This publication on endocrine disruption is part of a series on policy responses to contaminants of emerging concern (CECs) in freshwater. Previous work focused on pharmaceutical residues and microplastics. Building on these earlier publications, this publication focuses on endocrine disrupting chemicals (EDCs) in freshwater. This publication takes a different approach to water quality regulation: it explores the opportunity to complement a substance-by-substance approach of chemicals management with an effect-based approach, centred around the negative effects of EDCs on humans and wildlife. This chapter characterises the challenge of endocrine disruption in freshwater. It provides a typology of EDCs and their effects on human health, ecology and the economy. It also examines the sources, pathways and sinks of endocrine disrupting chemicals in freshwater. Lastly, this chapter provides an outlook of driversthat increase the future release of endocrine disrupting chemicals in freshwater.
Endocrine Disrupting Chemicals in Freshwater

1. The challenge of endocrine disruptors in freshwater
Abstract
1.1. Introduction
Worldwide, the production capacity for the chemical industry, plastics and pesticides has dramatically increased since 2000 (Figure 1.1: "Global production capacity"). Some of these chemicals, plastics and pesticides have properties that could have a negative effect on human and wildlife. One such property is their ability to alter function(s) of the endocrine system of organisms. These compounds are called endocrine disrupting chemicals (EDCs). A modification of function(s) of the endocrine system in the body can lead to adverse health effects, some of which may not manifest until many years after exposure. EDCs are associated with disease outcomes such as obesity, fertility loss, hormone-sensitive cancers, thyroid malfunctions and neurodevelopment impacts (Gore et al., 2015[1]). In wildlife, similar effects can occur. Moreover, in wildlife, endocrine disruptors can negatively affect populations - potentially contributing to biodiversity loss and undermining the provision of ecosystem services.
Figure 1.1. Trends of chemical industry production capacity between 2000 and 2017 (expressed as the relative growth)
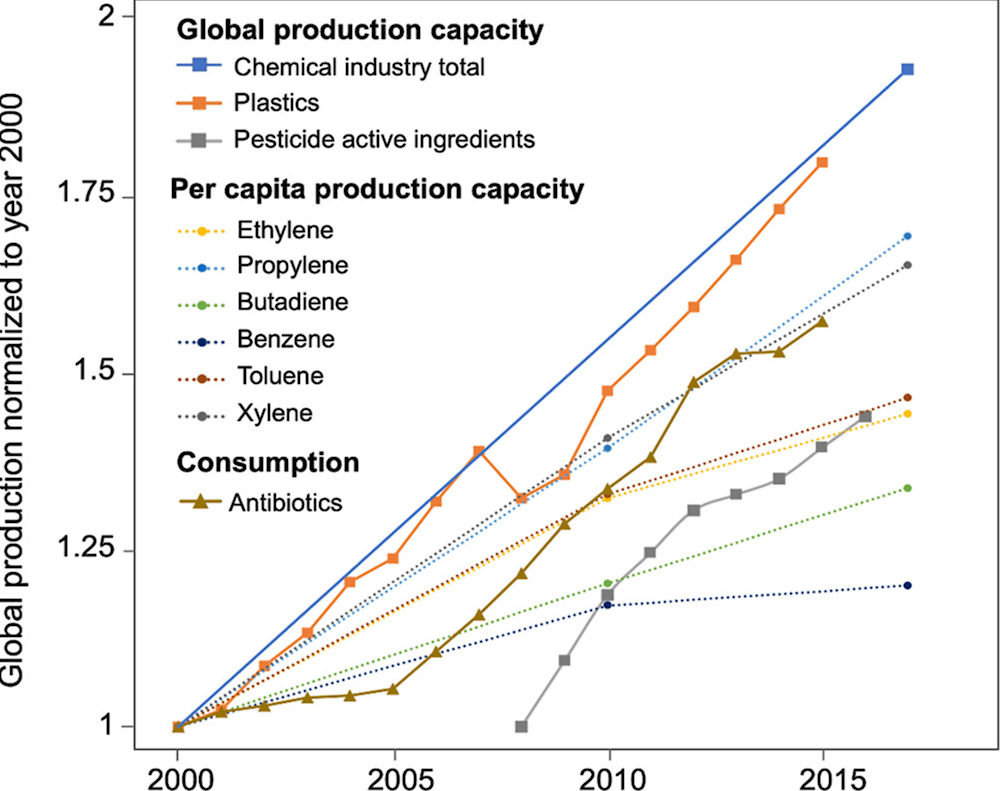
Note: 1. Global production capacity; 2. Per capita production capacity, 3: Global consumption of antibiotics.
Source: (Persson et al., 2022[2])
Endocrine disruptors are ubiquitous in the environment – that is, in water, air and soil (Section 1.5). Moreover, the impacts of climate change, environmental degradation and global population growth are drivers for an even more ubiquitous presence and effect on human health and ecosystems (Section 1.8).
Endocrine disruptors are not extensively regulated in OECD countries to date. This Chapter characterises some of the challenges to manage EDCs in the freshwater environment, which can be summarised as:
1. Endocrine disruptors are not “ordinary” chemicals. EDCs can work at low doses (ng/l), in mixtures with other chemicals and the dose does not always compare to the level of toxicity (Section 1.2).
2. Regulators have limited control over the release of EDCs into the environment, as they are not completely removed by wastewater and drinking water treatment plants, and they are directly released into the environment through diffuse sources or by upstream activities in other countries and continents (Section 1.4).
3. Endocrine disruption is characterised by uncertainty. Causal relationships between exposure and adverse effects on humans and wildlife are not fully understood and many chemicals are not recognised or even suspected as endocrine disruptors (Sections 1.5 and 1.6).
4. The chemicals circumvent our traditional ways of monitoring as they can trigger adverse effects at very low doses (ng/l), below threshold values. Moreover, chemicals that interfere with the endocrine system may comprise close to 800 chemicals (WHO-UNEP, 2013[3]), most of which are not routinely monitored in water. In comparison, the European Union Water Framework Directive (WFD) currently regulates a total of 45 priority substances (Chapter 2).
5. EDCs stem from a very diverse group of uses, products and processes. The cross-sectoral, transboundary and multidisciplinary nature of this problem demands attention across multiple policy domains, such as those related to water resources management, chemical safety, public health, agriculture and food, environment and biodiversity, industry, trade, and waste management. Countries face a major challenge in attempting to holistically address the issue, and regulatory efforts to date have been fragmented (Section 1.5 and Chapter 2).
Knowledge of adverse effects of chemicals in the environment is evolving, with better-characterised health effects of better-studied pollutants (Landrigan et al., 2018[4]). With improved knowledge and advanced technologies, countries are better equipped to respond to pollution challenges. This is also the case of endocrine disruptors. This publication comes at a time where there is a technological and epistemic readiness to respond to challenges posed by endocrine disruption in freshwater.
1.2. Endocrine disruption and endocrine disruptors
The World Health Organization’s (WHO) International Programme on Chemical Safety (IPCS) defines an endocrine disruptor as “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations” (IPCS, 2002[5]). Various actors globally have since applied the WHO’s definition, such as the OECD (2018[6]) and the European Commission (EU Regulations 2017/2100 and 2018/605). The Environmental Protection Agency of the United States (US EPA) instead employs a more specific definition that notes the biological effects of EDCs, stating that they interfere with the “synthesis, secretion, transport, binding, action or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behaviour” (US EPA, 1997[7]).
While EDCs cause adverse effects, acting through an endocrine mode of action, endocrine active substances (EASs) can interfere with the endocrine system with or without an adverse effect. EASs have “the inherent ability to interact or interfere with one or more components of the endocrine system resulting in a biological effect, but need not necessarily cause adverse effects” (EFSA, 2013[8]).
Box 1.1. The endocrine system: an overview
The endocrine system allows to control various functions in the body through a complex system of messages orchestrated by the endocrine glands and their hormones. Endocrine glands are organs that synthesise and release hormones in the blood stream. The main endocrine glands are illustrated in Figure 1.2. They comprise the hypothalamus, the pituitary, the gonads (ovaries or testes), the thyroid, the parathyroid, the pancreas, the adrenal gland, and the pineal gland. Other organs can also secrete hormones, such as the gastrointestinal tract, the heart, the kidney, the thymus, and the adipose tissue. Hormones are chemical messengers that are released by an endocrine gland into the blood stream. Homes will then travel to their target organ and tissue. To deliver their message, hormones will bind to their receptor.
Figure 1.2. Scheme of the endocrine system with its main glands and organs and their respective hormones
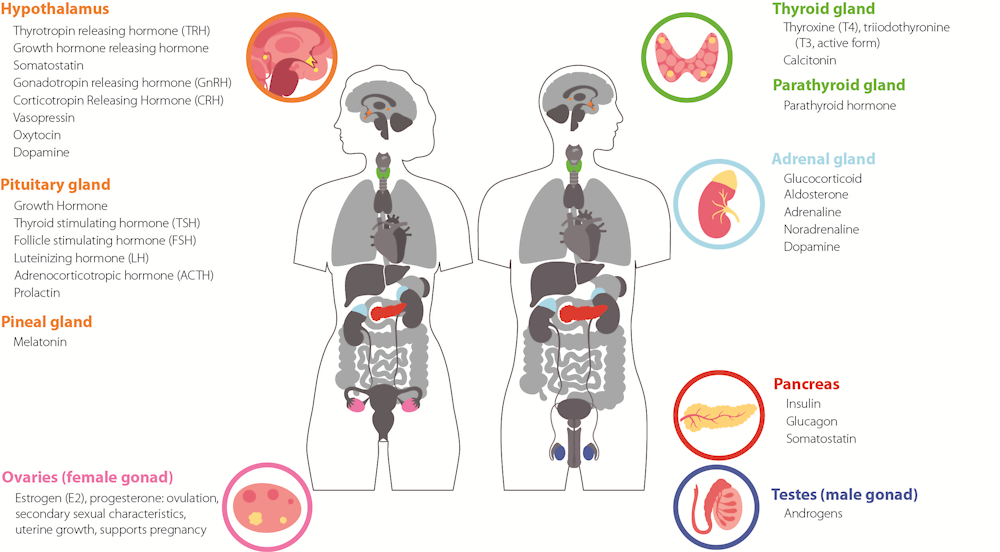
Note: Most of the hormones presented are conserved among vertebrates and some are also conserved in invertebrates.
Source for image: Authors, with drawings adapted from Pikovit through Adobe Stock
Source for information: (Norris and Carr, 2020[9]; WHO-UNEP, 2013[3])
EATS modalities/pathways refer to estrogen (E), androgen (A), Thyroid (T) and Steroidogenesis (S). The EATS modalities are the most studied and well understood pathways for endocrine disruption and have the most developed methodologies (OECD, 2018[6]). In the revised OECD Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption, the juvenile hormone (Jh) and the ecdysteroids (Ec) modalities were added to include invertebrate hormones. It should be recognized that EATSJhEc modalities, while important, are not the only ones that can be affected by endocrine disruption as many more hormones exist such as seen in Figure 1.2.
The endocrine system regulates and controls the release of hormones in humans and animals (Box 1.1, Figure 1.2). Well-known organs within the endocrine system are the ovaries and prostate, but also glands such as the pituitary, parathyroid, thyroid, adrenal and pancreas are part of the endocrine system (CCOHC, 2022[10])(Figure 1.2). Endocrine disruptors or endocrine active substances can work in roughly three ways: 1) they can either imitate the endocrine system (“agonist”), 2) work against the endocrine system as antagonist, 3) or interfere with the synthesis of the hormone, its transportation to the receptor, or its metabolism (CCOHC, 2022[10]; Kabir, Rahman and Rahman, 2015[11]; WHO-UNEP, 2013[3]). When the endocrine system is stimulated or inhibited by the action of EDCs on specific hormonal pathways, EDCs can act like a natural hormone and bind to a receptor or stimulate or inhibit the production or the transport of the natural hormone. It may then give the same or a more powerful signal than the “original” hormone, or give a signal at the “wrong” time, or disturb the signal at the appropriate time.
To help identify health hazards, La Merrill et al. (2020[12]) defined ten key characteristics of endocrine disrupting chemicals, summarised in Box 1.2.
Box 1.2. Ten key characteristics of endocrine disrupting chemicals
La Merrill et al. (2020[12]) made a scientific consensus statement on the ten key characteristics (KCs) of the impact of endocrine disrupting chemicals on the endocrine system. The key characteristics can help in hazard identification of EDCs to humans and animals, support in group assessments of chemicals, provide a basis for chemical risk assessments and support in prioritising knowledge and data gaps. This overview is not a checklist; EDCs may share one or a few KCs.
