This chapter reviews Australia’s frameworks relating to chemical management, with a particular focus on industrial chemicals. It includes legislation and policies across all tiers of government, provides an overview of the main challenges associated with chemical management and discusses strengths and weaknesses of the system. While it is too soon to know the effects of state/territorial and Commonwealth legislative and policy reforms currently being developed, the chapter looks into how they may address gaps in the risk management system and what else could be done.
OECD Environmental Performance Reviews: Australia 2019

Chapter 5. Chemical management
Abstract
The statistical data for Israel are supplied by and under the responsibility of the relevant Israeli authorities. The use of such data by the OECD is without prejudice to the status of the Golan Heights, East Jerusalem and Israeli settlements in the West Bank under the terms of international law.
Introduction
The 2007 Environmental Performance Review of Australia (OECD, 2007) did not include an in-depth review of the chemical management framework. This chapter is intended as a starting point that can also be used to evaluate progress in future reviews. It comes at a time when states/territories and the Commonwealth are well advanced in the development of reforms to their chemical legislative and policy frameworks. In particular, the reform of the National Industrial Chemicals Notification and Assessment Scheme (NICNAS) and creation of a National Standard for Environmental Risk Management of Industrial Chemicals will set the direction for future management of such chemicals.
The chapter reviews Australia’s frameworks relating to chemical management, with a particular focus on industrial chemicals. It includes relevant legislation and policies across all tiers of government. It also provides an overview of the main challenges associated with chemical management and discusses strengths and weaknesses identified in the system. Although it is too early to evaluate how the reforms will be implemented across the country, the report looks into how they may address gaps in the risk management system and what else could be done.
This chapter has been prepared in collaboration with Canada, who participated as a reviewing country. To build upon the opportunity to share experience, the Canadian perspective is presented in the form of boxes throughout the chapter, focusing on specific aspects of the Canadian chemical management system (Box 5.2, Box 5.6, Box 5.7).
Pressures on health and the environment from chemicals
This section describes recent trends in chemical manufacture, export and import, along with challenges associated with continued growth of chemical imports. It also describes available tools to limit pressures on human health and the environment from emissions during chemicals’ production and use, and further progress that could be made.
The scope of “chemicals” as a term depends on the statistical collection used, more specifically the classification system(s) in each collection. It is thus subject to variation. Data in this section come from several types of collections, so notes are included to define the type of chemicals described.
Chemical production and trends
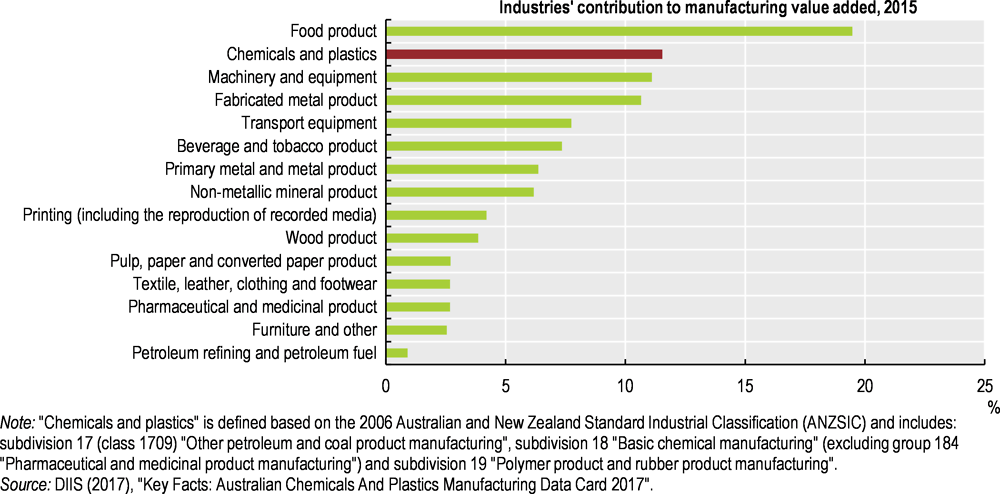
The chemical and plastic industry (including fertilisers and pesticides) is Australia’s second largest manufacturing industry, after food product manufacturing (Figure 5.1). It directly employs over 60 000 people and represents 11.5% of Australian manufacturing activity (DIIS, 2017); both figures have remained steady since 2006/07.
Figure 5.1. The chemical and plastic industry is the country’s second largest manufacturing sector
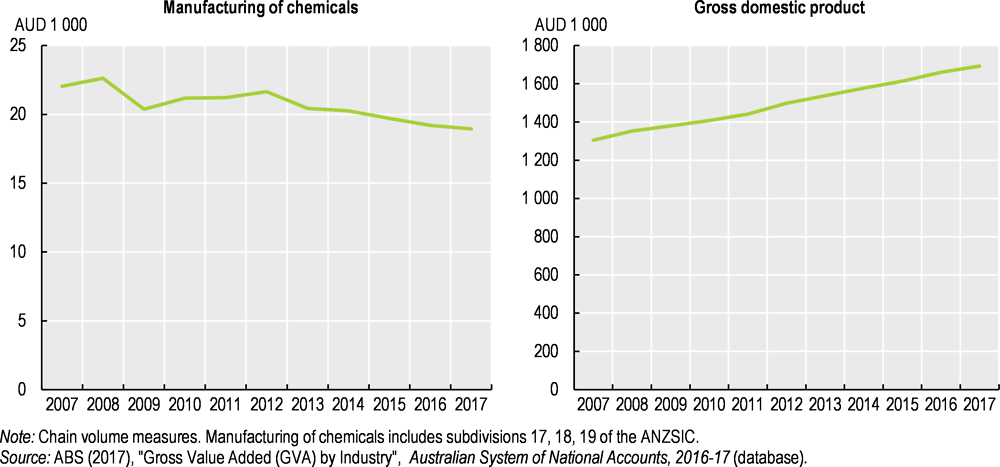
In 2014, 80% of outputs from the chemical industry became inputs to other industry sectors (PACIA, 2014) and the chemical industry contributed AUD 11.3 billion to gross domestic product (GDP) (DIIS, 2017), i.e. about 0.7% of GDP. However, GDP has steadily increased over the past ten years while the combined value of chemical and pharmaceutical production dropped by 14% (Figure 5.2).
Figure 5.2. The value of chemical and pharmaceutical production fell significantly in a decade of general economic growth
Australia’s manufacturing sector as a whole has been slowing since 2011 and is now one of the smallest in the OECD (OECD, 2018). Regarding chemical manufacturing in particular, the country has become a net importer of chemicals over the last decade as exports remained relatively stable while imports steadily increased. Since 2007, Australia’s trade deficit in chemical and plastic products has thus increased by 4.4% per year, on average (ABS, 2017), and the country has become increasingly dependent on imported chemical and plastic products.
It can be assumed that importation of articles containing chemicals would follow the same trend. A direct consequence could be an increase in the amount of chemicals entering Australia untracked and thus not subject to pre-market risk assessment. Like many other countries and regions, Australia does not impose information requirements on all substances entering the national market as part of imported articles. Chemicals contained in imported articles are regulated and assessed by NICNAS if they are designed to be released during use. Chemicals not designed to be intentionally released are not subject to registration or categorisation requirements, although they can be assessed in certain cases, e.g. if released from an article into the environment during use or disposal. However, importers do not have to declare the content of articles they import and often do not know their composition. With the rapid innovation shift to emerging economies (Lynn, 2007[9]), articles could contain new chemicals that have never been assessed in OECD countries, posing a potential threat to human health and the environment. Of particular concern are chemicals not intended for release that may leach from articles or enter waste streams. This is also a challenge for recycling.
Tracking chemical accidents and monitoring chemical emissions to determine pressures on human health and the environment
Most of Australia's population is concentrated in or near coastal areas. Chemical industry facilities follow the same pattern and are mainly located along the south and east coasts and in the vicinity of capital cities. In addition to being the most densely populated areas, these are also the most environmentally vulnerable.
To monitor and manage risks associated with chemical facilities – chemical accidents, release of chemicals to the environment during production and use, site contamination – Australia has various tools, such as a regulatory framework for management of risks related to what are known as major hazard facilities (MHF). It is based on the nature and quantity of chemicals present in a facility, and sets out enhanced security procedures and obligations. Another tool is the National Pollutant Inventory (NPI), a system monitoring emissions of polluting or harmful substances to the environment. However, as the chapter will show, these and other monitoring tools are not systematically applied or updated, and others lack co‑ordination.
As new challenges lie ahead, and human chemical exposure via the environment becomes of greater concern, more can be done to identify emerging contaminants and to support current risk assessment and management measures. Environmental monitoring and human bio-monitoring are important tools in this respect that can be used for monitoring progress and for decision making. They would benefit from increased harmonisation across states and territories for greater efficiency.
Reporting on chemical accidents
Australia does not maintain a national chemical accident database, nor does it systematically report to international accident databases. Chemical accident prevention is covered by state and territory regulations but is not co‑ordinated at the national level. The obligation to report accidents and incidents is not enforced identically by states and territories, although it is generally becoming more stringent. For example, Victoria is undertaking a major reform programme to better protect the environment, including tighter reporting requirements and penalties for not reporting.
Major hazard facilities
Since the early 2000s, progress in harmonising MHF legislation across states has been significant (Safe Work Australia, 2002). One major step was Safe Work Australia’s development of a set of model work health and safety (WHS) laws in 2011. The model laws have now been implemented and thus are legally binding in all jurisdictions except Victoria and Western Australia (Safe Work Australia, 2002), which have other legislation applying to MHF.
Reporting to the National Pollutant Inventory
One objective of pollutant release and transfer registers (PRTRs) is to achieve related UN Sustainable Development Goals by encouraging companies to adopt sustainable practices and reduce the amount of pollution released to the environment (OECD, 2017). Although Australia was an OECD country leader in the development of PRTR systems in the 1990s, the NPI, established in 1998, is now outdated and may not achieve the expected objective.
Among the 93 toxic chemicals that industrial facilities are meant to report on annually, only 70 are on the OECD harmonised list of 126 pollutants for PRTR reporting (OECD, 2014).
Environment Protection Authorities (EPAs) in states and territories are responsible for monitoring point source emission data provided by facilities in their jurisdictions. However, at the Commonwealth level, there is no mechanism for collective action or any prioritisation based on NPI data to show possible inconsistent outcomes in emission trends. For example, among nine key chemicals on the OECD harmonised list (OECD, 2014), NPI data reported across Australia between 2007 and 2016 showed emission decreases over time only for trichloroethylene, tetrachloroethylene and dichloromethane; stability of emissions for nickel and related compounds and for styrene; and a decrease, followed by an increase, for benzene, ethylbenzene and, marginally, di-(2-ethylhexyl) phthalate, while no trend could be derived for 1,2-dichloroethane.
Although the Commonwealth may take action on a case-by-case basis to deal with a particular concern, a systematic overarching monitoring mechanism is needed to address increases in emissions over time at the national level.
