As cancer is anticipated to become the leading cause of death in Europe by 2035, this report aims to provide policy insights for preventing cancer, ensuring timely identification of emerging cases, and improving care for diagnosed patients. This chapter highlights the key findings, beginning with an overview of trends in cancer incidence, mortality and survival, and assessing inequalities in these indicators. It offers an overview of the main behavioural, metabolic and environmental risk factors for cancer, and provides overarching recommendations for addressing them. The chapter also discusses key messages on cancer screening programmes, existing initiatives and emerging approaches to enhance screening reach. It concludes by highlighting major challenges to delivering high-quality cancer care, including workforce limitations, access to oncology medicines and care system organisation.
Beating Cancer Inequalities in the EU

1. Beating cancer inequalities: Current trends and key policy directions
Abstract
1.1. Cancer is a major public health concern across European countries
1.1.1. In 2022, one new cancer case was diagnosed every 11 seconds in European countries
Across the 27 European Union Member States (EU27) plus Iceland and Norway (EU+2 countries), an estimated 2.78 million new cancer cases were diagnosed in 2022 (ECIS, 2023[1]). This translates to about five people being diagnosed every minute, or one cancer case diagnosed every 11 seconds. Compared to 2020, the number of new cancer cases increased by 2.4% in 2022 (an increase of around 65 000 cases). It is estimated that new cancer diagnoses will increase by around 18% in the EU27 in 2040 compared to 2022.
Leukaemia is the most common cancer diagnosed in children (aged under 15), accounting for around 33% of cancer cases among boys and 30% among girls. In adults, the most common cancers among those estimated to have been diagnosed in the EU27 in 2022 were breast, prostate, colorectum and lung, which together represented 50% of all new cancer diagnoses in 2022 (Table 1.1). The same cancer sites, with the addition of pancreatic cancer, were the leading causes of death in 2020 – responsible for 52% all cancer deaths.
Table 1.1. Breast, prostate, colorectum and lung cancer are estimated to be the leading cancer sites in 2022
|
Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Estimated new cases |
Breast |
374 836 |
29% |
 |
 |
Prostate |
330 492 |
23% |
|
Colorectum |
158 698 |
12% |
Lung |
203 029 |
14% |
|||
|
Lung |
116 207 |
9% |
Colorectum |
197 456 |
13% |
|||
|
Corpus uteri |
69 163 |
5% |
Bladder |
127 640 |
9% |
|||
|
Melanoma skin |
49 509 |
4% |
Kidney |
58 213 |
4% |
|||
|
Pancreas |
50 438 |
4% |
Melanoma skin |
51 998 |
4% |
|||
|
Non-Hodgkin lymphoma |
41 189 |
3% |
Non-Hodgkin lymphoma |
51 518 |
4% |
|||
|
Ovary |
40 714 |
3% |
Pancreas |
49 714 |
3% |
|||
|
Thyroid |
38 503 |
3% |
Stomach |
45 246 |
3% |
|||
|
Brain and other CNS |
19 539 |
2% |
Multiple myeloma |
18 808 |
1% |
|||
|
All cancer sites* |
1 276 601 |
All cancer sites* |
1 465 846 |
|||||
Note: CNS stands for central nervous system. * Includes all cancer sites except non-melanoma skin cancer. Estimates were calculated based on incidence and mortality trends before the COVID‑19 pandemic and may differ from observed rates in more recent years. Lung also includes bronchus and trachea.
Source: ECIS (2023[1]), European Cancer Information System, https://ecis.jrc.ec.europa.eu (accessed on 27 April 2023).
Estimated cancer incidence increased in 14 of the 24 countries with available data between 2010 and 2022. After adjusting for different population age structures, overall cancer incidence was highest in Norway and Denmark, at close to 28% higher than the EU27 average. Ireland, the Netherlands, Croatia and Hungary were also among the 20% of countries with the highest incidence (the highest quintile) among EU+2 countries, with incidence rates above 622 per 100 000 population. In Bulgaria and Austria, overall estimated cancer incidence was the lowest, with rates more than 14% lower than the EU27 average. Low incidence was also seen in Romania, Spain, Greece and Lithuania (all with estimated incidence below 542 per 100 000 – the lowest quintile). In the EU27, cancer incidence rates are estimated to vary near 2‑fold across countries.
1.1.2. Cancer mortality rates decreased by 10% between 2010 and 2020 in the EU27, with rates varying greatly across countries
In 2020, about one in four (22.5%) deaths were caused by cancer (Eurostat, 2023[2]). Cancer is the second leading cause of death in Europe after cardiovascular diseases, but it is anticipated to become the leading cause of death by 2035. However, between 2010 and 2020, the age‑standardised mortality rate for all cancer decreased by 10% in the EU27. Reductions in cancer mortality rates were observed in all 29 EU+2 countries except Bulgaria and Cyprus. The highest mortality rates occurred in Hungary (32% higher than the EU27 average), but high rates were also observed in Croatia, the Slovak Republic, Latvia, Slovenia and Poland. The lowest mortality rates occurred in Luxembourg (16% lower than the EU27 average), Cyprus, Finland, Malta, Sweden and Spain. Overall, cancer mortality rates varied 1.6‑fold across countries.
Mortality rate decreases were seen across almost all cancers, with stomach cancer mortality declining the most (at 27%). Significant decreases in mortality rates were also seen for cancers of the cervix uteri (‑16%), colorectum (‑15%), kidney (‑14%), and lung cancer (‑12%) (Figure 1.1).
Figure 1.1. Mortality rates decreased for most of the main cancer sites in the last decade
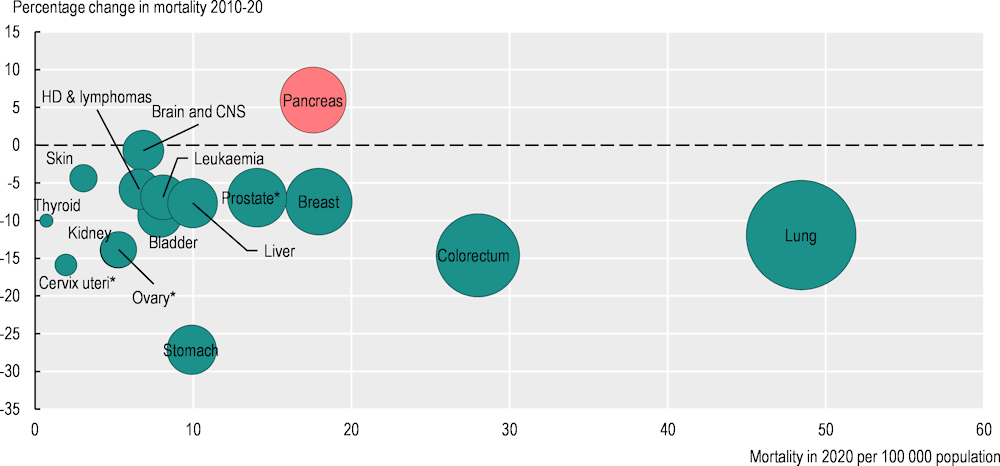
Note: The red bubble signals an increase in the percentage change in the cancer mortality rate during 2010‑20; green bubbles signal a decrease. The size of the bubbles is proportional to the mortality rate in 2020. The mortality rate for some of these cancers is low; hence, the percentage change should be interpreted with caution. * Percentage change for prostate, ovary and cervix uteri cancers refers to 2011‑20. HD stands for Hodgkin disease.
Source: Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
In 2020, cancer mortality rates varied greatly across the EU+2 countries, as seen in Table 1.2. This shows a per-cancer-site colour scale where dark red corresponds to the highest quintile of mortality rates and dark blue corresponds to the lowest quintile. The relative predominance of blue across the top indicates lower cancer mortality rates in Nordic and Western European countries, while the predominance of red across the bottom indicates higher cancer mortality rates in Central and Eastern European countries.
Table 1.2. Cancer mortality is consistently higher in Central and Eastern European countries
Age‑standardised mortality rate per 100 000 population, 2020, both sexes
|
Bladder |
Brain and CNS |
Breast |
Cervix uteri |
Colorectum |
HD & lymphomas |
Kidney |
Leukaemia |
Liver |
Ovary |
Pancreas |
Prostate |
Stomach |
Lung |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Sweden |
6.1 |
6.3 |
13.5 |
1.1 |
26.4 |
6.2 |
4.5 |
7.2 |
7.1 |
4.8 |
18.6 ↑ |
21.1 |
5.0 |
33.5 |
|
Luxembourg |
6.3 |
5.2 |
20.3 |
0.3 |
23.9 |
6.3 |
2.9 |
8.8 |
7.4 |
5.1 |
16.3 |
13.3 |
7.2 |
39.2 |
|
Spain |
8.5 |
6.5 |
12.8 |
1.3 |
29.4 |
6.0 |
4.2 |
6.6 |
10.0 |
4.1 |
14.9 ↑ |
11.2 |
9.7 |
44.8 |
|
Finland |
5.0 |
6.3 |
15.8 |
0.8 |
20.8 |
8.9 |
5.7 |
5.4 |
9.1 ↑ |
4.5 |
21.2 ↑ |
14.8 |
6.7 |
36.7 |
|
Belgium |
6.5 |
6.5 ↑ |
17.8 |
1.2 |
21.6 |
6.2 |
4.4 |
8.5 |
8.5 ↑ |
5.1 |
16.0 |
13.2 |
5.8 |
49.4 |
|
Norway |
6.0 |
6.5 |
12.3 |
2.1 ↑ |
32.2 |
6.3 |
5.1 |
6.6 |
6.6 ↑ |
5.0 |
16.0 |
20.2 |
5.8 |
44.6 |
|
France |
7.2 |
5.9 |
18.0 |
1.1 |
23.3 |
6.7 |
4.8 |
8.3 |
12.3 |
4.7 |
17.6 ↑ |
12.0 |
6.0 |
44.4 |
|
Cyprus |
5.9 |
8.8 ↑ |
16.3 |
1.4 ↑ |
18.6 ↑ |
6.8 ↑ |
2.6 |
10.8 ↑ |
8.2 ↑ |
6.4 ↑ |
14.6 ↑ |
12.5 |
8.5 ↑ |
40.9 ↑ |
|
Italy |
7.9 |
6.3 ↑ |
18.0 |
0.7 ↑ |
25.1 |
7.1 |
4.8 |
8.3 |
11.6 |
4.6 |
17.7 ↑ |
10.0 |
11.5 |
44.5 |
|
Portugal |
7.4 |
7.9 ↑ |
15.6 |
1.8 |
32.0 |
7.4 |
4.0 |
7.3 |
11.3 ↑ |
3.4 |
14.2 ↑ |
15.9 |
18.0 |
37.2 ↑ |
|
Malta |
9.3 |
8.6 |
20.1 |
0.8 |
25.6 |
6.0 |
6.6 ↑ |
7.8 |
5.9 |
7.1 |
22.8 ↑ |
8.6 |
5.9 |
35.6 |
|
Greece |
10.1 ↑ |
9.1 |
17.5 |
1.2 |
21.5 |
5.1 ↑ |
4.7 |
9.1 |
11.0 |
4.4 |
16.1 ↑ |
13.0 |
9.3 |
58.0 |
|
Austria |
6.1 |
6.8 |
18.3 |
1.6 ↑ |
23.4 |
7.1 |
4.1 |
9.3 |
9.4 ↑ |
5.4 |
20.4 ↑ |
15.4 ↑ |
8.2 |
44.7 |
|
Netherlands |
8.1 |
5.5 ↑ |
18.1 |
1.4 |
27.1 |
7.8 |
5.3 |
8.1 |
7.3 |
5.9 |
16.8 |
17.8 |
6.6 |
57.2 |
|
Germany |
6.0 |
6.5 |
19.4 |
1.7 |
25.2 |
7.4 |
5.3 |
8.6 |
8.8 |
5.5 |
19.5 ↑ |
15.5 |
8.7 |
47.5 |
|
Iceland |
7.9 |
8.7 |
18.1 |
2.0 ↑ |
25.6 |
6.9 |
6.9 |
6.7 |
7.5 ↑ |
5.4 |
16.7 ↑ |
23.4 ↑ |
6.1 |
50.1 |
|
Denmark |
7.7 |
7.6 |
18.5 |
1.5 |
28.8 |
6.0 |
4.2 |
8.6 |
7.7 |
5.5 |
19.5 ↑ |
23.9 |
7.2 |
57.2 |
|
Romania |
9.1 ↑ |
8.7 ↑ |
18.7 ↑ |
6.9 |
34.3 ↑ |
3.6 |
4.7 ↑ |
6.3 |
14.4 |
5.3 |
15.4 ↑ |
13.5 ↑ |
15.6 |
49.1 |
|
Bulgaria |
8.4 ↑ |
8.9 ↑ |
19.3 ↑ |
4.8 ↑ |
36.0 ↑ |
4.2 ↑ |
5.1 ↑ |
5.8 |
9.1 |
6.0 ↑ |
16.3 ↑ |
16.8 ↑ |
14.4 |
44.8 ↑ |
|
Ireland |
6.9 |
7.8 ↑ |
19.9 |
1.7 |
27.3 |
8.5 |
5.5 |
7.1 |
10.5 ↑ |
7.4 |
16.1 |
17.4 |
7.8 |
52.1 |
|
Czechia |
8.8 |
7.2 |
17.1 |
2.7 |
33.3 |
6.1 ↑ |
8.7 |
9.5 |
8.2 |
6.0 |
21.9 |
15.1 |
9.3 |
48.8 |
|
Poland |
11.8 ↑ |
8.3 |
19.9 ↑ |
4.1 |
35.6 |
5.3 ↑ |
6.7 |
8.0 |
5.9 |
7.4 |
13.8 |
16.9 ↑ |
13.4 |
60.5 |
|
Lithuania |
8.7 |
9.3 ↑ |
19.1 |
6.4 |
30.4 |
5.8 ↑ |
7.8 |
9.3 |
7.9 ↑ |
8.9 |
17.4 ↑ |
18.1 |
20.8 |
41.4 |
|
Estonia |
7.8 |
8.1 |
18.9 |
4.6 |
29.8 |
7.0 |
9.5 ↑ |
8.9 |
9.6 ↑ |
7.2 |
18.7 ↑ |
17.4 |
18.9 |
44.6 |
|
Hungary |
10.7 |
6.6 ↑ |
22.9 |
3.8 |
50.5 |
5.3 |
7.8 |
8.4 |
8.2 |
7.0 |
22.0 ↑ |
14.2 |
13.3 |
81.0 |
|
Slovenia |
11.3 ↑ |
7.1 |
21.9 |
1.8 |
30.9 |
9.8 ↑ |
7.4 |
8.6 |
13.6 ↑ |
4.9 |
18.9 |
20.5 |
14.4 |
53.3 |
|
Slovak Republic |
9.8 ↑ |
8.5 ↑ |
23.8 ↑ |
3.5 |
46.3 |
7.0 ↑ |
8.8 ↑ |
9.8 ↑ |
9.1 |
6.9 ↑ |
20.6 ↑ |
17.7 |
13.7 |
47.5 |
|
Croatia |
10.9 ↑ |
9.7 |
16.8 |
2.9 ↑ |
47.6 |
7.3 |
8.2 |
9.1 |
11.0 |
7.2 ↑ |
17.6 ↑ |
18.4 |
15.3 |
63.0 |
|
Latvia |
11.1 ↑ |
10.0 ↑ |
22.4 ↑ |
5.6 |
33.3 |
6.6 |
9.8 |
8.1 |
9.1 ↑ |
10.5 |
20.6 ↑ |
21.1 ↑ |
20.0 |
46.8 |
|
EU27 average |
7.9 |
6.9 |
18.0 |
2.0 |
28.0 |
6.6 |
5.3 |
8.1 |
10.0 |
5.3 |
17.6 ↑ |
14.0 |
9.9 |
48.4 |
Notes: CNS stands for central nervous system. The colours correspond to quintiles of mortality among the 29 countries, where blue is the quintile with the lowest mortality rate, light blue the second quintile, white the third quintile, light red the fourth quintile and dark red the quintile with the highest mortality rate. The order of countries in the table is determined by the average position of annual mortality rates for each cancer. In Iceland, the 2020 mortality rate is a five‑year rolling average (2016‑20) and the 2010 mortality rate is a four‑year rolling average (2006‑09) (no data for 2010). Arrows indicate an increase greater than 3% in mortality rates between 2010 and 2020; except for Iceland and Denmark, and for cervix uteri, ovary and prostate cancers, which show the difference between 2011 and 2020. EU27 averages include only EU Member States and are calculated as population-weighted averages.
