regarding their movements is sent by text message to residents living nearby15. There are also a number of privately run services which track confirmed infected patients on the map. Similarly, Israel is using mobile data to locate people who have been in contact with patients who test positive and to send them a text message that they are legally required to quarantine themselves for 14 days16. Their mobile data is monitored to ensure they remain at home. In some countries, in-bound travellers are provided with electronic tools (e.g. a QR code to scan to an on-line form about their flight origin and travel history over the past 14 days) to make collection of data about their travel history in digital format easier (Wang, Ng and Brook, 2020[5]). High-risk patients are quarantined.
While these approaches help with efforts to contain the spread of the virus, they can raise issue about the right to privacy and personal freedoms. In all of these countries, the tracking of movements is a privacy intrusion and it is crucial to ensure that such intrusions are both necessary and time limited so that the fundamental rights of people in democratic societies are not eroded.
Digital technologies are also emerging to increase the speed and volume of testing of suspected cases. AI models developed in China learn from CT scans to support faster interpretation of images17. Portable “lab on a chip” testing kits for COVID‑19 process samples more quickly than traditional laboratory methods and have received provisional authorisation for use in Singapore health settings18.
It is crucial to strike a balance between making these new digitally enabled diagnostics widely available, especially in low-resource contexts, while continuing to monitor and evaluate their accuracy, robustness and validity in real-world settings.
Prevent: Take advantage of digital technologies to advise the public and limit physical contacts
Telehealth has many potential benefits in the context of COVID‑19, as people with mild symptoms can consult from their homes – avoiding potentially infecting others, including much needed health workers, or even themselves if they do not have the virus – and reserving physical capacity in health care units for critical cases and people with serious health conditions unrelated to the outbreak. Telehealth – the use of information and communication technologies to promote health at a distance, including non-clinical services and education – has been used in previous disease outbreaks like Ebola and Zika19, and supplies a set of tools and applications to prevent spread, including not only videoconsultations with health professionals but also automated chatbots that can both enquire about symptoms and give up-to-date advice20,21. It is essential that chatbots be updated often though, as outbreaks are extremely fluid situations (e.g. new travel alerts and treatment guidelines being issued repeatedly).
While the use of telemedicine in the OECD is currently low (Oliveira Hashiguchi, 2020[6]) , France, England, Japan and the United States are relaxing regulatory barriers (see Figure 8), and senior government officials and health care leaders are actively urging its use in the current context. For example, restrictions on reimbursement have been lifted in France and the United States so that patients can now consult remotely with any doctor that uses telemedicine, whether or not they have consulted that doctor face-to-face in the past, and the United States Department of Health and Human Services has now waived certain requirements for use of telemedicine under Medicare22. Yet, some barriers to wider use, like access to broadband, will be difficult to tackle in the short-term, highlighting the need to strengthen health care provision in rural and low-resource settings.
Figure 8. Selected barriers and enablers of telemedicine use highlighted by experts, by number of reporting countries
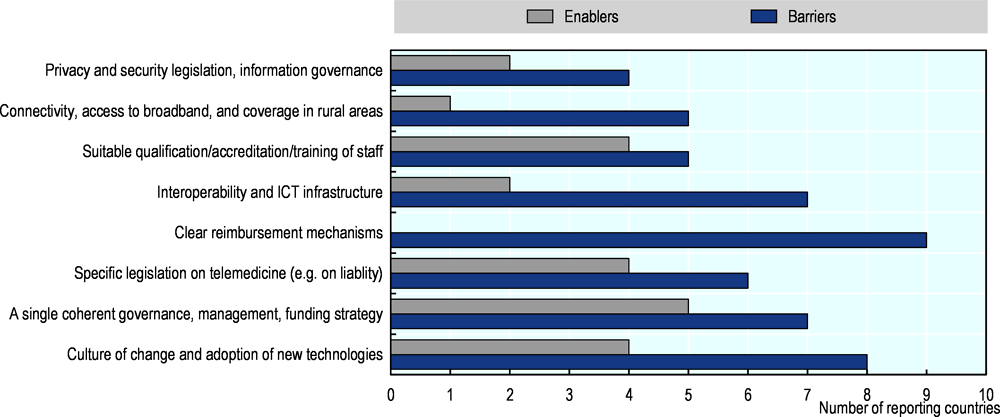
Source: Oliveira Hashiguchi (2020[6]), “Bringing health care to the patient: An overview of the use of telemedicine in OECD countries”, http://dx.doi.org/10.1787/8e56ede7-en.