Ten key characteristics of endocrine disrupting chemicals
KC1. Interacts with or activates hormone receptors
KC2. Antagonizes hormone receptors
KC3. Alters hormone receptor expression
KC4. Alters signal transduction in hormone- responsive cells
KC5. Induces epigenetic modifications in hormone- producing or hormone responsive cells
KC6. Alters hormone synthesis
KC7. Alters hormone transport across cell membranes
KC8. Alters hormone distribution or circulating hormone levels
KC9. Alters hormone metabolism or clearance
KC10. Alters fate of hormone- producing or hormone-responsive cells
Source: (La Merrill et al., 2020[12])
Close to 800 chemicals are known or suspected to be capable of interfering with hormonal processes (WHO-UNEP, 2013[3]). EDCs can be clustered in different ways (WHO-UNEP, 2013[3]; Metcalfe et al., 2022[13]; Karthikeyan et al., 2019[14]; Kassotis et al., 2020[15]; Kabir, Rahman and Rahman, 2015[11]). Common clusters are pharmaceuticals for humans and livestock, pesticides, and additives to plastics (e.g. to make plastics fire proof, extra flexible, coloured, hardened or resistant against UV radiation). For the purpose of conducting a water policy analysis, Table 1.1 presents a typology based on product groups and their most common EDCs.
Table 1.1. Examples of EDCs per product group
|
Product group |
Examples of EDCs (suspected or recognised) |
|---|---|
|
Consumer products (e.g. children products, electronics, textiles) |
Flame retardants, bisphenols, phthalates, perfluorooctanoic acid (PFOA) |
|
Cosmetics, personal care products |
DBP, benzophenones, parabens, triclosan, DEET, phthalates |
|
Food contact materials (e.g. plastic food containers, food wrappers, baby bottles) |
Bisphenols, perfluorooctanoic acid (PFOA) |
|
Industrial chemicals |
Bisphenol A, PCBs, triphenyl phosphate, PBDEs, TCDD |
|
Metals |
Lead, cadmium, mercury, arsenic |
|
Pesticides (including herbicides, fungicides) |
Chlorpyrifos, chlorotriazine, pyraclostrobin, DDT, PCBs, atrazine, vinclozolin |
|
Pharmaceuticals (for humans and livestock) |
Trenbolone acetate, ethinylestradiol (EE2, synthetic estrogen), dexamethasone, levonorgestrel, rosiglitazone, non-steroidal synthetic estrogen |
|
Synthetic and naturally occurring hormones |
Progesterone, testosterone, cortisol, oestrone |
Notes: 1. Some of the chemicals listed above are under investigation or suspected of having endocrine active properties, such as in EDC assessment programmes or scientific journals, but they are not officially classified as EAC or EDC under national legislation; 2. Some substances are banned or restricted, but still appear in environment as legacy compounds; 3. This list is not exhaustive; 4. Glyphosate has been mentioned as endocrine active substance in sources used to produce this table (Kabir, Rahman and Rahman, 2015[11]; Kassotis et al., 2020[15]). However, further research by US-EPA and EFSA has shown that there is no indication that glyphosate is an endocrine disruptor (U.S. EPA, 2015[16]; EFSA, 2023[17]). EFSA notes that no firm conclusions can be drawn concerning the risks for biodiversity (EFSA, 2023[17]).
Endocrine disrupting chemicals are a subset of contaminants of emerging concern (CECs), micropollutants and persistent organic pollutants (POPs). EDC pollution may therefore be covered in action plans and strategies that do not necessarily carry the title of endocrine disruption (Table 1.2).
Table 1.2. A typology of pollutants and their relation to EDCs
|
Typology |
Definition |
Link to EDCs |
|---|---|---|
|
Contaminants of emerging concern (CECs) Also known as “emerging contaminants” |
A vast array of contaminants are of recent concern because they have only recently been introduced in water, or because they have only recently been detected at, or their risk to human and ecosystem health is only recently acknowledged (OECD, 2019[18]; Houtman, 2010[19]). |
Many endocrine disruptors are CECs. Examples include pharmaceuticals, industrial and household chemicals, personal care products, pesticides, manufactured nanomaterials, and their transformation products (OECD, 2019[18]). |
|
Micropollutants |
Natural or synthetic chemicals that exist in the environment at very low concentrations (microgram to nanogram per litre) and that are of toxicological concern (Schwarzenbach et al., 2006[20]). |
Many EDCs are micropollutants. Particularly relevant to EDCs as they can be harmful at a low dose or through mixture effects. |
|
Persistent Organic Pollutants (POPs) Also known as ‘forever chemicals’ |
Pollutants that stay in the environment for long periods of time (where the half-life of the chemical in water is greater than two months), that bio-accumulate or bio-concentrate in organisms and that have adverse effects on human or environmental health. |
The majority of POPs are endocrine disruptors or endocrine active substances (WHO-UNEP, 2013[3]). The Stockholm Convention recognises some EDCs as POPs, such as aldrin, BDE, chlordane, DDT, HBCD, HCB, HCH, PCB, PCDD, PCDF, PFOA, PFOS (Metcalfe et al., 2022[13]). Other substances, such as long-chain PFCAs, are undergoing a risk management evaluation as part of the POP review process (UNEP, 2022[21]). Not all EDCs are persistent, but some are continuously released into the environment (NORMAN Network and Water Europe, 2019[22]). |
Note: EDCs may fall under multiple pollutant categories.
1.3. The distinctive dynamics of EDCs
EDCs can catalyse adverse effects on humans and wildlife in multiple ways. Four areas of critical complexity of EDCs affect the application of traditional toxicology and risk assessment methods:
The cocktail effect or mixture effect1: robust evidence has emerged over the last 15 years that shows that EDCs can work together to produce combined effects, with the result that they can produce adverse effects when combined, even when they occur at concentrations wherein no effect from the individual EDC has been observed (Kortenkamp, 2007[23]; Carvalho et al., 2014[24]) (Box 1.3). This is particularly pertinent for EDCs in water sources, as chemical mixtures are likely to occur in water bodies (Gosset, Polomé and Perrodin, 2020[25]). Mixing with other pollutants can have an additive effect, and in some cases even synergistic (Kabir, Rahman and Rahman, 2015[11]). From a policy perspective, mixture effects in water imply the need to shift from traditional approaches of targeted chemical analysis (focusing on individual chemicals) towards monitoring of endocrine effects (Ministère de la transition écologique et solidaire, 2019[26]; WHO-UNEP, 2013[3]). This challenges the regulatory practice of many countries, which currently take a substance-by-substance approach to analysis and regulation.
The low-dose effect: EDCs are believed to have what is referred to as a low-dose effect (Welshons et al., 2003[27]; WHO-UNEP, 2013[3]). This is based on evidence that implies that there is no safe threshold of minimal exposure (i.e. the dose below which no adverse effect is expected to occur) and that monitoring conducted on this basis would be insufficient (ANSES, 2013[28]; Vandenberg et al., 2012[29]).
The non-monotonic dose-response relationship: related to the low-dose effect, it has been appraised that some EDC dose responses are non-linear and potentially non-monotonic (Vandenberg et al., 2012[29]; WHO-UNEP, 2013[3]). It is suspected, albeit with uncertainty, that EDCs may follow “inverted curves”, i.e., can exhibit greater or even opposite effects at low doses compared to those observed at high doses. This means that traditional toxicology, which hinges on the premise that high-dose toxicity testing will proportionally inform us about low-dose exposures, sometimes does not hold (Vandenberg et al., 2012[29]).
Continuous release into the environment causing chronic exposure: not all CECs, including endocrine disruptors, are persistent: they can be broken down. However, as some chemicals are continuously released into the environment, they are routinely found in the environment and food webs and could form a source of chronic exposure (NORMAN Network and Water Europe, 2019[22]; Windsor, Ormerod and Tyler, 2018[30])
Box 1.3. The cocktail effect: how substances mix
Endocrine disrupting compounds can co-exist and have a joint endocrine disrupting effect when combined: the cocktail-effect or mixture effect. The point is that, while individual substances may not be harmful or toxic, their combination is. This is particularly challenging from a regulatory perspective. Such mixtures are formed through different pathways, including in the environment. Mixtures are grouped in the following categories:
1. Intentional mixtures: manufactured formulations e.g., commercial mixtures of industrial substances; technical mixtures; product formulations.
2. Discharge mixtures: substance combinations that are emitted by a single industrial site e.g., effluent of a production site.
3. Coincidental mixtures: substances from different sources occurring in a medium e.g., combination of substances applied dermally from use of two or more product formulations.
4. Environmental mixtures: substance combinations in the environment e.g., substances found in soil from various exposure sources (application of product formulation, deposition from air, water run-off, etc.).
Mixtures can comprise multiple categories, e.g., a coincidental mixture that mixed in a freshwater body is also an environmental mixture.
In this context, combined exposure is another important concept as it relates to the exposure of humans and environment to mixtures. Combined exposure is defined by the OECD (2018[31]) as “exposure to multiple chemicals by a single route and exposure to multiple chemicals by multiple routes, from one or multiple sources of release and/or use(s)”.
Source: cited from (OECD, 2018[31])
1.4. Sources, environmental pathways and sinks of EDCs in freshwater
Endocrine disruptors are ubiquitously present in water bodies; they have been observed in aquatic organisms, freshwater bodies, soil, sediments, cryosphere and the ocean. EDCs are released into the environment through point sources and diffuse sources. The environment further transports EDCs through atmospheric currents, river flows, ocean currents, groundwater-surface water exchange and fish spawning. Table 1.3 provides a summary of sources, pathways and sinks.
Table 1.3. Summary of sources, environmental pathways and sinks of EDCs in freshwater and oceans
|
Sources |
Entry pathways into the environment |
Sinks |
|---|---|---|
|
Households and consumer uses E.g. Cleaners, Electronics, Food packaging, Personal care products, Pharmaceuticals, Plastics, Toys Agriculture and aquaculture E.g. Treated sewage sludge, Pesticides, Pharmaceuticals, Poultry and fish feed Industrial production E.g. Combustion, Disinfection by-products, Metals, Plasticizers Transportation E.g. Fossil fuel combustion, Ships |
Point sources Wastewater treatment plants Diffuse sources Agricultural runoff Urban runoff Industrial outfalls Waste disposal Leaching (wastes, septic tanks) Environmental migration Atmospheric currents River flows Ocean currents Groundwater-surface water exchange Fish spawning |
Aquatic organisms (biological retention) Freshwater bodies (rivers, lakes, groundwater) Soils Sediments Cryosphere Oceans |
Source: Authors
1.4.1. Sources
The key sources of EDCs in the environment are (Metcalfe et al., 2022[13]; Pironti et al., 2021[32]; Karthikeyan et al., 2019[14]):
Households and consumer uses. Cleaners, personal care products and (occasionally) pharmaceuticals are drained through sinks and showers; pharmaceuticals, leachates from food packaging and metabolites are excreted; electronics, packaging, toys and other consumer products end up in waste collection sites or landfills.
Agriculture and aquaculture. Pesticides and remaining EDCs in recycled effluents are discharged into the freshwater system as runoff; poultry feed, pharmaceuticals and their metabolites are excreted by livestock; or directly enter the aquatic ecosystem through fish farming.
Industrial production. Combustion can release EDCs into the atmosphere before deposition on land or water bodies; drinking water production can release EDCs from disinfection by-products or as a leachate from pipe systems with endocrine-active additives.
Transportation. Fossil fuel combustion can release EDCs into the atmosphere before deposition on land or water bodies; ships contain anti-fouling coatings that are released into the environment (many harmful anti-fouling coatings have been phased out).
Next to current-use sources, legacy chemicals are still present in the environment and organisms, in spite of global use restrictions or bans. Legacy chemicals are still found in the environment and humans due to their ability to dissolve in fats and their persistence (Yilmaz et al., 2020[33]). Some legacy chemicals are causing “more severe and widespread damage to many wildlife species than current-use chemicals” (Matthiessen, Wheeler and Weltje, 2018[34]). For example, tributyltin (TBT) is an antifouling paint. Since its ban in 2008 the levels of TBT have declined in the marine environment, but TBT is still present in sediments and marine species although some species have recovered (Marty et al., 2017[35]; Metcalfe et al., 2022[13]).
1.4.2. Entry pathways to freshwater ecosystems
EDCs enter the environment through point sources and diffuse sources. They are also transported from one ecosystem to another through environmental migration. Figure 1.3 provides an overview of EDCs entry pathways into surface water bodies.
Figure 1.3. Pathways of endocrine disrupting compounds into surface water bodies
Point sources
Direct discharge from municipal wastewater treatment plants (WWTPs) is one of the primary sources of emission of EDCs into the environment (Kasprzyk-Hordern, Dinsdale and Guwy, 2008[36]; NORMAN Network and Water Europe, 2019[22]; Luo et al., 2014[37]; WHO-UNEP, 2013[3]; Ruhí et al., 2016[38]; IPCP, 2017[39]; Wee et al., 2021[40]). WWTPs do not remove all pollutants. As a consequence, some EDCs are released into the freshwater environment (Box 1.4). The removal of micropollutants, including EDCs, depends on the properties of the pollutant (hydrophobicity, biodegradability, and volatility), the treatment process and the composition of the wastewater itself (pH and temperature) (Luo et al., 2014[37]). Removal rates also differ across countries and even within countries (Tran, Reinhard and Gin, 2018[41]). Yet the risk for human and wildlife health does not only depend on the concentration of EDCs discharged. Chemicals can be diluted through the main water system which reduces their concentration, although some EDCs remain active even at very low concentrations.