Diffuse source emissions (i.e. non-industrial sources such as transport, domestic heating and the use of pesticide) (Box 5.1) are of growing concern because they potentially have a greater impact on human health than point source emissions. While point source emissions are often emitted away from major population centres, population exposure to air pollution in metropolitan areas mainly comes from diffuse source emissions (Caiazzo et al., 2013).
Thus far, however, no diffuse source emission data have been regularly collected in the context of a national framework. States and territories model diffuse source emissions, especially to air, and some have produced emission estimates from a range of diffuse sources in their air emission inventories. However, most diffuse emission data in the NPI come from a study completed in 1998/99 (DEE, 2018a, 2018b), and the most recent addition dates from 2008. Little work has been completed on the level of pollutants found in ambient conditions in other media, such as water.
This situation needs to be taken into account in the review of the NPI (DEE, 2016) currently being conducted by the National Environment Protection Council (NEPC), which is considering emerging substances of concern, better use of data and diffuse source monitoring, as well as the scope for improving the performance of the NPI.
Box 5.1. The use of pesticides increases by an average of 5% a year
Total sales* of agricultural pesticides (in volume) have increased since 1990 at an annual rate of 5%. Although extensive agriculture is predominant in Australia, so the fertiliser and agrochemical footprint is relatively small compared to other OECD countries, agricultural pesticides are an increasing source of diffuse chemical pollution that is difficult to monitor and control.
* Data on pesticide sales are used as a proxy for pesticide use.
Source: OECD (2018), “Environmental performance of agriculture”, OECD Agriculture Statistics; OECD (2015), Innovation, Agricultural Productivity and Sustainability in Australia.
Remediation of contaminated sites
Soil and groundwater contamination associated with past land use is of growing concern. Commonwealth, state and territory legislation requires potentially contaminated sites to be assessed for contamination under the National Environment Protection Measure for Assessment of Site Contamination. Management remains reactive, however; for example, after NICNAS published alerts related to the effects of per- and poly‑fluoroalkyl substances (PFAS) on human health and the environment, it took 14 years for a nationally co‑ordinated framework on remediation of PFAS-contaminated sites to enter into force (Box 5.8). Mechanisms are needed for rapid action when emerging legacy contamination is detected.
Monitoring under the Stockholm Convention
Australia has conducted several successful monitoring activities since 2001 to support its obligations under the Stockholm Convention and to inform the Global Monitoring Plan (UNEP, 2018). The Pilot Monitoring Programme on persistent organic pollutants (POPs), for example, which ran from 2010 to 2015, contained core representative data from all regions. It focused on 40 chemicals, not only POPs but also other chemicals of concern. They were measured in human media, including blood, milk and urine, and in the environment (air, water). Monitoring and bio-monitoring showed most POPs concentrations had decreased since previous testing, thus supporting implementation of policies for POPs elimination (WEOG, 2015) (Box 5.7).
It is unfortunate that the POPs pilot monitoring programme was not continued. States and territories undertake ad hoc monitoring programmes in response to particular situations, but Australia has no national human bio-monitoring or environmental chemical monitoring programme, except for air quality (see below). There is willingness to engage further in bio-monitoring at the national level, however, and Australian health ministers recently agreed to conduct a feasibility study for a national bio-monitoring programme. This work is being led by the Victorian Department of Health and Human Services.
Monitoring ambient air quality data
Less extensive than the POPs monitoring programme but more sustainable, the national framework for air quality management, developed by the NEPC, includes two National Environment Protection Measures (NEPMs): the Ambient Air Quality NEPM and the Air Toxics NEPM, which set national standards for outdoor air quality and goals for key pollutants, and mandate monitoring and reporting requirements. Data, reported annually to the NEPC, are published.
Box 5.2. Monitoring and bio-monitoring, the Canadian perspective
Canada has a robust programme, the Chemicals Management Plan, aimed at reducing risks posed by chemical substances to human health and the environment. A key element of the plan is monitoring and surveillance of levels of harmful chemicals in Canadians and their environment. Monitoring and surveillance are essential to identify and track exposure to hazards in the environment and associated health implications.
Canada’s environmental monitoring programme focuses on media such as air, water, sediment and biota. Environmental monitoring is used to quantify exposure levels and generate science-based information to help identify risks and inform risk management. It is also used to understand the environmental fate and behaviour of chemicals and evaluate performance of control actions.
Canada also undertakes human bio-monitoring as part of its chemical management programme. Human bio-monitoring is used to establish baseline levels of chemicals in Canadians, detect trends in exposure over time and by geographical region, identify populations that may have higher levels of certain substances and may be at higher risk of adverse health effects, and identify substances not previously thought to be of concern or to accumulate in people.
Legal, policy and institutional framework, including domestic co-operation, for managing risks to health and the environment from chemicals
The primary policy objective of the chemical management system in Australia is to protect human health and the environment. Additional objectives are to protect trade and ensure national security.
These policy objectives are pursued through regulatory responsibilities shared between all levels of government, as well as through separate chemical regulatory regimes depending on the sector of chemical use. The result is a complex matrix, described in this section, involving over 19 agencies at the Commonwealth level, 34 at the state/territory level and many local councils, which are responsible for managing chemicals throughout their life cycle to protect public health, worker health and the environment. This regulatory framework contains gaps, particularly regarding environmental protection, and is challenging in terms of harmonisation among states and territories and consistency of the framework as a whole.
Chemical management: roles and responsibilities
The chemical regulatory framework operates across the Commonwealth, state/territory and local government levels, involving multiple policy departments, assessment agencies and regulatory decision makers.
Policy regarding regulation of chemicals is determined by ministerial councils. Commonwealth responsibilities primarily relate to risk assessment and risk management standard setting, while implementation of chemical risk management resides with the state and territory governments. Table 5.1 describes in more detail the breakdown of responsibilities at each level (OECD, 2015; DIIS, 2016).
Table 5.1. Government roles and responsibilities in chemical management: a multilayer framework
|
Level of government |
Regulatory responsibility |
|---|---|
|
Ministerial councils |
• Elaboration of the policy for regulation of chemicals |
|
Commonwealth |
• Maintenance of the national inventory • Registration (companies introducing chemicals) • Hazard and risk assessment of chemicals • Delivery of permits or certificates (introduction of new chemicals; see Section 5.5) • Implementation of international agreements and regulation of international trade |
|
States and territories |
• Risk management, including: a) Control of use of agricultural/veterinary (agvet) and industrial chemicals b) Protection of public health c) Work health and safety d) Transport (by road and rail) and storage of dangerous goods e) Environmental protection (emissions and disposal) |
|
Local |
• Land use planning and waste disposal (powers delegated by the relevant state) |
Source: Adapted from DIIS (2016), Chemicals business checklist; and OECD (2015), Preliminary analysis of policy drivers influencing decision making in chemicals management.
Chemical assessment and registration programmes
Chemicals are regulated according to use, with separate regimes for chemicals in therapeutic products, food ingredients, agvet chemicals and industrial chemicals (Box 5.3). The corresponding four chemical assessment and registration programmes at the Commonwealth level are as follows (Figure 5.3):
Therapeutic Goods Administration (TGA): The TGA, part of the Commonwealth Department of Health, regulates chemicals of therapeutic use.
Food Standards Australia New Zealand (FSANZ): Also part of the Department of Health, FSANZ sets standards for chemicals in food and food additives.
Australian Pesticides and Veterinary Medicines Authority (APVMA): Under the Department of Agriculture and Water Resources, the APVMA regulates agvet chemicals and products through the National Registration Scheme for Agricultural and Veterinary Chemicals, which sets out the regulatory framework for the management of these chemicals and products.
National Industrial Chemicals Notification and Assessment Scheme: Under the Industrial Chemicals (Notification and Assessment) Act 1989 (ICNA Act), new industrial chemicals are notified to and assessed by NICNAS, a statutory entity administered by the Office of Chemical Safety within the Department of Health.
Figure 5.3. The chemical regulatory system: a complex matrix
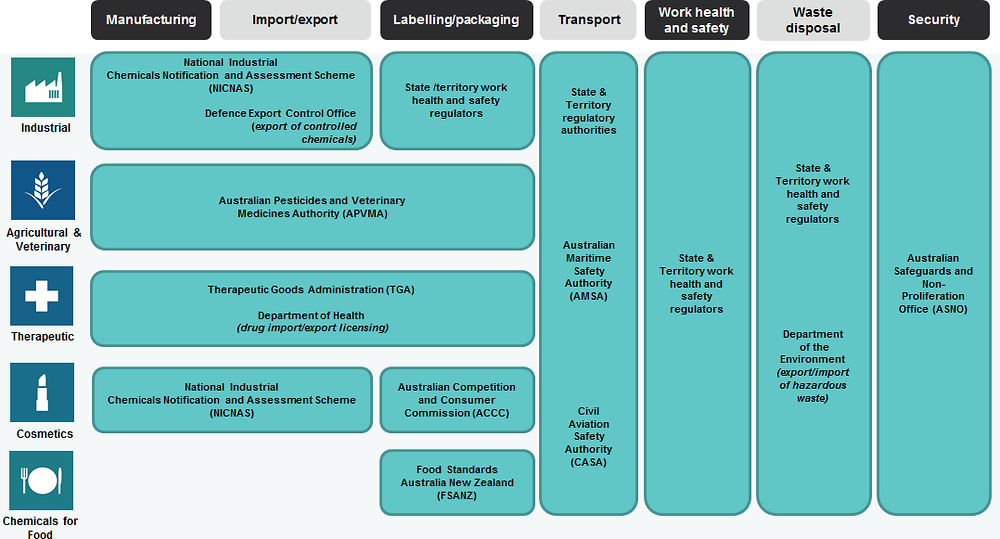
Source: DIIS (2016), Chemicals business checklist.
Box 5.3. Industrial chemicals: a category defined by exclusion
In Australia, industrial chemicals are defined by exclusion from other categories of chemicals. Industrial chemicals are all chemicals not used in medicines (human and animal), pesticides, foods and food additives. Thus, industrial chemicals are those used in everything else, from mining and manufacturing processes to domestic cleaning and cosmetic products. Certain biocides that do not meet the definition of an agvet chemical are regulated under the ICNA Act.
The role of NICNAS
Under NICNAS, Department of Health officers carry out occupational health and safety and public health assessments. Officers from the Department of the Environment and Energy (DEE) conduct environmental assessments for NICNAS under a service agreement and report back to the director of NICNAS.