Source: Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
Variations in cancer mortality between EU+2 countries are wide. In 2020, breast cancer mortality rates varied almost two‑fold, and the mortality rates for colorectal, liver, prostate, stomach and lung cancer varied between more than two‑fold and four‑fold.
As with the improvement in cancer mortality rates over the last decade, five‑year estimated survival probabilities for most cancers have improved (or changed very little) in most countries for people diagnosed between 2010 and 2014 compared to people diagnosed between 2005 and 2009, mostly because of earlier diagnosis (through better imaging, biomarkers and screening strategies) and new treatments. Among countries, there are major differences in estimated cancer survival probabilities. Western European and Nordic countries such as Belgium, Norway, Sweden, Iceland, Germany and Portugal consistently have survival estimates in the top quintile (the best performing) for most cancers. Cyprus also has survival estimates in the top quintile for 8 of the 11 cancers examined. Bulgaria, the Slovak Republic, Czech Republic (hereafter “Czechia”), Croatia, Poland, Romania and Lithuania have some of the lowest estimated five‑year survival estimates across the 11 cancer sites, with estimates in the lowest quintile for at least 5 cancer sites, suggesting important room for improvement.
1.2. There are large gaps in the cancer burden within countries by geographical region, gender and socio‑economic group
1.2.1. Cancer mortality rates vary by up to 37% between regions within a country
Large geographical disparities in cancer incidence, cancer survival and cancer mortality rates exist, and cancer outcomes can vary dramatically within different regions of the same country (Figure 1.2). The largest within-country differences in overall cancer mortality by European NUTS2 regions can be found in Romania, where Bucuresti-Ilfov had 37% higher cancer mortality rates than Sud-Vest Oltenia in 2020. There were also large regional disparities in overall cancer mortality in Poland, France, Spain and Germany, with at least a 30% variation in mortality rates. By contrast, relatively small countries such as Slovenia, Ireland, the Slovak Republic and Lithuania had smaller geographical disparities in cancer mortality in 2020.
Figure 1.2. Cancer mortality rates vary considerably by region in Romania, Poland, France, Spain and Germany
Age‑standardised cancer mortality rate (ASMR) per 100 000 population by NUTS2 regions
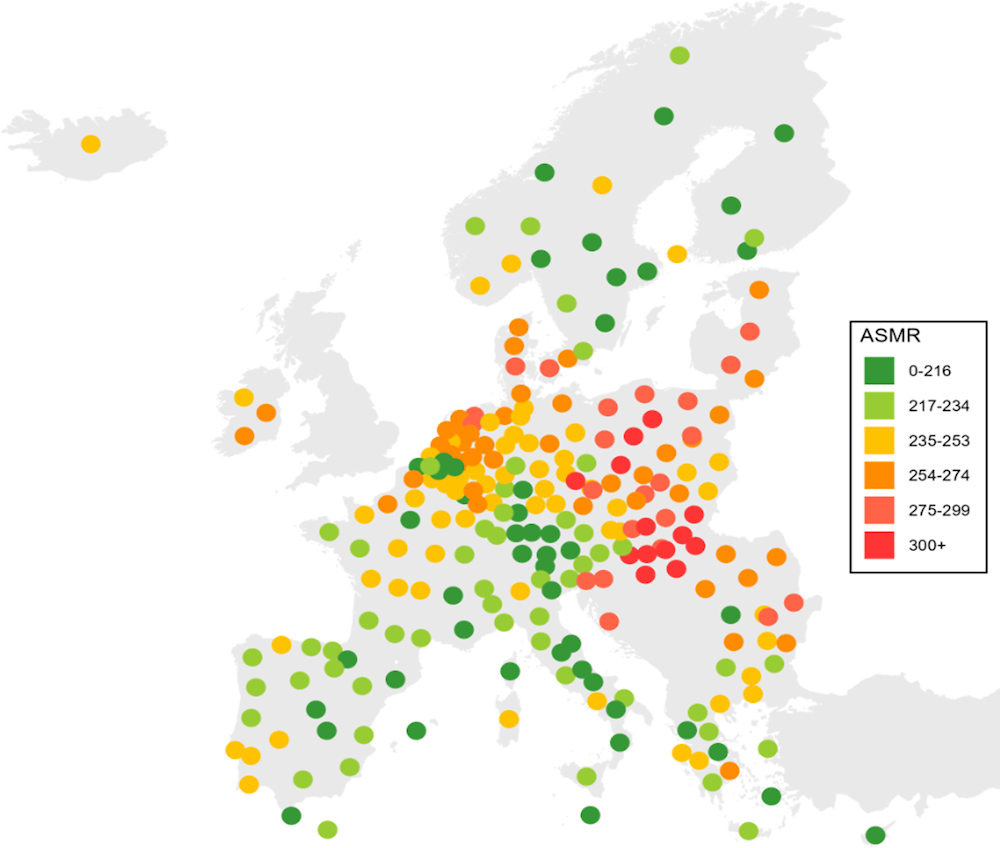
Note: The map is based on cancer mortality rates in 2020. In Iceland, the 2020 mortality rate is a five‑year rolling average (2016‑20).
Source: Eurostat (2023[3]), Causes of Death – Standardised Death Rate by NUTS 2 Region of Residence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ASDR2__custom_6414996/default/table.
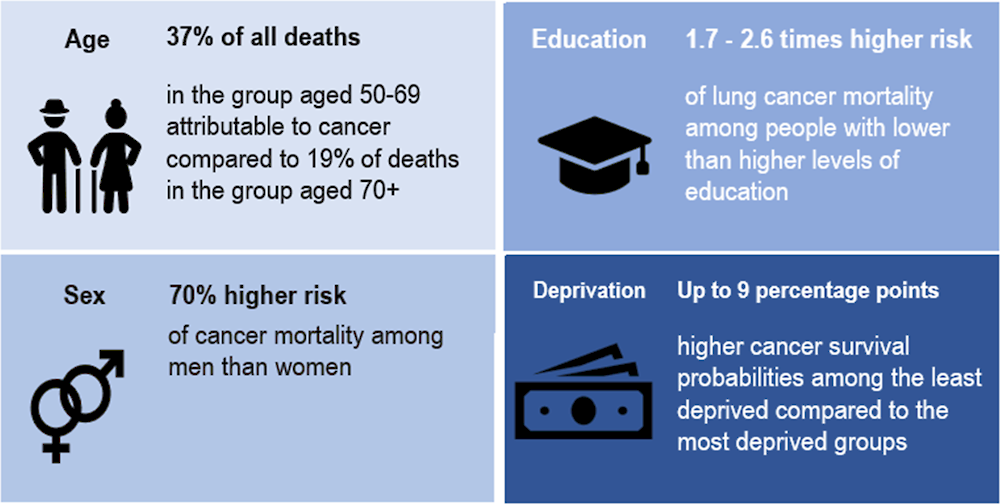
1.2.2. Men have a higher overall cancer mortality rate than women by 70%
Among the EU27, age‑adjusted cancer incidence in 2022 was 40% higher among men, while cancer mortality rates in 2020 were almost 70% higher among men than women. The gender gap in both cancer incidence and mortality rates has decreased over time. These figures vary widely by country, however. EU+2 countries with the highest gender gaps in cancer mortality were the Baltic countries (Lithuania, Latvia and Estonia), Portugal and Spain, while Nordic countries (Iceland, Denmark and Sweden) and Ireland had the smallest gender gaps.
While the majority of cancer deaths occur in the oldest age group, the proportion of cancer deaths among all deaths is highest in the group aged 50‑69 (at 37%), compared to 19% among those aged 70‑85 in 2020.
1.2.3. Lung cancer mortality rates were higher among women and men with lower education levels than among their counterparts with higher education levels
Systematic differences in cancer incidence, survival and mortality are observed between social groups – most often assessed on the basis of education levels (Vaccarella et al., 2023[4]; Launoy, Zadnik and Coleman, 2021[5]). A recent study of 18 European countries confirmed that people with lower education levels diagnosed during 1990‑2015 had higher mortality rates for nearly all cancer types than their more educated counterparts (Vaccarella et al., 2023[4]). This is especially notable for tobacco-related and infection-related cancers. Preliminary findings from the EUCanIneq study show that lung cancer mortality rates were 2.6 times as high among men with lower than higher levels of education (Figure 1.3), and 1.7 times as high among women with lower than higher levels of education. Figure 1.4 offers a summary of population groups vulnerable to cancer.
Figure 1.3. Lung cancer mortality rates among men vary with education level in all countries
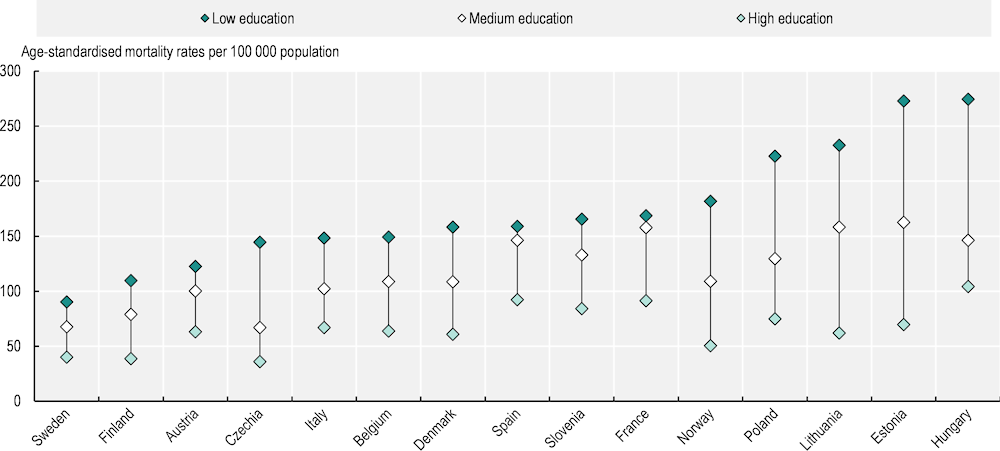
Note: Caution is recommended when interpreting results, as figures are based on predictions for 2015‑19, with different methodology across countries and varying levels of population coverage.