Hospitals are other point sources of pharmaceutical EDCs, although the household contribution of pharmaceutical residues is higher (OECD, 2019[18]). Industries can also release EDCs through point sources, such as paper and pulp mills and chemical manufacturers (WHO-UNEP, 2013[3]; Ussery et al., 2021[42]).
Diffuse sources
Among diffuse sources, storm runoff from agricultural fields and livestock activity and leaching from waste disposal sites or landfills account for a significant amount of EDCs in aquatic environments (WHO-UNEP, 2013[3]; Luo et al., 2014[37]). Storm runoff from agricultural fields can contain EDCs from pesticides (Pironti et al., 2021[32]), animal excretion (oestrogens, for example) (Matthiessen et al., 2006[43]) and recycled effluents (Schapira et al., 2020[44]; Edwards et al., 2009[45]). The estrogen release into the environment from livestock is possibly twice as large as the estrogen release from humans (Adeel et al., 2017[46]). Aquaculture may also potentially release EDCs in the environment, such as through disinfectant formulations (Ahmad et al., 2022[47]).
EDCs also enter the freshwater environment through the urban water cycle. Diffuse urban entry points are wet and atmospheric deposition, storm water runoff, direct discharge of untreated wastewater and sewage overflow from combined sewers (Pal et al., 2014[48]; Pironti et al., 2021[32]; König et al., 2017[49]). In a study on the occurrence of endocrine disruptors in the urban water cycle of Bogotá, Colombia, plasticisers (e.g. phthalates and bisphenol A) occurred the most in the water samples taken from aquatic media, while the pharmaceutical carbamazepine contributed with the highest concentrations (Bedoya-Ríos et al., 2018[50]).
Leaching from landfills and septic tanks is another diffuse entry pathway. EDCs used in industrial and household products can leach from landfills into surface water, sediments, and groundwater. Leaching of flame-retardants, such as PBDEs that have been banned in many jurisdictions, from e-waste dumpsites is a case in point (Alcock et al., 2003[51]; Oloruntoba et al., 2022[52]). Plastic additives, such as PBDE, phthalates, nonylphenols (NP), bisphenol A (BPA) and antioxidants, also leach into the environment during production, usage and disposal (Hermabessiere et al., 2017[53]).
Environmental migration
EDCs migrate through the environment and across ecosystems, with transboundary pollution as a result. For example, perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are distributed globally by ocean currents (WHO-UNEP, 2013[3]). EDCs can also be transported from oceans to freshwater ecosystems by anadromous fish living in saltwater and returning to freshwater bodies to spawn (Nilsen et al., 2019[54]). Air currents also transport and deposit EDCs. This is particularly the case for highly persistent, semi-volatile compounds such as PCBs, DDTs, pesticides and predecessors of PFOS and PFCA (WHO-UNEP, 2013[3]). Certain hormones can travel long distances through rivers to seas and oceans. For example, the Jordan river carried testosterone, estrogen (and to a lesser extent ethinylestradiol and estriol) up to 100 km from the source of pollution, although concentrations dropped going downstream (Barel-Cohen et al., 2006[55]). Groundwater-surface water exchange is another inter-ecosystem pathway of groundwater pollution (Lapworth et al., 2012[56]). As EDCs are deposited in water bodies through many environmental media, water bodies provide perfect conditions for mixture effects to appear (see also Box 1.2).
Box 1.4. Figures on the removal efficiency of wastewater treatment plants
EDCs represent a wide range of compounds, some of which are better removed than others. Removal efficiency is highly context-specific. Figure 1.4 gives an impression of the discrepancies and general removal efficiency of a range of micropollutants.
Figure 1.4. Removal efficiency of selected micropollutants in WWTPs
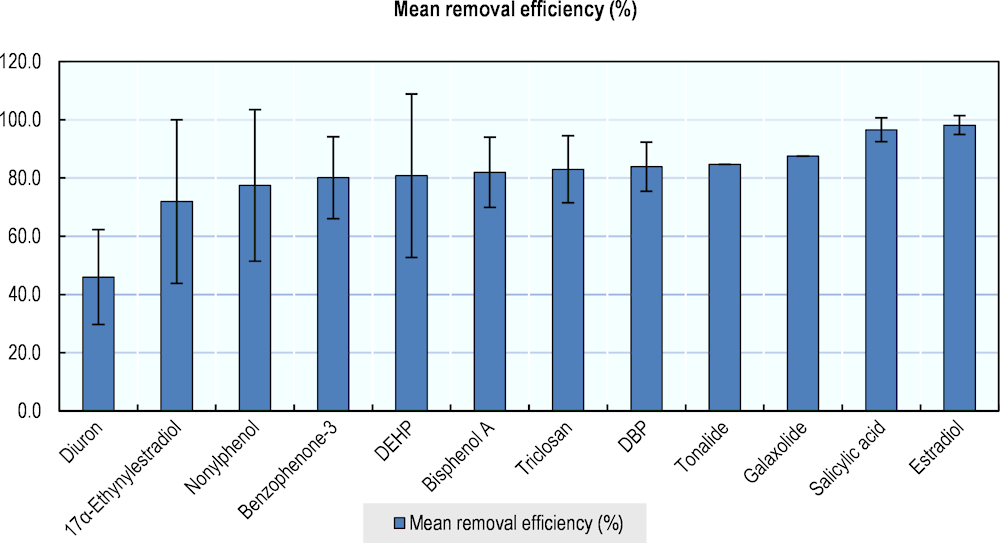
Note: Mean removal efficiency (bars) and standard deviations (error bars). Data were taken from WWTPs in 14 countries/regions, including OECD countries. Micropollutants were selected based on their status as endocrine disruptor, endocrine active, or under evaluation as endocrine active, largely based on (edlists.org, n.d.[57]).
Source: (Luo et al., 2014[37])
Luo et al. (Luo et al., 2014[37]) developed a classification of the removal efficiency of several compounds:
Table 1.4. Simple classification of micropollutants based on removal efficiency
|
Degree of removal |
Compounds |
|---|---|
|
Poorly removed (< 40%) |
Atrazine, carbamazepine, diazinon, diclofenac, erythromycin, metoprolol, mefenamic acid, tris(2-carboxyethyl)phosphine (TCEP), tris chloroisopropyl phosphate (TCPP) |
|
Moderately removed (40–70%) |
Atenolol, bezafibrate, clofibric acid, durion, ketoprofen, nonylphenol, sulfamethoxzole, tebuconazole, trimethoprim |
|
Highly removed (> 70%) |
Acetaminophen, benzophenone-3, bisphenol A, caffeine, clotrimazole, dibutyl phthalate, N,N-diethyl-meta-toluamide (DEET), Di(2-ethylhexyl) phthalate (DEHP), dimethyl phthalate (DMP), estradiol, estriol, estrone, ethinylestradiol, galaxolide, gemfibrozil, ibuprofen, naproxen, nonylphenol, octylphenol, salicylic acid, tonalide, triclosan |
Note: The actual removal is highly context-specific
Source: (Luo et al., 2014[37])
Freshwater sinks
EDCs are found in surface water bodies, groundwater bodies, drinking water and the marine environment. EDCs are also detected in aquatic organisms. Since EDCs represent a broad group of chemicals, there is no consolidated analysis of EDC concentrations and distribution in freshwater systems. Few international comparative analyses exist for individual substances, such as estrogens (Figure 1.5) and PFOS and PFOA emissions and concentrations in Europe (Pistocchi and Loos, 2009[58]).
Many monitoring initiatives focus on WWTP effluents at the outlet. Downstream concentrations and distribution, including impacts on downstream aquatic organisms, are much less studied (Windsor, Ormerod and Tyler, 2018[30]). This forms a considerable knowledge gap.
Figure 1.5. Global distribution of estrogens in river and surface water sites
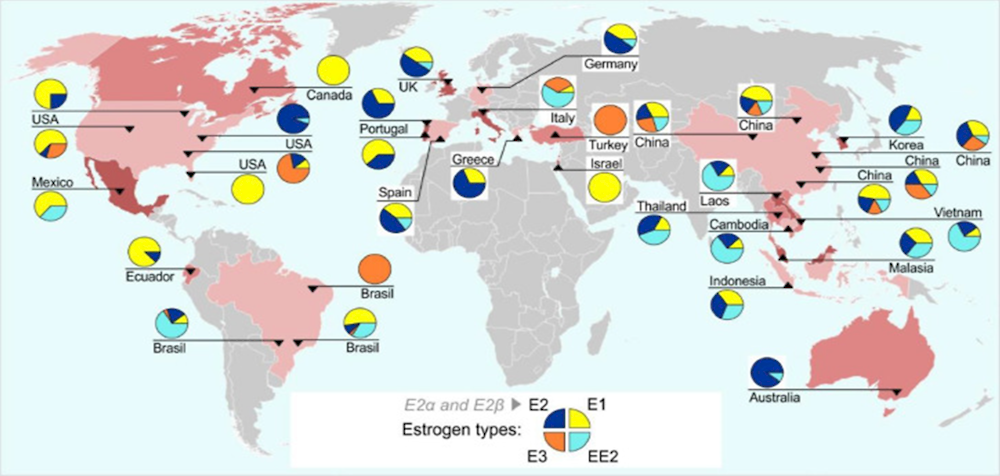
Note: Each pie chart comprises the concentrations (ng/l) of E1, E2, E3 (natural estrogens) and the EE2 (synthetic estrogen applied in birth control pills) as percentages (%) of total at each site. Year of data collection is unknown.
Source: (Ciślak et al., 2023[59]) based on (Adeel et al., 2017[46])
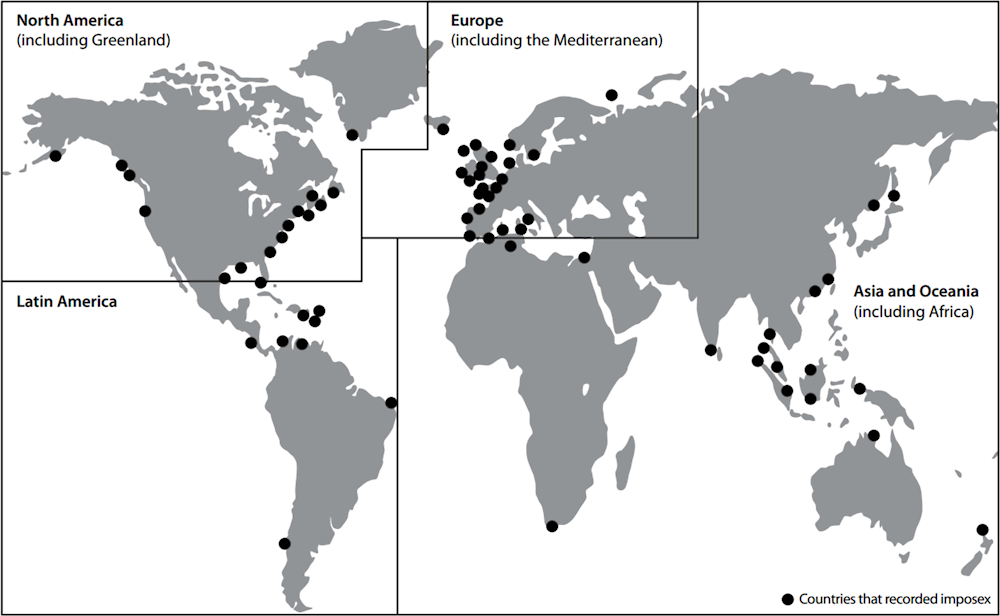
To get an understanding of the sinks of EDCs it is meaningful to look at the global distribution of endocrine-related effects within freshwater organisms. See for example Figure 1.6, showing detected signs of masculinisation of female gastropods (snails and slugs), associated with exposure to TBT - an antifouling paint applied on ships in the 1970s-1990s (WHO-UNEP, 2013[3]). EDCs and pharmaceuticals can bioaccumulate in organisms (Ruhí et al., 2016[38]), forming an additional exposure route through diets within the food web. Knowledge gaps exist regarding the distribution of EDCs. Recent literature does not report on the regional or global distribution of EDCs in freshwaters and freshwater organisms.
Figure 1.6. Geographic regions where female gastropods were reported as affected by imposex, intersex and ovo-testis
The understanding of endocrine disruptors in groundwater is limited compared to other freshwater bodies, even though groundwater is an important drinking water source for many regions in the world. Leaching from septic tanks, wastes and landfills, wastewater effluents, livestock activities, and groundwater-surface water exchange are common pathways of groundwater pollution (Lapworth et al., 2012[56]). Whether a compound can be transferred to groundwater depends on its physiochemical properties (Luo et al., 2014[37]). In a meta-analysis of EDCs in groundwater in several OECD countries, estrone, E2, NP and bisphenol A were the most frequently reported EDCs (Lapworth et al., 2012[56]). Personal care products, pesticides, plastic additives, fragrances and pharmaceuticals have also been reported in groundwater (Lapworth et al., 2012[56]; Jurado et al., 2012[60]). A study in Spain found that contaminant concentrations including EDCs were sometimes higher in aquifers than in their respective rivers, although generally groundwater was significantly less polluted than other water bodies (Jurado et al., 2012[60]). This may suggest that some contaminants can be persistent in groundwater.