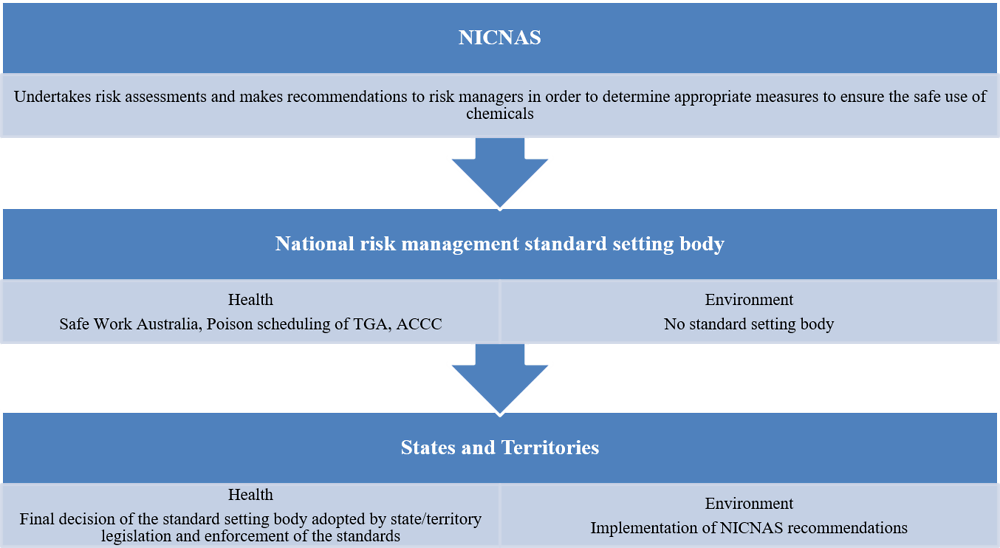
Depending on the outcome of an assessment, NICNAS makes recommendations on the safe use of chemicals. For the protection of human health, recommendations are submitted to the following statutory authorities at the Commonwealth level, in charge of chemical management (Figure 5.4):
Safe Work Australia: An independent Commonwealth agency, it has the primary responsibility of improving work health and safety across the country.
Standard for the Uniform Scheduling of Medicines and Poisons, under the TGA: The Poisons Standard applies to all chemicals that are available to the general public, including medicines, agricultural chemicals and consumer goods. It promotes uniformity in the scheduling of substances and in labelling and packaging requirements across Australia.
Australian Competition and Consumer Commission (ACCC): An independent Commonwealth statutory authority in charge of consumer product safety and responsible for regulation of consumer goods. The ACCC and state/territory consumer product safety regulators are particularly involved in (i) investigating potential chemical hazards in consumer products and (ii) developing bans and mandatory standards when evidence shows a consumer product has caused or could cause injury, illness or death.
As a Commonwealth entity, NICNAS does not have the constitutional authority to enforce its risk recommendations. The Commonwealth statutory authorities listed above are responsible for considering the NICNAS recommendations and determining any necessary risk management measures to control the use, release and disposal of industrial chemicals. States and territories are then responsible for adopting these measures into their regulatory frameworks.
There is no standard-setting body in charge of chemical management for environmental protection. Recommendations from NICNAS have not been implemented for the environment in any standardised way. The objective of the proposed National Standard for Environmental Risk Management of Industrial Chemicals (or National Standard) being developed by environment agencies is to close this gap by developing pre-established risk management measures to be implemented across the country (Figure 5.4, Box 5.4, Section 5.5.1).
Figure 5.4. The current process presents a gap in the environmental risk management
Co‑ordination of the chemical management framework
The chemical management system can be seen as a multilayer framework where more co‑ordination is needed to ensure a coherent regulatory system:
vertical co‑ordination between the assessment entities (Commonwealth) and the risk management entities (states and territories)
horizontal co‑ordination between risk management entities (i.e. between states and territories)
overall co‑ordination between regulatory regimes (depending on chemical use).
Australia has several vertical co-operation mechanisms. The Council of Australian Governments co‑ordinates inter-ministerial responsibilities. It initiates, develops and monitors implementation of policy reforms that are of national significance and that require co‑operative action by governments at various levels. Co‑ordination of responsibilities and promotion of greater jurisdictional coherence in regulatory practice and policy are ensured by high level meetings (e.g. Meeting of Environment Ministers), intergovernmental agreements or high-level forums such as Heads of EPAs. For the environment in particular, the NEPC is responsible for establishing NEPMs, which are sets of legal instruments designed to assist in protecting or managing particular aspects of the environment in a uniform and consistent way across jurisdictions (NEPC, 2018); NEPMs deal, for example, with Air toxics and Ambient air quality (see Monitoring ambient air quality data in Section 5.2.2), Assessment of site contamination, Movement of controlled waste and the NPI. Feedback from the states and territories to the Commonwealth on how NICNAS recommendations are implemented in terms of risk management measures is limited, though more transparency is expected with the relevant reform (see The role of NICNAS in the context of risk management in Section 5.6.1).
Horizontal co‑ordination is more difficult to achieve. Because each state or territory can implement laws in its jurisdiction according to its own timeline and to reflect its individual needs, chemical regulations may vary from one state or territory to another. With uneven application of risk management measures, some environments and populations remain unprotected. In addition, the complexity of the regulatory framework means the lack of harmonisation between states and territories results in additional administrative and financial burden for regulated entities. The need for greater national consistency in the chemical sector was raised in the research report of the Productivity Commission on Chemicals and Plastics Regulation (Productivity Commission, 2008). This report formed the basis of reform efforts, including those administered by NICNAS and the APVMA, and the development of the National Standard by DEE (Box 5.4).
To progress towards harmonisation among states and territories, a system of mirror legislation has been used in some cases to implement laws developed at the Commonwealth level. One example is the WHS laws developed by Safe Work Australia in 2011 to be implemented across Australia in order to provide a nationally consistent framework to secure the health and safety of workers and workplaces. The model WHS laws were implemented in 2012 in all states and territories except Western Australia, and Victoria which already had equivalent legislation. Some jurisdictions made minor variations to ensure the legislation was consistent with their own laws and processes (Safe Work Australia, 2018a). The National Standard is intended also to develop harmonised measures for the management of risk to the environment posed by industrial chemicals. These measures, in turn, will need to be implemented by the states and territories in their own legislation.
Horizontal mechanisms include bilateral meetings between jurisdictions, various meetings across states and territories, and working groups that ensure dialogue and co‑operation on specific issues. The Regulatory Science Network, established in 2011, is a network of government agencies responsible for regulating chemicals (including radioisotopes) and/or biological agents. Its objective is to discuss regulatory scientific issues among member agencies and improve interagency co‑operation.
Although differences between state regulations increase costs for business, the independence of states and territories can also be seen as a way of instilling competition to improve health and environmental protection. For example, a ban on lightweight single-use plastic bags was organised at the local and state/territory level rather than nationally. It was prompted by pressure from states, starting with South Australia in 2009, and implemented in all states and territories except New South Wales.
Box 5.4. Reforms to the industrial chemical framework are a way of addressing gaps
The two major reforms to the industrial chemical management framework were launched following recommendations made in 2008 by the Productivity Commission Research Report on Chemicals and Plastics Regulation.
The reform to NICNAS addresses risk assessment processes for the introduction of industrial chemicals in Australia, under the Department of Health.
Objective: Introduce a more proportionate risk-based framework for risk assessment, focusing on chemicals of greater risk to humans and the environment and making greater use of information from assessments performed by comparable regulatory agencies in other countries.
The centrepiece of this reform is a package of six bills: the Industrial Chemicals Bill 2017 and associated legislation, introduced in the House of Representatives and Senate. It will establish the framework of a new Australian Industrial Chemicals Introduction Scheme (AICIS), which will replace NICNAS.
The National Standard for Environmental Risk Management of Industrial Chemicals addresses risk management approaches, under DEE.
Objective: Reform Australia’s approach to the management of environmental risks posed by industrial chemicals.
DEE is working closely with NICNAS to ensure that the objectives of both reforms are met and that both are implemented in an integrated manner.
Source: Australian Government (2016), “National Standard for Environmental Risk Management of Industrial Chemicals”; NICNAS (2018), Reforms Cost Recovery Model discussion paper; Parliament of Australia (2018), Industrial Chemicals Bill 2017; Productivity Commission (2008), “Research Report on Chemicals and Plastics Regulation”.
Factors influencing decision making for chemical management
Role and input of stakeholders
Strong stakeholder engagement is an integral part of chemical management in Australia. It is achieved through information dissemination, consultations, and involvement and collaboration with stakeholders. Formal mechanisms, e.g. the Regulation Impact Statement (RIS), ensure that stakeholders are consulted on key national policies and contribute to the discussion in a transparent manner.
Pressure from the public has a significant influence on regulatory decisions. A key example is a ban on cosmetic testing on animals, which will be achieved through the future AICIS. An important driver of the introduction of the ban was the message of strong public support, along with the fact that it will bring Australia into line with EU countries and others banning such testing (Department of Health, 2018a).
Economic analysis
The government is committed to conducting cost-benefit analysis (CBA) when a new policy is under consideration. CBA helps the government move towards transparent regulatory design and support decision making (APRA, 2018). States and territories also use economic analysis to support decision making.
Resourcing of chemical management programmes
Chemical management functions are broad and include a variety of funding structures. In line with recommendations by the United Nations Environment Programme (UNEP, 2015), the APVMA, TGA and NICNAS are primarily funded through cost-recovery mechanisms, i.e. fees and charges paid by industry. The Hazardous Waste (Regulation of Exports and Imports) Act 1989 regulates waste import, export and transit across Australia’s national borders through a system of cost-recovered permits. FSANZ, however, is primarily funded by appropriation and only a small part of its work plan is cost recovered (APVMA, 2015). Australia’s participation in the Stockholm, Rotterdam, Basel and Minamata conventions is funded by the federal government, except provision of technical input provided by regulators, which is funded through cost recovery from industry.
Great differences exist between the states/territories regarding funding mechanisms, and resources to implement chemical management measures depend upon their legislation. In some states and territories, emission fees, based on the polluter-pays principle, are one cost-recovery mechanism. The principle, although not established at the Commonwealth level, seems to have been adopted in most states and territories and is implemented under state/territory environmental protection laws.
Although cost-recovery mechanisms are key to a sustainable legal and institutional framework for sound chemical management (UNEP, 2015), the full cost-recovery funding system of NICNAS may have potential side effects. Industry perceives the high cost of introducing chemicals in Australia as disproportionate, a potential hindrance to competitiveness and innovation, and a limitation on incentives to move to newer, safer chemicals. In addition, because not all substances are available on the Australian market, companies tend to manufacture articles in other countries, which increases the trade deficit (Section 5.2.1).
⇒ Focus on the NICNAS reform:
The reform will not change the government position that the full cost of regulatory activities is to be recovered through fees and charges paid by regulated entities (NICNAS, 2018a). But it will drive some input on substitution by safer chemicals (Box 5.10).
⇒ Focus on the National Standard:
In line with government policy on charges, DEE is exploring the option of recovering the costs to the Commonwealth associated with the establishment and administration of the National Standard. If the government decides cost recovery is appropriate, the department will develop a statement outlining potential cost-recovery arrangements.
Performance measurement
Performance measurement framework for cost saving
As the government is committed to reducing the cost of potentially unnecessary or inefficient regulation, a performance measurement framework was put in place in 2014 to increase the transparency and accountability of Commonwealth regulators. Regulators such as NICNAS and the APVMA are required to assess their performance through public annual self-assessment reports that demonstrate performance against key generic indicators. The framework is not specific to chemical management.