Respond: Monitor people who have been diagnosed
Telehealth has been used to monitor the health and wellbeing of people who have been diagnosed with COVID‑19, both less severe patients who are able to stay at home and more critical cases who need to be hospitalised. With tele-monitoring already being used to follow mostly chronic patients in at least 14 OECD countries (Oliveira Hashiguchi, 2020[6]), Korea23, Israel24 and Hong Kong China25 are using wearables and communication technologies to remotely monitor patients with COVID‑19 at home, catching signs of possible deterioration, and adding to health researchers’ understanding of how the disease develops. In China26, Israel27 and the United States28, hospital physicians are using robots to reduce physical interactions to essentials. Robots can also help with food delivery and even sterilising rooms29.
Recover: Learn from this crisis and build resilience for the next outbreak
When the crisis abates, countries should draw lessons from COVID‑19 to prepare for future outbreaks, as Korea did following the SARS epidemic of 2003 (Wang, Ng and Brook, 2020[5]). Global health emergencies illuminate the importance of coherent, comparable and timely data across borders, within and between countries. The health system is woefully behind other sectors in developing a harmonised approach to data governance and global standards for health data terminology and exchange (OECD, 2019[7]). In many countries, the consequence is that when data sharing and linkage are most needed, data are trapped in silos, difficult to exchange in their entirety and shared with significant delays. This is particularly pertinent to decentralised federated health systems, in which subnational areas have developed their own health information infrastructure and governance, typically not in alignment with other regions, and thus incapable of informing a unified response (Carinci, 2020[8]).
Health systems must be strengthened to become capable of providing national and global data that are useable and available in near real-time for surveillance and emergency response, across national and regional borders. Health data governance frameworks are also needed to safeguard privacy, including having systems for secure data exchange, automatic data extraction from clinical records, and secure data access mechanisms for research. As with any problem of the commons, it is unlikely that a few countries alone can tackle this issue, multilateral action is essential, for example to track spread across borders, share information on containment and treatment interventions that work, and make sure international supply chains of medical supplies keep going.
Implementing effective research and development policy for vaccines and treatments
An impressive effort to develop diagnostics, vaccines and treatments is underway since the outbreak of COVID‑19. The novel coronavirus pathogen sequence was shared as early as 11/12 January 2020. Researchers, health tech and pharmaceutical companies have come together. Collaborations involving private companies and the public sector have been announced, many of which build on existing networks such as CEPI (Coalition for Epidemic Preparedness Innovations) and Europe’s IMI (Innovative Medicines Initiative). There are several new clinical trials registered since 1 January 2020, some taking place already and some in the planning phase, of which most are on medicines. There is hope that development of a vaccine and treatment will be quicker than usual, because of prior work on SARS, MERS.
While the scientific effort is impressive, vaccines are unlikely to become available in time to respond to the current wave of the pandemic. Disposables, material for intensive care units are urgent in the short term (see above), as are rapid diagnostic tests, as well as developing evidence on the efficacy of existing medicines that could be used to treat the disease. R&D efforts, boosted by the current pandemic, need also to be sustained over time and products (treatments, tests, eventually vaccines) that are developed need to be made available where they are needed most.