The marine environment is important to mention in this context, as it receives EDCs from land-based activities via rivers. Endocrine disrupting chemicals from land-based activities have been found in estuaries, such as industrial xenoestrogens and natural and synthetic estrogens (Rocha et al., 2019[61]). Killer whales carry high levels of PCBs in their tissues originating from river runoff and atmospheric deposition, posing a potential risk to future killer whale populations (Desforges et al., 2018[62]). Similarly, endocrine disrupting POPs stemming from industrial and agricultural activities have reached polar bear populations through air and ocean currents (Routti et al., 2019[63]). Other EDCs found in the marine environment originate from marine-based activities, such as EDCs stemming from antifouling coatings on ships (Birch, Scammell and Besley, 2014[64]).
1.5. Human health impacts
This is a brief overview of the impacts of endocrine disrupting chemicals on the human body and public health. With the purpose of providing policy guidance to the environmental sector, this section simplifies exposure routes and disease impacts on humans. To provide adequate policy guidance for the health sector, a dedicated review is appropriate.
Humans may be exposed to endocrine disrupting chemicals through consumption of food and water, skin contact, inhalation, intravenous routes or biological transfer to the human foetus or newborn from the placenta and breast milk (Kabir, Rahman and Rahman, 2015[11]). At present, EDCs have been identified in human urine, blood, sweat and breast milk (Azzouz, Rascón and Ballesteros, 2016[65]; Shekhar et al., 2017[66]).
Endocrine disruption is a mode of action, i.e. it catalyses a change within an organism resulting from chemical exposure, that could lead to different health outcomes. In other words, endocrine disruption is not a health effect in itself. There are still knowledge gaps about the impacts of EDC exposure on human health, owing to the difficulty of separating their specific contribution from other potential causes (i.e. the heavy toll of establishing causality) in tandem with a dearth of epidemiological and experimental toxicology studies. Nonetheless, research over the last decade has made significant steps ahead in deepening our understanding and identifying an increasing number of potential new exposure-outcome associations.
The diseases induced by exposure to endocrine disrupting chemicals may comprise (based on a grouping by Kahn et al. (2020[67])):
Birth defects. They include disrupted foetal development and growth (Kahn et al., 2020[67]), reduced birthweight (Steenland, Barry and Savitz, 2018[68]), preterm birth (Gao et al., 2019[69]; Ferguson, McElrath and Meeker, 2014[70]; Latini et al., 2003[71]), and reduced anogenital distance in males (Bornehag et al., 2015[72]; Swan et al., 2015[73]).
Neurodevelopment conditions, such as attention-deficit hyperactivity disorder, autism, and cognitive and behavioural changes and dysfunction (Ghassabian and Trasande, 2018[74]).
Male and female reproductive health. The literature documents reproductive system disorders such as infertility (Kahn et al., 2020[67]), congenital malformations of the male reproductive system (Goodyer et al., 2017[75]), endometriosis (Kim et al., 2015[76]) (Kim et al., 2015), polycystic ovarian syndrome, breast cancer (Cohn et al., 2020[77]; Mancini et al., 2020[78]; Bonefeld-Jørgensen et al., 2014[79]), testicular cancer (Soto and Sonnenschein, 2010[80]), prostate cancer (Soto and Sonnenschein, 2010[80]; Kachuri et al., 2017[81]; Meyer et al., 2007[82]), and poor sperm quality and function (Li et al., 2011[83]; Omran et al., 2018[84]).
Obesity and metabolic diseases. Increased incidence of metabolic syndromes, such as obesity, insulin resistance, type 2 diabetes and cardiovascular diseases (Casals-Casas and Desvergne, 2011[85]; Giulivo et al., 2016[86]). Diabetes has been associated with PFAS exposure in Swedish and American cohorts (Lind et al., 2014[87]; Sun et al., 2018[88]; Cardenas et al., 2019[89]), whereas the strongest associations have been found with bisphenols BPA (Li et al., 2018[90]; Duan et al., 2019[91]; Murphy et al., 2019[92]; Rancière et al., 2019[93]; Sun et al., 2014[94]). However, increased PFAS exposure does not always cause increased diabetic outcomes (Karnes, Winquist and Steenland, 2014[95]).
Other endocrine disruptors including BPA, pesticides and flame retardants (e.g. PCBs, PBBs) have consistently shown thyroid disrupting properties (Boas, Feldt-Rasmussen and Main, 2012[96]; Murk et al., 2013[97]).
The most sensitive window of exposure to EDCs pertains to the critical periods of development, such as embryonic development, perinatal development, puberty, pregnancy and lactation periods, and menopause, i.e. periods during which organisms are more sensitive to hormonal disruption (Woodruff et al., 2008[98]; WHO-UNEP, 2013[3]). This implies a greater degree of risk for foetuses, infants, adolescents, pregnant women and the elderly (Leung et al., 2013[99]). Importantly, concerns for infants and young children have increased dramatically as they were found to be subject to much higher EDC exposure compared to adults through inter alia dust and particulates (Lunder et al., 2010[100]; Wormuth et al., 2006[101]). Moreover, early (especially prenatal) exposure can have health impacts at a later life stage (WHO-UNEP, 2013[3]).
Freshwater bodies potentially serve as a vehicle for transmitting EDC exposure from the environment to humans, mainly through contaminated drinking water, although causal linkages have not been established with certainty. Some of the interlinkages between human exposure to EDCs from freshwater are:
Consumption of untreated or contaminated drinking water collected from polluted freshwater sources. It is established that EDCs are not completely removed from drinking water treatment processes (Wee and Aris, 2017[102]; Kuch and Ballschmiter, 2001[103]; Benotti et al., 2009[104]). However, the health risk of consuming the very low levels of EDCs present in treated drinking water is likely to be low (Pironti et al., 2021[32]). For example, Wee et al. (2021[40]) found no risk for different age groups via tap water consumption in Malaysia, in spite of the presence of several endocrine disrupting chemicals. Similarly, endocrine-disrupting chemicals may be present in drinking water as by-products resulting from the process of water disinfection with chlorine, so-called chlorinated by-products (Gonsioroski, Mourikes and Flaws, 2020[105]; Liu, Dang and Liu, 2021[106]). The spray-on-lining of aged piping systems, specifically those with epoxy coating which contains bisphenol A (BPA), can leach from the pipes into to the drinking water supply (Rajasärkkä et al., 2016[107]).
Food consumption, for instance when food products are cultivated using recycled effluent or when EDCs bioaccumulate in crops, fish and seafood. Recycling of wastewater for irrigation could have an impact on human health. A study in Israel detected an association between vegetable consumption and relatively high concentrations of the carbamazepine drug (although carbamazepine is a CEC, it is not with certainty associated with endocrine disruption) in urine of people living in areas with extensive recycled effluent irrigation (Schapira et al., 2020[44]).
Bathing water, such as pools, ponds, lakes or seas, have not been identified as a vehicle for transmitting EDCs from the environment to humans. A possible explanation is that bathing water is not a source of chronic exposure. However, phenols, oestrogens, caffeine and progestogens have been detected in swimming pool water in China (Zhou et al., 2020[108]). A risk assessment done as part of the same study suggests that swim water skin contact is a more dominant exposure route than ingestion.
1.6. Ecological impacts
There are concerns about EDCs as drivers of biodiversity loss and ecosystem degradation, terrestrial, freshwater and marine species included (Harrison, 2022[109]). The European Environment Agency concludes that “on average 20 % of aquatic species are lost due to exposure to chemical mixtures” (European Environment Agency, 2020[110]). However, there is still a limited understanding of the effects of EDCs on biodiversity.
Pollution of water, even at low concentrations, is an important source of EDC exposure for wildlife. Effects of exposure to EDCs have been observed in a breadth of aquatic species: alligators, fish, frogs, minks/otters, mussels, polar bears and snails (Hotchkiss et al., 2008[111]; Orton et al., 2018[112]; Rodil et al., 2019[113]). Fish take up endocrine disruptors through their gills, while birds and mammals are exposed mainly through drinking water (WHO-UNEP, 2013[3]). Diet (trophic transfer through the food web) is another exposure route for aquatic organisms, as EDCs can bioaccumulate in organisms (Ruhí et al., 2016[38]). Aquatic plant species, such as algae, duckweed and wetland macrophytes can accumulate estrogens, thereby removing them from water (Shi et al., 2010[114]; Adeel et al., 2017[46]).
Ecosystems respond in different ways to contamination by endocrine disruptors (Table 1.5). Understanding the degree of impact of EDCs on wildlife and broader ecosystems is crucial yet quite challenging. Effects do not limit themselves to an individual organism, instead they can affect all levels of biological organisation. Endocrine contamination may not only affect the physiology of an organism, but can also change behaviour, fitness and evolution. Moreover, direct impacts on one species could cascade onto other species, so-called “indirect effects” (Saaristo et al., 2018[115]). Effects can be lethal or sublethal to some organisms, while other species can adapt or be(come) resistant. The effects of endocrine disrupting chemicals on wildlife and ecology are explained briefly in the following sections.
Table 1.5. Typology of effects of EDCs on wildlife and ecology
|
Lethality |
Effects |
Level of biological organisation |
Temporal aspects |
Coping mechanisms |
|---|---|---|---|---|
|
Lethal Sublethal |
Direct Physiology Behaviour Fitness Evolution Indirect |
Molecules Cells Tissues Organs Organisms Population Community Ecosystem |
Delayed effects Generational effects |
Resistance Adaptation Recovery |
1.6.1. Lethality
While chemical pollution can be lethal to wildlife, many species survive toxic exposure and experience more subtle effects that can still be harmful. Such sublethal toxic effects can change survival, growth and reproductive capabilities of organisms, ultimately affecting individual organisms, populations and communities (Beiras, 2018[117]; Saaristo et al., 2018[115]). Exposure to low doses of chemicals, sometimes over longer periods, can trigger sublethal effects (Nilsen et al., 2019[54]).
1.6.2. Direct and indirect effects
Endocrine disrupting chemicals can directly affect the physiology, behaviour or fitness of organisms:
Physiology: Among the adverse physiological effects in aquatic organisms (alligators, fish, frogs, minks/otters, mussels, polar bears and snails) are, inter alia: immune system damage, alterations of the hormonal system, disruption of homeostasis, reproductive dysfunctions (embryo malformation, hatchability, sex ratio alteration, sperm alteration), feminisation of male fish (Marty et al., 2017[35]; Zhou, Cai and Zhu, 2010[118]).
Behaviour: Chemical contaminants can affect the behaviour of individual organisms, which can, in turn, affect populations, communities and ecosystems (Ford et al., 2021[119]; Windsor, Ormerod and Tyler, 2018[30]). There are many uncertainties regarding EDC-induced behavioural changes. Zala and Penn (2004[120]) recorded several cognitive and neurological effects in aquatic organisms (Table 1.6). Changing feeding behaviour, avoidance of contaminated areas and changed migration routes have also been reported (Saaristo et al., 2018[115])
Fitness: Fitness-related traits of species are body size, growth and locomotor skills which affect the ability to move from one place to another, such as swimming performance of fish (Arendt, 2003[121]). Fitness of aquatic species can be affected by pollution and can have consequential effects on the population growth rates of species (Egea-Serrano and Tejedo, 2014[122]; Hamilton et al., 2017[123]). The fitness parameters that are affected by endocrine disruption are largely unknown.
Table 1.6. Cognitive and behavioural effects of EDCs present in the environment on aquatic species
|
Species |
Behaviour |
Changes |
EDC |
|---|---|---|---|
|
Mosquitofish |
Reproductive behaviour and sex characters |
Females masculinized Precocious and aggressive (males) |
Paper mill effluent* |
|
Dominance |
Increased |
||
|
Brown pelican |
Reproductive behaviour |
Aberrant |
Chlorinated hydrocarbon* |
|
Common tern |
Reproductive behaviour |
Aberrant |
DDT metabolites* |
|
Gulls |
Mate choice |
Homosexual in females |
DDT |
|
Guppies |
Sexual behaviours |
Decreased (males) |
4-t-octylphenol, vinclozolin |
|
Three-spined sticklebacks |
Aggression |
Decreased (males) |
Ethinyl oestradiol* |
|
Courtship and nesting |
Abnormal (males) |
||
|
Atlantic salmon |
Mating (response to females' pheromones) |
Inhibited (males) |
Cypermethrin (low doses) |
|
Mallard ducks |
Response to maternal calls |
Decreased |
Methyl-mercury exposure in utero or as adult |
|
Response to fright stimulus |
Increased |
||
|
Oviposition |
Laid more eggs outside nest |
||
|
Laid fewer eggs |
|||
|
Common tern |
Behaviour |
Altered |
Lead |
|
Herring gulls |
Begging |
Decreased |
Lead |
|
Balance |
Decreased |
||
|
Righting responses |
Decreased |
||
|
Individual recognition |
Decreased |
Note: *EED chemical tested at levels found in the environment; Only aquatic wildlife species are presented in this table; Experimental and correlational evidence are presented.
