With cancer expected to become the leading cause of death in the EU by 2035, countries are examining opportunities to prevent cancer and decrease its incidence at a population level. This chapter provides an overview of trends in the leading known and modifiable risk factors for cancer, such as tobacco, alcohol, high body weight, unhealthy diet, physical inactivity, environmental risk factors and viral infections such as human papillomavirus and hepatitis B and C. It examines the gaps in prevalence of these risk factors between countries and between socio‑economic groups and sexes. Finally, the chapter provides insights into the current practices, policies and programmes countries are implementing to prevent cancer.
Beating Cancer Inequalities in the EU

3. Preventing cancer: Identifying risk factors and related inequalities
Abstract
Key findings
Globally in 2019, 50.6% of cancer deaths among men and 36.3% among women were attributable to modifiable risk factors.
At a population level, the largest risk factor for cancer deaths in the 27 European Union Member States (EU27) plus Iceland and Norway (EU+2 countries) is tobacco, with more than 25% of cancer deaths attributed to it in 2019. Alcohol is the second leading cancer risk factor (6.3% of cancer deaths), followed by dietary risks – such as diets high in red or processed meat and low in fruits and vegetables (6.2%), occupational risks (5.9%), high body mass index (5.7%), high blood sugar (5.6%), air pollution (2.0%), physical inactivity (1.2%) and human papillomavirus (HPV) infection (1.2%).
Compared to 2011, there has been an improvement in some of the top risk factors for cancer, including a reduction in daily smoking rates and higher rates of HPV vaccination. Prevalence of self-reported daily smoking decreased in all but four of the EU+2 countries in the decade leading to 2021, with a reduction of 30% or more in seven countries. However, the prevalence of overweight and obesity increased by 3% in the EU27 between 2014 and 2019, while consumption of fruit and vegetables remained low, and physical inactivity remained prevalent.
Variation between countries is high. Average annual alcohol consumption was twice as high in Latvia and Lithuania as in Greece. More than 90% of girls in Iceland, Portugal and Norway received the recommended doses of HPV vaccine to prevent cervical cancer in 2021 – more than double the rates in Bulgaria, France, Luxembourg, Slovenia and Latvia.
Socio‑economic inequalities can be seen in most risk factors, to the detriment of those with lower levels of education or income. In 2019, those with lower levels of education were more likely to report living with overweight and obesity, smoking cigarettes daily, low fruit and vegetable consumption, or physical inactivity. As some risk factors are decreasing more rapidly in groups with higher socio‑economic characteristics, inequalities in daily cigarette smoking and low fruit and vegetable consumption appear to be increasing.
Disparities in behavioural risk factors by gender to the detriment of men are also large – notably for cigarette smoking, alcohol consumption, overweight and obesity, and low fruit and vegetable consumption. In addition, 85% of occupational cancer deaths in 2019 in EU+2 countries were among men (mostly due to exposure to asbestos).
Given the persistent inequalities in exposure to risk factors, policies should be selected and designed to reduce gaps between population groups. Pricing policies such as higher taxation (on tobacco, unhealthy food and alcohol) are effective in reducing demand – particularly among groups with lower socio‑economic characteristics – and thus helping to close gaps. Accompanying these with subsidies to increase affordability of healthy food options remains an underutilised avenue to affect nutrition, while tax increases should be linked to inflation to ensure that their impact does not decrease over time.
Policies that change the environment can affect behaviours without requiring an active or conscious change. Comprehensive smoking bans in public spaces and workplaces are an important environmental lever used increasingly by most EU+2 countries, as these restrict opportunities to smoke and reduce exposure to second-hand smoke. Mandated reductions in availability and accessibility of alcohol – such as age restrictions or regulations on outlet density – support lower consumption. Food reformulation helps to make products healthier, although most efforts in place in EU+2 countries are perceived to have limited impact as they are not mandatory. A supportive environment for clean air delivers synergies with increasing physical activity levels through planning that promotes active transport and creation of green spaces.
Messages that reach the population should be health-promoting, while restrictions on marketing of unhealthy products can reduce risks. Countries can affect messages about tobacco, alcohol and nutrition through advertising limitations, product labelling requirements and targeted communication of public health messages. Use of standard, plain packaging with visual warnings for cigarettes has been implemented in nine of the EU+2 countries, and easy-to‑understand front-of-pack labelling for food products in 12 countries. Given that population-level health promotion may be more effective among less vulnerable populations (e.g. those with higher education levels or native‑born populations), several countries are tailoring communication to engage specific groups with relevant content, in the language and format that suits them, thereby reducing risk factors and improving health literacy.
Improving health literacy can help reduce some risk factors for cancer. However, nearly half of the respondents to the European Health Literacy Population Survey 2019‑21 had insufficient levels of health literacy, and those with lower socio‑economic characteristics scored lower on average. To reach vulnerable populations, increasing attention is given to the role of healthcare organisations in facilitating understanding and use of health information via training staff on health literacy and communication techniques; dedicating sufficient time to patient communications; providing translated materials; and using plain language and visual materials created in partnership with their target patient populations.
As vulnerable groups may be less likely to benefit from measures targeted at the whole population, many countries develop specific interventions adapted to reach at-risk groups. Using mobile vaccination buses, delivering vaccines in school settings and allowing vaccinations by non-physician healthcare professionals can help HPV vaccination programmes reach people who may be missed in the healthcare system. To prevent transmission of hepatitis B and hepatitis C, most at-risk groups – including migrants, people who inject drugs and men who have sex with men – can be reached with hepatitis B vaccination, harm-reduction programmes and testing and treatment for hepatitis B and C.
Co‑operation between countries in areas such as taxation, product formulation, advertising and labelling can add to the effectiveness of these measures. Large differences in tobacco taxation and pricing across countries, and continuous output of new products by the industry reduce the benefits of existing EU-wide tobacco directives. Co‑ordination of alcohol taxation or reformulation of food across European borders is limited. In many cases, media transcend borders, and while EU-wide advertising rules restrict alcohol marketing to minors, they do not limit advertising to general audiences. Furthermore, advertising of alcohol and unhealthy food through social media is at best only partially or voluntarily restricted at the country level, while international co‑operation on such restrictions is lacking.
Policies and interventions to target cancer risk factors must be implemented across various settings where people spend time – in schools, workplaces and healthcare facilities, among others. Schools are a key setting for reducing disparities in exposure to risk factors, as they can reach and educate students, informing them about risks, and facilitating HPV vaccinations and a healthy food environment. Primary care can connect smokers to cessation support services, conduct screenings and brief interventions on alcohol and reduce overweight and obesity through counselling on nutrition or prescription of physical activity. Some of the most effective evidence‑based practices for HPV vaccination involve healthcare providers offering recommendations, reminders and information on safety and effectiveness.
An effective approach for addressing each cancer risk factor requires a multi-component policy package tailored to reach its target populations. Integrated approaches between sectors aid development and implementation of comprehensive and effective policy packages tailored to target populations.
3.1. The impact of risk factors on cancer burden is substantial
Given the high and increasing burden of cancer (Chapter 2), it is important to consider that a large proportion of cancer cases could be prevented through action on modifiable risk factors. This section discusses the leading risk factors for cancer in the 27 European Union Member States (EU27) plus Iceland and Norway (EU+2 countries), using attributable deaths and disability-adjusted life‑years (DALYs) to quantify the cancer burden. One DALY represents the loss of the equivalent of one year of full health (Box 3.1). As such, DALYs extend the measures of mortality to include poor health, providing insight into the impact of each risk factor on the population by considering both deaths and the experience of those living with cancer.
3.1.1. Over half of cancer deaths among men and one‑third of cancer deaths among women are attributable to modifiable risk factors
Worldwide, 50.6% of cancer deaths among men and 36.3% among women in 2019 were attributable to risk factors, including behavioural, environmental and metabolic risks (GBD 2019 Cancer Risk Factors Collaborators, 2022[1]). The proportion of cancer deaths attributable to risk factors increased globally by 20.4% between 2010 and 2019. Table 3.1 shows the number of cancer deaths in 2019 in EU+2 countries attributed to the leading high-level risk factors for men and women.
Table 3.1. Significant numbers of cancer deaths were attributed to leading risk factors in EU+2 countries in 2019
|
Category |
Tobacco |
Alcohol |
Dietary risk |
Occupational risks |
Overweight and obesity |
High blood sugar |
Air pollution |
Physical inactivity |
HPV infection (cervical cancer) |
All cancer deaths |
|---|---|---|---|---|---|---|---|---|---|---|
|
Men |
266 398 |
60 718 |
46 429 |
69 733 |
39 087 |
41 910 |
19 191 |
6 140 |
N/A |
773 124 |
|
Women |
102 273 |
25 898 |
38 463 |
11 706 |
39 574 |
35 126 |
8 300 |
9 906 |
15 931 |
596 727 |
|
Total |
368 671 |
86 616 |
84 892 |
81 439 |
78 661 |
77 036 |
27 491 |
16 046 |
15 931 |
1 369 851 |
|
Of all cancer deaths, percentage attributed to the risk factor |
26.9% |
6.3% |
6.2% |
5.9% |
5.7% |
5.6% |
2.0% |
1.2% |
1.2% |
Note: N/A stands for not available. This table refers to estimated deaths due to neoplasms that are attributed to level-2 risk factors as defined by the Institute for Health Metrics and Evaluation (IHME) (see Box 3.1). All cancer deaths include both risk-attributable and non-risk-attributable cancer deaths. Deaths can be attributed to more than one risk factor, and thus the numbers and percentages are not summative. The burden of deaths from HPV infections shown in the table is an underestimate, as it only includes deaths from cervical cancer, while HPV can also cause anal, penile, vaginal, vulval and oropharyngeal cancer.
Source: GBD Compare Data Visualisation (IHME) (2023), http://vizhub.healthdata.org/gbd-compare.
In 2019, the estimated global burden of cancer attributable to all risk factors, measured in DALYs, reached a total of 105 million healthy life‑years lost due to cancer, for men and women combined. This figure accounts for approximately 42.0% of all cancer-related DALYs. Figure 3.1 shows the DALYs attributable to each of leading risk factors for cancer in the EU+2, by sex.
Notably, tobacco emerges as the largest risk factor for both sexes, contributing significantly to cancer-related deaths and DALYs, and surpassing other risk factors by a significant margin. For men, other major risk factors include alcohol consumption, occupational and dietary risk, high body mass index (BMI), high blood sugar and exposure to air pollution. For women, tobacco use is followed by high BMI, dietary risk, high blood sugar, alcohol use, HPV infection and occupational risks. Air pollution, insufficient physical activity and other environmental risk factors are also among the top ten risk factors for women, as are other environmental risks, drug use and physical inactivity for men (IHME, 2019[2]). The proportional burdens from deaths and DALYs do not match in all cases. For example, for men, occupational risks were attributed to the second highest number of deaths; however, alcohol came in as the second biggest risk factor for DALYs. This is likely to be influenced by the types of cancer associated with each risk factor.
Figure 3.1. The leading risk factors for cancer burden led to high numbers of DALYs in the EU+2
Age‑standardised cancer DALYs (in thousands) for leading cancer risk factors, by sex, 2019
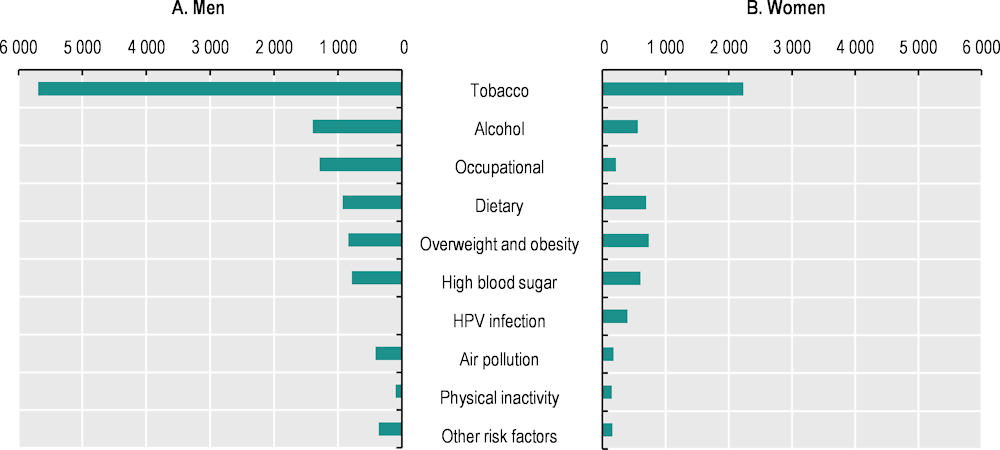
Note: Other risk factors include other environmental risks and drug use for both sexes.
Source: GBD Compare Data Visualisation (IHME) (2023), http://vizhub.healthdata.org/gbd-compare.
Box 3.1. Definitions used in section 3.1
DALY is a measure of overall disease burden, expressed as the loss of an equivalent of one year of full health, combining years lost due to premature mortality and due to living in states of less than full health.
Attributable burden is the reduction in current disease burden that would have been possible if past population exposure had shifted to an alternative or counter-factual distribution of risk exposure. Deaths and DALYs can be attributed to several risk factors at once and are not summative.
Risk factor estimates defined by the IHME model the risk factor attributions (used in Section 3.1):
Tobacco use includes estimates about smoking (current and former), second-hand smoke (at home, at work and in other public places) and chewing tobacco (use of primary chewing tobacco, non-chew smokeless tobacco and all other smokeless tobacco).
Alcohol use includes indicators of the proportion of current drinkers, alcohol consumption by current drinkers (in grams per day) and alcohol litres per capita stock, adjusted for the number of tourists in the location, their average length of stay and unrecorded alcohol stock.
Dietary risk includes factors that have associations with cancer such as diets low in fruit, vegetables, whole grains, milk and fibre, and high in red meat and processed meat. For policy measures (discussed in Section 3.3), other nutrients with health risks aside from cancer are included as a part of comprehensive efforts to improve diets.
Occupational risks include exposure to occupational carcinogens, with nearly 90% of deaths and 84% of DALYs in this category attributed to occupational exposure to asbestos.
Air pollution includes exposure to particulate matter that are 2.5 microns or less in diameter (PM2.5), including ambient (outdoor) and household (indoor) exposure.
Overweight and obesity – called “high BMI” in the Global Burden of Disease Study data – refers to BMI above 25 kg/m2.
High blood sugar – called “high fasting plasma glucose” (levels recorded after no eating or drinking for 8 hours) – is associated with several types of cancer through diabetes mellitus. It is defined as any level above the theoretical minimum risk exposure level of 4.8‑5.4 mmol/L.
Physical inactivity includes estimates of physical activity across all domains of life (leisure/recreation, work/household and transport).
Human papillomavirus (HPV) infection in the cervical area is caused by sexual contact, so in the IHME estimates, all cervical cancer cases are attributed to unsafe sex.
Note: The risk factors addressed in this report were chosen on the basis of the Global Burden of Disease Study 2019. Data on other known factors – e.g.; exposure to sunlight (ultraviolet radiation), infection with Helicobacter pylori and other well-known risks – are not available.
Source: For more detailed methodology, refer to the supplementary material in GBD 2019 Cancer Risk Factors Collaborators (2022[1]), www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)01438-6/fulltext.
3.1.2. Nearly 27% of all cancer deaths were attributed to tobacco use and 6.3% to alcohol use, most of which were among men
For both men and women, tobacco poses by far the largest risk for cancer DALYs and mortality (see Figure 3.1; Table 3.1). The majority of the cancer burden attributed to tobacco is from lung cancer, with the rest divided between various cancers, including digestive and reproductive system cancers. In EU+2 countries, tobacco smoking was attributed to 266 398 deaths among men and 102 273 deaths among women in 2019 (IHME, 2019[2]). Deaths from second-hand smoke exposure, however, were higher among women than men: almost one‑third of tobacco-related deaths in women were attributed to second-hand smoke exposure (WHO, 2023[3]).