R&D funding and activity has surged since the outbreak of COVID‑19
Similar to prior epidemics or pandemics, commitments to fund R&D have surged since the outbreak of COVID‑19. Based on announcements on Friday 13 March, various OECD governments and non-governmental entities have pledged at least USD 830 million in funding.30 This includes USD 100 million for vaccines pledged by the Coalition for Epidemic Preparedness Innovations (CEPI); USD 125 million for treatments by the Gates Foundation, Wellcome Trust and Mastercard COVID‑19 Therapeutics Accelerator; and more than USD 205 million by the European Union31 for both, vaccines and treatments, and various commitments by governments of OECD countries. This amount does not take into account all investments by the industry nor the amounts pledged by governments of non-OECD countries.
As a result, R&D activities related to SARS‑CoV‑2 and other coronaviruses that cause respiratory diseases have increased significantly since the beginning of 2020. To support rapid research advances, the genome sequence of the new coronavirus was released to the public by scientists in China in January 2020, shortly after the disease outbreak. Between 1 January and mid-March 2020, 361 clinical trials on coronaviruses were registered, compared to 14 between 2007 and 2019 (Figure 9).
Figure 9. Number of newly registered clinical trials related to corona viruses
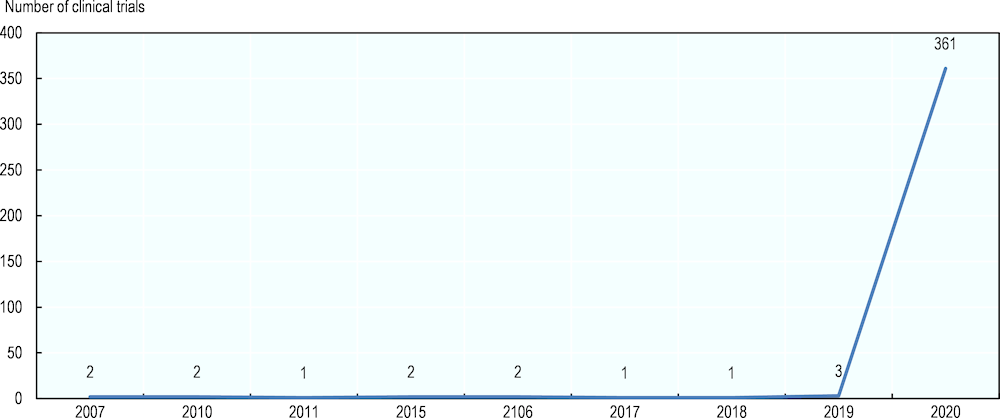
Note: 83% of the 361 trials in 2020 were registered in China.
Source: OECD analysis using the WHO International Clinical Trials Registry Platform (ICTRP) database (2020).
Progress is being made on various fronts
Trials are now underway on diagnostics, treatments and vaccines.
Diagnostic tests can be developed relatively quickly but there can be bottlenecks in capacity for manufacturing, distributing and administering them in sufficient quantities. Diagnostic tests are subject to 23 clinical trials registered in the ICTRP. They include technologies that accelerate the extraction of samples from patients and their processing, using current real-time RT-polymerase chain reaction (rRT-PCR) tests, as well as second-generation methods that could potentially lead to rapid tests that do not require processing at a lab and,32 therefore, increase test rates significantly. However, non-PCR testing methods are currently not yet sufficiently reliable. By 13 March 2020, the US FDA had granted Emergency Use Authorization (EUA) for four new diagnostic tests based on RT-PCR.33
One avenue of identifying treatments quickly is to test the efficacy in treating COVID‑19 of medicines currently used for other diseases. This means that developers can rely on early-phase trials that were already conducted previously to test dosage and safety, focusing more quickly on clinical trials assessing efficacy. Treatments represent the majority of clinical trials registered in the WHO International Clinical Trials Registry Platform (ICTRP) (225). They include trials of arbidol – an antiviral treatment for influenza infection, lopinavir and ritonavir – a fixed dose combination for the treatment and prevention of HIV/AIDS and tocilizumab – an immunosuppressive drug, currently mainly used for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children.
For vaccines, prior advances in R&D on previous emerging coronaviruses form the basis of current development. SARS‑CoV‑2 is 80% similar to SARS‑CoV‑1, the coronavirus that caused the 2003 SARS pandemic. A number of promising vaccine candidates for coronaviruses are already available (Chen et al., 2020[9]). Seven trials of vaccines against coronaviruses are now registered in the ICTRP and three are currently recruiting individuals.