Performance measurement framework for monitoring the impact of chemicals on the environment
Some OECD countries (e.g. Canada, Box 5.5) have formal mechanisms to evaluate the effectiveness of existing risk management measures. It is uncertain to what extent Australian states and territories have such mechanisms in place and their possible relation to the Commonwealth level. To go a step further towards performance measurement, it would be necessary to develop indicators to evaluate the current impact of chemicals on health and the environment so as to set a baseline against which performance of reforms could be measured in the future.
The 2008 Productivity Commission research report on chemicals and plastics regulation recommended: “Examination of the feasibility of developing a performance measurement framework for monitoring the impact of chemicals in the environment” (Recommendations 9.3) (Productivity Commission, 2008). It has not been determined yet whether this recommendation will be implemented. Once the National Standard is in place, the recommendation may gradually be acted upon (Commonwealth of Australia, 2017).
Successful implementation of a chemical management programme also requires funding for the collection of information related to monitoring and reporting, which may need to be secured by the federal government.
Box 5.5. Performance measurement, the Canadian perspective
Since 2016, Canada has taken a systematic approach to measuring the performance of new or amended risk management instruments. For each new or amended instrument, an implementation strategy is developed that documents the information that will be needed to measure progress in achieving risk management objectives. Timelines for the evaluation are also set.
Instrument-based performance measurement evaluates the effectiveness of an instrument in meeting specific risk management objectives that were set when the risk management tool was designed.
Substance-based performance measurement considers the performance of all final risk management instruments applied to a chemical substance to determine if human health and the environment were adequately protected from risks identified in risk assessment. To date, Canada has undertaken such measurement for four pilot substances. The results will help determine if additional risk management or assessment is needed.
The data sources Canada uses to measure the performance of its risk management instruments range from annual reports submitted by industry to information from the National Pollutant Release Inventory and environmental and bio-monitoring programmes. Information is also collected through mandatory surveys of industry stakeholders on the manufacture, import and use of chemicals in Canada.
Pollution Prevention Planning Notices are one type of risk management instrument that Canada has systematically used for performance measurement. They require the persons subject to them to prepare and implement a pollution prevention plan. They also require reporting used to gather data to measure overall progress in meeting the intended risk management objectives. A performance report is then prepared and posted on the notice's webpage after the reporting deadline. The report summarises the effectiveness of the notice in meeting the risk management objectives set out when the notices were designed.
Source: Government of Canada (2018), Pollution prevention notices performance results.
International obligations and co‑operation
On the international level, Australia plays an active role. Past and recent reforms contribute to reaching the goals of the Strategic Approach to International Chemical management, for sound management of chemicals by 2020, such as the (still partial) implementation of the United Nations’ Globally Harmonised System of Classification and Labelling of Chemicals (GHS). Australia is a signatory of the global environmental conventions, although there has been significant delay in ratification of amendments to the conventions, and of new conventions, due to the complexity of the legal framework. Mechanisms are needed to facilitate and accelerate domestic treaty-making processes.
Compliance with international conventions
Stockholm Convention on Persistent Organic Pollutants
Although Australia ratified the Stockholm Convention, becoming a party in 2004, it has not ratified recent amendments. As substances will continue to be added to the convention, a more responsive process for including amendments should be explored.
In 2006, the government developed a national implementation plan to meet its obligations under the convention (Department of the Environment and Heritage, 2006). In line with this plan, action has been taken in relation to the 12 original POPs. Because their production, import and, in most cases, use were already banned in Australia (DEE, 2018c), ratifying the treaty did not imply changing the law.
However, the implementation plan has not been updated and Australia is not in a position to act on some of the 16 additional POPs listed in annexes to the convention since 2009. This is because some of the new chemicals are still in use in Australia, so a complex domestic treaty-making process is needed to change the law before ratification (DEE, 2018d). A RIS was prepared before the 2009 Conference of the Parties, and the technical and regulatory implications of several of the listings are still being explored, including CBAs, as regulatory change is required to fulfil the management requirements, particularly with regards to management of waste materials and articles containing POPs. The National Standard will be key in this respect, as it will provide a legislative framework to implement the requirements. Given the nature of the process, it is difficult to predict ratification and these globally restricted chemicals may remain in use in Australia.
Actions that can be taken for agvet and industrial chemicals in this context are fundamentally different, as Boxes 5.7 and 5.8 show. Agvet regulations can prohibit certain activities in relation to these chemicals. The APVMA has legal powers to conduct reviews of approved active constituents and registered products. These powers include the authority to suspend or cancel active constituents and product registrations, e.g. for pesticide POPs. In the case of endosulfan (Box 5.7), the APVMA de-registration followed the nomination of endosulfan to the Stockholm Convention, and took effect in 2010, i.e. one year before endosulfan was formally listed in Annex A of the Convention.
Industrial chemicals are subject to a different regulation, making the process slow and inefficient. As the example of PFASs in Box 5.8 illustrates, the only means available to NICNAS are alerts and recommendations as incentives to take action, e.g. to restrict the use of chemicals and move to safer chemicals.
⇒ Focus on the NICNAS reform:
After the NICNAS reform, if risks cannot be properly managed, it will be possible to remove a chemical from the market, since the executive director of AICIS can stop its introduction, for example by cancelling the assessment certificate, varying the terms of the listing on the inventory or removing the chemical from the inventory (NICNAS, 2017a). However, it is uncertain whether the reform will be sufficient to facilitate ratification of the amendment in a timely manner.
Box 5.6. Ratification of international conventions, the Canadian perspective
Canada’s process for ratifying amendments to the Stockholm Convention is similar to that used by Australia, in that Canada must “opt in” by depositing an instrument of ratification. In the case of perfluorooctane sulfonate (PFOS), Canada ensured that its domestic regulations prohibiting the manufacture, import and use of PFOS were aligned with the requirements of the listing in the convention such that it claimed specific exemptions and permitted uses that aligned with those in its domestic regulation. Since 2011, Canada has been amending its domestic regulations to remove unneeded exemptions. Details on how Canada is implementing obligations for PFOS and other POPs under the convention are detailed in “Update to Canada’s National Implementation Plan under the Stockholm Convention on Persistent Organic Pollutants”, published in April 2013.
Source: Government of Canada (2018), Update to national implementation plan on persistent organic pollutants.
Other international conventions
Australia ratified the Rotterdam Convention on Prior Informed Consent in 2004 and has complied with its obligations. Import and export decisions for industrial chemicals and pesticides listed in Annex III of the convention reflect the current regulatory status of those chemicals in Australia and are administered by NICNAS and the Department of Agriculture and Water Resources, respectively.
Australia was an early signatory of the Vienna Convention and Montreal Protocol and has ratified all amendments, including the Kigali Amendment, which provides for a phase-down of hydrofluorocarbon (HFC) production and imports. Legislative requirements of the Ozone Protection and Synthetic Greenhouse Gas Management Act 1989 have reduced total imports of these chemicals, as well as domestic emissions, to meet Montreal Protocol requirements. Australia has met or exceeded its obligations under the protocol. It has an accelerated phase-out of hydrochlorofluorocarbons (HCFCs), which will see HCFC imports over 1996-2020 at a level 60% less than the Montreal Protocol prescribes. Australia began its HFC phase-down on 1 January 2018, a year ahead of the Montreal Protocol’s schedule and 25% below the baseline set by the protocol (DEE, 2018e).
Australia signed the Minamata Convention on Mercury in 2013, but is still not a party to the convention. The consultation (including RIS and CBA) on ratification, launched by DEE, closed in March 2017 (DEE, 2018f). In March 2018, the RIS was being finalised and the treaty-making process was under way. But as the lag of more than five years makes clear, the timeliness of the ratification process should be improved.
Box 5.7. Impact of the Stockholm Convention: example of success with a pesticide POP
The organochlorine pesticide endosulfan, which was widely used in Australia to control some insects and mites in crops, particularly cotton, showed a major decrease in concentration during the four years of monitoring at all sites in the Pilot Monitoring Programme for POPs (Figure 5.5). This decrease followed de-registration of endosulfan by the APVMA in 2010, which meant a ban on the use of endosulfan in Australia after a two year phase-out period. The cancellation of endosulfan registration followed the nomination of endosulfan to the Stockholm Convention, which facilitated collection of new environmental data.
Figure 5.5. Endosulfan concentrations at study sites in 2011-14
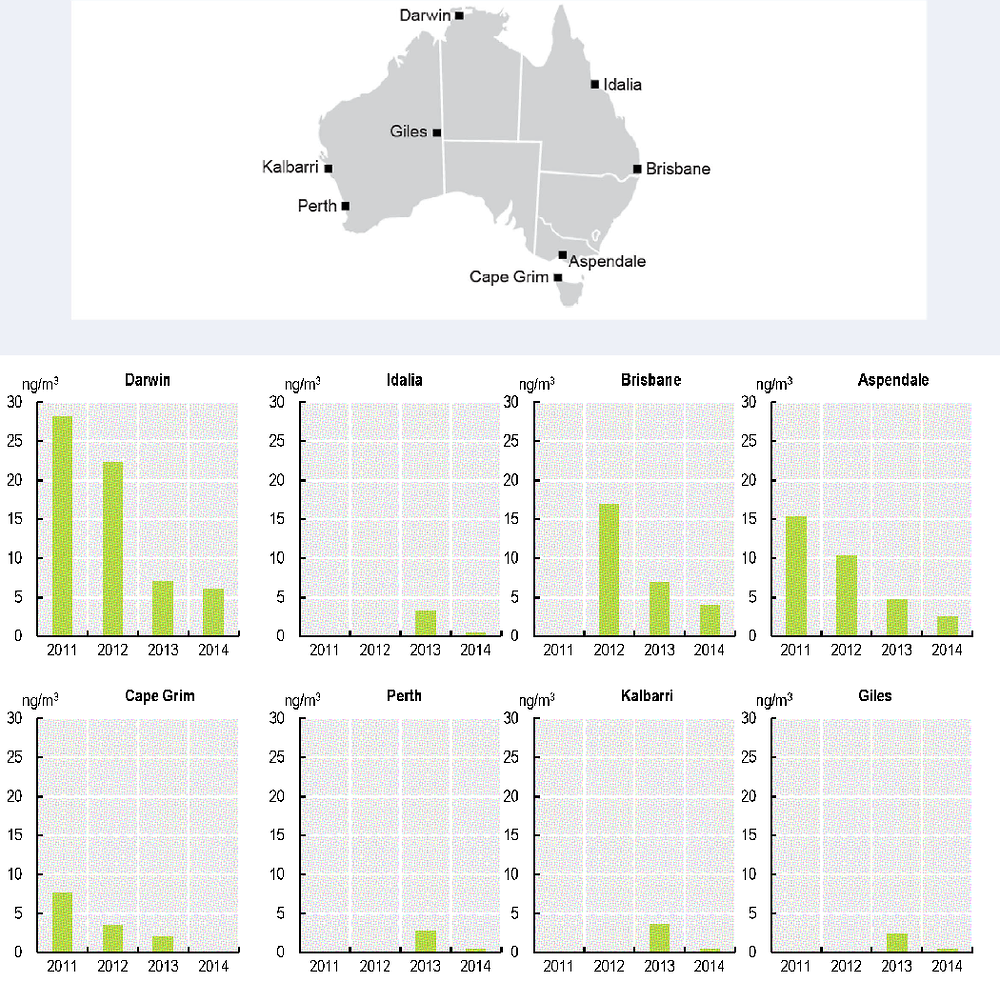
Source: Modified from Keywood, M., M. Hibberd and K. Emmerson (2017), Australia State of the Environment 2016: Atmosphere. Annual concentration of endosulfan measured in air, 2011-2014.