Although the most common mode of tobacco consumption is smoking, newer and emerging tobacco and nicotine products that are marketed as alternatives or supplementary to cigarettes are raising concerns. Smokeless tobacco use – such as oral use of snus common in Nordic countries – could increase the risks of some cancer types and of mortality after diagnosis compared to no tobacco use (Valen et al., 2023[4]). In recent years, use of e‑cigarettes has been increasing; this is a particular concern among young people (WHO, 2023[3]). Marketed as an alternative to tobacco, e‑cigarettes contain a variety of compounds with inconclusively characterised health effects. Given lag times in tobacco smoke data, not enough time has passed since market entry for clear evidence to emerge. An opinion statement published by the European Commission in 2021 concluded that strong evidence exists on the role of e‑cigarettes as a gateway to smoking, particularly among young people, while the addictive potential of the products is high because many of them contain nicotine (SCHEER, 2021[5]).
Despite recent reductions in many countries, Europe has historically had the highest level of per capita alcohol consumption in the world, which is reflected in a high burden of cancer attributable to alcohol. An estimated 4.1% of all new cancers globally in 2020 (and 5.6% in Central and Eastern Europe) were attributable to alcohol consumption (Rumgay et al., 2023[6]). In EU+2 countries, alcohol accounted for an estimated 86 616 cancer deaths in 2019, representing 6.3% of all cancer deaths, over 70% of which were among men (60 718 deaths) (see Table 3.1). Among women, 11% of all breast cancer deaths were attributable to alcohol consumption (IHME, 2019[2]). While there is no safe level of alcohol consumption with regard to cancer risk, the likelihood of developing alcohol-related cancers is influenced by various factors, including the quantity and frequency of alcohol consumption, individual susceptibility, and interaction with other risk factors such as smoking and genetic predisposition (Clinton, Giovannucci and Hursting, 2020[7]).
3.1.3. Dietary risk and physical inactivity are interconnected with metabolic cancer risk factors of overweight and obesity and high blood sugar
Diet is the third leading risk factor for cancer deaths in Europe, and is particularly associated with colon and rectum cancers (see Table 3.1). In EU+2 countries, 46 429 cancer deaths (6.2% of all cancer deaths) were attributed to dietary risk in 2019. Additionally, diet presents the third largest risk factor for cancer DALYs for women and the fourth largest for men (see Figure 3.1). Dietary factors with established links to certain types of cancer include low consumption of fibre, fresh fruit, vegetables and whole grains (Kerschbaum and Nüssler, 2019[8]). The International Agency for Research on Cancer (IARC) has classified consumption of processed meat as carcinogenic and unprocessed red meat as probably carcinogenic to humans, additionally noting inter-relationships between diet, overweight and obesity, and diabetes (IARC, 2018[9]). High sugar consumption, including in the form of sugar-sweetened beverages, is known to increase the risk of cancer indirectly through its associations with overweight and obesity.
In EU+2 countries, 16 046 cancer deaths (1.2% of all cancer deaths) were attributed to physical inactivity in 2019. Regular exercise has been heavily implicated in maintaining a healthy body weight, boosting the immune system and reducing systemic inflammation, all of which contribute to cancer prevention. Physical activity helps to regulate hormones like insulin and oestrogen that contribute to the growth of cancer cells, and enhances the efficiency of the digestive system, reducing the time it takes for the body to eliminate harmful substances (McTiernan, 2008[10]). Physical inactivity interacts with dietary factors such as consumption of red meat, contributing to an increased risk of overweight and obesity and high blood sugar, augmenting the risk of cancer.
Although also affected by the behavioural risk factors described above, overweight and obesity is classified as a metabolic risk factor for cancer and is affected by genetic predisposition and environmental influences. Overweight and obesity was attributed to 78 661 cancer deaths (5.7% of all cancer deaths) in 2019; it is the fourth leading risk factor for cancer deaths among women and the fifth among men. It is associated with a wide range of cancer types including breast and uterine cancers in women and oesophageal, colon and rectum cancers in both sexes. Overweight and obesity leads to cancer through various pathways – including systemic hormonal and inflammatory changes mediated by high adiposity (body fat) – which lead to an environment that favours tumour initiation and progression. Metabolic factors associated with obesity include increased levels of insulin and insulin-like growth factor, which promote development of cancer at several sites before the development of diabetes (Sami et al., 2017[11]; Gallagher and LeRoith, 2015[12]).
High blood sugar is a risk factor to which 77 036 cancer deaths (5.6% of all cancer deaths) were attributed in EU+2 countries in 2019. It is particularly associated with breast, pancreatic, lung and colorectum cancers. Various pathways have been implicated for the role of blood sugar in cancer initiation and progression, including increased availability of glucose for tumour growth. Diabetes, addressed in the Global Burden of Disease Study estimates through its associations with high blood sugar, is associated with both greater cancer incidence and cancer mortality through channels such as chronic inflammation and high insulin levels (due to insulin resistance) (Wang, Yang and Liao, 2020[13]) (Safiri et al., 2022[14]).
3.1.4. Around 2% of all cancer deaths were attributed to air pollution, while 5.9% were attributed to occupational exposures – mainly to asbestos
Chronic exposure to air pollution, particularly in the form of particulate matter (PM), can cause lung cancer. PM can be classified by size: PM2.5 refers to particles that are 2.5 microns or less in diameter and can enter deep into the respiratory tract to cause damage to the lungs. PM10, particles that are 10 microns or less in diameter, cause damage as well, but cannot penetrate as deep into the lung tissue (OECD/EU, 2020[15]). Ammonia emissions should also be considered, as ammonia undergoes chemical reactions in the atmosphere that lead to formation of PM2.5 particles. While around 1% of cancer cases are attributed to indoor and outdoor air pollution, this figure rises to more than 7% for lung cancer (EEA, 2022[16]). Among EU+2 countries, air pollution contributed to 27 491 cancer deaths in 2019 (2% of all cancer deaths) (see Table 3.1).
Exposure to air pollution can take place outdoors (ambient air pollution) and in indoor environments (household air pollution) due to use of solid fuels. Although the estimates in this chapter (see Box 3.1) refer to both indoor and outdoor pollution, the impact on population health in Europe from exposure to ambient air pollutants is much greater than that from household air pollutants; thus, this report focuses on discussion of exposure and policy options targeting outdoor air pollution (OECD/EU, 2020[15]).
Cancer is the main cause of work-related deaths. The International Labour Organization (ILO) has identified more than 200 substances – including chemicals, metals, dust, radiation and biological agents – as probable human carcinogens (ILO, 2021[17]). In 2019, 81 439 cancer deaths in the 29 EU+2 countries were attributed to occupational risks (see Table 3.1), while around 78% of occupational cancers were specifically related to asbestos – a naturally occurring fibrous substance widely used in industry in the past (European Commission, 2022[18]). Inhalation of small asbestos fibres is associated with a high risk of lung cancer and mesothelioma (a cancer almost always caused by exposure to asbestos), with an up to 30‑year delay between exposure and development of cancer.
3.1.5. Around 1.2% of all cancer deaths are due to cervical cancer attributed to human papillomavirus infections
HPV infection is an important cancer risk factor. According to 2019 estimates, 15 931 cervical cancer deaths attributable to HPV infections represented 1.2% of all cancer deaths among both sexes in EU+2 countries. The figure amounts to 2.7% of cancer deaths among women – excluding deaths in men, who are not considered at risk of cervical cancer (IHME, 2019[2]). This remains an underestimate of the total burden associated with HPV infections, as it does not include anal, penile, vaginal, vulval and oropharyngeal cancer associated with HPV among both women and men. The European Cancer Organisation estimates that 2.5% of cancer cases in Europe are attributable to HPV, up to 20‑30% of which are among men (European Cancer Organisation, 2020[19]).
HPV viruses are highly contagious, and more than 80% of the sexually active population could be exposed to this family of viruses during their lives (Chesson et al., 2014[20]). Most HPV-related cancer can be prevented by vaccination against the main HPV strains associated with cancer. Vaccinating both men and women against HPV provides protection for everyone by preventing transmission between sexual partners (Colzani et al., 2021[21]). Vaccination is a key recommended method for prevention due to its high efficacy and the possibility of targeting specifically carcinogenic HPV strains (Kamolratanakul and Pitisuttithum, 2021[22]). Ideally, vaccination should be offered before initial exposure to HPV, meaning before the onset of sexual activity (Meites et al., 2019[23]). As such, vaccination is generally targeted at children shortly after the age of 10, although older individuals can also benefit.
A meta‑analysis covering 40 studies from 14 countries suggested an 83% reduction in prevalence of the two most carcinogenic HPV types in girls aged 13‑19 when at least 50% vaccine coverage is achieved (Drolet et al., 2019[24]). According to the European Centre for Disease Prevention and Control (ECDC), reductions in prevalence of HPV strains covered by vaccines have been observed in vaccinated women in Australia, Belgium, Finland, France, Germany, Japan, the Netherlands, Spain, Sweden, Uganda and the United Kingdom (England and Scotland separately) (ECDC, 2020[25]). The two‑strain and four‑strain HPV vaccines currently licensed in Europe can potentially prevent 71% of cervical cancer cases, while the nine‑strain licensed vaccine can prevent up to 89% of cases (European Cancer Organisation, 2022[26]).
3.1.6. Liver cancer due to hepatitis B and hepatitis C is attributed to several risk factors
In 2019, hepatitis C virus (HCV)-related liver cancer accounted for around 16 400 deaths in EU+2 countries, and hepatitis B virus (HBV)-related liver cancer for around 4 600 (Cortesi et al., 2023[27]). Risk factors for contracting the viruses include high-risk sex, which is considered the leading risk factor for acute HBV in EU+2 countries (ECDC, 2022[28]) and the second leading risk factor for HCV after drug injections (ECDC, 2022[29]). Based on the IHME classification (see Box 3.1), some deaths and DALYs from HBV- and HCV-related liver cancer are attributable to risk factors that increase the probability of developing liver cancer following an acute viral infection, including tobacco, overweight and obesity and alcohol. HBV and HCV together account for about 55% of liver cancer deaths in EU+2 countries (ECDC, 2022[30]). Age‑standardised rates of HBV-related and HCV-related liver cancer remained relatively stable between 2010 and 2019, although incidence and prevalence of both HBV and HCV infections fell (Cortesi et al., 2023[27]). However, risk of HBV- and HCV-related liver cancer is unequally distributed across population groups (see Section 3.2.3).
3.2. Risk factors are inequitably distributed across and within countries
3.2.1. Behavioural and lifestyle‑associated risk factors vary widely between countries
Smoking rates vary almost three‑fold across countries, while education- and income‑related inequalities play a significant role in shaping within-country variations
Prevalence of self-reported daily smoking decreased in the EU27 from an average of 22.5% in 2011 to 18.7% in 2021 (or nearest years), yet the reduction was marked by inconsistencies across countries and population groups1. Norway and Iceland had the greatest reductions – of 50% or more – in overall daily smoking rates (falling from 17% to 8% of the population in Norway and from 14.3% to 7.2% in Iceland). Prevalence of daily smoking also decreased greatly in Estonia, Denmark, Finland, Germany and Ireland (by 30% or more). In Bulgaria, Luxembourg, Malta and the Slovak Republic, however, smoking rates increased slightly over the past decade. In 2021, the proportion of daily smokers varied almost three‑fold across countries: it was highest in Bulgaria (28.7%) and lowest in Iceland, Norway and Sweden (less than 10%). According to the Health Behaviour in School-aged Children (HBSC) study, tobacco experimentation among adolescents in Europe had fallen in 2022: 17% of 15‑year-olds in 2022 reported smoking at least once in the previous 30 days compared to 18% in 2018 and 22% in 2014. The proportion was slightly higher among girls than boys (HBSC, 2023[31]).
A higher proportion of men report smoking cigarettes daily compared to women in nearly all countries: across the EU27, the proportion of daily cigarette smokers is 51% higher among men than women. The gender gap is widest in Lithuania and Romania, where daily smoking is more than three times more common among men, and in Cyprus, Latvia and Portugal, where it is more than twice as common. By contrast, in Denmark and Sweden, the proportion of daily smokers is slightly higher among women. Between 2014 and 2019, the average gender gap across the EU27 in smoking rates remained unchanged (with a 7.5 percentage point difference in smoking prevalence rates between men and women).
Across the EU27, people with low levels of education are 46% more likely to smoke daily than those with high levels, and the social gradient is present in most European countries (Figure 3.2). On average in the EU27, the prevalence of daily cigarette smoking was highest among those with medium education levels. The largest education gaps in smoking rates are found in countries with a low prevalence of smoking among those with high levels of education, such as Norway (4.9% among those with high education levels vs. 17.4% among those with low education levels), Sweden (2.8% vs. 9.7%) and Iceland (3.9% vs. 9.9%). In absolute terms, Hungary, Czechia, Estonia, the Slovak Republic, Norway and the Netherlands all have an education gap of more than 12 percentage points. Education-related inequalities in cigarette smoking increased by 13% between 2014 and 2019. This is the result of smoking rates declining faster among people with high education levels (-6% during 2014‑19) than low education levels (-1% during 2014‑19).
Similarly, on average across the EU27, the proportion of daily cigarette smokers is 50% higher among individuals in the lowest (22.4%) than those in the highest income quintile (14.9%). Proportionally, the highest income‑related inequalities are found in the Netherlands (7% among those in the highest income quintile vs. 24.4% among those in the lowest quintile) and Sweden (3.4% vs. 11.5%), where smoking rates are more than three times higher among low-income than high-income groups. In absolute terms, the Netherlands, Hungary, Belgium and Germany all have income gaps of more than 12 percentage points. Overall, between 2014 and 2019, income‑related inequalities in smoking rates across the EU27 increased by 9%, as higher-income groups saw a faster decline in smoking (-8% during 2014‑19) than lower-income groups (-3% during 2014‑19).
While daily smoking rates continue to fall, concerns are emerging around the increasing use of e‑cigarettes (see Section 3.1.2), especially among adolescents and young people: 6.1% of those aged 15‑24 reported that they had used vaping products in 2021 on average across OECD countries, which is almost double the overall average of 3.2% among all those aged 15 and over (OECD, 2023[32]). In 2019, around 7% of 15‑16‑year-olds in Portugal and Sweden reported using e‑cigarettes in the last 30 days, while in Poland and Lithuania that figure was around 30% (ESPAD Group, 2019[33]). In 2021, the overall proportions of the population aged 15 and over who reported regular use of vaping products were highest in Czechia at 7.4% and Estonia at 10% (where the rate among young people reached more than 20% in 2022).
Figure 3.2. The proportion of daily cigarette smokers is 46% higher among people with low levels of education compared to those with high education levels
Percentage of people aged 15+ who smoke cigarettes on a daily basis, 2019
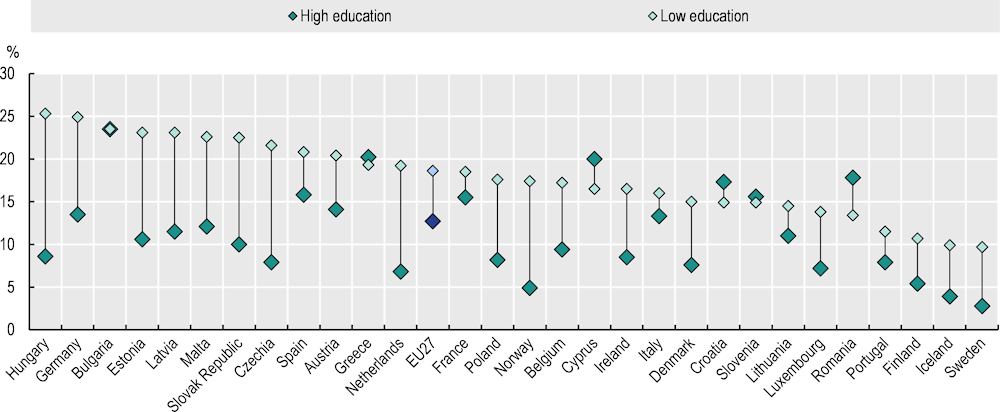
Note: EU27 is a weighted average. Low education is defined as people who have not completed secondary education (International Standard Classification of Education (ISCED) 0‑2), whereas high education is defined as people who have completed tertiary education (ISCED 5‑8).