Source: (Zala and Penn, 2004[120])
Contamination can cascade onto other organisms, populations and communities, too. Such indirect effects can, for example, arise when contaminants trigger changes of behaviour or populations, and subsequently changes competition or predator-prey relationships in the foodweb (Windsor, Ormerod and Tyler, 2018[30]; Saaristo et al., 2018[115]). Indirect effects can thus also affect endocrine-resistant species and ecosystems at large.
The indirect effects of estrogen 17a-ethinylestradiol (EE2) on an aquatic foodweb have been demonstrated in a whole-lake experiment by Kidd et al. (2014[124]). EE2 normally enters the environment via wastewater treatment plants, as a residue of the birth control pill. The introduction of small concentrations of EE2 (5–6 ng/L−1) to the lake led to the collapse of the fathead minnow, a freshwater fish often preyed upon by larger fish species such as trout. The biomass of trout subsequently declined as they lost their prey species. Moreover, moving down the foodweb, the zooplankton population increased as their predator - the fathead minnow - had disappeared.
Our current understanding of the indirect impacts of endocrine disrupting contamination on populations, communities and ecosystems is limited. Scientists therefore advocate for a better assessment of ecological risks, taking into account all levels of biological organisation (Kidd et al., 2014[124]; Saaristo et al., 2018[115]; Nilsen et al., 2019[54]; Windsor, Ormerod and Tyler, 2018[30]).
1.6.3. Levels of biological organisation
The effects of endocrine disruption on aquatic organisms can cascade from cell, molecular and individual effects, to populations, to communities up to the entire ecosystem and the food web (Figure 1.7). This raises concerns for ecosystem balances and biodiversity. The effects of pollutants are visible earlier and detected more easily on the lower levels of biological organisation. Adverse effects on higher levels of biological organisation are more difficult to detect. Sometimes there is a lag time between exposure and effect. A timely understanding of effects at lower levels of the biological organisation can prevent significant negative effects on populations, which are much harder to recover (Wernersson et al., 2015[125]).
Figure 1.7. Effects of exposure to pollutants on different levels of biological organisation
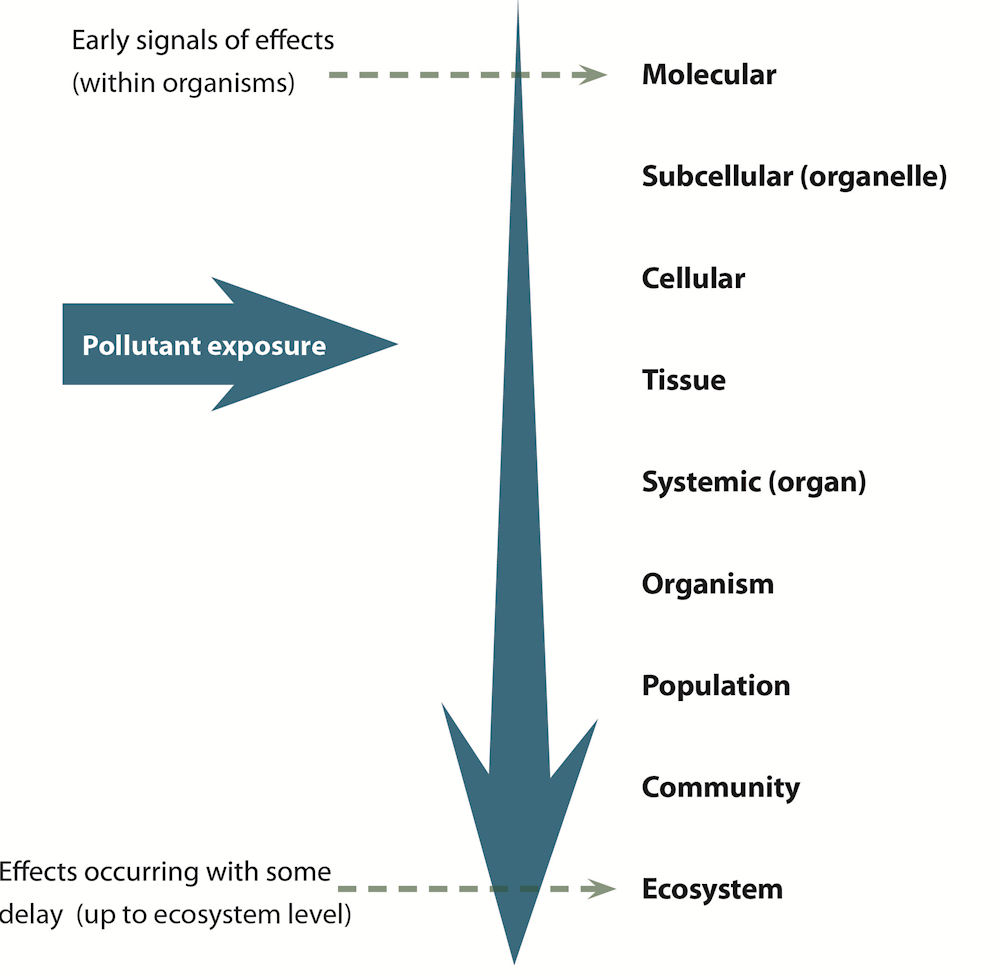
Note: The effects of pollutants are visible earlier and detected more easily on the lower levels of biological organisation before adverse effects are observed in higher levels of biological organisation.
Source: Authors, based on (Van der Oost, Beyer and Vermeulen, 2003[126])
1.6.4. Temporal aspects
The effects of EDC exposure are not always acute, but can be delayed or deferred to future generations. Effects of exposure to endocrine disrupting chemicals can be delayed when exposure happens in a period when an organism is vulnerable to contamination, but the effects will only manifest in another development stage, even if the exposure has already stopped (Parrott et al., 2017[116]; Matthiessen et al., 2017[127]). This is particularly true for endocrine disrupting chemicals, as they tend to affect the reproductive system during critical development stages.
Multigenerational effects become visible in the subsequent generation or generations (Parrott et al., 2017[116]). The type of impact may be different for the parental generation and the successive generations (Windsor, Ormerod and Tyler, 2018[30]). In a study on transgenerational effects of EE2 and BPA exposure on medaka fish, no irregularities were observed with the parental and subsequent generation (Bhandari, Vom Saal and Tillitt, 2015[128]). However, fertility dropped, and embryo survival dropped two and three generations later. The causes of such multigenerational effects vary. Factors include the duration of exposure, the timing of exposure (during a critical development window when the species are vulnerable to chemical exposure), or effects are carried over from one generation to the next, for example as a result of embryo exposure (Parrott et al., 2017[116]).
However, understanding of delayed and intergenerational effects is still limited (Parrott et al., 2017[116]; Saaristo et al., 2018[115]; Nilsen et al., 2019[54]; Windsor, Ormerod and Tyler, 2018[30]). The extent to which populations and evolution will be threatened is uncertain, as species may adapt, become resistant or recover from exposure (Windsor, Ormerod and Tyler, 2018[30]).
1.6.5. Coping mechanisms and recovery
Endocrine disruption does not always lead to permanent adverse effects. Species can be resistant or become resistant to endocrine disruptors, effects can be reversed, or species can adapt (Windsor, Ormerod and Tyler, 2018[30]). Species can recover from endocrine disruption, for example when the contaminant is removed from a water body (Marty et al., 2017[35]; Blanchfield et al., 2015[129]). The oyster population in the Sydney estuary recovered in a period of 10 years, following a partial ban of tributyltin as an antifouling coating (Birch, Scammell and Besley, 2014[64]). A study on impacts of pulp mill pollution in Jackfish Bay, in the Great Lakes of Canada, showed the ability of species to improve or recover from adverse effects on the endocrine system (Ussery et al., 2021[42]). In this study, three interventions over a period of 30 years had positive impacts on impairment and population size: introduction of secondary effluent treatment (1989), change in production processes (1990s) and a series of temporary closures of the mill (2000s). At the onset, adverse effects were observed in the white sucker fish species, such as smaller gonads, delayed sexual maturation, and changed production of sex steroids. After introduction of the measures, changes in “body size, liver size, gonad size and condition” reduced, but persisted (Ussery et al., 2021[42]). Some effects, such as enlarged liver sizes, bounced back to reference levels. Reproductive effects, however, persisted and can only be further reduced with mill closure. Nevertheless it was estimated that with the current measures, population levels could restore to over 93% of the lake’s carrying capacity, and improvements in population levels were observed.
Effects of EDCs differ across species, as some species are more resistant to contamination than others. The same study by Ussery et al. notes that some fish species are more sensitive to pulp and paper effluent exposure than others (Ussery et al., 2021[42]). Physical changes can also be observed following contamination, but without changing apical endpoints, i.e., without leading to any state of disease. Other species adapt to the contaminated environment and become resistant to EDCs, although these mechanisms have not been well documented (Windsor, Ormerod and Tyler, 2018[30]). Lastly, developing resistance to mixtures of chemicals with a broad range of modes of action is a much slower process than for similar-working chemicals (Saaristo et al., 2018[115]).
Uncertainty remains as to what extent the species can bounce back from the impacts of endocrine disruptive contamination. For example, Marty et al. (2017[35]) point out that after a global drop of TBT usage, some irreversible effects have been observed in female sea snails (nucella lapillus), while global snail populations have recovered.
1.7. Economic impacts
Environmental pollution from chemicals has substantial economic effects and costs (Landrigan et al., 2018[4]; Fuller et al., 2022[130]), in spite of the benefits that chemicals can offer. A handful of studies have assessed the economic costs of endocrine disruption and EDCs. These economic evaluations strive to estimate the disease costs of EDCs by hinging on a range of health expenditures and health outcomes (see Box 1.5).
Box 1.5. The costs of pollution-related disease
The Lancet Commission on Pollution and Health (Landrigan et al., 2018[4]) calculate the costs of pollution-related disease as a factor of:
1. “Direct medical expenditures, including hospital, physician, and medication costs, long-term rehabilitation or home care, and non-clinical services such as management, support services, and health insurance costs;
2. Indirect health-related expenditures, such as time lost from school or work, costs of special education, and the cost of investments in the health system (including health infrastructure, research and development, and medical training);
3. Diminished economic productivity in persons whose brains, lungs, and other organ systems are permanently damaged by pollution;
4. Losses in output resulting from premature death.”
Source: (Landrigan et al., 2018[4])
Trasande et al. (2016[131]) estimate a median annual cost of EUR 163 billion in the European Union stemming from exposure to EDCs, which amounts to 1.28% of EU GDP. In the same analysis, the largest burden per capita is found to be borne by Luxembourg (€791 per capita), Ireland (€583 per capita), and the Netherlands (€411 per capita). The study also estimated different probability-scenarios. Looking at the lower EDC exposure cost scenario, Trasande et al. estimate a 5% probability that costs are less than €22.5 billion/year. There is a 10% probability of the higher annual cost scenario of €215 billion/year.
For the US, Attina et al. (2016[132]) compute a cost of USD 340 billion/year (equal to 2.33% of US GDP). The lower cost scenario estimates, with 5% probability, that the cost of exposure to EDCs are less than $43.3 billion/year. The higher cost scenario calculates a 10% probability of costs amounting to $512 billion/year. The cost of exposure to EDCs are estimated to amount CAD 24.6 billion in Canada, or 1.25% of the Canadian GDP (Malits, Naidu and Trasande, 2022[133]).
Economic and social cost estimations have also been made for specific EDCs, such as PFAS and bisphenol A (BPA). The Nordic Council of Ministers (Goldenman et al., 2019[134]) estimated the socioeconomic costs from the use of PFAS in Denmark, Finland, Iceland, Norway and Sweden, covering health-related costs and environment-related costs to mitigate contamination. The annual health-related costs of exposure to PFAS have been estimated to range from EUR 2.8 – EUR 4.6 billion in the Nordic Countries, and EUR 52 - EUR 84 billion for all EEA countries. The estimated environment-related costs ranged from EUR 46 million – EUR 11 billion per country over a period of 20 years. Soil remediation measures constituted to be the highest expense, followed by upgraded treatment works and maintenance. Trasande (2014[135]) estimates the social costs of childhood obesity and adult coronary heart disease as a consequence of BPA exposure in the US at USD 2.98 billion in 2008.
Yet, the economic burden appraised via these approaches is deemed to be underestimated as only a limited subset of potential chemical exposure-outcome routes are taken into account (Malits, Naidu and Trasande, 2022[133]; Fuller et al., 2022[130]). Moreover, only the routes for which sufficient evidence of causation exists are considered. Lastly, the economic impact may be larger than the calculated direct and indirect costs, such as impacts on quality of life (Kassotis et al., 2020[15]; Malits, Naidu and Trasande, 2022[133]) or loss of property value near contaminated sites (Cordner et al., 2021[136]; Goldenman et al., 2019[134]).