Box 5.8. Substantial delays in taking action on industrial POPs: the example of PFAS
The release of per- and poly- fluoroalkyl substances into the environment is a concern globally, as some of these chemicals are highly persistent, bioaccumulate, can be transported long distances in the environment and are linked to adverse effects in humans and some plants and animals. PFAS contamination has been found at many sites, including where firefighting foams containing PFAS have been used.
2002: Early warning from NICNAS
Since 2002, NICNAS has published six alerts to inform importers, users and the general public about the known effects of some commonly used PFAS on human health and the environment, recommending that PFOS and related PFAS be restricted to essential uses for which no suitable and less hazardous alternatives are available, in order to minimise dispersal into the environment.
One NICNAS recommendation urged industry to seek alternatives and phase out PFAS and PFAS-related substances of concern. Industry has phased out some PFASs in certain consumer products and the trend among global manufacturers and users is to replace long-chain PFAS with shorter-chain acids that are less toxic and less bioaccumulative, although some essential uses of PFAS still exist.
2009: Listing under the Stockholm Convention
PFOS, its salts and perfluorooctane sulfonyl fluoride were listed under the Stockholm Convention for restriction in 2009, while pentadecafluorooctanoic acid or perfluorooctanoic acid, its salts and related compounds, as well as perfluorohexane sulfonic acid, its salts and related compounds were proposed for listing.
2017: The late start of policy responses to PFAS contamination
DEE published the Commonwealth Environmental Management Guidance on PFOS and PFOA in 2016, and in 2017 released a RIS on options for a national phase-out of PFOS and related chemicals. In 2018, Australia put in place a co‑ordinated framework across states and territories for the environmental regulation of PFAS-contaminated materials and sites, establishing a PFAS National Environmental Management Plan and an Intergovernmental Agreement on a National Framework for Responding to PFAS Contamination).
Although Australia has not ratified the treaty amendment to the convention, some actions have been taken to address this global issue. However, ratifying the amendments would have further supported risk management of these chemicals earlier on.
Source: DEE (2016), “Commonwealth Environmental Management Guidance on Perfluorooctane Sulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA)”; EPA Victoria (2018), PFAS National Environmental Management Plan; NICNAS (2018), Per- and poly-fluoroalkyl substances (PFASs).
Implementation of the Globally Harmonised System of Classification and Labelling
The GHS is a global standard for classifying and communicating chemicals’ hazardous properties. Its implementation is the responsibility of states and territories. It is now mandatory in most states and territories in the workplace (overseen by Safe Work Australia) and transport (under the Department of Infrastructure and Regional Development) (UNECE, 2018). Model WHS regulations, passed by most jurisdictions in 2012, were key in promoting GHS implementation. The GHS became mandatory for occupational settings on 1 January 2017 (except in Victoria and Western Australia, where it is accepted but not mandatory).
However, the GHS has not been implemented in all sectors in Australia and workplace hazardous chemicals subject to other labelling laws are exempted either partially or completely from workplace GHS labelling requirements to avoid regulatory inconsistency or duplication. Agvet chemical products are not required to comply fully with GHS requirements and have a specific, risk based labelling system approved by the APVMA. Hazardous chemicals that are labelled for consumer use and only used in the workplace in a quantity and way consistent with household use do not need to comply with GHS labelling requirements either, as these are subject to regulation under Australian consumer laws. Therapeutic goods are labelled in accordance with therapeutic goods laws (including the Poisons Standard) and therapeutics labelling is exempted from GHS labelling requirements when in the form intended for administration to humans.
In addition, GHS labelling, when implemented, has not been applied for environmental hazards. More needs to be done to expand GHS implementation and thus improve hazard communication and enhance the protection of human health and the environment during the handling, transport and use of chemicals.
Assessment of the costs and benefits of introducing environmental labelling for industrial chemicals was recommended in the 2008 Productivity Commission research report on chemical and plastic regulation (Productivity Commission, 2008), which DEE may consider implementing once the National Standard is established. The GHS criteria for the environment have been used in development of the draft National Standard, particularly in determination of scheduling criteria, and are used by NICNAS, which presents environmental classification under the GHS for information purposes, where sufficient data are available.
Systematic investigation of chemicals
Risks to occupational health and safety, public health and the environment from industrial chemicals are assessed by NICNAS, while the APVMA and its external advisory agencies assess agvet chemicals. This section describes the current systems, highlighting potential areas for improvement. It also looks at the NICNAS reform, including how the reform plans to address the current issues and what may remain to be done.
Assessment of chemicals
Although NICNAS and APVMA assessments cover both human health and the environment, the potential indirect impact of chemicals in the environment on human health is not systematically reported; risk assessments would benefit from distinguishing more clearly between risks from consumer products and risks to humans exposed via the environment. Environmental risk assessment is not conducted across all regulatory regimes (Box 5.9). Thus, chemicals in food and food additives are not subject to environment risk assessment, nor are pharmaceuticals, despite increasing concern worldwide regarding the fate and effect of pharmaceuticals in the environment.
The regimes associated with industrial and agvet chemical assessment date from the 1990s. Despite efforts to work through the backlog of chemicals present on the market before then, many remain unassessed or may need to be screened for potential reassessment based on progress in the science over the last 25 years.
Agricultural chemicals
Agvet chemical active constituents and most products require approval and registration, respectively, before they can be legally sold in Australia, although limited use of an unregistered chemical may be allowed by permit (APVMA, 2018a). There are separate arrangements for some products that are of low regulatory concern (APVMA, 2018b). Over 10 000 agvet products have been registered for use (APVMA, 2018c). Active constituents and products are recorded in the Record of Approved Active Constituents and Register of Agricultural and Veterinary Products, respectively.
The National Registration Scheme for Agricultural and Veterinary Chemicals was implemented in 1995, with a view to achieving national uniformity in the registration process. There were then over 5 000 agvet product registrations granted under earlier arrangements by the states and territories (APVMA, 2018d). To re-examine these chemicals, the Chemical Review Program, under the APVMA, was put in place and priority lists were established. Further effort is needed to complete assessment of chemicals on the initial priority list. In addition, the full list of active ingredients on the market before 1995 may need to be screened for potential reassessment of priority levels given progress in the science since the 1990s, particularly on endocrine disruption.
Industrial chemicals
NICNAS classifies industrial chemicals as "existing" or "new", using the Australian Inventory of Chemical Substances (AICS) as the relevant regulatory tool. AICS describes conditions of use for certain chemicals. For purposes of regulation under the ICNA Act, any chemical on AICS is considered an existing chemical when used within any specified conditions of use, while any chemical not on AICS, or used outside of any specified conditions of use, is considered a new chemical. In addition, AICS describes conditions of use of chemicals, if any.
Every “introducer” of industrial chemicals, i.e. manufacturer and/or importer, must be registered with NICNAS prior to introducing any industrial chemicals (Department of Health, 2018b). Once a chemical is listed on AICS, anyone who is registered with NICNAS can introduce it without notification and assessment, subject to conditions of use and secondary notification requirements.
Existing chemicals
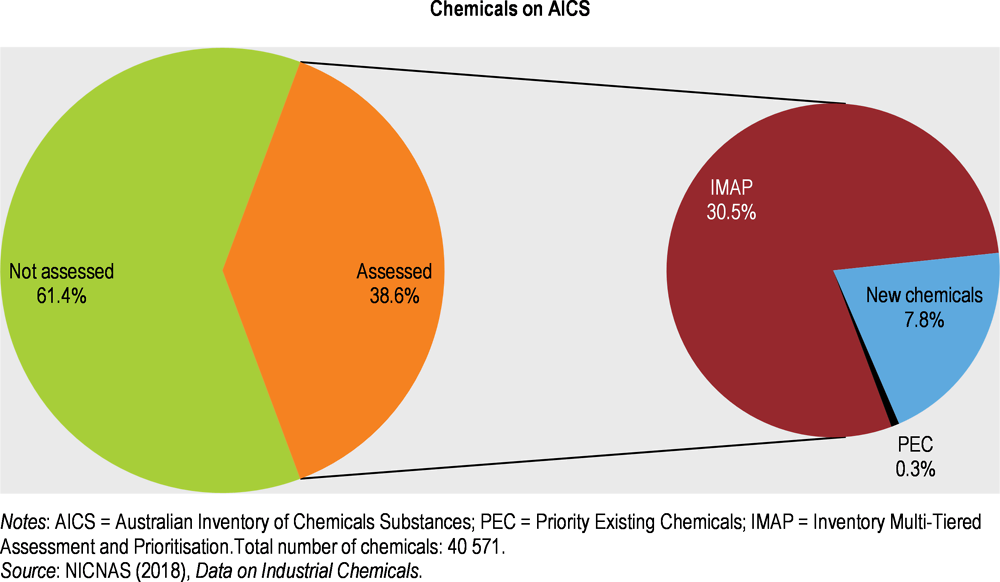
Of the approximately 40 500 chemicals listed on AICS (Department of Health, 2018b), a minority have been assessed (Figure 5.6). While efforts to fill this gap are continuing, it is uncertain how the reform would help in assessing the 25 000 still unassessed industrial chemicals within a reasonable time.
The chemicals on AICS are:
all industrial chemicals in use in Australia between 1 January 1977 and 28 February 1990
chemicals listed five years after an assessment certificate was granted under the ICNA Act
chemicals that were regulated under other Australian regimes and later became industrial chemicals.
Most chemicals on AICS were grandfathered in when the inventory was established, but had not been assessed by NICNAS. Before 2012, to fill this gap, NICNAS predominantly used the Priority Existing Chemicals (PECs) process, described in the ICNA Act, to assess industrial chemicals of concern. Nominated PECs are screened against criteria including volume of use, potential exposure and severity of effects on occupational health and safety, public health and the environment (NICNAS, 2018b). Although it provides a legislative framework for assessment, the process is slow and arguably marginal: only a few hundred PECs have been assessed to date (Figure 5.6).
In 2012, in response to concerns regarding the need to accelerate and prioritise assessment of existing chemicals, NICNAS developed a science- and risk-based framework for chemicals on AICS, the Inventory Multi-tiered Assessment and Prioritisation (IMAP) framework (Australian Government, 2012). Its objectives are to (i) identify and rapidly assess existing chemicals of concern and (ii) support risk management of industrial chemicals by enhancing the flow of chemical safety information. IMAP relies extensively on assessments performed abroad – particularly in Canada, the United States and EU – to inform NICNAS assessments (NICNAS, 2018c).