Source: Eurostat (2023), “Daily smokers of cigarettes”, European Health Interview Survey.
Alcohol consumption varies about two‑fold between the highest and lowest consuming countries
Overall recorded alcohol consumption, measured through sales data, stood at almost 10 litres of pure alcohol per capita on average across the EU27 in 2021 (Figure 3.3). Recorded consumption was highest in Baltic countries (Latvia and Lithuania) and Central and Eastern European countries (Czechia and Bulgaria), at more than 11 litres per adult. By contrast, Greece, Norway, Iceland, Sweden and Italy had relatively lower consumption, at 7.7 litres or less. Over the past decade, alcohol consumption has decreased in most EU27 countries, with the largest reductions (by more than 15%) in Finland, France, Greece, Ireland and Lithuania. However, per capita consumption increased by more than 10% in Bulgaria, Latvia, Malta, Norway and Romania, although in Malta and Norway it remained well below the EU27 average.
In addition to total alcohol consumed, it is relevant to look at drinking patterns. In 2019, countries in Northern and Western Europe (Denmark, Luxembourg, Germany and Belgium) and Romania reported more heavy episodic drinking (defined as six or more standard drinks per drinking session at least once per month). Patterns of alcohol consumption vary across population groups. Men drink more than women in all EU27 countries: 26.3% of men and 11.4% of women reported heavy episodic drinking at least once a month in the EU27 in 2019. The largest gender gaps were reported in Romania (53.1% vs. 18.0%), followed by Luxembourg, Denmark and Lithuania (all with over 20 percentage point gaps). On average across the EU27, the gender gap decreased between 2014 and 2019 due to a slightly larger reduction among men (-1.6 percentage points) than women (-0.5 percentage points). Reductions in the gap by more than 10 percentage points were observed in Ireland and Estonia, where decreases in heavy episodic drinking were larger among men. The proportion of 15‑year-olds who reported having been drunk more than once in their life decreased between 2018 and 2022 for both genders. Among boys, the reduction was larger, resulting in similar proportions among girls (17%) and boys (18%) (HBSC, 2023[31]).
Figure 3.3. Alcohol consumption varies across countries and population groups
Alcohol consumption (2021), Percentage of people aged 15+ reporting heavy episodic drinking (monthly or more, 2019)
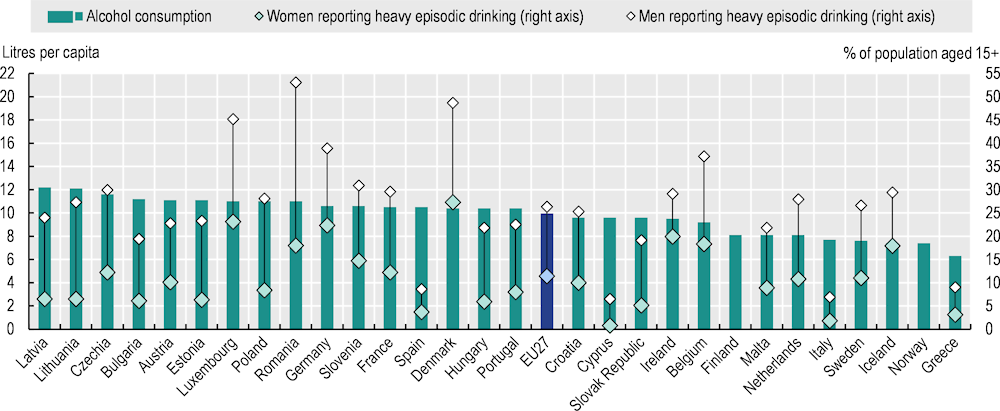
Note: Alcohol consumption is based on annual sales data in 2019 for Belgium, Bulgaria, Croatia, Germany, Greece, Italy, Luxembourg, Portugal and Romania, and in 2020 for Iceland. For alcohol consumption, the EU27 average is unweighted. For the percentage of heavy episodic drinking, the EU27 average is weighted.
Source: OECD Health Statistics 2023, https://doi.org/10.1787/health-data-en; Eurostat (2023), “Frequency of heavy episodic drinking”, European Health Interview Survey.
Socio‑economic status has been associated with differences in alcohol-related health effects. However, a clear and consistent pattern in self-reported heavy episodic drinking does not emerge: on average across the EU27, both men and women with medium levels of education report heavy drinking most often, and people in the highest income quintile are more likely to report heavy episodic drinking at least once a month than those in the lowest income quintile. Nevertheless, studies have consistently shown that groups with lower socio‑economic characteristics suffer greater harms from drinking, such as higher risk of alcohol-related mortality and greater likelihood of alcohol dependence, as well as comorbid psychiatric disorders – possibly due to compounding and comorbid vulnerabilities (Collins, 2016[34]).
More than half of adults were living with overweight and obesity in 2019, with rates growing by 3.2% during 2014‑19
The prevalence of overweight and obesity (BMI >25) has substantially increased in recent decades due to increases in the consumption of calorie‑dense and processed food, as well as increasingly sedentary lifestyles (OECD, 2022[35]). In 2019, more than half of adults in EU27 countries were living with overweight and obesity – a 3.2% increase compared to 2014. In Malta, Croatia and Iceland, the proportion exceeded 60%. Men are more likely than women to be living with overweight and obesity in all EU+2 countries. The gender gap is particularly large in Czechia and Luxembourg (with a difference of over 18 percentage points), and smallest in Latvia, Lithuania, Finland and Estonia. Among adolescents, the proportion reporting overweight increased by more than 12% between 2018 and 2022, remaining higher among 15‑year-old boys (26%) than girls (16%).
People with lower socio‑economic characteristics, such as lower income or education levels, are more likely to experience obesogenic environments, characterised by limited access to healthy foods or affordable healthy food options, living in neighbourhoods with limited spaces for physical activity and widespread advertising of unhealthy products. Moreover, stressors associated with lower socio‑economic conditions – such as financial strain, food insecurity and psychosocial stress – can contribute to unhealthy eating behaviours and hinder weight management (OECD, 2022[35]).
Although prevalence of overweight and obesity is increasing in all population groups, the proportion living with overweight and obesity in 2019 was higher among those with low (53.8%) than high education levels (44.3%). The education gap is uneven between genders. Women with low education levels are more likely to be living with overweight and obesity in all EU+2 countries, with an average difference of 16.7 percentage points (Figure 3.4). In contrast, prevalence of overweight and obesity among men with low education levels was only 2.1 percentage points higher than among men with high education levels, and about half the countries had a reverse gradient. Compared to 2014, the average education gap across EU+2 countries in 2019 remained at a similar level for men but decreased for women.
Figure 3.4. In all EU+2 countries, prevalence of overweight and obesity is higher among women with low education levels than high education levels
Percentage of women aged 15+, 2019
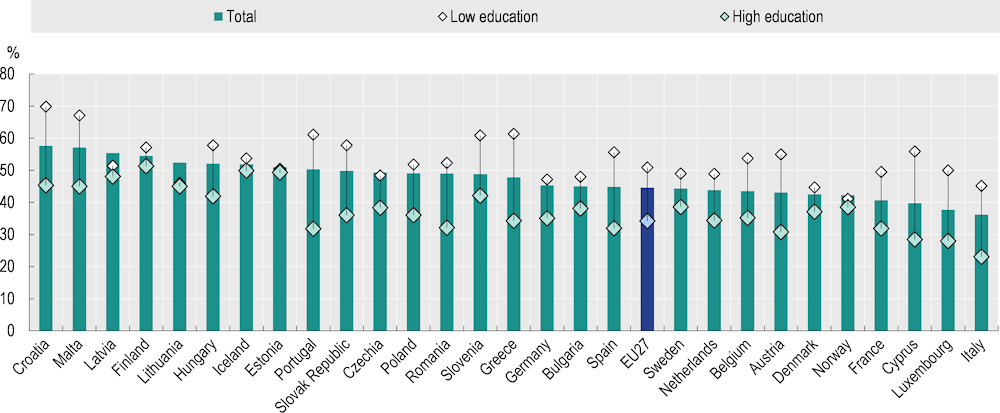
Note: The EU27 average is weighted. Low education is defined as people who have not completed secondary education (ISCED 0‑2), whereas high education is defined as people who have completed tertiary education (ISCED 5‑8).
Source: Eurostat (2023), “Body mass index”, European Health Interview Survey.
Only one in eight Europeans reported eating the recommended five portions of fruit and vegetables per day
Despite diet being an important risk factor for cancer (see Section 3.1.3), only about two‑thirds of the population across the EU27 reported consuming at least one portion of fruit or vegetables on a daily basis. Only 12.4% reached five portions per day, in line with WHO’s recommendation of a daily minimum of 400 g of fruit or vegetables (WHO, 2023[36]). The proportion eating five portions was lower among people living with overweight and obesity. Differences between countries emerge, as low consumption (less than five portions) was reported by 67% of the population in Ireland, but by more than 95% in Romania and Bulgaria.
Disparities in nutrition exist not only across but also within countries, where people with lower-income populations have more limited access to healthy food options (OECD, 2022[35]). Across the EU27, a higher proportion of women than men (14.9% vs. 9.8%) report consuming the recommended five portions of fruit and vegetables. Both men and women with high education levels are more likely to meet the recommendation, and those in the highest income quintile report meeting the recommendation more often.
A third of people in the EU27 (33.9%) report consuming sugar-sweetened beverages on a weekly basis, and a tenth do so daily. The share of people drinking soft drinks (many of which are sweetened with sugar, while others may contain artificial sweeteners) at least once per week is higher among men (41.2%) than women (27.1%). Importantly, more than 60% of teenagers aged 15‑19 report consuming soft drinks weekly or more, and 16.2% drink them daily, with the proportion declining with age. This highlights the importance of policy levers focusing on young people (see Section 3.3.3).
Rates of physical activity vary widely across Europe
On average across EU+2 countries, 67% of people report engaging in less than the recommended 150 minutes of health-enhancing (non-work-related) physical activity per week. Stark disparities can be seen between countries. Northern Europeans are most likely to meet the recommendation, with rates of people not meeting the recommendation below 50% in Norway, the Netherlands, Sweden, Iceland and Denmark, while the figure rises to more than 80% for some Southern, Central and Eastern European countries (Figure 3.5). Among 15‑year-olds, boys are twice as likely to engage daily in 60 minutes of moderate‑to-vigorous physical activity as girls (20% vs. 10%). These patterns persist to adulthood as across EU+2 countries a lower proportion of women than men meet the WHO recommendation.
Key drivers of physical inactivity are urbanisation and the increasing prevalence of sedentary lifestyles, including in occupational settings such as office work. Socio‑economic characteristics have also been found to be consistently associated with levels of physical activity: individuals with low income levels often face barriers such as limited access to recreational facilities, living in unsafe neighbourhoods and time constraints due to demanding work schedules, which prevent them from engaging in regular leisure physical activity (OECD/WHO, 2023[37]). It should be noted, however, that some low-income jobs may be more manual, involving physical activity throughout the day. Nevertheless, work-based physical activity is not always health-enhancing and can affect the individual’s capacity for physical activity outside working hours.
The average proportion not meeting the weekly physical activity recommendation was higher among those with high levels of education (by 31%) and income (by 17%), and this pattern persisted across all EU+2 countries. In Ireland, Belgium, Austria, Luxembourg and Estonia, the difference between those in the lowest and highest income quintiles was more than 20 percentage points (Figure 3.5). The level of inequality in physical activity between both education and income groups remained at similar levels between 2014 and 2019.
Figure 3.5. Proportions of the population meeting the recommendation of 150 minutes or more per week on physical activity vary by income level
Percentage of people aged 15+ spending <150 minutes or more per week on physical activity, 2019
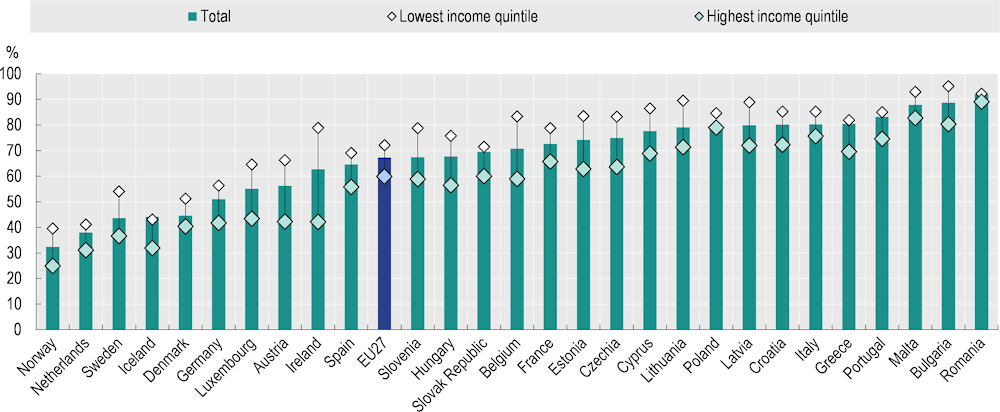
Note: The EU27 average is weighted.
Source: Eurostat (2023), “Health-enhancing (non-work-related) physical activity”, European Health Interview Survey.
3.2.2. Environmental and occupational exposure to carcinogens is substantial
Average outdoor air pollution has decreased over time, but varies almost four‑fold across EU+2 countries
The 2021 WHO Global Air Quality Guidelines, a set of evidence‑based recommendations of pollutant limits, lowered annual air pollution limits for PM2.5 to 5 μg/m3 and for PM10 to 15 μg/m3 (WHO, 2021[38]). In 2020, all EU+2 countries except Finland exceeded the WHO limit threshold set for PM2.5, although Iceland, Sweden, Norway and Estonia were very close to this level. Poland, Bulgaria, Croatia and the Slovak Republic had the highest annual average exposure – three times higher than the WHO recommendation. In 2021, 97% of the EU27 urban population was exposed to PM2.5 and 76% to PM10 levels exceeding WHO’s recommendations (EEA, 2023[39]).
Population exposure to PM2.5 decreased by 38% between 2000 and 2020 across the EU27 (Figure 3.6). The largest reductions were seen in Baltic countries (Estonia, Latvia and Lithuania), with an almost 50% drop in exposure during 2000‑20. The lowest reductions – of around 30% – were in the Slovak Republic, Croatia and Poland. It should be noted that cancer can develop decades after the initial exposure to air pollution, meaning that historical exposure continues to affect incidence of cancers now and in the years to come.
Figure 3.6. Mean population exposure to PM2.5 in 2020 was 38% lower than in 2000
PM2.5 exposure in micrograms per cubic metre (μg/m3), in 2000 and 2020
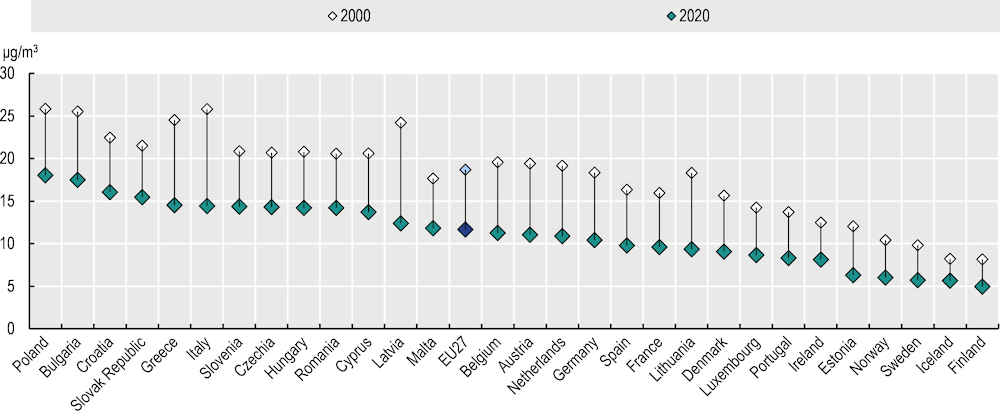
Note: The EU27 average is unweighted.
Source: OECD Environmental Database, Exposure to PM2.5, https://stats.oecd.org/Index.aspx?DataSetCode=EXP_PM2_5.