Policy should prioritise the most urgent needs for pandemic response while sustaining R&D efforts in the longer term
Despite these impressive efforts, vaccines and treatments are unlikely to be available in time to contain the current wave of the pandemic or help treating patients, even if they are developed quicker than usual. Repurposing medicines already approved, re-starting development of compounds that have been shelved, more efficient clinical trial design, and fast-track approval can certainly accelerate development of vaccines and treatments for COVID‑19. There is a strong need for policy action to encourage and sustain R&D effort in the development of diagnostic tests, treatments and vaccines, in at least the following areas.
First, there is a need to allocate more resources for R&D, and improve R&D by using new approaches
Policy responses to the pandemic should not assume that new products will be available quickly, and more resources may be necessary. As mentioned, lot of money has already been put into efforts to develop vaccines and new treatments, including efforts by CEPI, IMI, and other initiatives, and further funding stages may be necessary.
Beyond funding commitments, as health systems race to find treatments, there are opportunities to leverage new approached, including through Artificial Intelligences, to accelerate and improve the effectiveness of R&D efforts. A machine learning model developed in London has discovered that a drug used in rheumatoid arthritis may be effective against the virus, and a Hong Kong-China-based company reported that its artificial intelligence (AI) algorithms have designed six new molecules that could halt viral replication (McCall, 2020[10]). While promising, there is a long way to go from computer models to human trials and market approval (see next section). This is uncharted territory and it is yet uncertain whether AI will help deliver a vaccine for COVID‑19, or whether it will divert resources needed for frontline care (e.g. ventilators and masks) with potentially high opportunity costs (Freedman, 2019[11]).
Second, there is a need to accelerate approval
Governments can prepare now to make new products available quickly where needed once development is complete, and agree on using available fast-track procedures to clear any vaccine or treatment. Fast-track regulatory and emergency approval pathways can be prepared to clear new diagnostic tests and treatments. Regulatory agencies should also agree internationally that they will co-ordinate their efforts to ensure that evidence used for approval in one jurisdiction is sufficient for others, rather than applying different standards.
Third, public funding in exchange for commitments to make vaccines and treatment widely available and accessible once approved is needed
Governments should allocate public funding to build capacity to produce vaccines and treatments before regulatory approval, in exchange for commitments to make products widely available and accessible at moderate prices once approved.
Governments should allocate public funding to build capacity to produce vaccines and treatments before regulatory approval, in exchange for commitments to make products widely available and accessible at moderate prices once approved
There is a need to avoid a situation where investment in plant to produce large quantities of a medicine or vaccine is delayed until regulatory approval. Public funding can help encourage production capacity and scaling up production, so as to reduce and delay between regulatory approval and the time a treatment or vaccines is produced.
Fourth, there is a need for countries to avoid ‘me first’ behaviour to get access to new drugs
Some countries may be tempted to engage in export restrictions to protect domestic supply. Some evidence points to some such restriction already been implemented in some countries on export of pharmaceuticals, such as in India. There is also a temptation to impose limits to the export of protective medical equipment, which are in very short supply in some countries, for example in Europe. As a drug or vaccine is developed, there is a need to avoid international competition to access the first lots of the vaccines or treatment, before others. An international commitment to avoid such policies in the COVID‑19 period would be highly desirable to ensure that any effective vaccine or treatments is first directed where need is the highest and where it can have most impact.
Fifth, there is a need to finish the development process to prepare for future crises.