Source: Preliminary findings from the EUCanIneq study.
There is a crucial lack of research on inequalities in cancer outcomes by ethnicity or migrant population because of a lack of information on ethnicity, nationality or country of birth in many cancer registries. In Denmark, Finland, Iceland and Norway, non-Western immigrant women have a lower risk than the native‑born population of developing breast (‑29%), colorectal (‑28%) and lung cancer (‑45%) initially after migration; however, the likelihood increases with the length of stay in the host country (Lamminmäki et al., 2023[6]). These results corroborate the so-called “healthy migrant effect”, which suggests that migrants are often in better health than the native‑born population on arrival in the host country, but that their health deteriorates with length of residence. This worsening health status over time may occur as a result of lifestyle changes (wherein migrants change from more traditional to Westernised lifestyles), challenges in access to healthcare for migrants (including cost, language and cultural barriers, poor health literacy and discrimination) (Bradby, Hamed and Lebano, 2019[7]) or lower socio‑economic status and weaker social networks (Berchet and Jusot, 2012[8]). Using data from the Survey of Health, Ageing and Retirement in Europe, new analysis also suggests a healthy migrant effect in countries with available data, with non-citizen populations less likely to report a cancer diagnosis than citizens of the country of residence.
Nevertheless, given the higher prevalence of infection-driven cancer risks in migrants (such as hepatitis C and hepatitis B virus infections), as well as exposure to unhealthy environments in the host country (such as air pollution, poor nutrition or lack of physical activity) and reduced access to prevention and other healthcare services, the health risks faced by migrant populations in Europe warrant targeted consideration (Chapter 3).
Figure 1.4. Certain population groups experience disparities in cancer mortality
Sources: Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table; preliminary findings from the EUCanIneq study; Zadnik et al. (2022[9]), “Cancer patients’ survival according to socioeconomic environment in a high-income country with universal health coverage”, https://doi.org/10.3390/cancers14071620; Finke, I. et al., (2021[10]), “Small‐area analysis on socioeconomic inequalities in cancer survival for 25 cancer sites in Germany”, https://doi.org/10.1002/ijc.33553; Bambury, N. et al. (2023[11]), Cancer Inequalities in Ireland by Deprivation, 2004-2018: A National Cancer Registry report, NCRI, Cork.
1.2.4. In order to make data-driven decisions to improve outcomes and close gaps, countries need to link socio‑economic data to cancer registries
Cancer registries in Europe have evolved into indispensable instruments for assessing the cancer burden and facilitating evidence‑based decision making in cancer control. Their near-universal coverage and potential for data linkages enable comprehensive monitoring of the cancer burden and research on its treatments. A national cancer registry exists in 24 of the 29 EU+2 countries, while 5 (France, Greece, Italy, Romania, Spain) do not have a national cancer registry covering the entire population. The French Senate approved a law supporting the creation of a national cancer registry in June 2023, to be implemented in the near term. Among countries with cancer registries, however, the scope of information and extent of data quality, timeliness and utilisation of the registries varies widely. Mortality and diagnosis data are contained or linked in at least 26 EU+2 countries, while stage and survival data are contained or linked in 25 and 26 countries respectively, and treatment data captured in 24 countries. On the other hand, genetic information and patient-reported outcomes or experiences are more rarely included or linked to cancer registry information. Cancer registries are particularly helpful when integrated with national screening databases and information on socio‑economic characteristics, but this poses challenges in some European countries. Only 18 of the 29 EU+2 countries report that their cancer registries contain or link to screening data (for positive cases only). Linking of screening data to the cancer registry is critical to allow effective evaluation of national screening efforts. In addition, although a number of countries report national incidence information by region, only a few do so by socio‑economic status or deprivation level (France, Ireland, Italy and Sweden). Ensuring that key socio‑economic information is included or linked to cancer registries would facilitate better monitoring and addressing of disparities in cancer care.
1.3. Comprehensive prevention policy packages are needed to reduce risk factors associated with cancer
With the number of cancer diagnoses increasing, and cancer expected to become the leading cause of death in Europe by 2035, countries are exploring what can be done to prevent it. Effective policy making requires an in-depth understanding of the known and modifiable risk factors for cancer, of which population groups are most affected, and of the most effective approaches to address the risks.
1.3.1. Over half of cancer deaths among men and a third of cancer deaths among women are attributable to modifiable risk factors
Globally in 2019, 50.6% of cancer deaths among men and 36.3% among women were attributable to behavioural, environmental, occupational and metabolic risk factors. By far, the leading risk factor for cancer burden in disability-adjusted life‑years (DALYs) and deaths in the EU+2 countries is tobacco, with more than a quarter of all cancer deaths attributed to it in 2019. Alcohol is the next leading cancer risk factor (accounting for 6.3% of cancer deaths), followed by dietary risks such as diets high in processed and red meat and low in fruit and vegetables (6.2%), occupational risks – mainly through asbestos exposure (5.9%), overweight and obesity (5.7%), high blood sugar (5.6%), air pollution exposure – mostly through fine particulate matter (PM2.5) exposure (2.0%), physical inactivity (1.2%) and human papillomavirus (HPV) infection (1.2%; cervical cancer only). While addressed through similar interventions as nutrition and physical activity, the metabolic factors of overweight and obesity and high blood sugar (associated with diabetes) are considered independent cancer risk factors. Furthermore hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, which are concentrated in certain risk groups, are also risk factors for cancer.
Table 1.3 shows the prevalence of selected factors that put individuals at higher risk of cancer across EU+2 countries, alongside an indication of changes over time. Compared to 2011, there has been a reduction at the population level in the prevalence of some of the risk factors for cancer, including a reduction in smoking and alcohol use, and lower exposure to PM2.5 pollution. However, prevalence of overweight and obesity grew by 3% in the EU between 2014 and 2019, and low fruit and vegetable consumption remained prevalent. In 2019, more than half of adults in EU+2 countries were living with overweight and obesity. Large variation in cigarette smoking, alcohol consumption, overweight and obesity, dietary risk, physical inactivity, levels of HPV vaccination and exposure to PM2.5 can be seen across EU+2 countries.
Table 1.3. Prevalence and trends for selected cancer risk factors (or associated measures) vary across EU+2 countries
|
SMOKING |
ALCOHOL |
OVERWEIGHT AND OBESITY |
DIETARY RISK |
PHYSICAL INACTIVITY |
LOW LEVELS OF VACCINATION |
AIR POLLUTION |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Daily smokers (% population aged 15+; change 2011‑21) |
Litres consumed per capita (% population aged 15+; change 2011‑21) |
Population with BMI≥25 (% population aged 15+; change 2014‑19) |
Fruit and vegetable consumption < 5 portions per day (% population aged 15+; change 2014‑19) |
Less than 150 minutes per week (% population aged 15+; change 2014‑19) |
Not receiving all doses of HPV vaccine (% of girls aged 15; change 2012‑22) |
Mean population exposure to PM2.5 (micrograms per m3; change 2010‑20) |
||||||||
|
EU27 |
18.8 |
↓ |
10.0 |
↓ |
51.3 |
↑ |
87.6 |
→ |
67.3 |
↓ |
36.41 |
↓ |
11.6 |
↓ |
|
Austria |
20.6 |
↓ |
11.1 |
↓ |
51.1 |
↑ |
94.4 |
↑ |
56.2 |
↑ |
47.0 |
↓ |
11.0 |
↓ |
|
Belgium |
15.4 |
↓ |
9.2 |
↓ |
48.8 |
↑ |
84.9 |
↓ |
70.7 |
30.0 |
↓ |
11.3 |
↓ |
|
|
Bulgaria |
28.7 |
↑ |
11.2 |
↑ |
53.4 |
↑ |
95.0 |
↓ |
88.7 |
↓ |
91.0 |
↑ |
17.5 |
↓ |
|
Croatia |
22.1 |
↓ |
9.6 |
↓ |
63.8 |
↑ |
90.2 |
↓ |
80.1 |
↓ |
16.0 |
↓ |
||
|
Cyprus |
21.2 |
↓ |
9.6 |
↓ |
48.5 |
↑ |
92.1 |
↑ |
77.6 |
↑ |
36.0 |
↓ |
13.7 |
↓ |
|
Czechia |
17.6 |
↓ |
11.6 |
↑ |
58.4 |
↑ |
92.3 |
↑ |
74.9 |
↑ |
14.3 |
↓ |
||
|
Denmark |
13.9 |
↓ |
10.4 |
↓ |
48.8 |
↑ |
77.1 |
↑ |
44.6 |
↓ |
18.0 |
↓ |
9.1 |
↓ |
|
Estonia |
17.9 |
↓ |
11.1 |
↓ |
55.1 |
↑ |
86.7 |
↑ |
74.2 |
↓ |
40.0 |
↓ |
6.3 |
↓ |
|
Finland |
12.0 |
↓ |
8.1 |
↓ |
57.7 |
↑ |
86.5 |
↓ |
33.0 |
↓ |
5.0 |
↓ |
||
|
France |
25.3 |
↓ |
10.5 |
↓ |
45.4 |
↓ |
80.5 |
↓ |
72.6 |
↓ |
58.0 |
↓ |
9.6 |
↓ |
|
Germany |
14.6 |
↓ |
10.6 |
↓ |
52.1 |
↑ |
89.1 |
↓ |
51.0 |
↓ |
46.0 |
↓ |
10.4 |
↓ |
|
Greece |
24.9 |
↓ |
6.3 |
↓ |
56.2 |
↑ |
87.6 |
↓ |
80.4 |
↓ |
14.5 |
↓ |
||
|
Hungary |
24.9 |
↓ |
10.4 |
↓ |
58.3 |
↑ |
91.8 |
↑ |
67.7 |
↓ |
20.0 |
↓ |
14.2 |
↓ |
|
Iceland |
7.2 |
↓ |
7.4 |
↑ |
60.1 |
↑ |
90.9 |
↑ |
44.1 |
↑ |
6.0 |
↓ |
5.6 |
↓ |
|
Ireland |
16.0 |
↓ |
9.5 |
↓ |
67.1 |
↓ |
62.7 |
↓ |
17.0 |
↓ |
8.1 |
↓ |
||
|
Italy |
19.1 |
↓ |
7.7 |
↑ |
44.7 |
↑ |
89.5 |
↑ |
80.3 |
↓ |
39.0 |
↑ |
14.4 |
↓ |
|
Latvia |
22.6 |
↓ |
12.2 |
↑ |
56.7 |
↑ |
92.8 |
↑ |
79.8 |
↑ |
56.0 |
↑ |
12.4 |
↓ |
|
Lithuania |
18.9 |
↓ |
12.1 |
↓ |
55.0 |
↑ |
84.1 |
↓ |
79.1 |
↓ |
29.0 |
↓ |
9.3 |
↓ |
|
Luxembourg |
19.2 |
↑ |
11.0 |
↓ |
47.1 |
↑ |
86.4 |
↑ |
55.1 |
↓ |
57.0 |
↑ |
8.7 |
↓ |
|
Malta |
19.4 |
↑ |
8.1 |
↑ |
63.9 |
↑ |
88.4 |
↑ |
87.8 |
↑ |
22.0 |
↑ |
11.8 |
↓ |
|
Netherlands |
14.7 |
↓ |
8.1 |
↓ |
48.3 |
↑ |
70.5 |
↓ |
38.0 |
34.0 |
↓ |
10.9 |
↓ |
|
|
Norway |
8.0 |
↓ |
7.4 |
↑ |
49.6 |
↑ |
91.4 |
↓ |
32.4 |
↓ |
8.0 |
↓ |
6.0 |
↓ |
|
Poland |
17.1 |
↓ |
11.0 |
↑ |
56.7 |
↑ |
91.4 |
↑ |
79.7 |
↓ |
18.0 |
↓ |
||
|
Portugal |
14.2 |
↓ |
10.4 |
↓ |
54.5 |
↑ |
85.6 |
↑ |
83.1 |
↑ |
6.0 |
↓ |
8.3 |
↓ |
|
Romania |
18.7 |
↓ |
11.0 |
↑ |
56.4 |
↑ |
97.6 |
↑ |
92.0 |
↑ |
14.2 |
↓ |
||
|
Slovak Republic |
21.0 |
↑ |
9.6 |
↓ |
57.8 |
↑ |
91.5 |
↑ |
69.5 |
↓ |
15.5 |
↓ |
||
|
Slovenia |
17.4 |
↓ |
10.6 |
→ |
56.6 |
↑ |
94.7 |
↑ |
67.4 |
↑ |
56.0 |
→ |
14.4 |
↓ |
|
Spain |
19.8 |
↓ |
10.5 |
↑ |
52.3 |
↑ |
89.1 |
↑ |
64.6 |
↓ |
14.0 |
↓ |
9.8 |
↓ |
|
Sweden |
9.7 |
↓ |
7.6 |
↑ |
49.6 |
↑ |
92.4 |
↑ |
43.6 |
↓ |
15.0 |
↓ |
5.7 |
↓ |
Note: BMI stands for body mass index. For smoking, alcohol, HPV vaccination and air pollution the EU27 averages are unweighted while for overweight and obesity, dietary risk and physical inactivity, the EU27 averages are weighted. The EU average for HPV vaccination is calculated based on 21 EU countries. Green indicates the prevalence of the risk factor is lower than the median of the EU+2 countries by 1 median absolute deviation (MAD) or more; blue indicates that the prevalence is close to the EU+2 median (less than 1 MAD); red indicates the prevalence is worse than the EU+2 median (by 1 MAD or more). For all risk factors, ↓ indicates a reduction in the risk factor over time, regardless of magnitude, ↑ an increase over time and → indicates no change. Change refers to the specified years; data for the nearest years available were used where data from the specified years were not available.
