Such savings are not just monetary in nature. By reducing the need for duplicative testing of chemicals due to the OECD MAD system, almost 33 000 less animals are needed every year to test new industrial chemicals. While not quantified in this report, due to the much greater amount of testing needed for biocides and pesticides, it is expected that an even more significant number of animals will not need to be sacrificed to assess the safety of these chemicals.
In developing this report, it was not possible to quantify all of the benefits of the EHS Programme’s work. However, these unquantified benefits are just as real, likely and important as the quantified benefits (see Chapter 3). Some examples of work which leads (or will lead) to non-quantified benefits for governments and industry are:
ensuring safer nanomaterials by developing harmonised tools for testing and assessment
harmonising the safety assessment methodologies for products of modern biotechnology
providing harmonised tools to identify the risks of endocrine disrupters
reducing the need for national government inspections of test facilities in other countries which test chemicals
enhancing hazard assessment methods and limiting the use of animals in chemical testing
facilitating the exchange of information on chemical accidents to support prevention, preparedness and response
advancing harmonisation of biocides regulations and testing
reducing repeat testing for new pharmaceuticals
counteracting the illegal trade of pesticides and thus reducing the chance that unregulated, unsafe and ineffective products are used on crops.
Also excluded are the benefits to industry of avoiding delays in marketing new products. According to industry sources, these could represent similar amounts to those saved by avoiding duplicative testing (for example, delays in the registration of a pesticide might lead to missed sales for a full growing season). Also excluded are the added benefits to health and the environment of governments working together to be able to evaluate and manage more chemicals than they would if they worked independently. Finally, while pharmaceuticals were not the subject of this analysis, it is expected that due to the extensive non-clinical testing required for such products, and because many of these test methods may fall within the MAD system, the benefits of the EHS Programme for these products could be extensive.
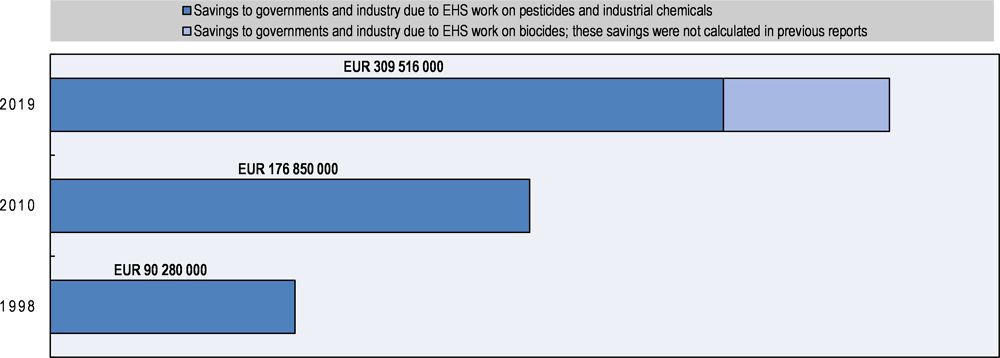
With more than 40 years of experience and a vast area of work, the EHS Programme ensures safer and more efficient chemicals policies and promotes more sustainable development in OECD member countries and key partner countries around the world. This report has demonstrated that the programmes’ benefits to society amount to more than EUR 309 million and tens of thousands of animal lives saved every year, in addition to numerous non-quantifiable benefits. With the more recent parts of the EHS Programme evolving and better methodologies being developed, many of the qualitative benefits may be quantifiable in the future.