Spikes in funding cannot replace sustained investment to prepare for future outbreaks. Recent industry announcements claim that it will take at least 12-18 months to test a new vaccine, which may be optimistic. Development is risky and expensive. Most importantly, it takes time. Estimates of the probability of approval of vaccines that enter phase 1 clinical trials range from 12 to 33%, after some 7 to 9 years of testing (Pronker et al., 2013[12]; Wong, Siah and Lo, 2019[13]; Sertkaya et al., 2014[14]). A typical successful drug candidate for infectious diseases undergoes approximately 7 years of testing, of which 2 to 3 years are needed for phase 3 trials of efficacy alone, and only 1 in 4 candidates that enters phase 1 is approved (Wong, Siah and Lo, 2019[13]). These estimates do not include time needed for pre-clinical R&D. Without accounting for the opportunity cost of capital, an estimated USD 700 million is needed to develop a single new medicine (Wouters, McKee and Luyten, 2020[15]) and estimates for vaccines are in the same order of magnitude.
While a vaccine to prevent COVID‑19 and new medicines to treat the disease may not be available in time to respond to the first wave of the current pandemic, products developed in this effort can help contain future outbreaks. Should SARS‑CoV‑2 disappear or become a seasonal infection, there will still be a need for continued efforts to develop vaccines in the future. More than 15 years hence since the 2003 SARS pandemic, no effective vaccine or treatment is available. While it has been known that coronaviruses present in animals pose a risk for humans (Menachery et al., 2015[16]), researchers could not attract funding for clinical testing in the years between the 2003 and the current pandemic.34 Had development of a vaccine for the SARS‑CoV‑1 been completed at the time, development of a vaccine for the current outbreak could have been much faster. New incentive mechanism, such as global innovation funds, market entry rewards and advance purchase commitments, may need to be considered to correct this failure.
References
[8] Carinci, F. (2020), “Covid-19: preparedness, decentralisation, and the hunt for patient zero”, BMJ, p. bmj.m799, http://dx.doi.org/10.1136/bmj.m799.
[9] Chen, W. et al. (2020), “The SARS-CoV-2 Vaccine Pipeline: an Overview”, Current Tropical Medicine Reports, http://dx.doi.org/10.1007/s40475-020-00201-6.
[2] European Centre for Disease Prevention and Control (ECDC) Public Health Emergency Team, P. et al. (2020), “Rapidly increasing cumulative incidence of coronavirus disease (COVID-19) in the European Union/European Economic Area and the United Kingdom. 1 January to 15 March 2020.”, Euro Surveill, Vol. 25/11, https://doi.org/10.2807/1560-7917.ES.2020.25.11.2000285.
[11] Freedman, D. (2019), “Hunting for New Drugs with AI The pharmaceutical industry is in a drug-discovery slump. How much can AI help?”, Nature, Vol. 576, pp. S50-S53.
[10] McCall, B. (2020), “COVID-19 and artificial intelligence: protecting health-care workers and curbing the spread”, The Lancet Digital Health, http://dx.doi.org/10.1016/S2589-7500(20)30054-6.
[16] Menachery, V. et al. (2015), “A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence”, Nature Medicine, Vol. 21/12, pp. 1508-1513, http://dx.doi.org/10.1038/nm.3985.
[4] Oderkirk, J. (2017), “Readiness of electronic health record systems to contribute to national health information and research”, OECD Health Working Papers, No. 99, OECD Publishing, Paris, https://doi.org/10.1787/9e296bf3-en.
[1] OECD (2020), Containment and mitigation policy actions are key to fight the COVID-19 pandemic, OECD, Paris.
[7] OECD (2019), Health in the 21st Century: Putting Data to Work for Stronger Health Systems, OECD Publishing, Paris, https://doi.org/10.1787/e3b23f8e-en.
[6] Oliveira Hashiguchi, T. (2020), “Bringing health care to the patient: An overview of the use of telemedicine in OECD countries”, OECD Health Working Papers, No. 116, OECD Publishing, Paris, http://dx.doi.org/10.1787/8e56ede7-en.
[12] Pronker, E. et al. (2013), “Risk in Vaccine Research and Development Quantified”, PLoS ONE, Vol. 8/3, http://dx.doi.org/10.1371/journal.pone.0057755.
[3] Rhodes, A. et al. (2012), “The variability of critical care bed numbers in Europe”, Intensive Care Medicine, Vol. 38/10, pp. 1647-1653, http://dx.doi.org/10.1007/s00134-012-2627-8.