Source: OECD Health Statistics 2023, European Health Interview Survey (Eurostat 2023); WHO (2023[12]), Global Health Observatory database, www.who.int/data/gho; OECD Environment Statistics 2023.
1.3.2. Prevalence of smoking is almost 50% higher among people with lower education levels compared to those with higher education levels
A socio‑economic gradient can be seen in most risk factors, as people with lower education and income levels are more likely to use tobacco, be overweight, have unhealthy diets and be physically inactive than people with higher education or income levels in EU+2 countries (Figure 1.5). The gap between socio‑economic groups grew for tobacco, alcohol and poor diets between 2014 and 2019.
Figure 1.5. Socio‑economic gaps to the detriment of people with lower education levels are found for several cancer risk factors
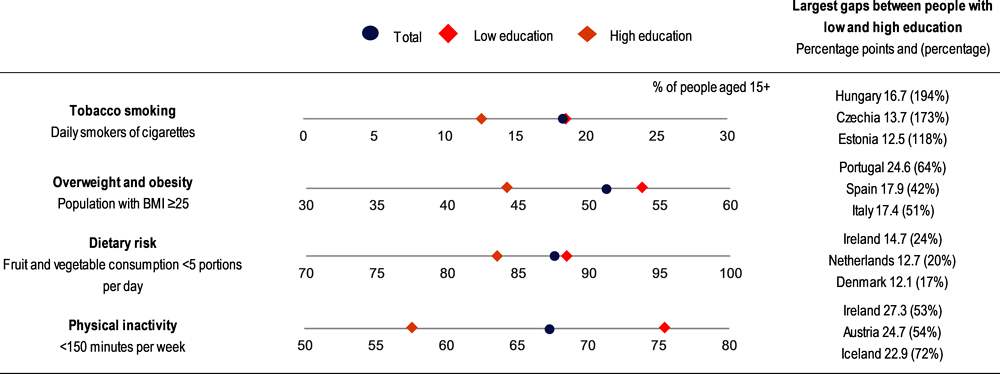
Note: The percentages refer to the population aged 15+ in the EU27. Low education is defined as people who have not completed secondary education (ISCED 0‑2), whereas high education is defined as people who have completed tertiary education (ISCED 5‑8).
Source: Eurostat (EHIS, 2019).
People with low education levels are nearly 50% more likely to smoke daily (18.6%) than those with high education levels (12.7%), but those with a medium level of education had the highest smoking prevalence, at 21.9%.
Across the EU27, people with low education levels are 21% more likely to be overweight and obese and 31% more likely not to undertake the recommended minimum of 150 minutes of health-enhancing physical activity per week than people with high education levels.
For air pollution, a systematic review of available evidence in European countries suggests that higher socio‑economic deprivation is generally associated with higher levels of exposure to particulate matter and nitrogen oxides (Fairburn et al., 2019[13]). Evidence from some European countries indicates that minority groups and foreign-born populations may be more exposed to air pollution.
Data from the Netherlands, Denmark, France, Sweden and Poland indicate lower rates of HPV vaccination or lower confidence in HPV vaccine among people with lower socio‑economic characteristics and migrant groups.
1.3.3. Men across the EU27 are more than twice as likely to report heavy alcohol drinking as women
Similarly, there are gaps in some risk factors between genders that align with the greater cancer incidence and mortality among men. Men smoke cigarettes more than women in nearly all countries. The highest gender gaps are in Lithuania and Romania, with daily smoking more than three times as common among men, and in Cyprus, Latvia and Portugal, where it is more than twice as common. Similarly, 26.3% of men compared to 11.4% of women reported heavy episodic drinking at least once a month in the EU27 in 2019. Men are also more likely to be living with overweight and obesity, and to have diets with insufficient fruit and vegetables compared to women, while women have higher rates of physical inactivity. Between 2014 and 2019 in the EU27, gender gaps in smoking, overweight and obesity, and dietary risk stayed steady, but they decreased for alcohol consumption and physical inactivity. In addition, 85% of occupational cancer deaths in 2019 in EU+2 countries were among men (mostly due to exposure to asbestos).
Certain groups are at higher risk of HBV and HCV infection, which can become chronic and lead to liver cancer. People who inject drugs, people who engage in high-risk sex, prisoners and people who have migrated from endemic areas may be particularly vulnerable. Age constitutes an additional factor worth consideration, as some emerging potential risks – such as e‑cigarette use – are particularly common (and growing in prevalence) among young people.
Engagement in cancer prevention behaviours is linked to health literacy – the knowledge and skills that people have to access, understand, appraise and use information to promote health. Concerningly, around 50% of respondents to the European Health Literacy Population Survey 2019‑21 had an inadequate level of health literacy. A social gradient (considering education, perceived social status and financial deprivation) in health literacy was also demonstrated in all participating countries, to differing degrees.
1.3.4. Cancer prevention requires risk-factor-specific interventions, but key themes and lessons transcend risks
The most effective approach to address each cancer risk factor is a comprehensive prevention policy package
A variety of policy actions have been shown to reduce specific cancer risk factors such as tobacco and alcohol consumption, unhealthy diets and physical inactivity, and to increase HPV vaccination and engagement in prevention or treatment of viral hepatitis (B and C). These include population-level regulatory and fiscal policies that modify prices, availability and advertising of products associated with cancer risk, and information and communication measures to affect attitudes around the risk factors. Organisational and systems design policies include measures delivered to groups or individuals in places where people spend their time, such as schools, workplaces, and the healthcare system. A comprehensive set of policy levers is needed to tackle each of the top cancer risk factors (Figure 1.6).
Figure 1.6. A comprehensive prevention policy package is needed to address cancer risk factors
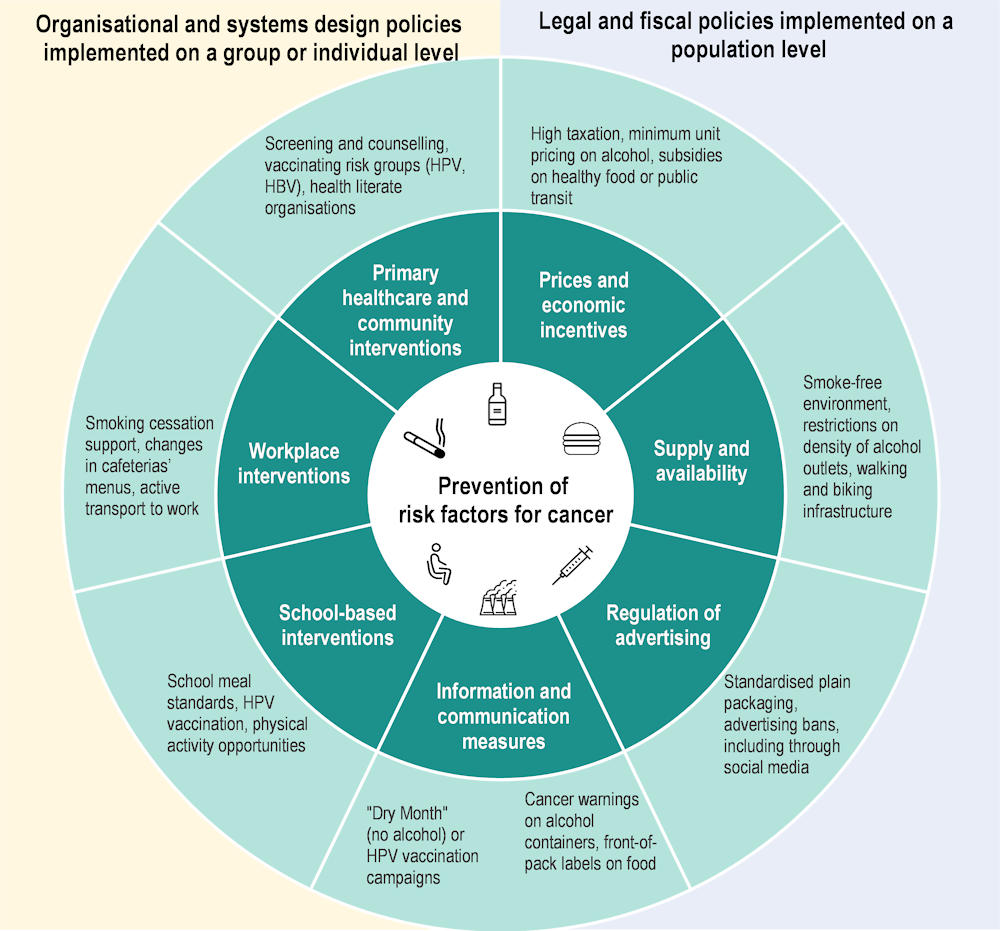
Note: Only selected policy examples are included.
Policy packages to reduce tobacco use include high taxation on tobacco products, banning smoking in a range of places, investing in public awareness campaigns, using clear visual health warnings, restrictions on advertising and providing cessation support to those interested in quitting. Based on these measures, Ireland, France and the Netherlands had the strongest tobacco control policies in 2021, while Bulgaria and Germany had the weakest policies. Almost all the 29 EU+2 countries have increased their tobacco restrictions over the past decade. Importantly, countries with a higher tobacco control score in 2010 experienced a greater reduction in smoking prevalence in the following decade. Similarly, a cross-sectoral alcohol policy comprising a combination of effective and cost-effective interventions is associated with larger gains in prevention of alcohol-related cancer than single interventions in isolation, with the greatest expected impact in the Baltic, Central and Eastern European countries (OECD, 2021[14]). Table 1.4 classifies key risk-factor-specific interventions by general themes, emphasising the applicability of lessons learned to a range of risk factors.
Table 1.4. Tackling the main cancer risk factors requires an integrated policy approach
|
Risk factor |
Prices and financial measures |
Information and communication |
Regulatory measures |
Primary care and healthcare organisations |
Country examples |
|---|---|---|---|---|---|
|
Tobacco |
High cigarette taxes Financial coverage for smoking cessation programmes & support |
Visual pictorial warning labels Language‑ and culture‑specific targeted campaigns and online tools Operating a quitline/ awareness campaigns |
Comprehensive smoking bans Standardised packaging/ warning labels Advertising bans |
Physician recording of smoking status & initiation of cessation discussion Referral to smoking cessation resources |
Denmark, Estonia, Finland: highest taxes as a share of average retail selling price Cyprus, Ireland, Romania: full reimbursement of nicotine replacement therapies Denmark, Finland, Iceland, Ireland, Norway: ban on tobacco advertising across all mediums, sponsorships, point of sale or product display Iceland: highest per capita national spending on anti-tobacco campaigns |
|
Alcohol |
Excise, value added taxes (adjusted for inflation) and minimum unit pricing |
Health-related warning labels Awareness campaigns |
Restrictions on density of outlets Advertising bans Minimum legal age |
Screening and brief interventions |
Iceland, Ireland, the Slovak Republic: minimum unit pricing on alcohol Belgium, France, Italy, Romania, Spain: alcohol taxes adjusted for inflation Cyprus: restriction on density of both on- and off-premise alcohol outlets |
|
Dietary risk, physical inactivity, overweight and obesity, high blood sugar |
Taxes on unhealthy food Subsidies on healthy food |
Front-of-pack labelling Awareness campaigns |
Advertising bans Reformulation School meal standards or school-based sales restrictions |
Counselling on nutrition and physical activity Physical activity prescription |
Belgium, Croatia, Denmark, Finland, France, Hungary, Ireland, Latvia, Poland, Portugal: excise tax on sugar-sweetened beverages Belgium, France, Germany, Luxembourg, the Netherlands: Nutri-Score front-of-pack labelling Estonia, Finland, Sweden: free school meals for primary and secondary school children |
|
Environmental and occupational exposure |
Cap & trade taxes Subsidies for cleaner fuel, appliances & retiring old cars (means-based) Subsidies for public transit |
Energy efficiency labelling on appliances Active transit campaigns Asbestos awareness and safety campaigns |
Standards set for fuel, appliances and industrial plants Low-emission zones Strict asbestos occupational exposure limits |
– |
Austria, Germany, Luxembourg, Malta, and the Netherlands: long-term network tickets valid on all or most modes of transit Austria, Finland, Germany, Ireland, Luxembourg, the Netherlands, the Slovak Republic: national government programmes to support active transit to both school and workplaces Poland: national programme for safe removal of asbestos & asbestos database |
|
HPV infection; low HPV vaccination coverage |
Free universal vaccination for both boys and girls Free vaccination of high-risk groups |
Campaigns to promote confidence around vaccines Culturally adapted community/peer education efforts |
Shift to one‑dose vaccination regimen School-based vaccination programmes |
Reminders to physicians and/or parents Bundling with other vaccinations Vaccination by nurses, pharmacists and mobile vaccination clinics |
Austria, Belgium*, Croatia, Cyprus, Estonia, Finland, France, Hungary, Iceland, Ireland, Norway, Slovenia, Spain, Sweden: school-based HPV vaccination programmes Ireland: one‑dose HPV vaccine regimen Denmark, Iceland: pharmacists able to provide HPV vaccine the Netherlands: HPV vaccination buses and pop-up vaccination stops France: Extended HPV vaccination target age for certain high-risk groups |
|
HBV and HCV infection |
Free vaccination (HBV) for all children and risk groups |
Sexual health programmes Awareness campaigns |
– |
Antenatal screening (HBV) Harm reduction for people who inject drugs (HBV, HCV) |
France, Greece: screening for HBV/HCV and linkage to services of vulnerable groups Hungary: school-based HBV vaccination programme |
Notes: * Belgium’s school-based HPV vaccination programme is in the Wallonia-Brussels region only. The policies and examples highlighted here do not include all those available.