In comparison, the economic costs of antimicrobial resistance (AMR), another One Health issue associated with the freshwater environment, amount to $55 billion every year in the US (CDC, 2013[137]), €1.5 billion in the EU (ECDC, 2009[138]) and an annual GDP decline of between CAD 13-21 billion in 2050 in Canada (CAC, 2019[139]). It should be noted that these figures cannot be directly compared to the figures on endocrine disruption due to differences in methodology, underlying assumptions, uncertainties and disease pathways considered in each model. Still, the figures tell us that economic costs of endocrine disruption are likely to be at par or higher than the economic costs associated with AMR.
It should be noted that the cost estimates in the previous paragraphs cannot be fully attributed to environmental causes. Other exposure routes significantly contribute to the burden of disease, such as through food contact materials, working in occupations with high chemical exposure, or breathing in contaminated air.
Economic costs are not always easily defined when it comes to the loss of biodiversity and ecosystem services caused by endocrine disruption. There have been no attempts to quantify such costs.
1.8. Drivers for future endocrine disruption in the environment
1.8.1. Climate change
Climate change-related stressors can have important implications with respect to EDCs’ impacts on wildlife and ecosystems. Higher average temperatures may increase the rate of volatilisation (evaporation) and dilution of endocrine-disrupting chemicals in water (Godfray et al., 2019[140]). It has also been shown that higher water temperatures can affect organisms, as it may lead to intensified feminising effects of zebrafish following exposure to the synthetic oestrogen EE2 (Luzio et al., 2016[141]); increased vitellogenin production of fish exposed to EDC mixtures (Brian et al., 2008[142]); induced female biased sex ratio (Dang and Kienzler, 2019[143]); and, more broadly, negative impacts on fish survival, development and reproduction where there is concurrent exposure to EDCs, eventually leading to population declines (Brown et al., 2015[144]). Nonetheless, the interactive effects of co-exposure to EDCs and warming and/or acidification are not clear-cut. What is certain is that an alteration takes place, which may lead to either the enhancement or inhibition of responses to EDCs (Maulvault et al., 2019[145]).
According to Godfray et al. (2019[140]), climate change has the potential to lead to reduced precipitation in certain regions and at certain times, driving reduced flows in water bodies and less dilution of wastewater, in turn enhancing EDC concentrations in water. Or, conversely, climate change may elicit changes in extreme rainfall events that in turn increase agricultural runoff and sewer overflows into river water. Furthermore, endocrine disrupting chemicals trapped in glacial ice may be released upon melting (Godfray et al., 2019[140]). Soil erosion due to rainwater and certain types of land use has also been found to cause greater EDC pollution of nearby water bodies (Issaka and Ashraf, 2017[146]).
Climate change can also have an indirect effect on pollution. In regions where climate change causes intensified or more frequent droughts, countries may resort to alternative water resources such as wastewater recycling (California EPA, 2018[147]). Recycled effluents, however, can discharge EDCs into the environment through agricultural runoff (Schapira et al., 2020[44]; Edwards et al., 2009[45]). Moreover, an increased demand of biofuels to accommodate the energy transition, combined with increased food production, could lead to a doubling of pesticide and fertiliser use by 2050 (Harrison, 2022[109])
1.8.2. Societal changes
Urbanisation and increased population density can reduce water quality by generating a higher concentration of EDCs in water bodies, especially in those with modest dilution capacities (Miller and Hutchins, 2017[148]; Gabor et al., 2018[149]; Godfray et al., 2019[140]; Li, Zhang and Shan, 2019[150]).
Among other major global trends, various demographic and economic changes are affecting EDC releases into water sources and the broader environment. Growing populations will trigger a higher release of EDCs, even if per capita consumption remains constant, as increasing wealth is associated with greater consumption. Trends such as population ageing and the non-communicable diseases epidemic are also expected to increase the discharge of pharmaceuticals into waterways (Godfray et al., 2019[140]).
In this context of societal changes, it should also be noted that substances are being phased out in several regions of the world (Metcalfe et al., 2022[13]). This potentially has a positive effect on human health and ecosystem recovery, although legacy compounds are still found in the environment. Partial bans (e.g. based on geography or usage) cannot eradicate the transboundary movement of substances, and substitutes can still be harmful (Matthiessen, Wheeler and Weltje, 2017[151]; Barton-Maclaren et al., 2022[152]).
References
[46] Adeel, M. et al. (2017), “Environmental impact of estrogens on human, animal and plant life: A critical review”, Environment International, Vol. 99, pp. 107-119, https://doi.org/10.1016/J.ENVINT.2016.12.010.
[47] Ahmad, A. et al. (2022), “Contaminants of emerging concern (CECs) in aquaculture effluent: Insight into breeding and rearing activities, alarming impacts, regulations, performance of wastewater treatment unit and future approaches”, Chemosphere, Vol. 290, p. 133319, https://doi.org/10.1016/J.CHEMOSPHERE.2021.133319.
[51] Alcock, R. et al. (2003), “Understanding levels and trends of BDE-47 in the UK and North America: an assessment of principal reservoirs and source inputs”, Environment International, Vol. 29/6, pp. 691-698, https://doi.org/10.1016/S0160-4120(03)00120-X.
[28] ANSES (2013), ANSES’s work and involvement in the area of endocrine disruptors, https://www.anses.fr/en/content/ansess-work-and-involvement-area-endocrine-disruptors (accessed on 31 May 2022).
[121] Arendt, J. (2003), “Reduced burst speed is a cost of rapid growth in anuran tadpoles: Problems of autocorrelation and inferences about growth rates”, Functional Ecology, Vol. 17/3, https://doi.org/10.1046/j.1365-2435.2003.00737.x.
[132] Attina, T. et al. (2016), “Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis”, The Lancet Diabetes & Endocrinology, Vol. 4/12, pp. 996-1003, https://doi.org/10.1016/S2213-8587(16)30275-3.
[65] Azzouz, A., A. Rascón and E. Ballesteros (2016), “Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography-mass spectrometry”, Journal of Pharmaceutical and Biomedical Analysis, Vol. 119, https://doi.org/10.1016/j.jpba.2015.11.024.
[55] Barel-Cohen, K. et al. (2006), “Monitoring of natural and synthetic hormones in a polluted river”, Journal of Environmental Management, Vol. 78/1, pp. 16-23, https://doi.org/10.1016/J.JENVMAN.2005.04.006.
[152] Barton-Maclaren, T. et al. (2022), “Innovation in regulatory approaches for endocrine disrupting chemicals: The journey to risk assessment modernization in Canada”, Environmental Research, Vol. 204, https://doi.org/10.1016/j.envres.2021.112225.
[50] Bedoya-Ríos, D. et al. (2018), “Study of the occurrence and ecosystem danger of selected endocrine disruptors in the urban water cycle of the city of Bogotá, Colombia”, Journal of Environmental Science and Health, Part A, Vol. 53/4, pp. 317-325, https://doi.org/10.1080/10934529.2017.1401372.
[117] Beiras, R. (2018), “Sublethal Toxicity at the Level of Organism”, Marine Pollution, pp. 233-245, https://doi.org/10.1016/B978-0-12-813736-9.00014-3.
[104] Benotti, M. et al. (2009), “Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water”, Environmental Science and Technology, Vol. 43/3, pp. 597-603, https://doi.org/10.1021/ES801845A/SUPPL_FILE/ES801845A_SI_001.PDF.
[128] Bhandari, R., F. Vom Saal and D. Tillitt (2015), “Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes”, Scientific Reports 2015 5:1, Vol. 5/1, pp. 1-5, https://doi.org/10.1038/srep09303.
[64] Birch, G., M. Scammell and C. Besley (2014), “The recovery of oyster (Saccostrea glomerata) populations in Sydney estuary (Australia)”, Environmental Science and Pollution Research, Vol. 21/1, https://doi.org/10.1007/s11356-013-2168-x.
[129] Blanchfield, P. et al. (2015), “Recovery of a Wild Fish Population from Whole-Lake Additions of a Synthetic Estrogen”, Environmental Science & Technology, Vol. 49/5, pp. 3136-3144, https://doi.org/10.1021/es5060513.
[96] Boas, M., U. Feldt-Rasmussen and K. Main (2012), Thyroid effects of endocrine disrupting chemicals, https://doi.org/10.1016/j.mce.2011.09.005.
[79] Bonefeld-Jørgensen, E. et al. (2014), “Breast cancer risk after exposure to perfluorinated compounds in Danish women: a case–control study nested in the Danish National Birth Cohort”, Cancer Causes and Control, Vol. 25/11, https://doi.org/10.1007/s10552-014-0446-7.
[72] Bornehag, C. et al. (2015), “Prenatal phthalate exposures and anogenital distance in swedish boys”, Environmental Health Perspectives, Vol. 123/1, https://doi.org/10.1289/ehp.1408163.
[142] Brian, J. et al. (2008), “Evidence of temperature-dependent effects on the estrogenic response of fish: Implications with regard to climate change”, Science of the Total Environment, Vol. 397/1-3, https://doi.org/10.1016/j.scitotenv.2008.02.036.
[144] Brown, A. et al. (2015), “Climate change and pollution speed declines in zebrafish populations”, Proceedings of the National Academy of Sciences of the United States of America, Vol. 112/11, https://doi.org/10.1073/pnas.1416269112.
[139] CAC (2019), When Antibiotics Fail The Expert Panel on the Potential Socio-Economic Impacts of Antimicrobial Resistance in Canada, Council of Canadian Academies, Ottawa.
[147] California EPA (2018), Water quality control policy for recycled water, State Water Resources Control Board, California Environmental Protection Agency.
[89] Cardenas, A. et al. (2019), “Associations of perfluoroalkyl and polyfluoroalkyl substances with incident diabetes and microvascular disease”, Diabetes Care, Vol. 42/9, https://doi.org/10.2337/dc18-2254.
[24] Carvalho, R. et al. (2014), “Mixtures of Chemical Pollutants at European Legislation Safety Concentrations: How Safe Are They?”, Toxicological Sciences, Vol. 141/1, pp. 218-233, https://doi.org/10.1093/toxsci/kfu118.
[85] Casals-Casas, C. and B. Desvergne (2011), “Endocrine disruptors: From endocrine to metabolic disruption”, Annual Review of Physiology, Vol. 73, https://doi.org/10.1146/annurev-physiol-012110-142200.
[10] CCOHC (2022), OSH Answers Fact Sheets - Endocrine Disruptors, Canadian Centre for Occupational Health and Safety, https://www.ccohs.ca/oshanswers/chemicals/endocrine.html (accessed on 31 May 2022).
[137] CDC (2013), Antibiotic resistance threats in the United States, 2013, Centers for Disease Control and Prevention, https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 29 April 2022).
[59] Ciślak, M. et al. (2023), “Estrogen pollution of the European aquatic environment: A critical review”, Water Research, Vol. 229, p. 119413, https://doi.org/10.1016/j.watres.2022.119413.
[77] Cohn, B. et al. (2020), “In utero exposure to poly− and perfluoroalkyl substances (PFASs) and subsequent breast cancer”, Reproductive Toxicology, Vol. 92, https://doi.org/10.1016/j.reprotox.2019.06.012.
[136] Cordner, A. et al. (2021), The True Cost of PFAS and the Benefits of Acting Now, https://doi.org/10.1021/acs.est.1c03565.
[143] Dang, Z. and A. Kienzler (2019), “Changes in fish sex ratio as a basis for regulating endocrine disruptors”, Environment International, Vol. 130, p. 104928, https://doi.org/10.1016/j.envint.2019.104928.
[62] Desforges, J. et al. (2018), “Predicting global killer whale population collapse from PCB pollution”, Science, Vol. 361/6409, https://doi.org/10.1126/science.aat1953.
[91] Duan, Y. et al. (2019), “Association between phthalate exposure and glycosylated hemoglobin, fasting glucose, and type 2 diabetes mellitus: A case-control study in China”, Science of the Total Environment, Vol. 670, https://doi.org/10.1016/j.scitotenv.2019.03.192.
[138] ECDC (2009), The bacterial challenge: time to react, European Centre for Disease Prevention and Control, Stockholm, https://doi.org/10.2900/2518.
[57] edlists.org (n.d.), Endocrine Disruptor Lists, https://edlists.org/ (accessed on 15 March 2023).
[45] Edwards, M. et al. (2009), “Pharmaceutical and personal care products in tile drainage following surface spreading and injection of dewatered municipal biosolids to an agricultural field”, Science of The Total Environment, Vol. 407/14, pp. 4220-4230, https://doi.org/10.1016/J.SCITOTENV.2009.02.028.
[17] EFSA (2023), EFSA explains the scientific assessment of glyphosate, European Food Safety Authority, Parma.
[8] EFSA (2013), “Scientific Opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment”, EFSA Journal, Vol. 11/3, https://doi.org/10.2903/j.efsa.2013.3132.