Between July 2012 and mid-2016, NICNAS assessed more than 3 000 chemicals using the IMAP framework. The choice of chemicals was based on three characteristics:
Chemicals for which NICNAS holds exposure data.
Chemicals identified as a concern for which action has been taken in other countries.
Chemicals detected in international studies analysing chemicals present in the blood in babies' umbilical cords.
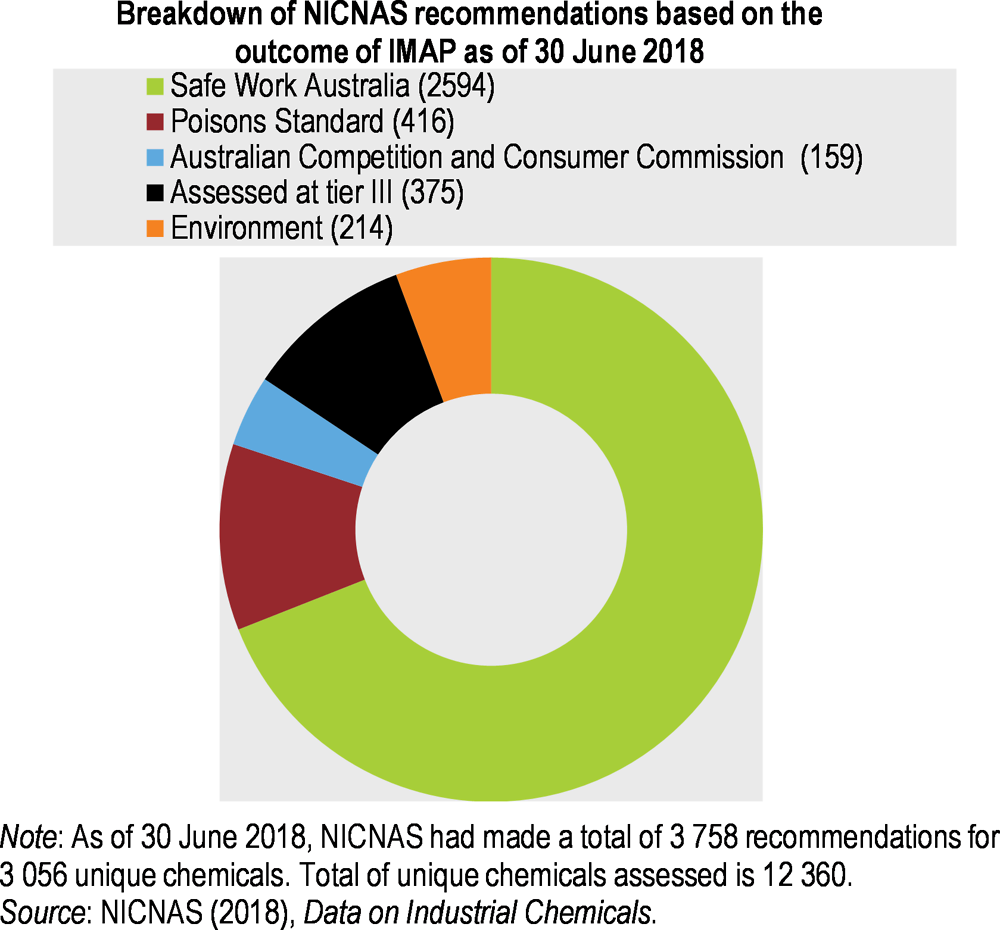
As of 30 June 2016, 62% of the 3 419 chemical assessments under IMAP had resulted in one or several NICNAS recommendations on risk management to the various standard-setting bodies for human health (NICNAS, 2018c).
In 2016, the government approved continued application of the IMAP framework while transitioning to new assessment arrangements as part of the NICNAS reform. Between 2016 and early 2018, NICNAS assessed over 8 500 additional substances under IMAP stage 2, reducing the gap from 92% unassessed chemicals on AICS in 2012 to 61% in June 2018 (Figure 5.6). The assessment focus shifted from identification of concerns (IMAP stage 1) to de-prioritisation (IMAP stage 2). Consequently, the more recent assessments resulted in significantly fewer recommendations than in stage 1, i.e. 11% of the chemicals assessed under stage 2. Overall, stages 1 and 2 led to recommendations on 25% of assessed chemicals (Figure 5.7).
Figure 5.6. Despite significant progress achieved with IMAP, a large share of existing chemicals remain unassessed
Figure 5.7. The IMAP framework is an effective means of industrial chemical assessment
⇒ Focus on the NICNAS reform:
The chemicals still unassessed on AICS, amounting to 61% of existing chemicals, fall into a grey zone of data-poor substances of unknown toxicity where the IMAP framework faces some limitations. Because IMAP is not a statutory tool, there is no legislative authority for NICNAS to conduct mandatory calls for information, which may be needed to assess these chemicals. The NICNAS reform will provide a legislative tool to request information from industry. In the new system that is supposed to replace both IMAP and the PECs process, it is expected that Australia will increase the use of international standards and assessment materials. The expected time frame for closing the gap remains unclear.
New chemicals
New chemicals are subject to a three-tier categorisation system: exemption, permit or certificate. The system is based on industry self-categorisation and primarily depends on introduction volumes (NICNAS, 2017b), although other criteria such as use, concentration, hazard and fate are also considered. Chemicals exempt from notification (i.e. the majority of new chemicals) are not assessed by NICNAS, but the introducer needs to comply with annual reporting and record-keeping requirements. Permits apply to lower-risk introductions, subject to a reduced assessment by NICNAS. Chemicals requiring a certificate undergo a more comprehensive assessment.
Since 2010, the annual number of exemptions, permits and certificates delivered was relatively stable, averaging 150-200 certificates, 100-150 permits and 10 000 chemicals reported exempt (largely cosmetics) (Department of Health, 2018b; NICNAS, 2018d). Exempt chemicals are reported each year; most of the 10 000 are thus the same year to year. About 13% of them were first reported in 2015/16 and 12.4% in 2016/17 (NICNAS, 2018d). As for the two other categories, around 300 industrial chemicals per year have been subject to a pre-introduction assessment by NICNAS, but the reform is expected to result in a 70% to 90% decrease to fewer than 100 pre-introduction assessments per year.
⇒ Focus on the NICNAS reform:
The current system largely relies on exposure-based criteria, such as annual introduction volume, as proxies for risk in determining how a chemical is regulated. A key driver of the NICNAS reform was the need to devise a system in which regulatory efforts would more proportionally reflect risk.
The reform will thus establish hazard and exposure criteria, which will be used by introducers to self-categorise industrial chemical introductions. Three categories have been defined: (i) exempted (very low risk), (ii) reported (low risk) and (iii) assessed (medium to high risk). The outcome of self-categorisation will allow risk-based regulation (NICNAS, 2017a). The reform will require introducers to declare exempted introductions annually. However, the chemicals introduced under this category will not be notified to AICIS prior to introduction and the declaration will not specify which chemicals were introduced. Like chemicals imported in articles, they would be allowed to enter the Australian market with no record from the regulatory authorities and no information on their chemical name or structure. This would make them difficult to manage in case of emerging risk.
In case of emerging risk it would be up to the introducer to consider any new hazard information that becomes available to them and then recategorise the introduction if needed. If the emerging hazard information was not available to the introducer, but was available to the executive director of AICIS, then targeted calls for information (to all those who have declared they are introducing under the exempted category) would need to occur to determine if any of the chemicals relevant to the new hazard information were being introduced.
The share of chemicals not requiring AICIS pre-market assessment is expected to grow significantly, with cosmetics and polymers mainly falling into the exempted or reported categories. The reduction in pre-market assessment will be balanced in the new system by increased AICIS post-market monitoring of lower-risk chemicals, enhanced compliance powers and increased regulatory efforts on higher-risk chemicals.
Auditing will also form an important part of the reformed regime, ensuring that introducers have categorised chemical introductions correctly.
Data requirements
To ensure that data are of sufficient quality for use in risk assessment, NICNAS and the APVMA require all new toxicity studies to be conducted according to the OECD Test Guidelines or other recognised test methods (e.g. EU, US, Japanese guidelines), and the standard of testing to obtain data should conform to the OECD Principles of Good Laboratory Practice.
The assessment programmes specify various sets of data requirements depending on the type and volume of chemicals being introduced. However, there are some gaps in the data requirements; in particular, potential effects from endocrine disrupters are not investigated systematically for industrial and agvet chemicals.
Agricultural chemicals
Any applicant for a new pesticide active ingredient or chemical product has to comply with defined data requirements. For toxicological data, the applicant should provide a comprehensive assessment comprising a full toxicology data package and a scheduling proposal for inclusion in the Standard for the Uniform Scheduling of Medicines and Poisons. Specific separate testing for endocrine disrupting potential is not required. The potential effects from endocrine disruptors however, are investigated through consideration of overall toxicological effects.
The APVMA can require an applicant to provide further information at any time during the evaluation process to address issues of concern, including toxicological concerns. Failure to do so is grounds for refusal (Sironis Pty Ltd, 2016).
Industrial chemicals
The ICNA Act specifies minimum information requirements (hazard, use and exposure data), which the notifier provides to NICNAS. The requirements are based on the notification category of a new chemical. There are 12 new-chemical notification categories (7 for certificate and 5 for permit), based on the type of chemical and amount introduced (NICNAS, 2018e). The data requirements and type and degree of risk assessment thus depend on the category of new chemical notification.
The data requirements nevertheless include gaps in the coverage of hazard end points, since neither reproductive and developmental toxicity nor carcinogenicity studies are included in the minimum data set, and screening for endocrine properties is requested only for the highest assessment category. These gaps may be filled in some circumstances via two mechanisms for submitting additional information: (i) a notifier with access to additional information must provide it with the application (NICNAS, 2018f) and (ii) the director of NICNAS can request additional data/testing from the notifier in case of a particular concern (e.g. structural alert). The power to require information is limited, however, since NICNAS cannot refuse a certificate application (Sironis Pty Ltd, 2016).
⇒ Focus on the NICNAS reform:
After the reform, industry will self-categorise chemicals based categories defined by a matrix of exposure parameters (defining exposure bands) and hazard parameters (defining hazard bands) (Figure 5.8; NICNAS, 2018g). Chemicals will be classified in exempted or reported categories if they don’t have any of the hazard characteristics described in the higher level hazard bands. However, carcinogenicity, reproductive and developmental toxicity, and adverse effects known to be mediated by an endocrine disruption mode of action are only to be reported if known (NICNAS, 2018g). Under the reform, generation of new data, if no data are available for these endpoints, is not required at least at the self-categorisation stage. Additional information may be required at the stage of AICIS pre-market assessment for chemicals that fall in the assessed category. For chemicals introduced in the highest exposure band this would include screening for endocrine properties as part of the repeated dose toxicity information requirement. Reproductive and developmental toxicity will be required for certain specified classes (such as introduction of polyhalogenated chemicals).