To promote equity, policies need a design that not only reduces overall risk factors but also narrows disparities among population groups
It is important to recognise that some policies can be effective to reduce risk factors for the population as a whole, yet lead to an increase in disparities through larger improvements in one group than another. For instance, mass media campaigns are effective at disseminating messages that help prevent cancer through improving health literacy and people’s awareness of cancer risk factors. However, people with higher levels of education may benefit more from mass media campaigns, as they may more effectively understand and act upon health information. Similarly, smoking bans that are not comprehensive are often more common and more stringently enforced in areas with higher socio‑economic characteristics. In contrast, higher taxation of unhealthy products (tobacco, unhealthy food, alcohol) has consistently been proved effective in reducing consumption among people with lower socio‑economic characteristics, such as lower incomes. To ensure that such measures do not lead to financial hardship for people with low incomes, price increases should be accompanied by measures to ensure access to cessation services (tobacco, alcohol), or by price decreases and subsidies for healthy products (food). Taxes affecting sugar-sweetened beverages are the most common nutrition-related tax but are present in only 13 EU+2 countries. School-based measures can intervene on health-promoting choices across a range of risk factors.
Of the 29 EU+2 countries, 19 set mandatory standards for healthy food in school meals and 12 restrict availability of sugar-sweetened beverages in schools. Some countries provide school meals free of charge to students to ensure access for all, including children from families with lower socio‑economic characteristics.
School-based programmes that provide HPV vaccines are in place in 14 of the EU+2 countries, helping to reach all children in the target group.
Policies can be tailored to the needs of hard-to-reach population groups or to be effective in underserved areas (Box 1.1). Although gender is an important determinant of cancer risk, evidence of the effectiveness of policies that reduce gaps by gender is scarce, highlighting an avenue that warrants more attention, given the underlying differences in motivations, behaviour and responses between men and women.
Box 1.1. Some countries are using effective policies and interventions to reach people who are most at risk
In the United Kingdom (Scotland), introducing a minimum unit price of GBP 0.50 per UK unit of alcohol was found to decrease weekly alcohol purchases by 7.6%, with a larger impact among low-income groups than high-income groups. In 2023, only three EU+2 countries had implemented minimum unit pricing (Iceland, Ireland and the Slovak Republic) and five (Belgium, France, Italy, Romania and Spain) had adjusted taxation to inflation to ensure that alcohol does not become more affordable in real terms over time.
Specific local interventions in Greece and France reach vulnerable populations (people who inject drugs, prisoners or migrants) to ensure screening coverage for hepatitis B and C, and link them to health services to prevent further health harms, including liver cancer.
The Netherlands operates mobile HPV vaccination teams that visit underserved or rural communities and temporary pop-up vaccination stops in locations frequented by young people.
The Flemish Government in Belgium prevents exposure to asbestos among workers by requiring owners of units built prior to 2001 to record asbestos present in the property, requiring asbestos removal as a precondition for solar panel installation and, from 2022, requiring certification on asbestos and its safe management and removal prior to the sale of a building.
Policies that promote healthy living through environmental changes can reduce risk factors without requiring an active change in behaviour
Creating environments conducive to healthy lifestyles is an effective policy to reduce cancer risks, leading to a reduction in prevalence without requiring active behaviour change among the population. Increasing the availability of healthy options in the environment while decreasing exposure to unhealthy ones is relevant for most risk factors.
Smoking bans reduce second-hand exposure to tobacco smoke in various settings, as evidenced by improvements in lung function among both non-smokers and smokers after the implementation of an indoor smoking ban in Denmark. In the Netherlands, the Smoke‑free Living for Everyone Programme takes a local, tailored approach to reducing smoking in vulnerable communities, designing interventions with local residents’ involvement, wherein smoking is tackled alongside other community challenges.
A reduction in the number and density of alcohol sales outlets is effective to reduce alcohol consumption, as well as associated socio‑economic inequalities, yet only 10 of the 29 EU+2 countries regulate this. The Nordic countries (Iceland, Norway, Sweden and Finland) effectively regulate sales of strong alcohol via state monopolies, and modelling studies suggest that dismantling of the monopolies would result in increases in alcohol consumption and mortality.
Policies to improve air quality by reducing road transit pollution include regulatory (low-emission zones), infrastructure (developing cycling and pedestrian routes) and financial (affordable public transit) measures, among others. Gaining additional health benefits from synergies with increasing physical activity levels, 17 EU+2 countries had national government support for active transport to school or work in 2023. For example, Ireland collaborates with employers via campaigns and educational materials to promote active travel to work.
To improve diet, regulatory limits on specific nutrients incentivise manufacturers to reformulate products, making them healthier. Indirect incentives for reformulation can include taxation of unhealthy nutrients or labelling of food nutritional content. Most EU+2 countries have agreements with the food industry on reformulation of food products, yet these remain mostly voluntary in nature, and thus potentially less effective than mandatory limits. Agreements to reduce fat content exist in 10 EU+2 countries, and sugar is targeted in 16.
Primary healthcare interventions are effective in reducing cancer risk factors while also improving health literacy
Reaching people across the population, a well-structured and accessible primary care system can have an important role in health promotion. For instance, primary healthcare can be an important venue to promote and provide vaccinations, including those against HPV or HBV. Healthcare workers can also initiate conversation about, and connect smokers or people consuming large amounts of alcohol to, cessation support services. Screening and brief interventions in primary care settings are cost-effective in most EU27 countries in reducing alcohol-attributable morbidity and deaths; however, their implementation varies across EU+2 countries owing to differences in policies and training given to healthcare providers.
On an organisational level, primary care providers can make navigating choices in health and healthcare easier for people with low health literacy by facilitating access, understanding and use of health information. This entails effective communication and support to patients, among others. Additionally, physical activity prescription programmes, wherein evidence‑based recommendations and community support are prescribed by healthcare providers, exist in 10 EU+2 countries. Portugal is leveraging its national healthcare system to deliver brief counselling on nutrition, and counselling and prescription of physical activity, with training provided to health professionals. Slovenia integrates health-promotion centres in all primary healthcare centres, thus providing free lifestyle interventions against key risk factors, and establishing cross-sectoral partnerships with different stakeholders, including social services and non-governmental organisations at the local-community level. As a result, more than half of Slovenia’s population have been screened for lifestyle and risk factors, while almost 50 000 patients per year take part in lifestyle interventions run by the centres.
Co‑operation between countries can deliver additional gains in reducing cancer risk factors
Co‑operation between countries can deliver important gains in comparison to individually implemented interventions. Given the transnational reach of air pollution, the EU is collaborating to achieve clean air through a mix of regulatory and financial incentives, product design standards, communication and education campaigns, and partnership programmes. Considering the economic integration of Europe and freedom of movement across borders, lessons from these actions are relevant for other measures.
While the EU already collaborates on tobacco control via several key directives, stronger harmonisation of tobacco pricing and taxation between European countries could mitigate cross-border trade challenges. Importantly, the industry’s release of new products such as e‑cigarettes and heated tobacco point to a need to revise existing EU-wide policies continually.
Alongside acting as a health information dissemination measure for consumers, co‑ordinated labelling on food packages can simplify compliance with regulations for food manufacturers. Although monochrome back-of-pack nutrition labels are mandatory in the EU, a range of front-of-pack labelling systems are in use, which are not applied by all manufacturers due to their voluntary nature. The largest evidence base in terms of understanding and use across different groups of consumers supports the Nutri-Score labelling system.
Given that much media content crosses borders, and forms of commonly used media can change over time, co‑ordination on comprehensive advertising restrictions between countries can make them more effective. Conversely, select or inconsistent bans can lead to reallocation of resources to advertising forms that are not yet restricted.
The EU restricts tobacco and alcohol advertising (that specifically targets minors or encourages excessive drinking) on various media platforms, but challenges persist, such as limited bans on alcohol marketing on social media. Social media use has been associated with more frequent alcohol consumption among young people, yet only Lithuania and Norway restrict alcohol advertising via social media. Importantly, countries cannot impose their advertising rules on content from other countries.
Owing to the cross-national reach of advertisements, most EU countries regulate direct advertising of unhealthy food or beverages to young people, yet only 11 countries do this through mandatory legislation, which is considered more effective than voluntary measures. In 2023, Norway announced a plan to fully ban all advertising of unhealthy food and beverages targeted at minors across media channels.
1.3.5. There is scope to increase spending on prevention
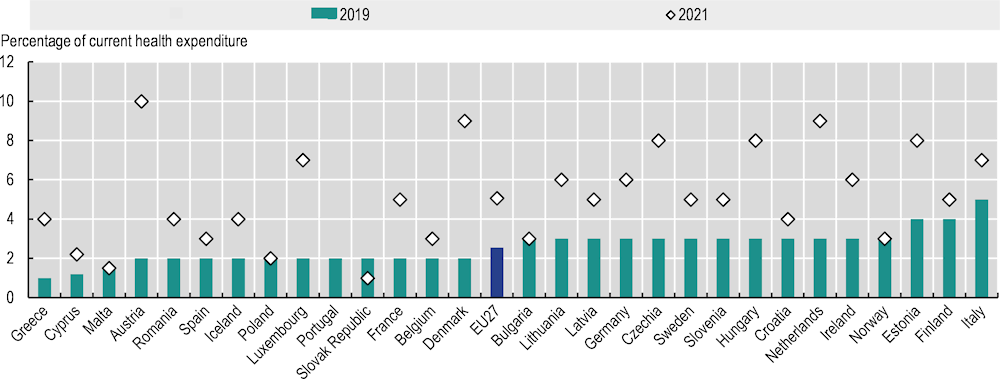
Despite the breadth of possible prevention activities, their cost – effectiveness and the vast benefits they can deliver through reduction in rates of chronic diseases – including cancer – prevention spending in EU+2 countries is generally perceived to be insufficient. Prior to the COVID‑19 pandemic in 2019, it accounted for an average of 2.5% of health expenditure across the EU27, ranging from 1% in Greece to 5% in Italy. During the COVID‑19 pandemic in 2021, it temporarily increased for nearly all countries owing to spending on COVID‑19 infection prevention and control, amounting to an average of 5.1% of health expenditure (Figure 1.7). As most of the additional spending went to vaccines, masks and other COVID‑19 prevention efforts and did not address underlying population health, there is potential to increase investments that effectively tackle factors that increase cancer risk (OECD/European Union, 2022[15]).
Figure 1.7. Spending on prevention as a share of current health expenditure is relatively low
1.4. Improving the reach of cancer screening and early diagnosis is a priority area to improve cancer outcomes
Along with preventing cancer by reducing risk factors, countries are planning to improve early detection of cancer. This effort comprises two key aspects: screening – or testing asymptomatic and apparently healthy individuals to potentially identify a precursor or early-stage cancer lesion in people without symptoms – and early diagnosis, which focuses on detecting symptomatic people as early as possible. In December 2022, a new EU Council Recommendation on Cancer Screening was adopted. This replaces and extends the scope of the previous Council Recommendation 2003/878/EC on cancer screening adopted in 2003, which encompassed recommendations for breast, colorectal and cervical cancer screening.
1.4.1. Population-based screening for breast, colorectal and cervical cancer are in place in most EU+2 countries, with varying eligibility and testing approaches
Aside from Bulgaria, Lithuania and Romania, all other EU+2 countries have population-based screening programmes in place for breast cancer. These are generally organised at the national level, except in Belgium, Denmark, Italy and Sweden, where they are organised by the regions. Consistent with the 2022 EU Council Recommendation, breast cancer screening programmes target women aged 50‑69 with a mammogram every two years in 18 countries (Table 1.5). Austria, Cyprus, the Czech Republic (hereafter “Czechia”), France, Hungary, Iceland, the Netherlands and Sweden conduct screening on a broader age range. In several countries, there are also plans to extend the age limits of the target population to 45‑74 (Cyprus, Germany, Malta, Poland and Spain). Among 22 of the 29 EU+2 countries, a population-based colorectal cancer screening programme is in place, organised at the national or regional level, but only 7 countries align with the EU Council Recommendation to perform faecal immunochemical testing (FIT) for those aged 50‑74. With the exception of Austria, which will target people aged 45‑75 when the recent recommendations are implemented, EU+2 countries include narrower age ranges, such as 60‑68 in Estonia, 59‑69 in Ireland and 55‑65 in Norway.