[122] Egea-Serrano, A. and M. Tejedo (2014), “Contrasting effects of nitrogenous pollution on fitness and swimming performance of Iberian waterfrog, Pelophylax perezi (Seoane, 1885), larvae in mesocosms and field enclosures”, Aquatic Toxicology, Vol. 146, https://doi.org/10.1016/j.aquatox.2013.11.003.
[110] European Environment Agency (2020), The European environment —state and outlook 2020. Knowledge for transition to a sustainable Europe, European Environment Agency, https://www.eea.europa.eu/soer/publications/soer-2020 (accessed on 27 June 2022).
[70] Ferguson, K., T. McElrath and J. Meeker (2014), “Environmental phthalate exposure and preterm birth”, JAMA Pediatrics, Vol. 168/1, https://doi.org/10.1001/jamapediatrics.2013.3699.
[119] Ford, A. et al. (2021), “The Role of Behavioral Ecotoxicology in Environmental Protection”, Environmental Science & Technology, Vol. 55/9, pp. 5620-5628, https://doi.org/10.1021/acs.est.0c06493.
[130] Fuller, R. et al. (2022), “Pollution and health: a progress update”, The Lancet Planetary Health, Vol. 0/0, https://doi.org/10.1016/S2542-5196(22)00090-0.
[149] Gabor, C. et al. (2018), “Urbanization is associated with elevated corticosterone in Jollyville Plateau salamanders”, Ecological Indicators, Vol. 85, https://doi.org/10.1016/j.ecolind.2017.10.047.
[69] Gao, H. et al. (2019), “Prenatal phthalate exposure in relation to gestational age and preterm birth in a prospective cohort study”, Environmental Research, Vol. 176, https://doi.org/10.1016/j.envres.2019.108530.
[74] Ghassabian, A. and L. Trasande (2018), “Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment”, Frontiers in Endocrinology, Vol. 9, https://doi.org/10.3389/fendo.2018.00204.
[86] Giulivo, M. et al. (2016), Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review, https://doi.org/10.1016/j.envres.2016.07.011.
[140] Godfray, H. et al. (2019), “A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife”, Proceedings of the Royal Society B: Biological Sciences, Vol. 286/1897, https://doi.org/10.1098/rspb.2018.2416.
[134] Goldenman, G. et al. (2019), The cost of inaction: A socioeconomic analysis ofenvironmental and health impacts linked to exposure to PFAS, TemaNord, Nordic Council of Ministers, Copenhagen K, https://doi.org/10.6027/TN2019-516.
[105] Gonsioroski, A., V. Mourikes and J. Flaws (2020), Endocrine disruptors in water and their effects on the reproductive system, https://doi.org/10.3390/ijms21061929.
[75] Goodyer, C. et al. (2017), “A case-control study of maternal polybrominated diphenyl ether (PBDE) exposure and cryptorchidism in Canadian populations”, Environmental Health Perspectives, Vol. 125/5, https://doi.org/10.1289/EHP522.
[1] Gore, A. et al. (2015), EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals, https://doi.org/10.1210/er.2015-1010.
[25] Gosset, A., P. Polomé and Y. Perrodin (2020), “Ecotoxicological risk assessment of micropollutants from treated urban wastewater effluents for watercourses at a territorial scale: Application and comparison of two approaches”, International Journal of Hygiene and Environmental Health, Vol. 224, p. 113437, https://doi.org/10.1016/j.ijheh.2019.113437.
[123] Hamilton, P. et al. (2017), Adaptive capabilities and fitness consequences associated with pollution exposure in fish, https://doi.org/10.1098/rstb.2016.0042.
[109] Harrison, J. (2022), Strengthening collaboration and coordination between biodiversity and chemicals and waste clusters, Nordic Council of Ministers, https://doi.org/10.6027/temanord2022-513.
[153] HELCOM (2022), Micropollutants in wastewater and sewage sludge. Baltic Sea Environment Proceedings Proceedings No. 185., Baltic Marine Environment Protection Commission – Helsinki Commission, Helsinki.
[53] Hermabessiere, L. et al. (2017), “Occurrence and effects of plastic additives on marine environments and organisms: A review”, Chemosphere, Vol. 182, pp. 781-793, https://doi.org/10.1016/j.chemosphere.2017.05.096.
[111] Hotchkiss, A. et al. (2008), Fifteen years after “wingspread” - Environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go, https://doi.org/10.1093/toxsci/kfn030.
[19] Houtman, C. (2010), “Emerging contaminants in surface waters and their relevance for the production of drinking water in Europe”, https://doi.org/10.1080/1943815X.2010.511648, Vol. 7/4, pp. 271-295, https://doi.org/10.1080/1943815X.2010.511648.
[39] IPCP (2017), Overview Report II: An overview of current scientific knowledge on the life cycles, environmental exposures, and environmental effects of select endocrine disrupting chemicals (EDCs) and potential EDCs, International Panel on Chemical Pollution.
[5] IPCS (2002), Global Assessment of the state-of-the-science of Endocrine Disruptors, International Programme on Chemical Safety, Geneva.
[146] Issaka, S. and M. Ashraf (2017), “Impact of soil erosion and degradation on water quality: a review”, Geology, Ecology, and Landscapes, Vol. 1/1, https://doi.org/10.1080/24749508.2017.1301053.
[60] Jurado, A. et al. (2012), “Emerging organic contaminants in groundwater in Spain: A review of sources, recent occurrence and fate in a European context”, Science of The Total Environment, Vol. 440, pp. 82-94, https://doi.org/10.1016/J.SCITOTENV.2012.08.029.
[11] Kabir, E., M. Rahman and I. Rahman (2015), “A review on endocrine disruptors and their possible impacts on human health”, Environmental Toxicology and Pharmacology, Vol. 40/1, pp. 241-258, https://doi.org/10.1016/j.etap.2015.06.009.
[81] Kachuri, L. et al. (2017), “Cancer risks in a population-based study of 70,570 agricultural workers: Results from the Canadian census health and Environment cohort (CanCHEC)”, BMC Cancer, Vol. 17/1, https://doi.org/10.1186/s12885-017-3346-x.
[67] Kahn, L. et al. (2020), Endocrine-disrupting chemicals: implications for human health, https://doi.org/10.1016/S2213-8587(20)30129-7.
[95] Karnes, C., A. Winquist and K. Steenland (2014), “Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid”, Environmental Research, Vol. 128, pp. 78-83, https://doi.org/10.1016/J.ENVRES.2013.11.003.
[14] Karthikeyan, B. et al. (2019), “A curated knowledgebase on endocrine disrupting chemicals and their biological systems-level perturbations”, Science of The Total Environment, Vol. 692, pp. 281-296, https://doi.org/10.1016/J.SCITOTENV.2019.07.225.
[36] Kasprzyk-Hordern, B., R. Dinsdale and A. Guwy (2008), “The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK”, Water Research, Vol. 42/13, pp. 3498-3518, https://doi.org/10.1016/j.watres.2008.04.026.
[15] Kassotis, C. et al. (2020), “Endocrine-disrupting chemicals: economic, regulatory, and policy implications”, The Lancet Diabetes & Endocrinology, Vol. 8/8, pp. 719-730, https://doi.org/10.1016/S2213-8587(20)30128-5.
[124] Kidd, K. et al. (2014), “Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen”, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369/1656, https://doi.org/10.1098/RSTB.2013.0578.
[76] Kim, S. et al. (2015), “Possible role of phthalate in the pathogenesis of endometriosis: In vitro, animal, and human data”, Journal of Clinical Endocrinology and Metabolism, Vol. 100/12, https://doi.org/10.1210/jc.2015-2478.
[49] König, M. et al. (2017), “Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis”, Environmental Pollution, Vol. 220, pp. 1220-1230, https://doi.org/10.1016/J.ENVPOL.2016.11.011.
[23] Kortenkamp, A. (2007), “Ten Years of Mixing Cocktails: A Review of Combination Effects of Endocrine-Disrupting Chemicals”, Environmental Health Perspectives, Vol. 115/Suppl 1, pp. 98-105, https://doi.org/10.1289/ehp.9357.
[103] Kuch, H. and K. Ballschmiter (2001), “Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC-(NCI)-MS in the picogram per liter range”, Environmental Science and Technology, Vol. 35/15, pp. 3201-3206, https://doi.org/10.1021/ES010034M/ASSET/IMAGES/LARGE/ES010034MF00003.JPEG.
[12] La Merrill, M. et al. (2020), “Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification”, Nature Reviews Endocrinology, Vol. 16/1, pp. 45-57, https://doi.org/10.1038/s41574-019-0273-8.
[4] Landrigan, P. et al. (2018), “The Lancet Commission on pollution and health”, The Lancet, Vol. 391/10119, pp. 462-512, https://doi.org/10.1016/S0140-6736(17)32345-0/ATTACHMENT/BFCFD5A0-F041-4475-B3E9-13C53824D76B/MMC1.PDF.
[56] Lapworth, D. et al. (2012), Emerging organic contaminants in groundwater: A review of sources, fate and occurrence, https://doi.org/10.1016/j.envpol.2011.12.034.
[71] Latini, G. et al. (2003), “In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy”, Environmental Health Perspectives, Vol. 111/14, https://doi.org/10.1289/ehp.6202.
[99] Leung, H. et al. (2013), “Pharmaceuticals in tap water: Human health risk assessment and proposed monitoring framework in China”, Environmental Health Perspectives, Vol. 121/7, https://doi.org/10.1289/ehp.1206244.
[90] Li, A. et al. (2018), “Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia”, Environmental Research, Vol. 166, https://doi.org/10.1016/j.envres.2018.06.040.
[83] Li, D. et al. (2011), “Urine bisphenol-A (BPA) level in relation to semen quality”, Fertility and Sterility, Vol. 95/2, https://doi.org/10.1016/j.fertnstert.2010.09.026.
[87] Lind, L. et al. (2014), “Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly”, Diabetologia, Vol. 57/3, https://doi.org/10.1007/s00125-013-3126-3.
[106] Liu, Z., Z. Dang and Y. Liu (2021), “Legislation against endocrine-disrupting compounds in drinking water: essential but not enough to ensure water safety”, Environmental Science and Pollution Research, Vol. 28/15, pp. 19505-19510, https://doi.org/10.1007/S11356-021-12901-1/TABLES/2.
[150] Li, Z., W. Zhang and B. Shan (2019), “The effects of urbanization and rainfall on the distribution of, and risks from, phenolic environmental estrogens in river sediment”, Environmental Pollution, Vol. 250, https://doi.org/10.1016/j.envpol.2019.04.108.
[100] Lunder, S. et al. (2010), “Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers”, Environmental Science and Technology, Vol. 44/13, https://doi.org/10.1021/es1009357.
[37] Luo, Y. et al. (2014), “A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment”, Science of The Total Environment, Vol. 473-474, pp. 619-641, https://doi.org/10.1016/j.scitotenv.2013.12.065.
[141] Luzio, A. et al. (2016), “Effects of 17α-ethinylestradiol at different water temperatures on zebrafish sex differentiation and gonad development”, Aquatic Toxicology, Vol. 174, https://doi.org/10.1016/j.aquatox.2016.02.003.
[133] Malits, J., M. Naidu and L. Trasande (2022), “Exposure to Endocrine Disrupting Chemicals in Canada: Population-Based Estimates of Disease Burden and Economic Costs”, Toxics, Vol. 10/3, p. 146, https://doi.org/10.3390/toxics10030146.
[78] Mancini, F. et al. (2020), “Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort”, International Journal of Cancer, Vol. 146/4, https://doi.org/10.1002/ijc.32357.
[35] Marty, M. et al. (2017), “Population-relevant endpoints in the evaluation of endocrine-active substances (EAS) for ecotoxicological hazard and risk assessment”, Integrated Environmental Assessment and Management, Vol. 13/2, pp. 317-330, https://doi.org/10.1002/IEAM.1887.
[127] Matthiessen, P. et al. (2017), “Recommended approaches to the scientific evaluation of ecotoxicological hazards and risks of endocrine-active substances”, Integrated Environmental Assessment and Management, Vol. 13/2, pp. 267-279, https://doi.org/10.1002/IEAM.1885.
[43] Matthiessen, P. et al. (2006), “Contamination of headwater streams in the United Kingdom by oestrogenic hormones from livestock farms”, Science of The Total Environment, Vol. 367/2-3, pp. 616-630, https://doi.org/10.1016/J.SCITOTENV.2006.02.007.
[34] Matthiessen, P., J. Wheeler and L. Weltje (2018), “A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations”, Critical Reviews in Toxicology, Vol. 48/3, pp. 195-216, https://doi.org/10.1080/10408444.2017.1397099.
[151] Matthiessen, P., J. Wheeler and L. Weltje (2017), “A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations”, Critical Reviews in Toxicology, Vol. 48/3, pp. 195-216, https://doi.org/10.1080/10408444.2017.1397099.