[14] Sertkaya, A. et al. (2014), “Analytical Framework for Examining the Value of Antibacterial Products”, Public Law Research Paper, No. 14-25, Boston University School of Law, Boston, http://ssrn.com/abstract=2641820www.erg.comhttps://ssrn.com/abstract=2641820.
[5] Wang, C., C. Ng and R. Brook (2020), “Response to COVID-19 in Taiwan”, JAMA, http://dx.doi.org/10.1001/jama.2020.3151.
[13] Wong, C., K. Siah and A. Lo (2019), “Estimation of clinical trial success rates and related parameters”, Biostatistics, Vol. 20/2, pp. 273-286, http://dx.doi.org/10.1093/biostatistics/kxx069.
[15] Wouters, O., M. McKee and J. Luyten (2020), “Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018”, JAMA, Vol. 323/9, pp. 844-853, https://jamanetwork.com/journals/jama/article-abstract/2762311.
Contact
Stefano SCARPETTA (✉ stefano.scarpetta@oecd.org)
Mark PEARSON (✉ mark.pearson@oecd.org)
Francesca COLOMBO (✉ francesca.colombo@oecd.org)
Frederico GUANAIS (✉ frederico.guanais@oecd.org)
Notes
← 3. Calculated with data from https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030.
← 4. Calculated with data from https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/testing-in-us.html.
← 5. https://www.wsj.com/articles/inside-the-south-korean-labs-churning-out-coronavirus-tests-11584610667.
← 8. Finland, Estonia, Singapore, Israel, Denmark, Austria, Canada, Slovakia and the United Kingdom.
← 9. Technical and operational readiness is based on nine indicators: electronic medical record coverage, information sharing among physicians and hospitals, defined minimum dataset, use of structured data, unique record identification, national standardisation of terminology and electronic messaging, legal requirements for adoption, software vendor certification, and incentives for adoption.
← 10. https://www.forbes.com/sites/tomtaulli/2020/02/02/coronavirus-can-ai-artificial-intelligence-make-a-difference/amp/.
← 11. https://www.technologyreview.com/s/615351/ai-could-help-with-the-next-pandemicbut-not-with-this-one/.
← 13. Denmark, Sweden, Finland, Norway, Netherlands, Korea, Israel, and Czech Republic.
← 14. Preliminary data from the OECD 2019-20 Survey of Health Data Use and Governance.
← 15. https://www.nature.com/articles/d41586-020-00740-y?error=cookies_not_supported&code=ce5e43cc-43af-4313-b49a-c99273cb871f.
← 16. https://techcrunch.com/2020/03/18/israel-passes-emergency-law-to-use-mobile-data-for-covid-19-contact-tracing/2020/03/18/israel-passes-emergency-law-to-use-mobile-data-for-covid-19-contact-tracing/.
← 18. https://www.mobihealthnews.com/news/asia-pacific/veredus-laboratories-verecov-detection-kit-obtains-provisional-approval-ivd-use.
← 19. https://mhealthintelligence.com/features/using-telehealth-technology-for-care-coordination-during-a-disaster.
← 20. https://mhealthintelligence.com/news/coronavirus-scare-gives-telehealth-an-opening-to-redefine-healthcare.
← 21. https://www.statnews.com/2020/02/05/chatbots-screening-for-new-coronavirus-are-turning-up-flu/.
← 22. https://www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html.
← 25. https://www.fiercehealthcare.com/tech/boston-startup-using-ai-remote-monitoring-to-fight-coronavirus.
← 26. https://www.newscientist.com/article/2236777-coronavirus-hospital-ward-staffed-entirely-by-robots-opens-in-china/.
← 28. https://www.vox.com/recode/2020/2/27/21156358/surveillance-tech-coronavirus-china-facial-recognition.
← 29. https://healthtechmagazine.net/article/2020/02/5-ways-healthcare-tech-helping-tackle-coronavirus.
← 30. Based on recent public announcements.
← 31. Including through the public/private Innovative Medicines Initiative (IMI).