Table 1.5. In many countries, the target age for the population-based cancer screening programme differs from the 2022 EU Council Recommendation
|
Category |
Breast cancer screening |
Colorectal cancer screening |
Cervical cancer screening |
|---|---|---|---|
|
Number of countries with population-based programmes |
26 EU+2 countries |
22 EU+2 countries |
21 EU+2 countries |
|
Target age and test in line with the 2022 EU Council Recommendation |
Belgium, Croatia, Denmark, Estonia, Finland, Germany, Greece, Ireland, Italy, Latvia, Luxembourg, Malta, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain |
Belgium, Cyprus, Czechia, Denmark, France, Portugal, Slovenia |
Estonia, Finland, France, Ireland and Netherlands |
Note: According to the 2022 EU Council Recommendation, breast cancer screening is recommended for women aged 50‑69; for colorectal cancer screening, the preferred screening test is quantitative FIT for people aged 50‑74; for cervical cancer screening, HPV testing is recommended for women aged 30‑65.
For cervical cancer, 21 EU+2 countries have a population-based screening programme in place, organised at the national or regional levels. Compared to breast and colorectal cancer screening, there is wider variation in age ranges of the population screened in EU+2 countries. Only Estonia, Finland, France, Ireland and the Netherlands perform HPV testing for women aged 30‑65, as recommended by the EU Council Recommendation. Some countries include lower age limits, such as Germany and Slovenia (20), and some include women until 69 (Norway) and 70 (Czechia, Latvia and Sweden). In addition, only seven countries have only HPV-based screening in place (Denmark, Finland, Ireland, the Netherlands and Portugal since 2020, Estonia since 2021, and Norway since 2023) although evidence supports the use of HPV-based screening as an effective method compared to the cytology test.
Importantly, an increasing number of countries offer self-sampling tests for colorectal and cervical cancer screening, sent by post or delivered in local pharmacies or by general practitioners (GPs), to improve participation rates. For colorectal cancer, 14 countries provide the option to self-test at home and send a sample to a laboratory for analysis (as in the Netherlands, Belgium, Denmark and Italy). Seven EU+2 countries provide the option of self-sampling for HPV testing: Czechia, Estonia, France, the Netherlands, Norway, Spain (in some regions) and Sweden. In Denmark, women who do not respond to the cervical cancer screening invitation are offered HPV self-sampling tests in the second reminder letter. A pilot programme in Czechia sent self-sampling HPV tests to women aged 50‑65 from vulnerable groups, such as women at risk of poverty and social exclusion in deprived areas.
1.4.2. Countries with higher participation in breast cancer screening programmes have better breast cancer outcomes
There is clear evidence that breast, colorectal and cervical cancer screening increases the likelihood of successful treatment – particularly when cancer is identified at an early-stage – and leads to a reduction in mortality rates (Zielonke et al., 2020[17]). Early diagnosis of cancer also leads to better survival probabilities, fewer complications and better quality of life (Hawkes, 2019[18]; Neal et al., 2015[19]).
Breast cancer screening rates based on programme data demonstrate that countries with higher participation rates among the eligible population in 2015 have better cancer outcomes in 2020, such as a lower ratio of breast cancer mortality to incidence rate (Figure 1.8). Among the 25 countries with available data, 8 had lower participation rates in breast cancer screening and a higher ratio of mortality to incidence (top left quadrant). A further 12 countries had higher participation rates in breast cancer screening and a lower ratio of mortality to incidence (bottom right quadrant).
Figure 1.8. Higher coverage rates for breast cancer screening programmes are associated with lower ratios of breast cancer mortality to incidence
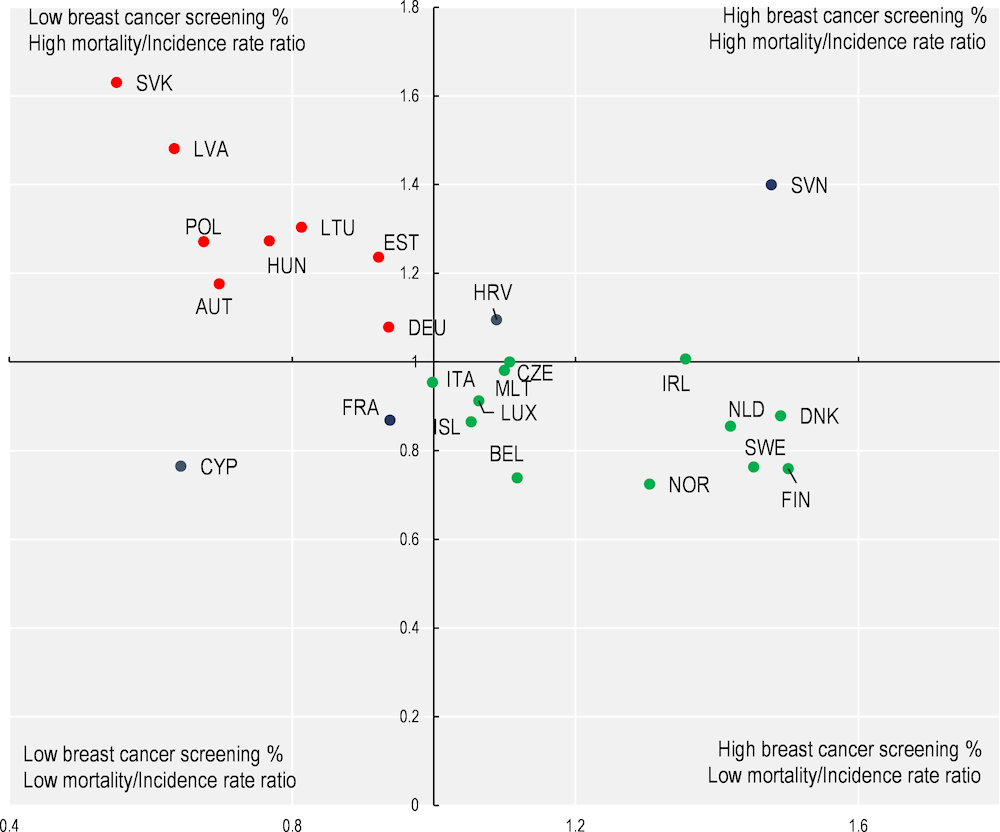
Notes: The quadrant chart shows the association between breast cancer screening rates (2015) and cancer outcome as measured by a breast cancer mortality to incidence rate ratio. Age‑standardised breast cancer mortality data are from 2020; age‑standardised breast cancer incidence rates are 2020 estimates from the Joint Research Centre; and breast cancer screening rates are based on programme data from 2015 (or nearest year). The centre of the quadrant chart is the EU average.
Source: OECD Health Statistics (2023[16]), https://doi.org/10.1787/health-data-en; Eurostat (2023[2]), Eurostat (2023), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table (accessed on 16 June 2023); ECIS (2023[1])., European Cancer Information System, https://ecis.jrc.ec.europa.eu.
1.4.3. Despite population-based programmes, cancer screening participation rates remain lower than 50% in at least one‑third of EU+2 countries
For the three cancer screening programmes, the proportions of the eligible population receiving the test vary widely across EU+2 countries. In 2021 (or the latest available year), the proportion of women aged 50‑69 who had had a mammography examination in the two preceding years ranged from a high of 83% of the eligible population in Denmark to a low of 9% in Romania. Similarly, the coverage rates for cervical cancer screening vary from 85% in Austria to 12% in Poland. Overall, participation rates among the eligible population in EU+2 countries are lower than 50% in 9 countries for cervical cancer screening programmes, in 11 countries for breast cancer screening programmes and in 21 countries for colorectal cancer screening programmes. Overall, only Austria, Denmark, Finland, the Netherlands and Slovenia, have participation rates above 50% for all three cancer screening programmes (Table 1.6).
Table 1.6. Only five EU+2 countries have above 50% participation rates in all three of the main cancer screening programmes
|
Less than 50% participation in… |
Above 50% for all three cancers |
||||
|---|---|---|---|---|---|
|
All three cancer screening programmes |
Cervical & colorectal cancer only* |
Mammography & colorectal cancer only* |
Cervical cancer only* |
Colorectal only* |
|
|
Germany |
Belgium |
Bulgaria |
Malta |
Croatia |
Austria |
|
Hungary |
Italy |
Cyprus |
Czechia |
Denmark |
|
|
Latvia |
France |
Estonia |
Finland |
||
|
Poland |
Lithuania |
Greece |
Netherlands |
||
|
Romania |
Luxembourg |
Iceland |
Slovenia |
||
|
Slovak Republic |
Ireland |
||||
|
Norway |
|||||
|
Portugal |
|||||
|
Spain |
|||||
|
Sweden |
|||||
Note: The data show the number of countries with participation rates for the three cancer screening programmes: mammography screening within the past two years (% women aged 50‑69), cervical cancer screening within the past three years (% women aged 20‑69), colorectal cancer screening coverage (% of population aged 50‑74 screened). The data refer to either programme or survey data (see Chapter 4), limiting the international comparability. Categories with an asterisk indicate that countries in the corresponding list have above 50% participation rates on the other cancer screening test(s) not listed. No countries had less than a 50% screening rate on mammography only.
Source: OECD Health Statistics (2023[16]), https://doi.org/10.1787/health-data-en.
1.4.4. People with low education levels are 15% less likely to receive mammography screening than people with high education levels
Overall, across EU+2 countries with available data, less well-off groups have a lower probability on average of screening for breast and colon cancer. For breast cancer screening, the likelihood of having received a mammogram is 54% among women with low education levels compared to 64% among those with high education levels (Figure 1.9). Inequalities in favour of better-educated people are observed in 19 out of 25 countries.
For colon cancer, only 31% of individuals with low education levels had received screening tests compared to 38% of people with higher education levels, and inequalities in favour of better-educated groups were observed in 18 out of 25 countries. Furthermore, it was found that while people with a migration background have a lower likelihood of accessing breast cancer screening, the relationship is entirely explained by lower education and income. In contrast, people living in rural areas also have a significantly lower likelihood of having been screened for breast and colon cancer than those living in urban areas even after considering socio‑economic factors.
Figure 1.9. Women with low education levels have a lower likelihood of receiving a mammogram in 19 EU+2 countries
Indirectly age‑standardised probability of having had a mammogram, by country and education level
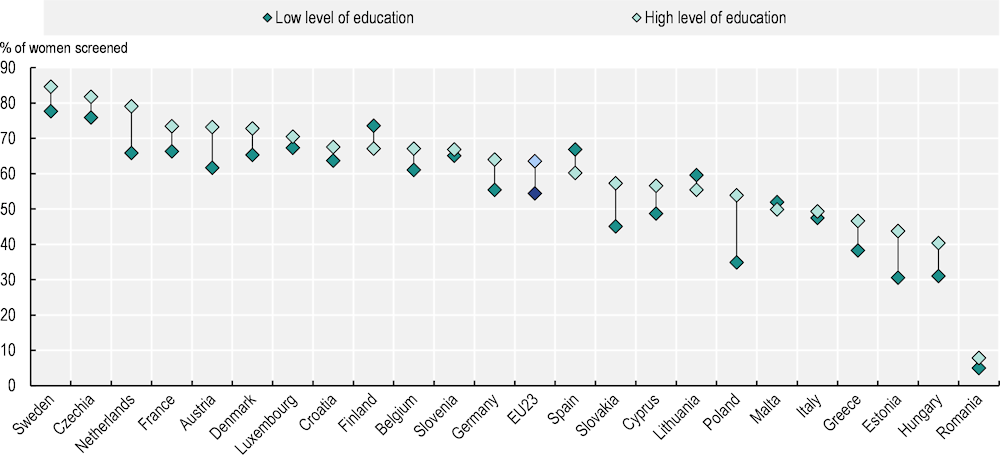
Note: Analysis based on 16 035 observations of women aged 50‑74 living in a private household in 25 countries. Probabilities are based on indirect age standardisation. Education level is built according to the International Standard Classification of Education (ISCED), with ISCED 0-2 for low level of education and ISCED 4-6 for high level of education.
Source: Survey of Health, Ageing and Retirement in Europe (wave 8).
1.4.5. A mix of strategies has proved effective at expanding screening and early diagnosis
Increasing awareness of cancer and the benefits of screening is key to raising screening participation rates
Greater awareness about cancer, the benefits of screening and cancer symptoms is key to greater participation in screening programmes and early diagnosis. However, cancer awareness varies across countries, and tends to be lower among those from lower socio‑economic groups and ethnic minority groups. Of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance1, 21 have awareness campaigns on screening, of which a number rely on media campaigns and information leaflets. More specifically, 18 countries reported that they have screening awareness campaigns and education initiatives to focus on hard-to-reach populations (Box 1.2).
Box 1.2. Of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance, 18 have initiatives targeting vulnerable or hard-to-reach populations
Slovenia and Sweden use peer-to-peer helpers who educate those within their community networks about screening.
France and Germany employ simple language and easy-to-read and -understand screening materials to ensure accessibility for people with low literacy levels.
Belgium (Flanders), Finland, Germany, Ireland, the Netherlands and Slovenia make invitations to screening, online education guides, video messages or other screening information available in various languages.