Exposure to outdoor air pollution is not equally distributed among population groups. A systematic review of available evidence in European countries suggests that higher socio‑economic deprivation is generally associated with higher levels of exposure to PM2.5 and nitrogen oxides (Fairburn et al., 2019[40]) Minority ethnic groups, immigrants and foreign-born populations also have higher exposure to air pollution in some European countries, including France, Spain, Sweden and Switzerland. Greater exposure to pollution among children with lower socio‑economic status arises from living in higher-traffic areas, nearer to waste sites and in poorer-quality housing (Bolte, Tamburlini and Kohlhuber, 2009[41]). Additionally, vulnerability to issues exacerbated by air pollution differs between population groups owing to differences in the prevalence of pre‑existing health problems, capacity and access to coping mechanisms, and complementary risks via other channels such as occupation or housing (Fairburn et al., 2019[40]).
Differences in historical use of asbestos between EU+2 countries continue to affect current cancer incidence and potential future exposure
Europe has some of the highest historical prevalence of exposure to asbestos worldwide owing to widespread use in manufacturing and construction, peaking in the 1950s to 1970s (Eurogip, 2006[42]). Although use of asbestos has been banned in European countries since the early 2000s, historical exposure continues to affect disease incidence decades later, and 60% of worldwide deaths from asbestos-related diseases (excluding lung cancer) between 1994 and 2010 were in Europe (Kameda et al., 2014[43]). Additionally, asbestos remains present in a large share of the 220 million buildings built in Europe before 2001; thus, workers engaged in demolition, construction, and building finishing (including plumbers, electricians, painters, carpenters and appliance specialists) remain at risk of exposure to asbestos during renovation efforts (Eurogip, 2006[42]; European Council, 2023[44]). As part of the European Green Deal, asbestos presence – along with other relevant factors such as age, energy savings potential and seismic risk – is a relevant factor in renovation prioritisation.
Total per capita asbestos use (defined as production plus import minus export) was highest in Cyprus, Luxembourg and Belgium during 1920‑70, and in Slovenia, Croatia, Luxembourg and Belgium during 1971‑2000 (Kameda et al., 2014[43]). Use of asbestos in residential buildings between 1920 and 2003 was highest in the Baltic countries, followed by Belgium and Cyprus. Presence of asbestos in residential buildings contributes to increased risk of future exposure as the buildings age or are exposed to natural disasters such as earthquakes in some regions (Kakoulaki et al., 2023[45]).
Among EU+2 countries, Belgium and the Netherlands were found to have the highest mortality rates from mesothelioma. However, it should be noted that owing to incomplete diagnostics and reporting of occupational diseases, the mortality in some countries may be underreported (Wilk and Krówczyńska, 2021[46]). Within-country patterns of disease can correspond to the use of asbestos, however. For example, in Slovenia, which had high use of asbestos during 1970‑2000, temporal and spatial trends in mesothelioma correspond to a 30‑year delay after peak use of asbestos (Zadnik et al., 2017[47]). Men are much more likely than women to experience asbestos exposure because of higher engagement in employment in sectors that use asbestos, such as construction and manufacturing. An estimated 85% of occupational cancer deaths in 2019 in the 29 EU+2 countries were among men (see Table 3.1).
3.2.3. Cancers caused by viral infections require targeted action
While human papillomavirus vaccination has been introduced in all EU+2 countries, coverage rates are well below the EU target
Prevalence of HPV infection varies greatly by country, but is estimated at about 14.4% for women in the European countries. It is slightly lower in Northern, Western and Southern Europe and substantially higher in Central and Eastern European countries (about 23.4%). Prevalence of HPV infection at any anogenital site is about 18.5% among men in the WHO European Region, and prevalence of high-risk HPV strains (those most likely to cause cancer) is slightly higher among men than women (European Cancer Organisation, 2022[26]).
Vaccination against HPV is included in national immunisation programmes in all EU+2 countries (see Section 3.3.5). Nevertheless, on average in 2022, 64% of girls had received all required doses by age 15, and in Latvia, Slovenia, Luxembourg, France and Bulgaria, the proportion was below 50%. Only Iceland, Portugal and Norway reached coverage of 90% among girls – the target set by WHO for all countries by 2030 (WHO, 2023[48]). As introduction of HPV vaccination for boys is more recent in most countries, coverage by age 15 was lower than that among girls, and only Norway had reached 90% (Figure 3.7). Additionally, the national estimates conceal regional inequalities in HPV vaccination coverage, as important variations may exist. For example, in Belgium, regional coverage estimates in 2016 ranged from 36% in the Brussels-Wallonia region to 91% in the Flemish region (Vermeeren and Goffin, 2018[49]; Vandermeulen et al., 2017[50]).
Coverage with HPV vaccination is affected by programme design, but also by beliefs, attitudes and confidence about vaccines. Compared to 2018, 2022 survey results indicate that the proportion of people reporting positive perceptions about the importance, safety and effectiveness of HPV vaccines had decreased in the majority of EU+2 countries. Differences between countries exist, however: around 90% of respondents agreed with the statements that the HPV vaccine is important, safe and effective in Portugal and Spain, while the lowest proportions were in Latvia, the Slovak Republic and the Netherlands. Given their role in prescribing, administering and advising on vaccines, perceptions of healthcare workers play a role in uptake. Among healthcare workers, confidence in HPV vaccines is generally high across the EU27 (de Figueiredo et al., 2022[51]).
Compared to other vaccines, uptake is also challenged by the lack of an immediate threat to adolescents from HPV (as cervical cancer can take a decade to develop after persistent infection), as well as by parental belief that vaccination against a sexual transmitted disease is not relevant for their young children, or that vaccination implies tacit approval for sexual activity (Jacobson et al., 2016[52]). As such, HPV vaccination coverage is often lower than that for other vaccines provided to adolescents, such as the combined tetanus, diphtheria and whopping cough and the meningococcal vaccines, according to the United States Centers for Disease Control and Prevention (CDC) (2021[53]).
Figure 3.7. Coverage of HPV vaccination varies across countries and by sex
Proportion of 15‑year-olds who received the last dose of HPV vaccine, by sex
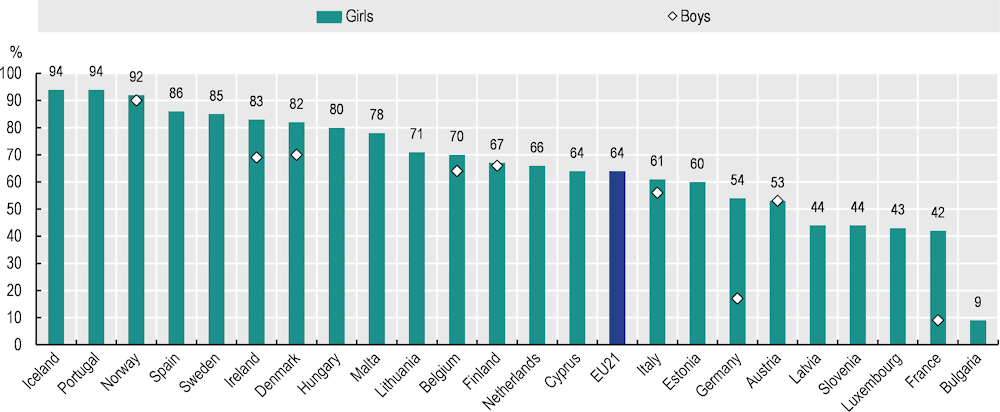
Note: The EU21 average is unweighted.
Source: WHO Global Health Observatory (2023), HPV vaccination coverage by age 15, last dose, https://immunizationdata.who.int/pages/coverage/hpv.html.
Several studies across EU and OECD countries have provided evidence of inequalities in uptake of HPV vaccination by socio‑economic status and migration background (coming from a family where at least one of the parents has migrated into the country). In the Netherlands, low uptake was found to be associated with living in an area with lower socio‑economic status (37% compared to 55% of those living in areas with high socio‑economic status) and having one or both parents born in Morocco or Türkiye (de Munter et al., 2021[54]). In Denmark, factors associated with lower HPV vaccination coverage for both boys and girls were migration background, and having an unmarried or unemployed mother with lower education and income levels (Slåttelid Schreiber et al., 2015[55]; Bollerup et al., 2017[56]). In France, survey data suggest that young women not vaccinated against HPV are more likely to be of low socio‑economic status (Guthmann et al., 2017[57]). Recent surveys in Sweden show that confidence around HPV vaccination is lower among people with lower education and income levels (Wemrell and Gunnarsson, 2022[58]), and those with migrant backgrounds (Wemrell, Perez Vicente and Merlo, 2023[59]). In Poland, parents’ positive attitudes towards HPV vaccination were found to be associated with higher education level and having had a conversation with a doctor about vaccination (Sypień and Zielonka, 2022[60]).
Risk of liver cancer due to hepatitis B and hepatitis C infection is concentrated among vulnerable groups
Transmission of HBV and HCV, which can lead to chronic hepatitis infection and liver cancer, has declined across EU+2 countries on average. Nevertheless, the ECDC estimates that population-level prevalence varies between countries, and is highest in Romania for both HBV surface antigen (4.5% of the population) and HCV ribonucleid acid (RNA) (2.3%). Based on available data, the most common known route of transmission for HBV in 2021 was sexual contact (heterosexual or sex between men), while for HCV it was injection drug use (followed by sex between men). Populations particularly at risk include people engaged in high-risk sex, people who inject drugs, prisoners and people who have migrated from endemic areas (ECDC, 2022[30]; WHO, 2017[61]). Although availability of data on imported cases greatly varies across countries, the ECDC (2022[28]) reports that migrants are particularly vulnerable in European countries. For example, migrant populations account for 80% of HBV cases in Germany, the Netherlands, Norway and Sweden, of which 68% are chronic cases presumed to be contracted before arrival. Chu et al. (2013[62]) found prevalence of HBV infection to be substantially higher among migrant populations than the general population in Western and Northern European countries.
A 2017 internet survey of European men who have sex with men (MSM) revealed that half of respondents had never been vaccinated against HBV. Similarly, vaccination coverage for other at-risk groups needs improvement. For example, the estimated percentage of people who inject drugs who had been vaccinated against HBV was less than 50% in Austria, France, Germany and Poland (ECDC, 2022[30]). These findings demonstrate that the burdens of HBV and HCV fall disproportionately on at-risk groups, and call for more targeted approach to prevention, detection and treatment strategies.
3.2.4. Health literacy levels influence preventive behaviours across risk factors
Health literacy encompasses the personal knowledge and competencies, mediated by organisational structures and availability of resources, that enable individuals to access, understand, assess and use information and services that enhance and sustain good health and well-being. (WHO, 2022[63]). Directly linked to health behaviour, low levels of health literacy are associated with higher prevalence of tobacco use, low levels of physical activity and consumption of unhealthy food. Health-literate organisations can help bridge the gap to make health knowledge more accessible and actionable (see Section 3.3.6).
The results of the European Health Literacy Population Survey 2019‑21, based on respondents from 17 countries (including 15 EU Member States), estimated that general health literacy was associated with more physical activity and fruit and vegetable consumption. However, the Survey found that nearly half of respondents had insufficient levels of health literacy. The proportion with low health literacy ranged from 25% in Slovenia to 72% in Germany. A social gradient emerged in all countries: on average, financially deprived groups and those with a low self-perceived role in society had 8% lower mean health literacy scores, while those with low education levels had 6% lower mean scores than those with higher levels. (M-POHL, 2021[64]). An analysis of the Survey results in Norway found that some groups of migrants are more likely than the general population to score the lowest level on health literacy, while migrants’ low health literacy related to health promotion and disease prevention was associated with their financial situation (low ability to pay bills and meet their expenses) (Le et al., 2021[65]). Health literacy is important across the cancer spectrum: among people diagnosed with cancer, lower health literacy is associated with greater difficulties in understanding and processing cancer-related information, poorer quality of life and poorer experience of care (Holden et al., 2021[66]).
3.3. Policy action is needed to reduce risk factors for cancer and target at-risk population
3.3.1. Measures to reduce tobacco use are in place in many countries
Europe’s Beating Cancer Plan aims to create a “tobacco-free generation” by 2040, with the goal to reduce use of tobacco to less than 5% of the population (European Commission, 2021[67]). This commitment, often presented in Member States alongside a national aspirational target for decreasing prevalence of smoking, is important for leveraging political, public and social support around specific tobacco control policies. The Netherlands and Portugal have set a goal that, from 2040, no new generations will smoke (Government of the Netherlands, 2019[68]; GECP, 2022[69]; Government of Portugal, 2023[70]). Other countries have set goals for a tobacco-free future, such as reducing the proportion of adolescents smoking to less than 3% (England, United Kingdom) or the proportion of adults to less than 5% (France) (Been et al., 2021[71]; Public Health France, 2023[72]). In the Netherlands, the framework of protecting future generations has been successful in driving stricter tobacco control policies (Willemsen and Been, 2022[73]).
Since 2010, most countries have strengthened their tobacco control policies, utilising a range of policy levers to reduce smoking prevalence
In 2003, the Framework Convention on Tobacco Control – the first international treaty under the auspices of WHO – was adopted (WHO, 2023[74]). This aims to facilitate demand-based reduction of tobacco consumption and to set the stage for a broad understanding that tobacco policies should be comprehensive and implemented as a package of different approaches. WHO’s MPOWER framework helps countries gauge their implementation of known cost-effective tobacco-related policies (Joossens and Raw, 2006[75]) by monitoring progress on tobacco usage; protecting people from smoke by regulating smoke‑free environments; offering help to quit tobacco use via access to cessation programmes; warning about the dangers of tobacco through prominent package labelling; enforcing bans on tobacco advertising, promotions, product placement and sales channels; and raising taxes on tobacco (WHO, 2021[76]). The Tobacco Control Scale (TCS) examines several policies, including those in the MPOWER framework, across a range of countries over time, weighting them on a total scale of 100 according to the known effectiveness of each of the measures. Figure 3.8 shows the 2021 country scores on the TCS (total and in the various policy categories) for EU+2 countries, and indicates their evolution since 2010.
Figure 3.8. Most countries have strengthened tobacco control policies since 2010
Tobacco Control Scale (TCS) scores by category in 2021, red arrows indicating a decline from 2010
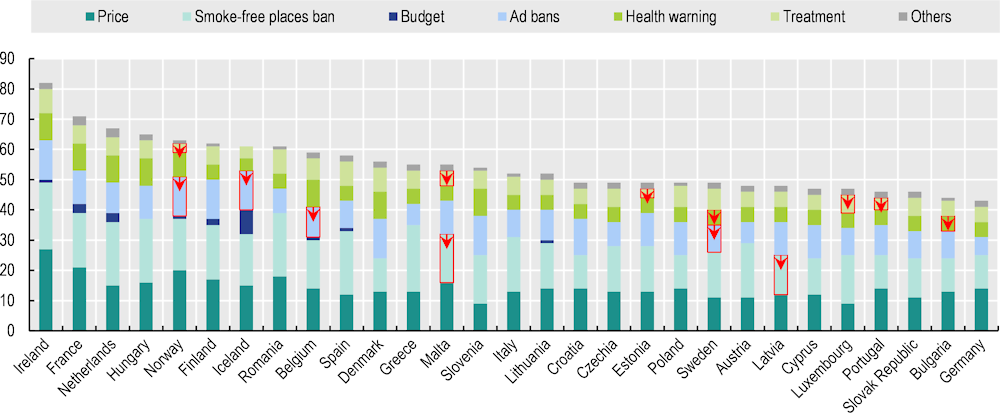
Note: The methodology to compute the score by policy domain is described in the TCS reports. “Others” includes the elimination of illicit trade and an alignment with Article 5.3 of the WHO Framework Convention on Tobacco Control. The maximum scores are 30 (Price), 22 (Smoke‑free place ban), 10 (Budget), 13 (Ad bans), 10 (Health warning) and 10 (Treatment).
Source: Tobacco Control Scale, www.tobaccocontrolscale.org/the‑reports/.