Figure 5.8. Determining the introduction category using the hazard band and the exposure band: Human health example
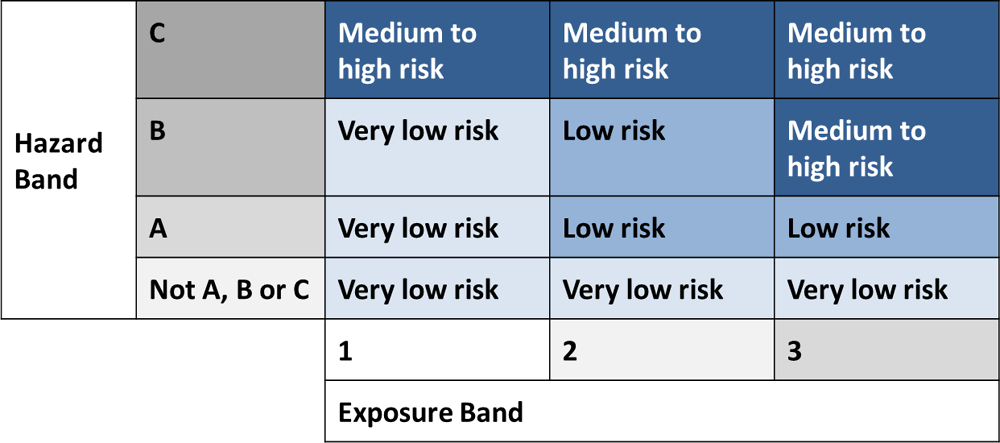
Source: NICNAS (2018), Notes on the draft General Rules.
Use of assessments performed in other countries
Acceptance of international assessments became a government principle in 2014, when the government, as part of the Industry Innovation and Competitiveness Agenda, adopted the principle that if a system, service or product has been approved under a trusted international standard or risk assessment, Australian regulators should not impose additional requirements for approval in Australia unless it can be demonstrated that there is good reason to do so (Sironis Pty Ltd, 2016).
The APVMA and NICNAS reforms are expected to take this principle further (see below), but both authorities already have the means in their regulatory frameworks to use information generated in other countries. The APVMA accepts trusted international data, standards and assessments in the assessment of agricultural and veterinary chemical applications, and provides guidance for applicants on the submission of international data and assessments (APVMA, 2018). The Office of Chemical Safety of the Department of Health has bilateral memoranda of understanding (co‑operative arrangements) with its counterparts in the European Union, the United States, Canada and New Zealand, and there are several arrangements by which NICNAS can use assessments from other countries when assessing new chemicals needing a certificate (NICNAS, 2018h). Assessments generated in other countries have also been extensively used since 2012 in the context of the IMAP framework (Section 5.5.1).
⇒ Focus on the NICNAS reform:
It is expected that with increased use of trusted international assessment materials, introducers will be permitted to use such materials for an industrial chemical to be downgraded from the assessed category to the reported category. This implies a significant reduction in regulatory costs to the Australian industrial chemical industry (NICNAS, 2018a; Box 5.10).
In the context of the use of assessments performed in other countries, intellectual property right provisions exist for reusing assessments and potentially protected data generated by companies in other countries. Thus, in line with the Recommendation of the OECD Council concerning the Protection of Proprietary Rights to Data Submitted in Notifications of New Chemicals, it is expected that forms approved by the executive director of AICIS under the Industrial Chemicals Bill 2017 will require certification by introducers that they are authorised to use the intellectual property inherent in all information relevant for the purposes of categorisation (and assessment) of unlisted introductions. In this context, the introducer should be able to provide such information to AICIS if requested during compliance monitoring and audit activities.
Box 5.9. Environmental risk assessment approach depend on the regulatory regime
Industrial chemicals: Environmental risk assessment is undertaken by the DEE, which reports the assessment outcome to the director of NICNAS.
Agvet: Environmental risk assessment is undertaken by the APVMA and external scientific experts, including DEE, on behalf of the APVMA.
Pharmaceuticals: Pharmaceuticals and products regulated as medical devices are not subject to environmental risk assessment.
Chemicals in food and food additives: FSANZ does not conduct, require or commission environmental risk assessments for substances added to food.
Systematic risk management of chemicals
Constitutionally, risk management is the responsibility of the states and territories. The Commonwealth wields no enforcement mechanism over them, resulting in some lack of harmonisation of risk management measures at the national level because each state has its own constitution and governance, and implements regulations independently.
This section describes current risk management systems, highlighting potential areas for improvement. It focuses on the major reforms under way, the reform of NICNAS and the National Standard, including how they are expected to improve the risk management framework, and describing remaining uncertainties regarding how the National Standard will be implemented in practice.
Implementation of risk management approaches
Industrial chemicals
Drawing on chemical risk assessment conducted at the Commonwealth level, states and territories are responsible for determining appropriate controls on the use, release and disposal of industrial chemicals within their regulatory frameworks for public health, worker health and safety, environmental management and land transport of dangerous goods (Sironis Pty Ltd, 2016).
For human health protection, NICNAS recommendations are submitted to national risk managers, i.e. Safe Work Australia, the Poisons Standard or ACCC, as appropriate (Figure 5.4), and the final decision of these standard-setting bodies is adopted and enforced in state and territory legislation. However, in this context it is uncertain which standard-setting body would be responsible for implementing potential recommendations on actions to protect humans from indirect exposure to chemicals via the environment, such as the establishment of air or water standards for health protection.
Nor does any standard-setting body currently exist for implementation of NICNAS recommendations on protection of the environment, which thus is the direct responsibility of the states and territories (Figure 5.4). Since recommendations that follow NICNAS assessment are not binding, this system results in uneven implementation of control risk measures in the various states and territories.
To fill this gap, and as an outcome of the 2008 Productivity Commission report (Productivity Commission, 2008), the Council of Australian Governments introduced a reform to establish the National Standard. The standard provides a framework for managing risk that industrial chemicals may pose to the environment. It describes a set of seven Environment Schedules or groups of concern, in which chemicals are categorised based on how they are of concern for the environment. It then outlines conditions describing how industrial chemicals in a given Environment Schedule are to be managed: each schedule corresponds a set of general outcome-based, pre-established management measures, covering all stages of a chemical’s life cycle, i.e. storage, handling, treatment and disposal, as well as more specific management measures aimed at protecting water, land and air (Australian Government, 2016).
The National Standard will be established under Commonwealth legislation but will need to be implemented through state and territory legislation. States and territories have several options for incorporating the standard into their legislation. They can adopt mirror legislation, as many states have done with the Work Safety Laws, for example, or they can adopt a different regulation that may set out a different scheduling system. Although there seems to be a high-level objective for consistency across the states, the second option is of concern to several stakeholders, industry in particular, which advocates more co‑ordinated legislation across states overall.
The design of the reform is still under discussion and uncertainty remains regarding the resources to be allocated for Environment Schedule categorisation of the more than 50 000 new and existing chemicals on the market. Moreover, neither the role of the states and territories in the new system, nor the monitoring or evaluation system that will be needed, has been fully defined. The states have outlined a need for the National Standard to develop performance metrics so that industry can demonstrate that the standards are met. In addition, a robust system of measurable indicators is fundamental to define a baseline against which results of the reforms can be measured (Section 5.3.6).
The role of NICNAS in the context of risk management
NICNAS interacts with its regulatory partners at the Commonwealth level (i.e. Commonwealth standard setters), but does not routinely interact directly with state and territory regulators, although they may ultimately use NICNAS assessments in controlling industrial chemicals at a jurisdictional level (Sironis Pty Ltd, 2016).
⇒ Focus on the NICNAS reform:
The government clearly expects that the new AICIS will continue to do risk assessment and make recommendations to risk managers. It will not be involved in risk management, except in circumstances where risk managers confirm they are unable to control identified risks within their respective risk management frameworks. Various mechanisms are expected to strengthen the relationship between assessment bodies and risk managers:
strengthening consultation with risk managers at the Commonwealth and state and territory levels, including mandatory consultation when AICIS proposes to impose conditions of use, to refuse to issue an assessment certificate or to stop introduction of a chemical on grounds that the risk cannot be adequately managed (NICNAS, 2017a)
increasing co‑ordination between risk management standard-setting bodies with the establishment of a new non-statutory committee of national risk managers, the Risk Management Advisory Committee, to facilitate information sharing among national risk managers (NICNAS, 2014)
increasing transparency on how AICIS recommendations are implemented in terms of risk control measures, with the maintenance by AICIS of a public register of responses by risk management agencies to AICIS recommendations (NICNAS, 2014).
Timelines associated with implementation of risk management measures
Import or manufacture of a new industrial chemical can begin after the introducer receives a permit or certificate. Timelines for implementing risk management measures vary by sector, depending on relevant legislation, processes and practices in states and territories. This means a chemical may be introduced in the market even if risk management measures may not yet be in place. While some jurisdictions can apply risk management measures relatively quickly (within eight weeks), it has been reported that others can take several years to consider NICNAS recommendations, complete the necessary regulatory tasks and apply appropriate risk management measures (Australian Government, 2012). By developing risk management measures to be implemented across the country, the National Standard will facilitate the application of measures for environmental protection and, ideally, reduce the time between introduction of a chemical and application of risk management measures.
Pesticides
While NICNAS does not have the constitutional authority to enforce risk management recommendations they provide, the situation is different for agvet, since the APVMA’s role extends to controlling how a product is to be used through the approval of label instructions. The APVMA regulates agvet chemicals up to and including point of sale, and the states and territories are responsible for after-sale risk management. The APVMA also administers the Adverse Experience Reporting Program, which assesses reports of adverse experiences associated with the registered use of agvet.
Compliance and enforcement activities regarding regulations on chemicals
EPAs and Safe Work bodies in states and territories undertake compliance and enforcement programmes for chemical-related activities. For example, EPAs regularly inspect facilities for compliance with licence conditions.
The National Compliance and Enforcement Policy was endorsed by Safe Work Australia members and the Workplace Relations Ministers’ Council in 2011. The regulators monitor compliance with WHS laws in several ways, including inspections and audits. The regulators also receive incident notifications and requests to respond to WHS issues. These notifications and requests are triaged to determine an appropriate regulatory response (Safe Work Australia, 2018b).
Initiatives to promote research and development on sustainable or green chemistry
Promoting R&D on sustainable or green chemistry initiatives is not an explicit objective of the ICNA Act and is not expected to be an objective of the chemical reforms. However, the reforms will increase incentives to promote development of safer chemicals (Box 5.10). Also, many initiatives on green or sustainable chemistry are conducted through partnerships between federal or state/territory governments and academic institutions. Examples are the Centre for Green Chemistry at Monash University in Melbourne, funded by the federal government, and the Commonwealth Scientific and Industrial Research Organisation, which in recent years has partnered with industry to deliver innovative chemical manufacturing processes.
Box 5.10. The chemical reforms will provide incentives to move to safer chemicals
The reforms of NICNAS and the National Standard, through a combination of means, convey incentives to move to safer chemicals.