Ireland and the Netherlands ensure access to and/or awareness and engagement of lesbian, gay, bisexual and transgender (LGBT+) communities in cervical and/or breast cancer screening.
Slovenia works with organisations that support people with disabilities and provides home screening assistance to increase screening participation.
Mobile screening units and expanding the role of pharmacists in screening help programmes reach remote populations
New delivery models have been adopted to reach socially vulnerable populations, rural and underserved groups in their local communities. Mobile breast cancer screening programmes have been implemented in a few countries (Croatia, Cyprus, Estonia, France, Iceland, Ireland, Norway, Slovenia and Sweden). In France, mobile mammography units have been found to increase participation in breast cancer screening, to reduce geographical and social inequalities, and to be more cost-effective than placing radiologist offices in underserved areas. Another approach takes advantage of pharmacies’ wide accessibility and familiarity with patients to increase screening in more remote areas. For example, France and Spain use pharmacies for distribution of and education on colorectal screening tests, and Norway engages them in skin cancer screening, with images of moles and pigmented lesions sent to dermatologists for assessment.
Primary healthcare providers play an important role in cancer screening and early diagnosis
Primary healthcare providers have a key role in early cancer detection – reminding their patients about screening, clarifying patients’ questions and referring symptomatic patients to specialist care. Their recommendations and reminders are helpful in increasing screening participation rates, and may be particularly important for individuals who have never been screened or are under-screened. Of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance, 15 rely considerably on primary healthcare providers to deliver cancer screening activities for cervical cancer, while 12 do so for colorectal cancer. For cervical cancer, the screening itself often takes place in primary care settings, whereas for colorectal cancer, specialists, hospitals or GPs are involved, depending on the country. Furthermore, optimising primary healthcare recognition and interpretation of symptoms is an important way to improve earlier diagnosis of cancer. GP-targeted cancer awareness campaigns, training and continuous medical education about referral guidelines have been shown to be effective in selecting patients for urgent cancer referral. Such training is part of the continuous medical education programme in Denmark, along with the United Kingdom and Australia. In addition, in the United Kingdom, primary care providers have access to decision-support tools within their software systems to help them identify relevant patients presenting with non-specific symptoms for cancer testing.
Fast-track pathways help to reduce delays in cancer diagnosis
Fast-track pathway policies help to reduce the time between cancer suspicion, cancer diagnosis and start of initial treatment to improve cancer prognosis. They have been developed in a few countries, including Denmark, Ireland, Latvia, Lithuania, Poland, Slovenia, Sweden and some regions in Spain. In Latvia, Lithuania and Poland, the fast-track pathways ensure that patients receive required diagnostic and care services within established national time limit guarantees (e.g. in Latvia, specialist consultation and diagnostic examination within ten working days of the date of referral). In Denmark, the pathway requires GPs to take a pre‑defined minimum panel of blood and urine tests from patients, and to assess the results of computerised tomography (CT) scans prior to further evaluation at hospitals. For all cancer patients, three-year relative survival increased from 45% to 54% after implementation of the cancer pathways in Denmark (Jensen, Torring and Vedsted, 2017[20]). Ireland’s system of rapid access clinics undertakes much of the country’s diagnostic work on breast, lung or prostate cancer, and an analysis of the initial patients assessed via this programme showed more than double the rates of lung cancer identified at early stages compared to figures from the country’s national cancer registry (Dunican et al., 2023[21]).
Monitoring inequalities helps to promote engagement with vulnerable groups and to build quality improvement cycles
Use of cancer screening data in quality assurance mechanisms could be improved to assess outcomes and monitor inequalities. Only 13 of the 26 EU+2 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance integrated information from both population-based and non-population-based screening into existing cancer screening databases, and only 16 acknowledged using screening data in quality improvement cycles. For example, in the Netherlands, screening data are translated into performance measures that are monitored at the local, regional and national levels. In Czechia, the National Oncological Registry provides epidemiological statistics, incidence by region and clinical stages of diagnosed cancers. While most countries responding to the OECD Policy Survey collect information on age and geography in their screening programmes, only 6 do so for socio‑economic information (France, Germany, Italy, the Netherlands, Slovenia and Sweden). Denmark, Italy and Sweden report collecting data about education.
1.4.6. Harnessing new technologies could improve early detection and patient experiences, but implementation should be evidence‑based
Innovations in cancer screening and early diagnosis – such as risk stratification, biomarker detection and use of artificial intelligence (AI) and machine learning (ML) algorithms – have the potential to improve the chances of early cancer detection. A stratified-risk screening approach follows a personalised screening decision, where the individual characteristics of each citizen are taken into consideration to determine screening frequency and test type rather than having screening determined exclusively based on sex and age. Risk stratification can also be guided by genomic testing, which can provide information on individual risk to help personalise prevention and early diagnosis. For breast cancer, research is under way on a risk-based approach based on family history, hormonal and reproductive aspects, mammographic breast density and common genetic variants. Similarly, for colorectal cancer, the possibility of sex-specific and age‑specific cut-off values for FIT and the possibility of tailoring screening intervals according to the results of prior FIT results is being researched. For cervical cancer, self-sampling tests targeting women at higher risk are being developed that can identify positive high-risk HPV infections that are relevant to cancer.
Implementation of risk-stratified approaches, however, faces relevant implementation challenges such as resource considerations, health literacy and support for informed decision making, as well as the need for workforce training and acceptability among healthcare professionals and the general population. Another potential screening innovation is use of liquid biopsies, which can detect certain types of cancer by analysing DNA fragments in a person’s blood that are released by cancer cells. While liquid biopsies have been effective in monitoring disease progression and treatment response, their use in early diagnosis of cancer is a subject of current research.
Image‑based risk prediction using ML on mammograms, X-rays and magnetic resonance imaging (MRI) has been studied to predict the likelihood of breast, lung and prostate cancers. New efforts are under way to establish large repositories for cancer images to aid in developing algorithms that can improve screening accuracy and early diagnosis. While only a few countries currently use AI for cancer screening, a number are engaging in discussions or pilot projects in this realm. In Germany, a project is using AI algorithms to support diagnosis of melanoma, while a joint collaboration project between universities in Latvia, Lithuania and Estonia – along with the Norwegian Cancer Registry – is focusing on the cost – effectiveness of specific AI tools in personalised cervical cancer screening. For colorectal cancer, a wireless ingestible capsule that utilises AI to analyse X-ray images is being researched, offering the opportunity to increase both the effectiveness and reach of colorectal screening. However, use of AI technology in cancer is in the early stages, and further work needs to be undertaken on regulatory, legal, ethical, clinical and economic aspects, including ensuring implementation without exacerbating existing inequalities.
1.5. In the context of the rising burden of cancer and growing cost pressures, countries need to ensure the sustainability of high-quality cancer care systems
In cases of positive cancer screening and early cancer detection, the focus shifts to the care system, which has its own set of challenges. To care for an increasing number of people with cancer, countries need to seek effective and efficient ways of delivering high-quality cancer care. Most European countries, however, face shortages of various types of professionals providing cancer prevention, diagnosis and care services, and difficulties in securing access to high-quality professionals across regions within countries. With emerging technologies in cancer medicines and medical equipment, EU+2 countries also face financial challenges in securing access to innovative treatments and in providing sustainable, high-quality cancer care.
1.5.1. Workforce shortages need to be addressed in order to safeguard the sustainability of high-quality cancer care
Most EU+2 countries face workforce shortages in the health sector as a whole and in cancer care, affecting the delivery of cancer prevention, screening, diagnosis, treatment and palliative care. Twenty-two countries responding to the 2023 OECD Policy Survey on Cancer Care Performance reported shortages of GPs, and most also reported shortages of oncology nurses, radiologists, radiation therapists and oncologists. Shortages of psychologists (Ireland, Slovenia and Sweden), palliative care professionals (Slovenia) and navigator nurses or survivorship co‑ordinators (Malta) were also noted. Furthermore, geographical distribution challenges are substantial, in both primary and cancer care. Austria, Czechia, Hungary, Italy, Latvia, Norway, Portugal and Romania reported inadequate geographical distribution of oncologists, affecting equitable delivery of cancer care.
Countries have implemented various policy levers to tackle workforce shortages (Figure 1.10). Half of the countries responding to the OECD Policy Survey have increased training capacity to improve availability of the workforce providing cancer care. In Slovenia, there has been an increase in training sites for clinical psychologists and palliative care, as the country plans to increase the number of mobile palliative care units and expand the availability of psychological support. About one‑third of countries pursued task substitutions and reallocations among healthcare professionals. To support pharmacists in providing high-quality cancer care and identifying their training needs, Ireland, for example, has developed the National Competency Framework, which outlines the behaviours, skills and knowledge required for pharmacists working in cancer care. Ireland has also developed a number of educational initiatives to equip various types of nurses with adequate knowledge, skills and competencies in areas such as anti-cancer therapy and psychosocial care to provide cancer care safely and effectively. Provision of financial incentives such as funding of training abroad or additional funds for staff working weekend shifts is another common approach taken to resolve health workforce shortages. These are used in 12 of the 26 countries responding to the OECD Policy Survey. In 2023, Denmark allocated funding to pay healthcare professionals for weekend shifts to improve workforce capacities in cancer care. Another policy option to address workforce shortages is to recruit foreign-trained health professionals; this has been implemented in 11 of the 26 responding countries. In Slovenia, alongside recognition of foreign-trained healthcare professionals, which has been in place for many years, the language requirements were relaxed recently to attract greater numbers.
Figure 1.10. Policy responses to address workforce shortages vary across EU+2 countries
Notes: Information is not available for Belgium, Cyprus and Denmark. The policies listed have not been adopted in Bulgaria, Greece, Italy or Malta.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
1.5.2. Unequal access to cancer medicines requires mechanisms to rationalise coverage decisions and encourage market entry of generics and biosimilars
Between 2004 and 2022, 152 new cancer medicines were granted centralised marketing authorisation by the European Medicine Agency (EMA), with a marked increase in the number of approved oncology medicines over time. Except for Cyprus and Slovenia, all EU+2 countries have established a health technology assessment (HTA) agency to inform decision making around pricing and coverage of cancer medications. This is particularly relevant given the rising prices of individual cancer medicines and rising expenditure on cancer pharmaceuticals as a share of cancer care costs.
An OECD analysis of a sample of indications with high clinical benefit in breast and lung cancer, with EMA marketing authorisation, shows that the proportion of indications reimbursed/covered varies substantially across countries. Germany reports coverage for all indications, while Malta, Cyprus and Latvia cover less than one‑third. However, it should be noted that not all eligible patients in clinical practice may have access to medication on a reimbursement list because of budget or other constraints; in parallel, early access schemes (as in Malta) or alternative medications in specific treatment settings may be available in cases where the specific medications examined are not reimbursed.
Actual market access to new medications depends both on when a company decides to file an application in a particular country (based on the company’s launch strategy, the size of the market and expected benefit) and on HTA and pricing processes. The time from EMA authorisation to issuing a reimbursement decision ranged from less than 100 days in Germany and Sweden to over 3 years in Cyprus, Latvia and Lithuania (Figure 1.11).
Figure 1.11. Shares of selected indications of cancer medicines that received public reimbursement/coverage vary across EU+2 countries
Notes: A total of 24 countries responded to the pilot data collection. Thirteen indications of ten cancer medicines used in the treatment of breast cancer and lung cancer with marketing authorisation by the EMA after 1 January 2016 and active authorisation on 26 March 2023, and with the highest clinical benefit according to the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring system (scores of A and 5), were included in the analysis. The shares show the inclusion status of the indications in the public reimbursement list on 1 April 2023.
Source: 2023 pilot data collection on access to cancer medicines in EU+2 countries.
Some countries may also choose to limit reimbursement based on health status of patients, stage of treatment, therapy length or cut-off value for gene expression. While 9 of the 24 EU+2 countries with available data did not restrict reimbursement beyond EMA authorisation, Estonia, France and Croatia reported that more than half of all reimbursed indications had restrictions, and Czechia reported that all indications had restrictions.
Timelines for assessment of new cancer medicines and extensions of their indications may experience improvements in the years to come. The adoption of Regulation (EU) 2021/2282 on HTA mandates joint clinical assessments and joint scientific consultations of patients, clinical experts and other relevant experts (European Commission, 2023[22]). This will apply to all new cancer medicines as of 12 January 2025. Joint European HTA and cross-border joint procurement (such as Beneluxa among Belgium, the Netherlands, Luxembourg, Austria and Ireland; and FINOSE among the Nordic countries excluding Iceland) are also good policy options to expedite public reimbursement/coverage decisions in the context of rising cancer medicines costs. At the same time, value frameworks (such as the ESMO-MCBS) have been developed to support the process of HTA and to assist in rationalising reimbursement decisions. By offering a grading system of new indications of cancer medicines and the relative magnitude of clinical benefit that can be anticipated based on data derived from pivotal clinical trials or meta‑analyses, the ESMO-MCBS value framework could be used as a tool to support the process of prioritisation of access to cancer medicines by national health authorities when resources are constrained (Cherny et al., 2015[23]; Cherny et al., 2017[24]). New medicines with a potentially high clinical benefit could be reviewed on a fast-track basis, whereas those with a potentially low clinical benefit could be de‑prioritised.