Ireland has the highest score on the 2021 TCS, at 82 points out of 100, with France in second place at 71 points. Ireland scores by far the highest on pricing (taxation) policies, and the maximum possible on comprehensive smoke‑free bans and advertising bans. Six other countries (the Netherlands, Hungary, Norway, Finland, Iceland and Romania) score over 60 points, while Germany (43 points) and Bulgaria (44 points) are at the bottom of the list. All EU+2 countries except Iceland – which already had a very high score – and Sweden, increased tobacco-related restrictions between 2010 and 2021. It is important to note that the score for smoke‑free places ban only relates to a specific selection of indoor bans that do not include recent outdoors bans. In Sweden, for example, new tobacco-related legislation came into force from 1 July 2019, covering smoke‑free outdoor environments; this applies to areas relating to public transport, play areas, sport activities and other public facilities, as well as serving areas of restaurants and cafés. On average, TCS scores increased by 24% between 2010 and 2021 among the 29 EU+2 countries.
Most TCS categories saw improvements across most countries during 2010‑21:
All countries had stronger product labelling requirements about the dangers of smoking.
Of the EU+2 countries, 22 strengthened bans on advertising cigarettes across different media.
Protection from tobacco smoke was increased in 21 countries by mandating more smoke‑free environments in places such as healthcare facilities, educational establishments, restaurants and public transport.
Countries are also increasingly offering more assistance to smokers looking to quit, by providing cessation programmes in a range of community or healthcare settings, nicotine replacement medications and/or a phoneline assisting people with quitting: 19 more countries offered cessation support in 2021 than 2010.
It is important to implement tobacco control policies as a comprehensive package, as France did with its 2016‑20 tobacco control interventions; these included a substantial tax increase, plain packaging for tobacco packages, a mass yearly cessation campaign and reimbursement of nicotine replacement products. Over 2023‑50, these combined interventions are expected to prevent 4 million cases of chronic disease, save EUR 578 million in healthcare expenses and return EUR 4 for each euro invested (Devaux et al., 2023[77]). As policy changes take time to take effect and influence population behaviour, Figure 3.9 plots countries’ scores on the TCS in 2010 and the change in smoking prevalence to 2021, finding a correlation between a higher TCS score – denoting stronger tobacco control policies – in 2010 and a reduction in adult daily smoking rates over the following decade.
Figure 3.9. A higher Tobacco Control Score in 2010 is associated with a larger reduction in daily smoking rates among adults during 2010‑21
Correlation between the 2010 Tobacco Control Scale score and the prevalence of daily smoking of cigarettes for those aged 15 years and more in 2010‑21
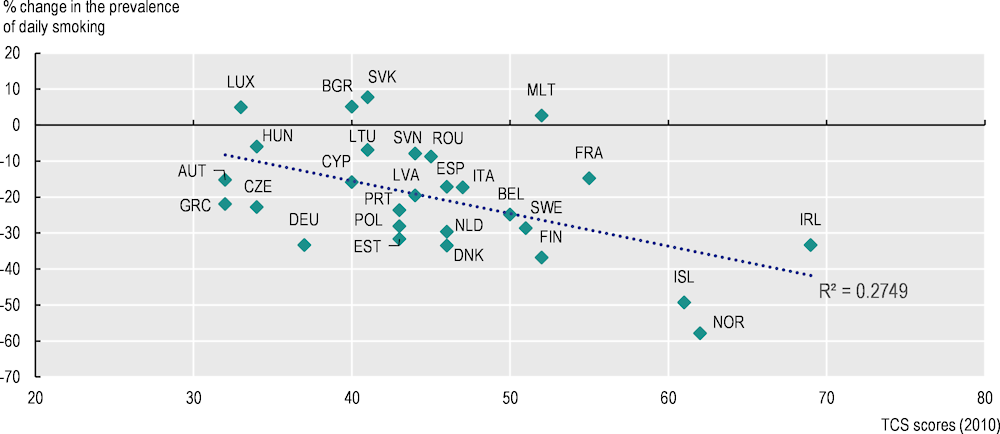
Note: Changes in the prevalence of daily smoking are based on the period from 2010 to 2021 (or the latest available data point).
Source: OECD Health Statistics 2023, https://doi.org/10.1787/health-data-en; Eurostat (2023), “Daily smokers of cigarettes”, European Health Interview Survey, Tobacco Control Scale (2021), www.tobaccocontrolscale.org/the‑reports/.
EU+2 countries are increasingly regulating e‑cigarettes and other electronic delivery systems via similar – though often weaker – policies to those used for tobacco, which are not reflected in the TCS scale (WHO, 2023[3]). Policy efforts include minimum age sales restrictions, taxes on electronic cigarettes, bans on smoking in indoor spaces, sales regulations and advertising restrictions (OECD, 2023[32]). In the EU27, e‑cigarettes are regulated by the 2014 Tobacco Products Directive. However, disposable e‑cigarettes, which are popular among younger people and are associated with substantial health and environmental impacts, are expected to be further restricted via national bans or EU-level legislation to ban single‑use disposable batteries (European Parliament, 2023[78]).
Tobacco taxation policies are cost-effective, but the tax level needs to be updated and aligned across categories of products and countries
Taxing tobacco is considered the most cost-effective tobacco control policy – especially among young people and low-income groups (Joossens and Raw, 2006[75]). On average, a 10% price increase will reduce consumption by 4% in high-income countries, while increasing tax revenues that can be used for tobacco control. In France, a 31% price increase in cigarettes in 2003 corresponded with a decrease in smoking prevalence by 5 percentage points that year. In the Netherlands, an 18% price increase in February 2004 was aligned with a drop in cigarette sales of almost 13% that year (Joossens and Raw, 2006[75]).
In 2022, excise duties and value added taxes on cigarettes in the EU27 ranged from less than EUR 3.00 in Bulgaria, Poland, Romania and the Slovak Republic to more than EUR 6.00 in Denmark, Finland, France and the Netherlands, to above EUR 11.00 in Ireland (Enache, 2022[79]). Estonia, Denmark and Finland have the highest taxes as a share of retail selling price, at over 85%. Luxembourg, Germany, Romania and Sweden have taxes below the 75% minimum level recommended by the WHO (2023[3]), (Enache, 2022[79]).
Ireland, Norway and France had the highest pricing scores in 2021.There have been some tax increases in EU+2 countries since the 2021 TCS score was assessed, but only the 1 January 2022 tobacco excise tax increase in Lithuania has been substantial in terms of its share of retail price (WHO, 2023[3]). While tobacco taxation is common, only a few EU+2 countries (Estonia, France, Iceland, Ireland, Lithuania and Romania) have earmarked a portion of tobacco taxes to go directly to tobacco control or other public health purposes (Campaign for Tobacco-Free Kids, 2021[80]).
An evaluation of the EU’s Tobacco Taxation Directive showed that major differences in taxation (and thus pricing) across Member States limit the benefits to public health – in particular, where cross-border sales are substantial. The evaluation also noted that newer products such as e‑cigarettes and heated tobacco were testing the limits of the existing Directive (European Commission, 2020[81]). In line with Europe’s Beating Cancer Plan, the Commission is reviewing not only the Tobacco Taxation Directive and the legal framework on cross-border purchases of tobacco by private individuals but also the Tobacco Products Directive. The Plan refers to the need to work in full transparency towards plain packaging and a full ban on flavours, using existing EU agencies to improve assessment of ingredients, extending taxation to novel tobacco products, and tackling tobacco advertising, promotion and sponsorship on the internet and social media (European Commission, 2021[67]).
Smoke‑free environments, information and advertising restrictions are of major importance in affecting individuals’ choices around tobacco consumption
Restrictions of smoking in public places including workplaces, public transport, restaurants and bars – along with policies covering outdoor locations such as playgrounds and public parks – are another very effective tool at reducing tobacco usage. A study from the United States found that comprehensive indoor smoking bans (restaurants, bars and workplaces) reduce smoking prevalence by 2‑3%. Furthermore, bans on smoking in bars were found to be particularly influential in reducing smoking among women, low-income groups, those under age 30 and heavy episodic drinkers (Carton et al., 2016[82]). A 2023 study that took advantage of the three‑year gap between implementation of indoor smoking bans in Denmark (2007) and Switzerland (2010) found that Denmark’s ban decreased smoking prevalence and that lung function improved among both non-smokers and smokers after implementation (Strassmann et al., 2023[83]).
Mass communication on tobacco control – entailing information, media campaigns and school-based programmes, depending on duration and scale – has been shown to reduce tobacco consumption. The 2021 TCS report recommends that governments spend at least EUR 2 per capita annually on anti-tobacco campaign efforts (TCS, 2022[84]). Iceland is the only country that came close to spending that amount in 2021: all other EU+2 countries except France and the Netherlands had low spending in this area. Iceland’s approach stems from its history of strong tobacco control policy, which has included use of earmarked tobacco taxes for tobacco prevention and education (WHO, 2016[85]). In recent years, Iceland has moved towards a holistic “Health-promoting Community” approach in municipalities and schools that includes tobacco education, with a larger focus on creating healthy lifestyles and overall well-being. Furthermore, since the “wake‑up call” peak in 1998 of 23% of adolescents reporting daily smoking and 42% reporting being drunk in the past month, Iceland initiated a major “Drug-free Iceland” intervention to improve the overall environment in which adolescents are raised. The research-driven approach focuses on parental monitoring, involvement, quality family time and adolescent participation in youth activities and sports. By 2006, rates among adolescents of daily smoking had fallen to 12% and of intoxication in the last month to 25%, alongside reported increasing rates of protective factors. Localities with the most interventions saw the greatest decreases in substance use (Sigfusdottir et al., 2008[86])
While three major EU-wide anti-tobacco campaigns took place between 2005 and 2016, the focus in recent years has been on more targeted and country-specific initiatives, such as efforts in Germany to make smoking cessation information available to specific population groups (European Commission, 2023[87]). The anti-tobacco and public health websites developed by Germany target migrants, providing information booklets in Russian, Turkish and Arabic on tobacco addiction support and services offered. In France, the TABADO Programme develops information materials and resources tailored to young populations. The Programme supports vocational high school students and apprentices with quitting smoking (Box 3.2).
Box 3.2. The TABADO Programme in France aims to reduce social inequalities in smoking among young people
France’s TABADO Programme, co‑ordinated by the National Institute against Cancer, aims to help students in vocational high schools and apprentices – populations with high prevalence of smoking – to quit. The Programme aims to reduce inequalities through inclusion and outreach to particularly vulnerable populations. It consists of raising awareness of the health risks associated with tobacco use, and provides support in quitting smoking. The Programme offers a three‑part intervention: a whole‑class information session on smoking and cessation; one or more individual counselling sessions with a tobaccologist/addictologist; and up to four motivational group workshops for young people enrolled in the Programme. It was found to be effective during its pilot phase in 2009, and was rolled out in 142 schools in 2019‑20.
Source: Cathelineau, F. et al. (2021[88]), “TABADO, un programme pertinent d’accompagnement des lycéens professionnels et apprentis à l’arrêt du tabac développé en milieu scolaire”, https://beh.santepubliquefrance.fr/beh/2021/8/pdf/2021_8_3.pdf.
Comprehensive bans on advertising and promotion of tobacco across all media have been shown to be effective at reducing demand, while partial bans (e.g. only on television or radio without addressing other media) have not (National Cancer Institute, 2008[89]). Northern European countries, which have the lowest smoking prevalence rates, have the highest possible 2021 TCS scores in this category, with bans on advertising across all media, sponsorships, points of sale and product displays, and on indirect advertising such as cigarette‑branded clothing. Germany had among the lowest scores in this area in 2020, but a new law was introduced in 2021 banning cigarette advertising on billboards and bus stops and in movies rated for under age 18 (alongside long-existing bans on television, radio and internet advertising), and prohibiting free cigarette samples (outside specialty stores). In 2022, following increases in use of tobacco-free nicotine products such as nicotine pouches, Iceland restricted their sales to minors as well as the advertising and marketing of such products (Government of Iceland, 2022[90]).
Large, visual health warnings covering most of the cigarette package have also been shown to discourage non-smokers from starting smoking and to encourage smokers to stop (Joossens and Raw, 2006[75]). Recent developments have centred around standardised cigarette packaging with no branding or logos to lower consumer interest: all cigarettes are sold with plain packaging, standardised visual warnings and the brand name written in plain text. Among EU+2 countries, Belgium, Denmark, Finland, France, Hungary, Ireland, the Netherlands, Norway and Slovenia have introduced standardised packaging. There are differences in standardised packaging requirements: all countries require it for cigarettes and some require it for all legal tobacco products. A few OECD countries also require standardised packaging for rolling paper (Belgium, Canada, and Israel) and for e‑cigarettes and e‑liquids (Denmark, Finland, Israel, the Netherlands and the Canadian province of British Columbia), whose visuals have tended to attract interest among young people (Moodie et al., 2022[91]; Campaign for Tobacco-Free Kids, 2023[92]). In 2023, Canada became the first country to take the concept to the next level, mandating that warning messages be printed on each cigarette, with the idea that the messages will reach newly initiating smokers who are handed a single cigarette (Government of Canada, 2023[93]).
Access to smoking cessation support, associated with actions in primary care settings, should be strengthened
Another WHO-recommended policy is increasing access to and financial coverage of smoking cessation aids, as many smokers report wanting to quit but finding it challenging to do so (El Asmar et al., 2022[94]). According to the TCS reports, most of the EU+2 countries have a national “quitline” that is widely available, and free network cessation support covering at least the major cities. However, in 2021, only three countries (Cyprus, Ireland and Romania) had full coverage of tobacco replacement medications, and about half do not provide any coverage (TCS, 2021[95]).
Healthcare professionals’ advice and aid in providing smoking cessation treatment has been shown to be cost-effective in helping smokers quit; however, there is an “evidence‑practice” gap, as physicians are too often not engaged in such efforts. A Dutch study showed that hearing about smoking cessation support from a healthcare provider in the previous year significantly increased the likelihood of a smoker using such services in their most recent cessation attempt (van Westen-Lagerweij et al., 2022[96]). One review examined 49 studies on implementation strategies to increase primary care engagement with smoking cessation. It included evidence that increasing insurance coverage for smoking cessation services in the United States corresponded with an increase in providers recording smoking status in the health record, providing cessation advice and prescribing cessation medications. Furthermore, financial incentives granted for “meaningful use” of health information technology in the United States and a pay-for-performance scheme in the United Kingdom showed that, under these incentive programmes, general practitioners (GPs) increased their recording of smoking status, and were more likely to report giving smoking cessation advice, although evidence regarding increases in prescription of cessation medications was mixed (Tildy et al., 2023[97]).
School-based programmes to prevent smoking and workplace interventions to offer cessation support have also been instrumental in reducing smoking prevalence. For example, a pooled analysis of 49 randomised controlled trials (RCTs) of school-based interventions showed a 12% statistically significant reduction in smoking rates at the longest available follow-up compared to controls (Thomas, McLellan and Perera, 2013[98]). A 2014 study that examined 31 moderate‑to-high-quality studies on workplace interventions provides strong evidence that individual therapy, group therapy, pharmacotherapy and multi-component interventions aimed specifically at smoking cessation were more successful than no or minimal intervention (Cahill and Lancaster, 2014[99]). A French study comparing free smoking cessation support to the existing EUR 50 coverage found that free cessation access was very cost-effective, with a base estimate of EUR 3 868 per life‑year gained (Cadier et al., 2016[100]).
Tobacco policies need to be implemented in ways that ensure they do not exacerbate existing socio‑economic gaps
Although countries have strengthened tobacco control policies and made headway at reducing overall prevalence, in many cases there are large inequalities in smoking rates across socio‑economic groups (OECD, 2019[101]). Thus, in addition to considering effective tobacco policies for the overall population, it is important to prioritise approaches that can be particularly effective among vulnerable subgroups. Of the 26 EU+2 countries that responded to the 2023 OECD Policy Survey on Cancer Care Performance,2 only 10 (Bulgaria, France, Germany, Latvia, Lithuania, Luxembourg, the Netherlands, Norway, Poland and Spain) reported having specific policies in place to address lifestyle risk factors among those with low socio‑economic status.