[145] Maulvault, A. et al. (2019), “Bioaccumulation and ecotoxicological responses of juvenile white seabream (Diplodus sargus) exposed to triclosan, warming and acidification”, Environmental Pollution, Vol. 245, https://doi.org/10.1016/j.envpol.2018.11.020.
[13] Metcalfe, C. et al. (2022), “An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment”, Environmental Research, Vol. 207, p. 112658, https://doi.org/10.1016/J.ENVRES.2021.112658.
[82] Meyer, T. et al. (2007), “A case-control study of farming and prostate cancer in African-American and Caucasian men”, Occupational and Environmental Medicine, Vol. 64/3, https://doi.org/10.1136/oem.2006.027383.
[148] Miller, J. and M. Hutchins (2017), The impacts of urbanisation and climate change on urban flooding and urban water quality: A review of the evidence concerning the United Kingdom, https://doi.org/10.1016/j.ejrh.2017.06.006.
[26] Ministère de la transition écologique et solidaire (2019), Second National strategy on endocrine disruptors – 2019-2022: Action Plan, Ministère de la transition écologique et solidaire and the Ministère des Solidarités et de la Santé, Paris, https://www.ecologie.gouv.fr/ (accessed on 31 May 2022).
[97] Murk, A. et al. (2013), Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals, https://doi.org/10.1016/j.tiv.2013.02.012.
[92] Murphy, L. et al. (2019), “Exposure to bisphenol A and diabetes risk in Mexican women”, Environmental Science and Pollution Research, Vol. 26/25, https://doi.org/10.1007/s11356-019-05731-9.
[54] Nilsen, E. et al. (2019), “Critical review: Grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs”, Environmental Toxicology and Chemistry, Vol. 38/1, pp. 46-60, https://doi.org/10.1002/ETC.4290.
[22] NORMAN Network and Water Europe (2019), “Contaminants of Emerging Concern in Urban Wastewater: Joint NORMAN and Water Europe Position Paper”.
[9] Norris, D. and J. Carr (2020), Vertebrate Endocrinology 6th edition, Elsevier, https://doi.org/10.1016/C2019-0-00957-0.
[18] OECD (2019), Pharmaceutical Residues in Freshwater: Hazards and Policy Responses, OECD Studies on Water, OECD Publishing, Paris, https://doi.org/10.1787/c936f42d-en.
[31] OECD (2018), Considerations for Assessing the Risks of Combined Exposure to Multiple Chemicals, Series on Testing and Assessment No. 296, OECD Environment, Health and Safety Division, Environment Directorate.
[6] OECD (2018), Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption, OECD Series on Testing and Assessment, No. 150, OECD Publishing, Paris, https://doi.org/10.1787/9789264304741-en.
[52] Oloruntoba, K. et al. (2022), “Polybrominated diphenyl ethers (PBDEs) concentrations in soil, sediment and water samples around electronic wastes dumpsites in Lagos, Nigeria”, Emerging Contaminants, Vol. 8, pp. 206-215, https://doi.org/10.1016/J.EMCON.2022.03.003.
[84] Omran, G. et al. (2018), “Potential hazards of bisphenol A exposure to semen quality and sperm DNA integrity among infertile men”, Reproductive Toxicology, Vol. 81, https://doi.org/10.1016/j.reprotox.2018.08.010.
[112] Orton, F. et al. (2018), “Exposure to an anti-androgenic herbicide negatively impacts reproductive physiology and fertility in Xenopus tropicalis”, Scientific Reports, Vol. 8/1, https://doi.org/10.1038/s41598-018-27161-2.
[48] Pal, A. et al. (2014), “Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle”, Environment International, Vol. 71, pp. 46-62, https://doi.org/10.1016/J.ENVINT.2014.05.025.
[116] Parrott, J. et al. (2017), “Uncertainties in biological responses that influence hazard and risk approaches to the regulation of endocrine active substances”, Integrated Environmental Assessment and Management, Vol. 13/2, pp. 293-301, https://doi.org/10.1002/IEAM.1866.
[2] Persson, L. et al. (2022), “Outside the Safe Operating Space of the Planetary Boundary for Novel Entities”, Environmental Science and Technology, Vol. 56/3, https://doi.org/10.1021/acs.est.1c04158.
[32] Pironti, C. et al. (2021), “Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure”, https://doi.org/10.3390/w13101347.
[58] Pistocchi, A. and R. Loos (2009), “A Map of European Emissions and Concentrations of PFOS and PFOA”, Environmental Science & Technology, Vol. 43/24, pp. 9237-9244, https://doi.org/10.1021/es901246d.
[107] Rajasärkkä, J. et al. (2016), “Drinking water contaminants from epoxy resin-coated pipes: A field study”, Water Research, Vol. 103, https://doi.org/10.1016/j.watres.2016.07.027.
[93] Rancière, F. et al. (2019), “Exposure to bisphenol a and bisphenol s and incident type 2 diabetes: A case-cohort study in the French cohort D.E.S.I.R.”, Environmental Health Perspectives, Vol. 127/10, https://doi.org/10.1289/EHP5159.
[61] Rocha, M. et al. (2019), “Presence of estrogenic endocrine disruptors in three European estuaries in Northwest Iberian Peninsula (Portugal)”, Toxicological and Environmental Chemistry, Vol. 101/3-6, https://doi.org/10.1080/02772248.2019.1692019.
[113] Rodil, R. et al. (2019), “Legacy and emerging pollutants in marine bivalves from the Galician coast (NW Spain)”, Environment International, Vol. 129, pp. 364-375, https://doi.org/10.1016/j.envint.2019.05.018.
[63] Routti, H. et al. (2019), State of knowledge on current exposure, fate and potential health effects of contaminants in polar bears from the circumpolar Arctic, https://doi.org/10.1016/j.scitotenv.2019.02.030.
[38] Ruhí, A. et al. (2016), “Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web”, Science of The Total Environment, Vol. 540, pp. 250-259, https://doi.org/10.1016/j.scitotenv.2015.06.009.
[115] Saaristo, M. et al. (2018), “Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife”, Proceedings of the Royal Society B, Vol. 285/1885, https://doi.org/10.1098/RSPB.2018.1297.
[44] Schapira, M. et al. (2020), “Involuntary human exposure to carbamazepine: A cross-sectional study of correlates across the lifespan and dietary spectrum”, Environment International, Vol. 143, https://doi.org/10.1016/j.envint.2020.105951.
[20] Schwarzenbach, R. et al. (2006), “The Challenge of Micropollutants in Aquatic Systems”, Science, Vol. 313/5790, pp. 1072-1077, https://doi.org/10.1126/science.1127291.
[66] Shekhar, S. et al. (2017), “Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population”, General and Comparative Endocrinology, Vol. 241, https://doi.org/10.1016/j.ygcen.2016.05.025.
[114] Shi, W. et al. (2010), “Removal of estrone, 17α-ethinylestradiol, and 17ß-estradiol in algae and duckweed-based wastewater treatment systems”, Environmental Science and Pollution Research, Vol. 17/4, https://doi.org/10.1007/s11356-010-0301-7.
[80] Soto, A. and C. Sonnenschein (2010), Environmental causes of cancer: Endocrine disruptors as carcinogens, https://doi.org/10.1038/nrendo.2010.87.
[68] Steenland, K., V. Barry and D. Savitz (2018), “Serum perfluorooctanoic acid and birthweight an updated meta-analysis with bias analysis”, Epidemiology, Vol. 29/6, https://doi.org/10.1097/EDE.0000000000000903.
[94] Sun, Q. et al. (2014), “Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: A prospective investigation in the nurses’ health study (NHS) and NHSII cohorts”, Environmental Health Perspectives, Vol. 122/6, https://doi.org/10.1289/ehp.1307201.
[88] Sun, Q. et al. (2018), “Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: A prospective investigation among U.S. women”, Environmental Health Perspectives, Vol. 126/3, https://doi.org/10.1289/EHP2619.
[73] Swan, S. et al. (2015), “First trimester phthalate exposure and anogenital distance in newborns”, Human Reproduction, Vol. 30/4, https://doi.org/10.1093/humrep/deu363.
[41] Tran, N., M. Reinhard and K. Gin (2018), “Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review”, Water Research, Vol. 133, pp. 182-207, https://doi.org/10.1016/j.watres.2017.12.029.
[135] Trasande, L. (2014), “Further Limiting Bisphenol A In Food Uses Could Provide Health And Economic Benefits”, Health Affairs, Vol. 33/2, pp. 316-323, https://doi.org/10.1377/hlthaff.2013.0686.
[131] Trasande, L. et al. (2016), “Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis”, Andrology, Vol. 4/4, pp. 565-572, https://doi.org/10.1111/andr.12178.
[16] U.S. EPA (2015), EDSP: Weight of Evidence Analysis of Potential Interaction With the Estrogen, Androgen or Thyroid Pathways. Chemical: Glyphosate, United States Environmental Protection Agency, Washington, D.C., https://www.regulations.gov/document/EPA-HQ-OPP-2009-0361-0047 (accessed on 18 July 2023).
[21] UNEP (2022), Report of the Persistent Organic Pollutants Review Committee on the work of its eighteenth meeting (Report of the Persistent Organic Pollutants Review Committee on the work of its eighteenth meeting (UNEP/POPS/POPRC.18/11), Persistent Organic Pollutants Review Committee, Stockholm Convention on Persistent Organic Pollutants.
[7] US EPA (1997), Special Report on Environmental Endocrine Disruption: an Effects Assessment and Analysis, Office of Research and Development, United States Environmental Protection Agency, Washington, DC.
[42] Ussery, E. et al. (2021), “A 30-Year Study of Impacts, Recovery, and Development of Critical Effect Sizes for Endocrine Disruption in White Sucker (Catostomus commersonii) Exposed to Bleached-Kraft Pulp Mill Effluent at Jackfish Bay, Ontario, Canada”, Frontiers in Endocrinology, Vol. 12, p. 369, https://doi.org/10.3389/FENDO.2021.664157/BIBTEX.
[126] Van der Oost, R., J. Beyer and N. Vermeulen (2003), Fish bioaccumulation and biomarkers in environmental risk assessment: A review, https://doi.org/10.1016/S1382-6689(02)00126-6.
[29] Vandenberg, L. et al. (2012), “Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses”, Endocrine Reviews, Vol. 33/3, pp. 378-455, https://doi.org/10.1210/er.2011-1050.
[102] Wee, S. and A. Aris (2017), “Endocrine disrupting compounds in drinking water supply system and human health risk implication”, Environment International, Vol. 106, pp. 207-233, https://doi.org/10.1016/J.ENVINT.2017.05.004.
[40] Wee, S. et al. (2021), “Tap water contamination: Multiclass endocrine disrupting compounds in different housing types in an urban settlement”, Chemosphere, Vol. 264, p. 128488, https://doi.org/10.1016/J.CHEMOSPHERE.2020.128488.
[27] Welshons, W. et al. (2003), “Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity.”, Environmental Health Perspectives, Vol. 111/8, pp. 994-1006, https://doi.org/10.1289/ehp.5494.
[125] Wernersson, A. et al. (2015), “The European technical report on aquatic effect-based monitoring tools under the water framework directive”, Environmental Sciences Europe, Vol. 27/7, https://doi.org/10.1186/s12302-015-0039-4.
[3] WHO-UNEP (2013), State of the Science of Endocrine Disrupting Chemicals 2012, https://apps.who.int/iris/handle/10665/78101.
[30] Windsor, F., S. Ormerod and C. Tyler (2018), “Endocrine disruption in aquatic systems: up-scaling research to address ecological consequences”, Biological Reviews, Vol. 93/1, https://doi.org/10.1111/brv.12360.
[98] Woodruff, T. et al. (2008), “Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: executive summary”, Fertility and Sterility, Vol. 89/2, https://doi.org/10.1016/j.fertnstert.2007.10.002.
[101] Wormuth, M. et al. (2006), “What are the sources of exposure to eight frequently used phthalic acid esters in Europeans?”, Risk Analysis, Vol. 26/3, https://doi.org/10.1111/j.1539-6924.2006.00770.x.
[33] Yilmaz, B. et al. (2020), Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention, https://doi.org/10.1007/s11154-019-09521-z.
[120] Zala, S. and D. Penn (2004), “Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges”, Animal Behaviour, Vol. 68/4, pp. 649-664, https://doi.org/10.1016/j.anbehav.2004.01.005.
[118] Zhou, J., Z. Cai and X. Zhu (2010), “Are endocrine disruptors among the causes of the deterioration of aquatic biodiversity?”, Integrated Environmental Assessment and Management, Vol. 6/3, pp. 492-498, https://doi.org/10.1002/ieam.47.
[108] Zhou, X. et al. (2020), “Assessment of water contamination and health risk of endocrine disrupting chemicals in outdoor and indoor swimming pools”, Science of The Total Environment, Vol. 704, p. 135277, https://doi.org/10.1016/J.SCITOTENV.2019.135277.
Note
← 1. The cocktail effect is not specific to EASs and EDCs. Mixtures of other, non-EDC related, groups of chemicals are possible.