The NICNAS reform will encourage introduction of lower-risk chemicals by:
reducing assessment cost of chemicals categorised as lower risk, which will not be subject to pre-market assessment
reducing assessment cost of innovative chemicals available in other countries due to the possibility of using assessments from abroad
refusing to grant market access to hazardous chemicals when risks cannot be adequately managed.
The National Standard, by providing more harmonised outcome-based risk management measures, will encourage continued innovation in environmental protection.
References
ABS (2017), “5204.0: Gross Value Added (GVA) by Industry”, Australian System of National Accounts, 2016-17, Australian Bureau of Statistics, Canberra, www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/5204.02016-17?OpenDocument.
ABS (2017), “International Trade in Goods and Services”, Australia Catalogue Number 5368.0, Australian Bureau of Statistics, Canberra www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/5368.0May%202017?OpenDocument (accessed 2017).
APRA (2018), Regulation Impact Statements, Australian Prudential Regulation Authority, Sydney, www.apra.gov.au/statement-expectations-2014.
APVMA (2018a), Registrations and Permits, Australian Pesticides and Veterinary Medicines Authority, Canberra, https://apvma.gov.au/node/1060 (accessed May 2018).
APVMA (2018b), Arrangements for Products of Low Regulatory Concern, Australian Pesticides and Veterinary Medicines Authority, Canberra, https://apvma.gov.au/node/171, (accessed September 2018).
APVMA (2018c), Chemicals in the news, Australian Pesticides and Veterinary Medicines Authority, Canberra, https://apvma.gov.au/node/18451 (accessed February 2018).
APVMA (2018d), “History of the Chemical Review Program”, Australian Pesticides and Veterinary Medicines Authority, Canberra, https://apvma.gov.au/node/10971 (accessed February 2018).
APVMA (2018e), Guidance for applicants – submission of international data, standards and assessments, Australian Pesticides and Veterinary Medicines Authority, https://apvma.gov.au/node/14186 (accessed September 2018)
APVMA (2015), “Employment of risk proportionate chemical regulatory regimes in Australia and selected international jurisdictions”, Australian Pesticides and Veterinary Medicines Authority, Canberra, https://apvma.gov.au/sites/default/files/publication/14301-risk-proportionate-regulatory-regimes-australia.pdf (accessed February 2018).
Australian Government (2016), “National Standard for Environmental Risk Management of Industrial Chemicals”, discussion paper, Department of the Environment and Energy, Canberra.
Australian Government (2012), “Review of the National Industrial Chemicals Notification and Assessment Scheme (NICNAS)”, discussion paper, Australian Government, Canberra.
Caiazzo et al. (2013), “Air pollution and early deaths in the United States. Part I: Quantifying the impact of major sectors in 2005”, Atmospheric Environment, Vol. 79, pp. 198-208.
Commonwealth of Australia (2017), “Environmental Risk Management of Industrial Chemicals: National Standard and Explanatory Report’, draft report unpublished as of 21 March 2018.
DEE (2018a), “Understanding NPI data”, Department of the Environment and Energy, Canberra, www.npi.gov.au/npi-data/understanding-npi-data (accessed 2018).
DEE (2018b), “National Pollutant Inventory”, Department of the Environment and Energy, Canberra, www.npi.gov.au/ (accessed 2018).
DEE (2018c), “Scheduled waste management”, Department of the Environment and Energy, Canberra, www.environment.gov.au/protection/chemicals-management/scheduled-waste#national (accessed January 2018).
DEE (2018d), “Nine new POPs and the treaty making process”, Department of the Environment and Energy, Canberra, www.environment.gov.au/protection/chemicals-management/pops/new-pops (accessed January 2018).
DEE (2018e), “Montreal Protocol on Substances that Deplete the Ozone Layer”, Department of the Environment and Energy, Canberra, www.environment.gov.au/protection/ozone/montreal-protocol.
DEE (2018f), “Minamata Convention on Mercury”, Department of the Environment and Energy, Canberra, www.environment.gov.au/protection/chemicals-management/mercury (accessed January 2018).
DEE (2016), “National Pollutant Inventory review 2017 terms of reference”, Department of the Environment and Energy, Canberra, www.npi.gov.au/resource/national-pollutant-inventory-review-2017-terms-reference (accessed May 2018).
Department of Health (2018a), Ban on cosmetic testing on animals, Department of Health, Canberra, www.health.gov.au/internet/main/publishing.nsf/Content/ban-cosmetic-testing-animals (accessed April 2018).
Department of Health (2018b), “Department of Health Annual Report 2016-17; Appendix 2: Report from the Director of the National Industrial Chemicals Notification and Assessment Scheme on the operation of the Industrial Chemicals (Notification and Assessment) Act 1989” , Department of Health, Canberra, www.health.gov.au/internet/main/publishing.nsf/AttachmentsByTitle/annual-report2016-17-attachments/$FILE/2016-17%20Department%20of%20Health%20Annual%20Report.pdf.
Department of the Environment and Heritage (2006), “Stockholm Convention on Persistent Organic Pollutants: Australia's National Implementation Plan”, www.environment.gov.au/protection/publications/national-implementation-plan.
DIIS (2017), “Key Facts: Australian chemicals and plastics manufacturing data card 2017”, Department of Industry, Innovation and Science, Canberra, https://industry.gov.au/industry/IndustrySectors/chemicalsandplastics/Pages/ChemicalsPlasticsManufacturingDataCard.aspx.
DIIS (2016), “Chemicals business checklist”, Department of Industry, Innovation and Science, Canberra, www.industry.gov.au/industry/IndustrySectors/chemicalsandplastics/RelatedLinks/Documents/ChemicalsBusinessChecklist.pdf.
NEPC (2018), “National Environment Protection Measures (NEPMs)”, National Environment Protection Council, http://nepc.gov.au/nepms (accessed May 2018).
NICNAS (2018a), Reforms Cost Recovery Model discussion paper, National Industrial Chemicals Notification and Assessment Scheme, Sydney, http://file:///C:/Users/Delrue_n/Downloads/Reforms_Cost_Recovery_Model_discussion_paper.pdf (accessed April 2018).
NICNAS (2018b), Priority Existing Chemical assessments, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.nicnas.gov.au/chemical-information/pec-assessments (accessed February 2018).
NICNAS (2018c), IMAP, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.nicnas.gov.au/chemical-information/imap-assessments (accessed February 2018).
NICNAS (2018d), “New chemicals reported”, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.nicnas.gov.au/notify-your-chemical/Annual-reporting/New-chemicals-reported (accessed February 2018).
NICNAS (2018e), Type of assessment, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.nicnas.gov.au/notify-your-chemical/types-of-assessments (accessed April 2018).
NICNAS (2018f), Data requirements, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.nicnas.gov.au/notify-your-chemical/data-requirements-for-new-chemical-notifications (accessed February 2018).
NICNAS (2018g), Notes on the draft General Rule, National Industrial Chemicals Notification and Assessment Scheme, Sydney, https://www.nicnas.gov.au/reforms/Rules-Guidelines/Draft-General-Rules/Draft-General-Rules-notes .
NICNAS (2018h), Use of overseas assessments, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.nicnas.gov.au/notify-your-chemical/types-of-assessments/assessment-certificate-categories/use-of-overseas-assessments.
NICNAS (2017a), Implementing reforms to the National Industrial Chemicals Notification and Assessment Scheme (NICNAS): Consultation paper 5, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.nicnas.gov.au/__data/assets/pdf_file/0004/50098/NICNAS-Reforms-CP5.pdf.
NICNAS (2017b), “Performance Framework Self-Assessment Report 2015-16”, National Industrial Chemicals Notification and Assessment Scheme, Sydney, www.health.gov.au/internet/main/publishing.nsf/Content/C3B10A392B150004CA258090000BB537/%24File/NICNAS_Self-Assessment-Report-2015-16.pdf.
NICNAS (2014), Regulation Impact Statement: Options for reforming the NICNAS, National Industrial Chemicals Notification and Assessment Scheme, Sydney.
OECD (2018), Industrial production (indicator), http://dx.doi.org/10.1787/39121c55-en. (accessed 7 May 2018).
OECD (2017), “Framework on the role of Pollutant Release and Transfer Registers (PRTRs)”, OECD Environment, Health and Safety Publications: Series on Pollutant Release and Transfer Registers n°21 - ENV/JM/MONO(2017)7.
OECD (2015), Preliminary analysis of policy drivers influencing decision making in chemicals management”, Series on Risk Management No. 28, OECD Publishing, Paris, ENV/JM/MONO(2015)21.
OECD (2014), “Global Pollutant Release and Transfer Register, Proposal for a Harmonised List of Pollutants”, Series on Pollutant Release and Transfer Registers No. 16, OECD Publishing, Paris, ENV/JM/MONO(2014)32.
OECD (2007), OECD Environmental Performance Reviews: Australia 2007, OECD Publishing, Paris, http://dx.doi.org/10.1787/9789264039612-en.
PACIA (2014), A Strategic Roadmap for the Chemicals and Plastics Industry, Plastics and Chemicals Industries Association, Melbourne, www.chemistryaustralia.org.au/docs_mgr/PACIA_Strategic_Industry_Roapmap_screen_V2.pdf.
Productivity Commission (2008), “Research Report on Chemicals and Plastics Regulation”, Productivity Commission, Canberra.
Safe Work Australia (2018a), Model WHS Laws, Safe Work Australia, Canberra, www.safeworkaustralia.gov.au/law-and-regulation/model-whs-laws (accessed February 2018).
Safe Work Australia (2018b), National Compliance and Enforcement Policy, Safe Work Australia, Canberra, www.safeworkaustralia.gov.au/doc/national-compliance-and-enforcement-policy (accessed February 2018).
Safe Work Australia (2002), MHF Guidance Material List of Key Differences between States, Safe Work Australia, Canberra, www.safeworkaustralia.gov.au/doc/mhf-guidance-material-list-key-differences-between-states.
Sironis Pty Ltd (2016), Opportunities for Structural Change in Commonwealth Chemical Assessment Functions Comparative Analysis, report for the Department of Industry, Innovation and Science, Canberra.
UNECE (2018), “GHS implementation”, United Nations Economic Commission for Europe, Geneva, www.unece.org/trans/danger/publi/ghs/implementation_e.html (accessed January 2018).
UNEP (2018), Global Monitoring Plan on Persistent Organic Pollutants, United Nations Environment Programme, Nairobi, www.pops-gmp.org (accessed May 2018).
UNEP (2015), UNEP Guidance document for the development of Legal and Institutional Infrastructures for the Sound Management of Chemicals and Measures for Cost Recovery of National Administrations (LIRA-Guidance), United Nations Environment Programme, Nairobi, http://wedocs.unep.org/bitstream/handle/20.500.11822/12224/LIRA_Guidance%20Report_PRESS.pdf.
WEOG (2015), GMP for POPs 2nd regional monitoring report for Western Europe and Others Group (WEOG) Region, United Nations Environment Programme, Nairobi, https://wedocs.unep.org/bitstream/handle/20.500.11822/19330/WEOG_Report_FINAL_2015_03_31.pdf.