A 2019 meta‑analysis of the impact of interventions for reducing tobacco disparities between socio‑economic groups showed that 17 interventions reduced socio‑economic gaps, 16 increased gaps and 1 was neutral, while the majority of studies (48) showed mixed or unclear results (Smith, Hill and Amos, 2021[102]). Higher tobacco pricing due to increased tobacco taxes is the main intervention that has consistently proved effective in reducing tobacco demand, particularly among vulnerable people – including young people and low-income groups, who tend to be more responsive to price increases (WHO, 2023[103]). Smoking cessation support and services also provide the necessary support for vulnerable individuals who may be looking to quit (Greenhalgh, Scollo and Winstanley, 2022[104]). Individual-level interventions, including quitlines, counselling and nicotine replacement therapy, have also been shown to be effective among vulnerable groups.
It is also important to adapt smoking cessation interventions to ensure their efficacy in all groups, accounting for cultural and linguistic differences. In a study on Bosnian and Turkish migrants in Austria who smoked, 78% preferred smoking cessation counselling in their native language. Furthermore, more migrants than non-migrant Austrians indicated a preference for the church or mosque as a location for receiving cessation support (Urban et al., 2015[105]). In the Netherlands, the Smoke‑free Living for Everyone Programme takes a local, tailored approach to reducing smoking in vulnerable communities. Integrated interventions are designed with local residents’ involvement, wherein smoking is tackled alongside other community challenges (Pharos, 2023[106]). In its Smokefree Aotearoa 2025 Action Plan, New Zealand has stated goals of providing tailored support for smoking cessation to its Pacific communities, and of ensuring co‑engagement via Māori leadership and involvement in the overall Plan (Ministry of Health, 2023[107]). In the United States, smoking cessation interventions in San Diego are conducted by community health workers (paraprofessionals working in primary healthcare with strong links to the community) to create supportive environments and address cultural-linguistic barriers for Latino communities, thereby helping to improve access to healthcare resources in the area of public health and cancer risk reduction programmes (Woodruff, Talavera and Elder, 2002[108]).
In contrast to taxation and smoking cessation programmes, studies found that smoke‑free regulations can increase inequalities in smoking. This is particularly the case where such bans are voluntary, partial or implemented in selected geographical locations (Brown, Platt and Amos, 2014[109]), as places with more vulnerable populations are less likely to adopt or enforce such bans. Comprehensive national bans that apply across the board are thus necessary to prevent widening inequalities in smoking. Mass media campaigns have shown mixed results in terms of equity, as groups with higher socio‑economic charcteristics may act more effectively on health information.
As such, policies applied in isolation may not be sufficient to reach all population groups and result in the highest possible health benefits. Recognising the benefits of a comprehensive approach over a narrow one, Hungary has taken multiple actions ranging from regulatory to counselling to reduce the prevalence of smoking (Box 3.3).
Box 3.3. Hungary is tackling high prevalence of smoking via strong tobacco control policies
Given its high prevalence of smoking and burden of tobacco-related diseases, Hungary has taken stringent action on tobacco control, ranking fourth among the EU+2 countries on the 2021 TCS thanks to the 1999 Anti-Smoking Law, a 2011 ban on smoking in workplaces and public spaces, a 2013 reduction of tobacco sales sites, and the introduction of uniform cigarette packaging in 2019. Smoking prevention efforts in Hungary start early: since 2018, about half the country’s kindergartens have participated in a programme that helps young children develop negative perceptions on smoking and take action to reduce exposure to second-hand smoke. A school-based “No Smoking” Programme reinforces the message via interactive videos, gaming tools and discussions, as well as a “smoking is bad” website with targeted materials for different age groups. The efforts are paying off: 74% of children aged 13‑15 were non-smokers in 2016 compared to 62% in 2008 (Demjén, Kimmel and Kiss, 2018[110]).
In terms of smoking cessation, Hungarians can visit lung clinics for cessation services without a referral, and there is a national quitline where callers are assisted via evidence‑based multi-session counselling (Demjén, Kimmel and Kiss, 2018[110]). In 2019, a new app called “Facing a problem? Don’t reach for the stick!” was introduced, which aims to sustain motivation by highlighting the health improvements and cost savings for former smokers. In 2021, a stop-smoking campaign was initiated, employing press releases, online advertisements and social media initiatives (European Commission, 2023[111]).
3.3.2. Policies to address harmful alcohol consumption vary across EU+2 countries
A comprehensive package of prevention policies is necessary to address harmful alcohol consumption, but implementation differs across EU+2 countries
The WHO Global Action Plan for the Prevention and Control of Noncommunicable Disease 2013‑20 (WHO, 2013[112]) aims to reduce harmful alcohol use by 10% through 11 policy interventions. Among these, taxation, restrictions on alcohol advertising and restrictions on the physical availability of alcohol are classified as “Best Buys” – policies considered the most cost-effective and feasible for implementation (WHO, 2021[113]).
Other interventions with potential to reduce alcohol consumption include blood alcohol concentration limits for drivers and penalties for driving under the influence of alcohol, brief interventions to detect and provide counselling to people who drink heavily, and other emerging policy interventions such as minimum unit pricing, labelling methods to communicate health warnings and nutritional content of alcohol, and mass media campaigns (WHO, 2017[114]). An OECD report using simulation models shows that investing in a comprehensive policy approach helps address harmful use of alcohol, reducing the burden of diseases and generating savings in health expenditure. While all policy interventions have a positive effect on population health, the results show that greater impact is achieved by combining established policy interventions (alcohol taxation, regulation of alcohol advertising, sobriety check points and alcohol counselling in primary care) and newer interventions (such as minimum unit pricing and bans on alcohol advertising targeting children); this is predicted to result in a gain of up to 4.6 million life‑years per year across all 48 countries examined. A comprehensive policy package is expected to reduce the number of alcohol-related cancer cases by 2 million by 2050, and to have a significant economic impact through healthcare expenditure savings and labour market outcomes. Overall, for each USD 1 invested in this comprehensive alcohol policy package, up to USD 16 is returned in economic benefits. The estimations also show that the impact of a comprehensive policy package on DALYs and life‑years gained would be greatest in the Baltic and Central and Eastern European countries (OECD, 2021[115]).
In 2021, all European countries had a series of policy actions in place to reduce harmful alcohol use. Using the categories of WHO’s Global Strategy to Reduce the Harmful Use of Alcohol, the OECD report reveals cross-country variation in the level of implementation of alcohol control policies. The clustering of countries shows that Finland, France, Italy and Sweden tend to have the highest levels of implementation, while Austria, Belgium, Croatia, Cyprus, Denmark, Greece, Hungary and Luxembourg show the lowest level of implementation for at least three policy areas. Table 3.2 highlights select alcohol policies, indicating scope in various countries to increase implementation of policy interventions to address harmful use of alcohol.
The remainder of the section presents some established and innovative policies to reduce alcohol consumption, including the three WHO Best Buys, minimum unit pricing and health warning labels. Particular attention is given to policies with potential to affect vulnerable or high-risk populations.
Table 3.2. Various alcohol interventions have been implemented across EU+2 countries
|
Country |
Pricing policies |
Availability restrictions |
Marketing regulations |
Consumer information |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
Taxation adjusted for inflation |
Minimum unit pricing |
Minimum legal age for purchasing |
Restrictions on sales by premise type (on- or off-premise) |
Restrictions on density of alcohol outlets |
Advertising on national television |
Advertising on social media |
Health warning labels |
Guidelines for school-based prevention |
|
|
Austria |
✗ |
✗ |
16‑181 |
Both types |
None |
Partial |
Voluntary |
✗ |
✗ |
|
Belgium |
✓ |
✗ |
16‑181 |
None |
Off-premise |
Partial |
Voluntary |
✗ |
✓ |
|
Bulgaria |
✗ |
✗ |
18 |
None |
Off-premise |
Partial |
Partial |
✗ |
✗ |
|
Croatia |
✗ |
✗ |
18 |
None |
None |
None2 |
None |
✗ |
✓ |
|
Cyprus |
✗ |
✗ |
18 |
Both types |
Both types |
Partial |
Voluntary |
✗ |
✓ |
|
Czechia |
✗ |
✗ |
18 |
None |
None |
Partial |
Partial |
✗ |
✓ |
|
Denmark |
✗ |
✗ |
16‑181 |
None |
None |
Partial |
Voluntary |
✗ |
✗ |
|
Estonia |
✗ |
✗ |
18 |
Off-premise |
None |
Ban |
Partial |
✗ |
✗ |
|
Finland |
✗ |
✗ |
18 |
Both types |
Off-premise |
Partial |
Partial |
✗ |
✗ |
|
France |
✓ |
✗ |
18 |
Both types |
On-premise |
Ban |
Partial |
✓ |
✓ |
|
Germany |
N/A |
✗ |
16‑181 |
None |
None |
Partial |
Voluntary |
✗ |
✗ |
|
Greece |
✗ |
✗ |
18 |
None |
None |
Voluntary |
Voluntary |
✓ |
✗ |
|
Hungary |
✗ |
✗ |
18 |
None |
None |
Partial |
Partial |
✗ |
✓ |
|
Iceland |
✗ |
✓ |
20 |
Both types |
Off-premise |
Ban |
None |
✗ |
✓ |
|
Ireland |
✗ |
✓ |
18 |
Both types |
Both types |
Partial |
Voluntary |
✗ |
✓ |
|
Italy |
✓ |
✗ |
18 |
Both types |
None |
Partial |
None |
✗ |
✓ |
|
Latvia |
✗ |
✗ |
18 |
Off-premise |
None |
Partial |
Partial |
✗ |
✗ |
|
Lithuania |
✗ |
✗ |
20 |
Both types |
None |
Ban |
Ban |
✗ |
✓ |
|
Luxembourg |
✗ |
✗ |
16 |
On-premise |
On-premise |
Partial |
Partial |
✗ |
✗ |
|
Malta |
✗ |
✗ |
17 |
Off-premise |
None |
Partial |
None |
✗ |
✓ |
|
Netherlands |
✗ |
✗ |
18 |
None |
None |
Partial |
Voluntary |
✗ |
✗ |
|
Norway |
✗ |
✗ |
18‑201 |
Both types |
Off-premise |
Ban |
Ban |
✗ |
✗ |
|
Poland |
✗ |
✗ |
18 |
None |
None |
Partial |
Partial |
✗ |
✗ |
|
Portugal |
✗ |
✗ |
18 |
Both types |
None |
Partial |
Partial |
✓ |
✓ |
|
Romania |
✓ |
✗ |
18 |
None |
None |
Partial |
Partial |
✗ |
✗ |
|
Slovak Republic |
✗ |
✓ |
18 |
None |
None |
Partial |
Voluntary |
✗ |
✗ |
|
Slovenia |
✗ |
✗ |
18 |
Off-premise |
None |
Partial |
Partial |
✗ |
✓ |
|
Spain |
✓ |
✗ |
18 |
Both types |
None |
Partial |
None |
✗ |
✗ |
|
Sweden |
✗ |
✗ |
18‑201 |
Both types |
Off-premise |
Ban |
Partial |
✗ |
✗ |
Notes: N/A stands for not available. Minimum unit pricing sets a mandatory floor price per unit of alcohol or standard drink. Minimum legal age for purchasing only concerns the age at which a young individual can purchase alcohol without parental supervision. On-premise refers to restaurants or bars; off-premise refers to establishments such as liquor stores. Partial regulations on advertising may refer to time and/or place and/or content.
1. In Austria, there are regional variations in minimum legal ages. In Belgium, Denmark and Germany, only drinks with low alcohol content can be sold to those aged 16-17. Similarly, only low-alcohol products can be sold to those aged 18-19 in Norway and Sweden.
2. Croatia has no restrictions on advertising on national television (except for beer).
Source: OECD (2021[115]), Preventing Harmful Alcohol Use, https://doi.org/10.1787/6e4b4ffb-en; OECD (2022[116]), Consumption Tax Trends 2022: VAT/GST and excise, core design features and trends, https://doi.org/10.1787/6525a942-en; European Commission (2021[117]), Excise Duty tables: Part 1 - Alcoholic beverages, https://taxation-customs.ec.europa.eu/system/files/2021-09/excise_duties-part_i_alcohol_en.pdf; Cyprus Bar Association (n.d.[118]) Law on the Sale of Alcoholic Beverages of Cyprus, https://www.cylaw.org/nomoi/enop/non-ind/0_144/full.html; Le service public fédéral (SPF) Santé publique, Sécurité de la Chaîne alimentaire et Environnement (2016[119]), Alcohol, https://www.health.belgium.be/fr/sante/prenez-soin-de-vous/alcool-et-tabac/alcool; WHO Global Health Observatory Database.
Taxation and minimum unit pricing are key levers to reduce alcohol consumption among low-income groups
Higher prices of alcoholic drinks have been shown to reduce alcohol consumption. A recent literature review shows that the mean of the elasticities varies from -0.5 for beer to -0.8 for spirits, meaning that a 10% price increase will reduce consumption by between 5% and 8% (Clements et al., 2022[120]). Despite clear evidence of the price elasticity of demand, 8 of the 29 EU+2 countries tax only beer and spirits, while 21 tax all beverage types (OECD, 2021[115]).
Beyond taxation, minimum unit pricing (MUP) is a policy intervention that sets a mandatory floor price per unit of alcohol or standard drink. While increasingly implemented globally, only three EU+2 countries have implemented MUP: the Iceland, Ireland and the Slovak Republic (WHO, 2022[121]). The Slovak Republic was the first EU27 country to implement a minimum pricing regulation on alcoholic beverages, forbidding sales of spirits at a price cheaper than the sum of value added tax (VAT), excise tax and the minimum unit price of EUR 0.86. Ireland implemented MUP in 2022 on all alcoholic products, making the lowest price that can be charged for a gram of alcohol EUR 0.10. Similarly successful examples were seen in the United Kingdom: MUP was introduced in Scotland in 2018, and in Wales in 2020, setting a floor price per unit of pure alcohol at GBP 0.50. In Scotland, this was associated with a 7.6% reduction in weekly purchases of alcohol, with a larger impact among low-income groups (O’Donnell et al., 2019[122]). Particularly targeting low-cost high-strength alcohol, the Scottish policy was associated with a 13% reduction in deaths wholly attributable to alcohol in nearly three years, with a particularly marked reduction among people living in the most socio‑economically deprived areas (Wyper et al., 2023[123]).
A systematic review of evidence from Australia, Canada and the United Kingdom similarly shows that taxation and MUP led to a reduction in overall alcohol consumption, with larger impacts among low-income populations (Kilian et al., 2023[124]). In Lithuania, the marked increase in alcohol excise taxation resulted in a decrease in education-related inequalities in mortality, driven by a stronger reduction of mortality rates among men with lower education levels (Manthey, Jasilionis and Jiang, 2023[125]). In Finland, a time‑series analysis found a negative association between higher minimum prices and alcohol-related mortality (Herttua, Makela and Martikainen, 2015[126]). It is important to note that the impact of taxation and MUP among socio‑economic groups varies according to the socio‑economic proxy used. Additionally, measures affecting the price of alcohol should be accompanied by measures to raise awareness of the change and support for decreasing consumption for heavy drinkers and those living with alcohol dependence, to prevent disproportionate financial strain from increased spending on alcohol.
Further restrictions on physical availability of alcohol and on alcohol advertising help to reach vulnerable populations
Restrictions on alcohol availability limit the opportunity for people to purchase and consume alcohol. According to the 2021 OECD report, 12 EU+2 countries restrict the hours and days for both on-premise (restaurants and bars) and off-premise (liquor stores) alcohol sales, and all countries set a minimum age at which people can purchase or consume alcohol legally (OECD, 2021[115]). While four EU+2 countries have set the minimum legal age at which people can purchase or consume alcohol with low alcohol content at 16, most allow purchasing of all alcohol at 18. The exceptions are Luxembourg and Malta, where all types of alcohol purchases are allowed at ages 16 and 17, respectively, and Iceland, Lithuania, Norway, and Sweden, where they are only allowed at age 20 (see Table 3.2).