Patent expirations in oncology are expected to alleviate part of the financial pressure. Here too, however, there are great differences by country in the share of biosimilars for cancer medicines that are publicly reimbursed, and in the time taken between EMA approval and reimbursement/coverage decisions. The mean time from EMA approval to public reimbursement/coverage of biosimilars exhibited great variation between countries, ranging from around 200 days in Germany and Spain to between 700 and 835 days in Greece, Iceland, Latvia, Lithuania and Slovenia, and almost 1 400 days in Cyprus. Encouraging market entry and use of generics and biosimilars when the originator product has gone off patent or lost market exclusivity is an important option to lower prices for oncology treatment, helping to redirect financial resources to pay for newer medicines with high clinical benefit and improve the financial sustainability of healthcare systems.
1.5.3. The ageing and unbalanced distribution of medical equipment needs to be addressed to tackle inequalities in cancer care
Availability of medical equipment has improved over the past decade. The supply of radiotherapy equipment per population has grown in all but eight EU+2 countries, and increased by 14% on average in the EU27. The availability of CT scanners and MRI units has also increased in almost all EU+2 countries over the last 10 years. However, while the use of outdated equipment is not recommended, old equipment is used in some countries. About one‑quarter of radiation therapy equipment is more than 15 years old in Belgium, Germany, Ireland, Italy, the Netherlands, Portugal and Spain. On average in the EU27, 17% of radiation therapy equipment is more than 15 years old.
In addition, uneven distribution of medical equipment – which leads to unequal access to medical technologies and the latest clinical procedures – is reported in a few countries. In Cyprus, for example, the majority of medical equipment is in private sector institutions, leading to long waiting times for public healthcare services and financial barriers to access for lower income groups (OECD, 2023[25]). In Spain, six provinces and the two autonomous cities (Ávila, Huesca, Palencia, Segovia, Soria, Teruel, Ceuta and Melilla) do not have radiotherapy units in their territories, creating substantial access barriers to cancer care among vulnerable groups as a result of long journeys or accommodation costs (OECD, 2023[26]).
1.5.4. Care concentration, structured networks, multidisciplinary teams and better availability of home care are critical to deliver high-quality cancer care
While the clinical benefits of concentrating cancer care on quality and health outcomes are well known (Weitz et al., 2004[27]; Morishima et al., 2013[28]), about half of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance had concentrated cancer care delivery (Figure 1.12).
Figure 1.12. Half of EU+2 countries have concentrated cancer care delivery, and over two‑thirds use multidisciplinary teams to enhance the quality of cancer care
Number of countries using each of the following practices for cancer quality improvement
Note: From 26 countries responding to the OECD Policy Survey on Cancer Care Performance.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
Several countries, including Austria, Hungary and Germany, have established vertically tiered cancer care delivery systems, with comprehensive centres of expertise, regional specialty centres and local certified cancer centres. In some countries (Czechia, France, Germany, the Netherlands and Spain), a volume norm is set to pay for cancer care or for a facility to be authorised to deliver certain treatment, leading to cancer care concentration. Countries such as Belgium and Portugal, which have cancer care systems that are mostly decentralised, also concentrate delivery of selected cancer surgical procedures and therapies. Some countries with small population sizes (including Austria, Denmark, Estonia, Iceland and Norway) make arrangements to allow for referrals abroad and international collaboration in cases of rare cancers or specific therapies to compensate for the lack of expertise within the country.
In addition, cancer care networks (which are associated with higher-quality care, including better compliance with evidence‑based guidelines) have been established in over half of the countries responding to the OECD Policy Survey. In some countries (Czechia, France and Italy), cancer care networks are organised horizontally across providers at regional levels to improve quality of cancer care, including care co‑ordination. Several countries have also developed networks for specific types of cancer (Poland) and for palliative care (Portugal). To promote high-quality cancer care across countries, the European Commission has committed to developing an EU Network of Comprehensive Cancer Centres; this plans to link recognised national centres in every Member State by 2025.
Multidisciplinary teams (MDTs) have been recommended to improve the quality of cancer care and outcomes, alleviate shortages in the health workforce, and facilitate provision of integrated cancer care. While MDTs – typically including oncologists, surgeons, radiologists and pathologists – entail considerable costs, 21 responding countries use them to provide high-quality cancer care in an efficient and effective manner (see Figure 1.12). In the Netherlands, all new diagnosed cancer cases are discussed in MDT meetings organised according to the type of cancer.
To respond to the needs of patients who prefer to receive care in the community where they live, countries are expanding availability of home care for cancer patients. Using video consultation, doctors and psychotherapists can provide follow-up care to their patients at home after surgery, examine the healing process of a surgical wound or have a psychotherapeutic consultation. An increasing number of countries are developing mobile palliative care for cancer patients at home. In Czechia, 15 accredited comprehensive cancer centres have a contract for palliative home care, and mobile palliative care teams providing home care are covered by the country’s health insurance funds (OECD, 2023[29]). France has made a large investment to expand home palliative care in recent years, including investments in mobile palliative care teams as part of a national plan to guarantee access to end-of-life care for all citizens as close as possible to where they live.
1.5.5. Promoting continuous quality improvement requires implementation of clinical guidelines, accreditation of providers and care monitoring
Clinical guidelines are key to ensuring standardised high-quality cancer care across providers throughout a country, and 20 the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance reported having developed clinical guidelines for cancer care (see Figure 1.12). Several countries, such as Iceland and Romania, benefit from clinical guidelines developed in other countries or at the international level. Provider accreditation or certification – which has been shown to be associated with a safety culture, hospital efficiency (Hussein et al., 2021[30]) and cancer care outcomes (Schroeder et al., 2022[31]) - is used in 16 EU+2 countries (see Figure 1.12). In Czechia, comprehensive cancer centres are subject to Ministry of Health accreditation every five years, based on criteria including staffing, availability of MDTs and minimum volume norms for selected treatments. In a few countries, such as Belgium, Bulgaria and France, cancer care providers need to be accredited in order to receive reimbursement.
Since timely access to cancer care is essential for good cancer outcomes, at least one‑third of EU+2 countries have set waiting time targets in areas such as diagnostic services, specialist referral and treatment initiation. In most cases, these are general guidelines across cancer sites; however, in some countries (Ireland and Luxembourg), the guidelines depend on the type of cancer. Furthermore, some countries, such as Finland, penalise providers if targets are not met. In Denmark, if a region cannot provide treatment within the maximum waiting time, it is obliged to refer patients to another hospital within the country or abroad that can do so.
Monitoring of cancer care quality also supports continuous improvement of access to and quality of cancer care in 16 countries. Poland undertakes systematic monitoring of cancer care with stakeholders, using indicators developed to measure the quality of oncological care and patient safety; shared ownership and patient involvement have enhanced rigorous monitoring. In some countries, including Denmark or Iceland, waiting times are monitored and assessed regularly to promote timely access to cancer care. To improve delivery of people‑centred cancer care, a growing number of countries – including Belgium, Estonia, Germany, Iceland, Ireland, Italy, Latvia, the Netherlands, Slovenia and Sweden – also collect and monitor patient-reported measures. Some countries with systematic monitoring of cancer care (Estonia, France, the Netherlands, Norway and Slovenia) also provide feedback at the provider level and publish the results.
References
[11] Bambury, N. et al. (2023), Cancer Inequalities in Ireland by Deprivation, 2004-2018: A National Cancer Registry report, NCRI, Cork.
[8] Berchet, C. and F. Jusot (2012), “Inégalités de santé liées à l’immigration et capital social: Une analyse en décomposition”, Économie publique/Public Economics, Vol. 24-25, pp. 73-100, https://doi.org/10.4000/economiepublique.8484.
[7] Bradby, H., S. Hamed and A. Lebano (2019), Migrants’ access to health care in Europe: A literature review, European Commission, Brussels, https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5c40201b2&appId=PPGMS.
[24] Cherny, N. et al. (2017), “ESMO-Magnitude of Clinical Benefit Scale version 1.1”, Annals of Oncology, Vol. 28/10, pp. 2340-2366, https://doi.org/10.1093/annonc/mdx310.
[23] Cherny, N. et al. (2015), “A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS)”, Annals of Oncology, Vol. 26/8, pp. 1547-1573, https://doi.org/10.1093/annonc/mdv249.
[21] Dunican, E. et al. (2023), “Outcomes of patients presenting to a dedicated rapid access lung cancer clinic”, Irish Medical Journal, Vol. 104/9, pp. 265-268, http://hdl.handle.net/10147/207263.
[1] ECIS (2023), European Cancer Information System, https://ecis.jrc.ec.europa.eu (accessed on 27 April 2023).
[22] European Commission (2023), Regulation on Health Technology Assessment, European Commission, Brussels, https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment_en (accessed on 12 July 2023).
[2] Eurostat (2023), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table (accessed on 16 June 2023).
[3] Eurostat (2023), Causes of Death – Standardised Death Rate by NUTS 2 Region of Residence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ASDR2__custom_6414996/default/table (accessed on 16 June 2023).
[13] Fairburn, J. et al. (2019), “Social inequalities in exposure to ambient air pollution: A systematic review in the WHO European Region”, International Journal of Environmental Research and Public Health, Vol. 16/17, p. 3127, https://doi.org/10.3390/ijerph16173127.
[10] Finke, I. et al. (2021), “Small‐area analysis on socioeconomic inequalities in cancer survival for 25 cancer sites in Germany”, International Journal of Cancer, Vol. 149/3, pp. 561-572, https://doi.org/10.1002/ijc.33553.
[18] Hawkes, N. (2019), “Cancer survival data emphasise importance of early diagnosis”, BMJ, Vol. 364, p. l408, https://doi.org/10.1136/bmj.l408.
[30] Hussein, M. et al. (2021), “The impact of hospital accreditation on the quality of healthcare: a systematic literature review”, BMC Health Services Research, Vol. 21/1, p. 1057, https://doi.org/10.1186/s12913-021-07097-6.
[20] Jensen, H., M. Torring and P. Vedsted (2017), “Prognostic consequences of implementing cancer patient pathways in Denmark: a comparative cohort study of symptomatic cancer patients in primary care”, BMC Cancer, Vol. 17, https://doi.org/10.1186/s12885-017-3623-8.
[6] Lamminmäki, M. et al. (2023), “A population-based cohort study on changes in breast, lung and colorectal cancer incidence and mortality among non-Western immigrant women”, BMC Cancer, Vol. 23/1, p. 665, https://doi.org/10.1186/s12885-023-11140-6.
[5] Launoy, G., V. Zadnik and M. Coleman (2021), Social Environment and Cancer in Europe: Towards an evidence-based public health policy, Springer, Cham, https://link.springer.com/book/10.1007/978-3-030-69329-9.
[28] Morishima, T. et al. (2013), “Impact of hospital case volume on quality of end-of-life care in terminal cancer patients”, Journal of Palliative Medicine, Vol. 16/2, pp. 173-178, https://doi.org/10.1089/jpm.2012.0361.
[19] Neal, R. et al. (2015), “Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review”, British Journal of Cancer, Vol. 31/112(S1), pp. S92–107, https://doi.org/10.1038/bjc.2015.48.
[25] OECD (2023), EU Country Cancer Profile: Cyprus 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/86732eb6-en.
[29] OECD (2023), EU Country Cancer Profile: Czech Republic 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/c37fd099-en.
[26] OECD (2023), EU Country Cancer Profile: Spain 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/260cd4e0-en.
[16] OECD (2023), Health Statistics, https://doi.org/10.1787/health-data-en.
[14] OECD (2021), Preventing Harmful Alcohol Use, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/6e4b4ffb-en.
[15] OECD/European Union (2022), Health at a Glance: Europe 2022: State of Health in the EU Cycle, OECD Publishing, Paris, https://doi.org/10.1787/507433b0-en.
[31] Schroeder, M. et al. (2022), “The impact of Commission on Cancer accreditation status, hospital rurality and hospital size on quality measure performance rates”, Annals of Surgical Oncology, Vol. 29/4, pp. 2527-2536, https://doi.org/10.1245/s10434-021-11304-3.
[4] Vaccarella, S. et al. (2023), “Socioeconomic inequalities in cancer mortality between and within countries in Europe: A population-based study”, The Lancet Regional Health – Europe, Vol. 25, p. 100551, https://doi.org/10.1016/j.lanepe.2022.100551.
[27] Weitz, J. et al. (2004), “Impact of volume and specialization for cancer surgery”, Digestive Surgery, Vol. 21/4, pp. 253-261, https://doi.org/10.1159/000080198.
[12] World Health Organisation (2023), Global Health Observatory database, https://www.who.int/data/gho.
[9] Zadnik, V. et al. (2022), “Cancer patients’ survival according to socioeconomic environment in a high-income country with universal health coverage”, Cancers, Vol. 14, p. 1620, https://doi.org/10.3390/cancers14071620.
[17] Zielonke, N. et al. (2020), “Evidence for reducing cancer-specific mortality due to screening for breast cancer in Europe: A systematic review”, European Journal of Cancer, Vol. 127, pp. 191-206, https://doi.org/10.1016/j.ejca.2019.12.010.
Note
← 1. Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, France, Germany, Greece, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, the Slovak Republic, Slovenia, Spain and Sweden responded to the 2023 OECD Policy Survey on Cancer Care Performance.