Restrictions on the number and density of outlets in a given area are also an effective policy intervention to reduce alcohol consumption, although less extensively implemented. Only four EU+2 countries have set on-premise outlet restrictions (Cyprus, France, Ireland and Luxembourg), while eight have off-premise outlet restrictions (Belgium, Bulgaria, Cyprus, Finland, Iceland, Ireland, Norway and Sweden). The remaining 19 EU+2 countries have no restrictions on the number or density of any alcohol outlets. Evidence suggests a positive association between alcohol outlet density, alcohol consumption and related harm and violence – particularly among young drinkers and those with lower socio‑economic characteristics. Reduction of outlet density has been found to decrease socio‑economic inequalities in alcohol consumption (Roche et al., 2015[127]). There is, however, large heterogeneity in policy design according to outlet type (such as bars, restaurants or liquor stores), alcoholic beverages and implementation level (national or local), for example. Nordic countries (Finland, Iceland, Norway, and Sweden) have a state monopoly to sell alcoholic beverages above a certain alcohol content that limits availability through lower retail outlet density and shorter opening hours (Box 3.4). Other OECD countries such as Australia, Canada, and the United States also have interesting examples of restricting outlet density, with implementation at the local level. All provinces of Canada (except Alberta), for example, have a retail alcohol monopoly (Room, 2021[128]).
Box 3.4. State alcohol retail monopolies are in place in Nordic countries
The Nordic countries (Finland, Iceland, Norway and Sweden) have implemented retail monopoly systems that are government-owned, allowing to control when, where and at what price alcohol is sold. The overarching objective is to limit the negative effects of harmful alcohol consumption on the population and society, and to reduce harm from alcohol.
In Iceland, access to alcohol is controlled through a state‑owned monopoly chain of liquor stores, which are the only retail sites allowed to sell alcoholic beverages containing more than 4.75% alcohol by volume.
The Norwegian Government has adopted policies that impose high prices, limit access and have a non-profit distribution model of alcohol containing more than 4.75% alcohol by volume through the Wine Monopoly.
Sweden restricts availability through the retail monopoly of state‑owned Systembolaget, which controls the sales of all alcoholic beverages containing more than 2.25% alcohol by volume, except beer with a maximum of 3.5% alcohol by volume. Systembolaget is also tasked with informing people about the risks of alcohol. It operates on a not-for-profit basis, and has limited operating hours. A modelling study estimated that dismantling the Swedish monopoly would increase alcohol consumption by up to 31% per year, which would lead to 1 234 more deaths each year compared to a 2014 baseline (Stockwell et al., 2018[129]).
Finland had granted a monopoly to a government-owned company for retail sales of alcohol products above 5.5% alcohol by volume, and limited opening hours. However, the government’s programme in 2023 has proposed changes to the policy, increasing the permissible alcohol content for alcohol sold in other stores. A modelling study (Sherk et al., 2023[130]) found that abolishing the state monopoly on alcohol sales would lead to a 9% increase in alcohol consumption and significant increases in alcohol-related economic costs and mortality compared to a 2018 baseline.
Source: OECD (2023[131]), EU Country Cancer Profiles, www.oecd.org/health/eu-cancer-profiles.htm.
Another effective policy intervention to target high-risk populations is reducing marketing that promotes favourable attitudes to alcohol. A handful of studies have shown a positive association between alcohol advertising and consumption (both initiation and hazardous drinking), which is particularly pronounced among young people. A recent systematic literature review confirmed the causal relationship between alcohol marketing and subsequent drinking behaviour among young people (Sargent and Babor, 2020[132]). While the European Audiovisual Media Services Directive sets restrictions on the content of alcohol advertising to televisions, radio and video-sharing platforms, EU+2 countries restrict alcohol marketing to varying degrees. In 2020, only six EU+2 countries had bans on national television advertising for beer and wine (Sweden, Norway, Lithuania, Iceland, France and Estonia) (see Table 3.2). In Sweden, advertising bans target television and radio, while marketing through other media (such as billboards or newspapers) needs to follow strict criteria on content, placement and inclusion of warning labels. For example, advertising cannot be aimed at or portray people aged under 25, cannot be shown in places where these groups are the main ones present, and can only portray the product and the produce used (e.g. grapes).
In addition, as both adults and children spend a significant amount of time on social media and other digital platforms, they are increasingly exposed to targeted alcohol advertising leading to drinking behaviour. The positive association between the amount of time spent on social media and alcohol use by young people has been reported in several countries. In Australia, a cross-sectional study suggested a significant association between interaction with alcohol content on three leading social networking sites and drinking levels (Gupta et al., 2018[133]). In the United Kingdom and Norway, social media use has been associated with more frequent alcohol consumption among young people (Ng Fat, Cable and Kelly, 2021[134]; Brunborg, Skogen and Burdzovic Andreas, 2022[135]). Those aged 10‑15 with four hours of social media use per day are twice more likely to drink at least monthly than those with less than one hour of social media use. Similar associations were found between greater use of social media and heavy episodic drinking among those aged 16‑19 (Ng Fat, Cable and Kelly, 2021[134])).
Despite such growing evidence on the increased risk of alcohol consumption associated with social media, very few countries have comprehensive bans on alcohol marketing on social media or other digital platforms. In 2020, only Lithuania and Norway had bans to restrict alcohol advertising via social media (see Table 3.2). By contrast, five EU+2 countries had no social media advertising restrictions (Croatia, Iceland, Italy, Malta and Spain). The remaining countries had partial (i.e. the restriction applies during a certain time of day or for a certain place, or to the content of events) or voluntary restrictions (i.e. the alcoholic beverage industry follows its internal voluntary rules). More effective regulation and international co‑operation are needed to implement and enforce further social media advertising restrictions.
Alcohol health warning labels have shown encouraging results to increase consumer awareness of the risks associated with drinking
Consumer knowledge of disease or injury risks due to alcohol consumption and behaviour change is mostly enhanced through mass media campaigns. These are common policy tools in all EU+2 countries, targeting driving under the influence of alcohol, awareness of the health risks associated with alcohol consumption and “dry month” campaigns promoting not drinking during one month (OECD, 2021[115]). However, labelling alcoholic beverages with health warnings is rarely implemented across EU+2 countries, although it provides further opportunities to increase awareness.
The literature on the impact of warning labels provides evidence of their effectiveness in increasing awareness. Effectiveness of reducing the level of alcohol consumption is still inconclusive, however, and depends on the evaluation method, the design and format of the labels and the timeframe of observation (WHO, 2021[136]). In Canada, a real-world experiment in the Province of Yukon provided evidence that exposing people to cancer warnings on alcohol containers was associated with a 7% reduction in per capita alcohol use (Zhao et al., 2020[137]). The labels were colourful, had multiple messages warning about the links between alcohol and selected conditions – including cancer – and provided information on the number of standard drinks and the Canadian low-risk drinking guidelines.
Based on key lessons from the use of health warning labels to address tobacco consumption and unhealthy diets, alcohol health warning labels could be an effective tool in the package of national prevention policies to address harmful alcohol consumption. In 2020, only three EU+2 countries used health warnings on alcohol products (France, Greece and Portugal). In Luxembourg, a warning label for pregnant women is used on alcoholic beverages produced nationally, but application is not mandatory. More recently, Ireland passed the Public Health Alcohol Act in 2018 and, in May 2023, became the first country in the world to mandate comprehensive health labelling for alcohol products, including cancer warnings (Government of Ireland, 2023[138]). Effective from May 2026, the new legislation requires alcohol product labels to specify calorie content, grams of alcohol, the risks associated with consuming alcohol during pregnancy and the risks of developing liver disease and fatal cancers from alcohol consumption. Australia, New Zealand and South Korea are examples of other OECD countries that have mandatory alcohol label warnings.
Implementation of screening and brief interventions is needed to modify lifestyles through information and education
The European Framework for Action on Alcohol 2022‑25 prioritises evidence‑based workplace, school and community interventions, emphasising the importance of evaluation and adaptation to reach target populations (WHO, 2022[139]). Effective interventions can be targeted at specific sectors or subgroups of employees, be delivered in a face‑to-face or web setting, and include dissemination of information or training. Overall, a review found that such workplace programmes are effective in reducing alcohol use – especially the quantity of drinking – including in European countries (Fellbaum et al., 2023[140])). Similarly, studies from Germany and Norway provide evidence on the efficacy of internet-based self-help alcohol interventions offered through the workplace (Boß et al., 2018[141]; Brendryen et al., 2017[142]). Interventions such as screening and brief interventions (SBIs) can also be delivered by independent healthcare professionals in the workplace, using tools such as the WHO Alcohol Use Disorders Identification Test-Concise (AUDIT-C) or Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) to identify employees at risk of harmful alcohol use (WHO, 2023[143])). Most countries have strict legislation on alcohol use by holders of specific jobs – such as drivers, physicians and construction workers, where being intoxicated can result in severe harm – while consumption of alcohol in other settings is often left up to employers (OECD, 2022[144]).
Beyond the workplace, SBIs could be implemented in primary healthcare settings. In Germany, for example, it is estimated that fewer than 3% of patients in primary healthcare are screened for alcohol use. Between 18% and 25% of individuals in Sweden and the Netherlands reported alcohol conversations in healthcare in 2017 (Abidi et al., 2020[145]). Survey data suggest that alcohol prevention efforts should be improved, including SBIs to reduce alcohol consumption and related harm in risky drinkers. A simulation model shows that large‑scale implementation of SBIs and referral to treatment in primary healthcare settings could yield large reductions in alcohol consumption in Germany. Accordingly, if one‑quarter of patients or more are screened once a year, a significant reduction in drinking levels among men and in the youngest age groups could be achieved (Manthey et al., 2021[146]). In Europe, SBIs in primary healthcare were found to be cost-effective in 24 out of 28 countries by reducing alcohol-attributable morbidity and deaths (Angus et al., 2017[147]). However as of 2020, Romania, Malta, the Slovak Republic and Greece had not developed or implemented national guidelines and standards of care for SBIs (OECD, 2021[115])).
Schools can also act as excellent locations for interventions to prevent alcohol use, traditionally by focusing on imparting knowledge about alcohol. However, more recent interventions take a more interactive approach, taking into account the social and cultural factors influencing students’ alcohol consumption (OECD, 2021[115]; Lee et al., 2016[148]). Although some studies show positive effects, evidence on the effectiveness of school-based intervention programmes is mixed (OECD, 2021[115]). The Unplugged Programme (Box 3.5), which is implemented in several European countries, was found to be effective in reducing alcohol-related behavioural issues, especially among children who had begun drinking before the intervention (Lee et al., 2016[148]).
Box 3.5. The Unplugged programme has been implemented in several EU27 countries
Unplugged is a school-based programme, designed for children aged 12‑14 and their parents, and delivered by trained teachers, that incorporates components focusing on coping with emotions and stress, normative beliefs and knowledge about the harmful health effects of alcohol use, illicit drugs and smoking. The curriculum consists of 12 one‑hour units taught once a week by class teachers who have attended a training course in the lessons and materials, and in how to teach them using methods that encourage interaction among pupils and between pupils and teachers, such as role‑play and giving and receiving feedback in small groups. This basic curriculum is ideally supplemented either by meetings led by pupils selected by their classmates, or by workshops for the pupils’ parents. As of 2023, the Unplugged Programme is implemented in ten EU27 countries, including Belgium, Croatia, Czechia, Germany, Greece, Italy, Romania, Slovenia, Spain, Sweden. Several studies have pointed to the effectiveness of the programme in different countries, while a systematic review of school-based prevention programmes concluded that the Unplugged Programme has the best evidence of effectiveness in Europe in prevention of alcohol use.
Source: EMCDDA (2023[149]), “Unplugged - a Comprehensive Social Influence programme for schools: life skills training with correction of normative beliefs,” www.emcdda.europa.eu/best-practice/xchange/unplugged_en; Agabio et al. (2015[150]), “A Systematic Review of School-Based Alcohol and other Drug Prevention Programs”, https://www.doi.org/10.2174/1745017901511010102.
3.3.3. Policies to improve diets, increase physical activity levels and address metabolic risk factors vary widely
Although unhealthy diet, physical inactivity and overweight and obesity constitute independent risk factors for cancer, efforts to address them are intertwined. Policies to tackle overweight and obesity must recognise it as a complex multi-faceted issue, while acknowledging that the primary mechanism leading to it relates to an imbalance between energy intake and expenditure. The evidence has catalysed most countries to develop initiatives to improve behavioural and metabolic factors related to diet and weight. All 29 EU+2 countries have implemented national dietary guidelines. All except Greece have an adult obesity strategy, and all except Austria, Croatia, France, Greece and Portugal have a child obesity strategy. Most EU+2 countries have guidelines on physical activity (OECD, 2022[35]). Table 3.3 outlines the national implementation status of selected policies on nutrition and physical activity. This table has been prepared using information from the World Cancer Research Fund International NOURISHING policy database, complemented by various alternative sources3.
Table 3.3. National-level policy implementation status varies by country in selected nutrition and physical activity policy areas
|
Economic tools |
Marketing |
Labelling |
Schools |
Healthcare |
|||
|---|---|---|---|---|---|---|---|
|
Health-related food taxes or tariffs |
Regulation of direct advertising to young people (unhealthy food and beverages)1 |
Voluntary Front-of-Pack labelling (positive and/or negative) |
Regulation of type of food and drink available in schools |
Restrictions on SSBs in schools |
Nutrition advice and counselling in healthcare , by target group |
Physical activity counselling, assessment, and prescriptions in primary care |
|
|
Austria |
No |
No |
No |
Voluntary |
No |
No |
General public |
|
Belgium |
Excise tax soft drinks |
Co-regulation |
Both |
Voluntary |
No |
No |
General public |
|
Bulgaria |
No |
Legislation |
No |
Mandatory |
No |
No |
General public |
|
Croatia |
Excise tax SSBs |
Legislation |
Positive only |
Mandatory |
No |
General public |
General public |
|
Cyprus |
No 2 |
N/A |
No 2 |
Mandatory 2 |
No 2 |
N/A |
No 2 |
|
Czechia |
No |
No |
No |
Mandatory |
No |
No |
No |
|
Denmark |
Excise tax sugar |
Self-regulation |
Positive only |
Voluntary |
No |
No |
Targeted groups |
|
Estonia |
No |
Self-regulation |
No |
Mandatory |
No |
Targeted groups |
No |
|
Finland |
Excise tax soft drinks |
Self-regulation 2 |
Both 3 |
Mandatory |
Mandatory |
General public |
Yes |
|
France |
Excise tax SSBs |
Legislation |
Both |
Mandatory |
Mandatory |
General public |
No |
|
Germany |
No |
No |
Both |
Voluntary |
Voluntary |
No |
Targeted groups |
|
Greece |
No |
No |
No |
Mandatory |
Mandatory |
General public |
Yes |
|
Hungary |
Excise tax multiple 4 |
Legislation |
No |
Mandatory |
Voluntary |
No |
General public |
|
Iceland |
No 2 |
N/A |
Positive only 2 |
N/A |
N/A |
N/A |
N/A |
|
Ireland |
Excise tax SSBs |
Self-regulation |
No |
N/A |
Voluntary |
Targeted groups |
No |
|
Italy |
No |
Self-regulation |
No |
Mandatory |
No |
Targeted groups |
General public |
|
Latvia |
Excise tax SSBs |
Co-regulation 2 |
No |
Mandatory |
Mandatory |
General public |
No |
|
Lithuania |
No |
Legislation |
Positive only |
Mandatory |
No |
General public |
General public |
|
Luxembourg |
No 2 |
N/A |
Both 2 |
Mandatory 2 |
Mandatory 2 |
N/A |
N/A |
|
Malta |
No |
Legislation |
No |
Mandatory |
No |
No |
No |
|
Netherlands |
No |
Self-regulation |
No |
Voluntary |
No |
Targeted groups |
General public |
|
Norway |
Ad valorem tax sugar |
Legislation |
Positive only |
Voluntary |
Voluntary |
Targeted groups |
Targeted groups |
|
Poland |
Excise tax SSBs |
Legislation |
No |
Voluntary |
No |
No |
No |
|
Portugal |
Excise tax SSBs |
Legislation |
No |
Mandatory |
Mandatory |
Yes |
General public |
|
Romania |
VAT soft drinks 2 |
Legislation |
No |
Mandatory |
No |
No |
No |
|
Slovak Republic |
No |
N/A |
No |
Mandatory |
Mandatory |
No |
Targeted groups |
|
Slovenia |
No |
Co-regulation |
Positive only |
Mandatory |
Voluntary 2 |
General public |
General public |
|
Spain |
Co-regulation |
No |
Voluntary |
No |
General public |
General public |
|
|
Sweden |
No |
Legislation |
Positive only |
Mandatory |
No |
Targeted groups |
General public |
Notes: N/A stands for not available; SSBs stands for sugar-sweetened beverages; VAT stands for value added tax. Only policies that are implemented and endorsed at the national level are included, while policies organised locally on the level of municipalities or schools are excluded. Cells marked in teal signal national implementation of a policy in line with best practices, while light teal and light red indicate presence of a measure with some differences from best practice. Red indicates the absence of nationally implemented measures. Targeted groups for nutrition and physical activity counselling include children and adolescents or children and adolescents with obesity-related issues.
1. Legislation refers to mandatory legislation and regulation, co-regulation refers to shared regulation between the government and industry, and self-regulation means any regulation is up to the discretion of the food industry. Alcoholic beverages are excluded.
2. Based on various alternative sources used to complement the information (see endnote 3)
3. Finland uses the heart symbol, classified as positive only, however an additional high-salt label is in use to signal negative assessment.
4. Hungary applies a specific excise tax on the salt, sugar and caffeine content of various food and soft drinks.
5. The region of Catalonia in Spain has a specific excise tax on SSBs.
Source: Where not otherwise stated, this material has been reproduced from the World Cancer Research Fund International NOURISHING policy database https://policydatabase.wcrf.org and nutrition policy index https://www.wcrf.org/policy/nutrition-policy/.
Well-designed and balanced interventions related to food product prices can reduce inequalities in nutrition
Interventions on the price of products, such as taxes, subsidies and other economic incentives, affect consumer behaviours. Taxation of unhealthy food products – including sugar-sweetened beverages and food with high sugar, salt or saturated and total fat content – is less common than taxation of tobacco and alcohol products; however, evidence shows that consumption is similarly affected by price changes. A systematic review found that a 10% decrease in price was associated with 12% increased consumption of healthy foods, while a 10% increase in the price of unhealthy products led to a 6% reduction in their consumption (Afshin et al., 2017[151]). Importantly, evidence suggests that high consumers of unhealthy food products could be more affected by price increases (Taillie et al., 2017[152]; Capacci et al., 2019[153]). The impact varies depending on programme design, the size of the tax and the extent of its pass-through to consumers (i.e. the extent to which producers increase the price of the taxed product). Additionally, substitution effects should be accounted for, whereby people may opt for other similarly unhealthy options if measures are applied unevenly (OECD, 2019[154]).
There are differences in the use of financial tools across the EU+2 countries. Health-related excise taxes, applied in 10 countries, are generally considered most effective, as they are applied to specific products, decreasing their affordability relative to other similar products (see Table 3.3). Taxes affecting sugar-sweetened beverages or soft drinks (which may or may not include added sugars and sweeteners) are most common – present in 13 countries. Latvia has applied an excise tax on non-alcoholic beverages since 2000, refining it most recently in 2022 by charging an increased rate on beverages with a sugar content above 8 g/100 ml. Catalonia (Spain) implemented a tax on sugar-sweetened beverages in 2017, which led to a marked reduction in consumption of taxed beverages in low-income neighbourhoods and heightened awareness of their health effects (Royo-Bordonada et al., 2019[155]). In Europe, only Hungary applies a wider health-related excise tax on food and drinks high in salt, sugar or caffeine. This measure was initially associated with a 3.4% decrease in consumption of processed food, with particularly marked improvements among poorer households (Bíró, 2015[156]). As an added benefit, well-applied tax measures can act as incentive to the food industry for product reformulation, with potential benefits to population health that do not rely on consumer behaviour change (Rogers et al., 2023[157])
Interventions on product prices at the point of sale – including increases and decreases – have been found to modify the choices of people with lower socio‑economic characteristics more than those with higher socio‑economic characteristics (OECD, 2019[154]). To ensure ethical implementation of tax increases on unhealthy products, they need to be accompanied by proportional price decreases for healthy products or targeted subsidies such as vouchers or discounts to offset economic hardship potentially imposed on low-income individuals. Evidence suggests that a combination of taxes and subsidies is more effective than either alone, while maximum efficacy could be achieved if each amounts to at least 10‑15% of the price of the product (Niebylski et al., 2015[158]; Saha et al., 2021[159]). To increase availability of healthy foods – such as fruit, vegetables and whole grains – and support a shift towards healthy proteins (including but not limited to plant-based ones), subsidies and food vouchers can act as an effective means to affect nutritional choices. Across Europe, targeted subsidies or initiatives to increase accessibility and affordability of healthy food are most commonly associated with school-based provision, remaining underutilised in other settings – such as targeting low-income areas or populations (WCRFI, 2023[160]).
Marketing restrictions affect nutrition throughout the life‑course, and are particularly important to prevent exploitation of children’s developmental vulnerabilities
Children and adolescents are exposed to a large amount of advertising for food and beverages, with child-oriented messaging commonly used to promote unhealthy products (Lavriša and Pravst, 2019[161]). Evidence shows a link between advertising exposure and short-term consumption, which is particularly strong in children under 12 and obese children (Delgado et al., 2022[162]). An Australian review concluded that regulation of television advertising of high-fat or high-sugar food and beverages to children was among the most cost-effective strategies to combat high BMI throughout the life‑course (Magnus et al., 2009[163]).
Children from lower socio‑economic groups have been shown to be more likely to follow an unhealthy diet, and to have high exposure to obesogenic marketing hazards, as well as higher responsiveness to advertising of unhealthy foods. Thus, interventions that reduce children’s exposure to promotional marketing of unhealthy healthy foods and beverages can act to reduce inequalities, as their impact may be stronger on these children (Lobstein, 2023[164]).
Regulations typically focus on restricting television advertising at peak viewing times for young children. Policies that target food and beverage advertising are implemented in nearly all countries, although the World Cancer Research Fund International (WCRFI) (2023[160]) suggests that there are substantial gaps due to the voluntary nature of restrictions in many countries and the fact that the bans are often limited to young children. Across the 25 EU+2 countries with available information, 11 have implemented legislation to restrict advertising to young people, while 10 rely on co-regulation or industry self-regulation (see Table 3.3). The majority of advertising restrictions focus on children aged under 12, yet data on poor nutrition habits among adolescents (see Section 3.2.1) highlight a need to include older age groups (WHO, 2020[165]). Only six countries have extended measures to protect adolescents over 12. Norway has announced plans to take one of the most comprehensive approaches to regulate advertising by banning all forms of advertising of unhealthy food and beverages to children under 18. Outside Europe, Chile has similarly instated mandatory restrictions for marketing to younger age groups, with evidence of efficacy on targeted outcomes (Box 3.6).
Box 3.6. Norway and Chile have instituted strict regulations on advertising to minors
Norway
In 2023, Norway announced a plan to ban all advertising of food and beverages deemed unhealthy targeted at children under 18 via legislation going into effect in 2024. A previous self-regulation scheme was deemed insufficient, leading to a renewed effort to protect children from commercial marketing (Safe Food Advocacy Europe, 2023[166]). Although the details of the full ban were not public as of September 2023, the previous voluntary measure included regulation of advertising though various media channels, including online and broadcast advertising, direct marketing, product placement, sponsorship and marketing in/around schools – among the most comprehensive coverage in Europe (WCRFI, 2023[167]).
Chile
A policy package implemented in Chile included a restriction on child-directed marketing of unhealthy foods. A Chilean study examining cereal packaging following the ban found that cereals classified as unhealthy had accordingly reduced their use of child-directed marketing strategies, such as use of characters, children and child-like figures, cartoons or references to children’s daily lives and games. Meanwhile, cereals not classified as unhealthy had, in contrast, increased such practices (Mediano Stoltze et al., 2019[168]). This finding indicates that the regulation was effective in favourably changing the types of products designed to appeal to children.
A recent review (Lobstein, 2023[164]) found that voluntary industry-driven limitations on advertising are challenging to oversee, are subject to swift changes or removal, and could exacerbate health disparities if weakened or eliminated. It suggested that statutory or coregulatory measures can be more effective. A WHO-UNICEF-Lancet Commission (Clark et al., 2020[169]) and a review (Galbraith‐Emami and Lobstein, 2013[170]) also concluded that advertising restrictions relying on self-regulation by the industry can be insufficient to affect children’s exposure to food advertising, largely due to a lack of compliance. The WCRFI (2023[160]) suggests that enforcing mandatory regulatory measures that affect various media platforms could have a significant positive impact on health.
Comprehensive policy design should take a broader approach to marketing restrictions, and should address areas including sponsorships, point-of-sale settings, marketing through product design and packaging, and location – such as restricting advertising around schools. Claims made in advertisements should be regulated, requiring them to be based on evidence and led by health-promoting motives (WCRFI, 2023[160]). Given the range of marketing media used by young people, regulation of television advertising alone is insufficient to prevent exposure. To address targeted advertising, policies may need to restrict paid content in posts generated through web-based communities and influencers (Kelly, Bosward and Freeman, 2021[171]). A comprehensive approach should consider both the types of foods whose marketing should be restricted and the techniques and channels through which marketing can take place. The Joint Research Centre has developed a toolkit providing guidance to countries on implementing well-designed codes of conduct to restrict marketing of food and beverages, comprising a checklist of the main aspects and specific actions that a comprehensive marketing code should include, and emphasising the importance of addressing digital marketing due to its cross-border nature, which requires collaboration (Grammatikaki et al., 2019[172]).
Food labelling works to affect consumer choice, but is most effective across population groups when simple intuitive labels are used widely
Food labels inform buyers about the nutritional content of foods – commonly including energy content, salt, sugar and saturated or trans fat content, or healthy aspects like amount of dietary fibre. Empowering consumers to make well-informed decisions, labelling schemes for prepackaged foods and menus are effective to reduce consumption of unhealthy foods, leading to overall improvements in the nutritional quality of diets (WHO, 2015[173]; OECD, 2019[154]). Labelling can act as an incentive for food companies to reformulate their products through decreases in energy density or sugar and salt content, to fit into healthier categories (Ni Mhurchu, Eyles and Choi, 2017[174]; Nohlen et al., 2022[175]); this can be cost-effective as a measure to improve population health. Mantilla Herrera et al. (2018[176])suggest that gains can be substantially larger for mandatory than for voluntary programmes. Key policy levers include mandating back- or front-of-pack labelling; on-shelf labelling; calorie, nutrient and warning labels on menus; and regulations on nutrient and health claims. It is vital that health claims on packaging are evidence‑based: evidence from Chile has found a higher prevalence of general health claims, child-directed characteristics and nature/fruit references on packaging of less healthy products (Stoltze et al., 2018[177]).
In the EU27, Regulation (EU) 1169/2011 on the provision of food information to consumers came into effect in 2014. An obligation to provide nutrition information has applied since December 2016, mandating energy value, fat saturates, carbohydrates, sugars, protein and salt content to be listed on prepackaged foods in a legible tabular format, often provided on the back of food packaging (OECD, 2019[154]; European Commission, 2023[178]). Nevertheless, the majority of consumers do not make optimal use of back-of-pack labels, as these can be hard to see and complex, and it takes time and effort to make informed choices (Nohlen et al., 2022[175]). The Regulation also allows countries to recommend front-of-pack (FoP) nutrition labelling to help consumers identify healthier foods – a key priority of the WHO Food and Nutrition Action Plan 2015‑20 (WHO, 2015[173]). Simple intuitive FoP labelling is more effective than back-of-pack labels, and is estimated to decrease average daily caloric intake by 1.16% (OECD, 2019[154]). It is generally valued by consumers as a quick and easy way to acquire nutrition information when making purchase decisions (Nohlen et al., 2022[175]). Informative FoP food labels have been shown to regulate cognitive biases arising from health claims on packaging better than back-of-pack labels, which are only effective if the consumer chooses to take the time to view and interpret them (Talati et al., 2017[179]). The WCRFI (2023[160]) recommends that labels should contain both positive and negative information. Across EU+2 countries, none have mandatory FoP labelling schemes, though 12 apply voluntary ones (see Table 3.3). Further, Finland, Ireland and Slovenia have implemented menu labelling in restaurants (WCRFI, 2023[167]).
In the choice of a harmonised FoP labelling system, the European Public Health Association (EUPHA) and IARC recommend a simple graded traffic-light labelling system such as the Nutri-Score, which is in use in several European countries (Box 3.7) (EHPHA, 2023[180]; IARC, 2021[181]). The second most common labelling system in Europe is the Keyhole marking for healthy products, established in Sweden in 1989 and subsequently adopted in Denmark, Lithuania, Norway and Iceland. While evidence of the impact of food labelling on people with low socio‑economic characteristics is scarce and inconclusive (Løvhaug, Granheim and Djojosoeparto, 2022[182]), some findings suggest that people from all socio‑economic groups are more likely to pay attention to simplified, colourful and evaluative summary FoP labels such as Nutri-Score than to more complex back-of-pack labels (Nohlen et al., 2022[175]; Shrestha et al., 2023[183]).
Box 3.7. The Nutri-Score is used in various European countries
In 2017, Santé Publique France developed an official non-compulsory “Nutri-Score” food label, which provides easy-to‑understand information on the overall nutritional quality of food products. In 2020, nearly 60% of people reported that they had modified aspects of their food purchasing behaviour with the help of the label (Santé Publique France, 2021[184]). Building on this experience, Belgium, France, Germany, Luxembourg, the Netherlands, Spain and Switzerland established a cross-country co‑ordination mechanism in 2021 to adopt a single Nutri-Score label, although not all have implemented it at a national level. One of seven labelling programmes currently in use in the EU27, the Nutri-Score is the only programme meeting the recommendations for 1) use of colour to increase salience and draw attention; 2) simplicity for easy interpretation; and 3) a clear grading structure summarising information on both positive and negative nutritional aspects (EHPHA, 2023[180]). The nutrient profile system that underlies the Nutri-Score is considered to be the most validated and the easiest to compute. It takes into account several nutrients known to be involved in the development of obesity and chronic diseases, including cancer (IARC, 2021[181]). An experimental study found that in the 12 countries examined, the Nutri-Score was associated with the highest objective understanding by consumers (Egnell et al., 2020[185]), although there are calls to revise the Nutri-Score to ensure that the algorithms behind it place heavier penalties on ultra-processed foods (Eureporter, 2023[186]).
Reformulation can affect the whole food supply, but programmes need to contain sufficient (dis)incentives to induce compliance
The nutritional quality of foods available for sale forms the core foundation of the whole food environment. Reformulation through a variety of measures to create a healthier food environment is considered among the most cost-effective strategies to drive consumers to adopt healthy food choices, as it does not require behaviour change and simultaneously targets all consumers (Lehmann et al., 2017[187]). Many countries have instituted standards to regulate food composition, including mandatory or voluntary restrictions limiting or removing specific nutrients in food products. An EU-level pilot project to monitor the effectiveness and progress of reformulation efforts was implemented recently, resulting in the development of the EU Food and Beverages Labels Explorer (FABLE), which allows consumers and policy makers to monitor the nutritional quality of foods on the shelf easily across countries and over time (European Health and Digital Executive Agency, 2022[188]).
The only EU-wide mandatory regulation on nutritional content to date is Regulation (EU) 2019/649, which entered into force in 2021 and mandates limits on trans fat in foods. Eight countries also implement mandatory salt limits for bread – the most targeted food globally for salt reduction (Trieu et al., 2015[189]). In addition, Greece and the Netherlands extend mandatory salt limits to other selected products. The majority of limits and reduction targets in Europe, however, are voluntary: 21 of the EU+2 countries have voluntary agreements with industry on specific nutrients. Sodium (in salt) remains the most commonly targeted nutrient (19 countries), followed by sugar (16 countries) and total fat (9 countries). Eight countries have not implemented any nutrient limits or targets, apart from the Regulation on trans fat (Figure 3.10).
Figure 3.10. Country-specific limits or reduction targets have been set for food producers on selected nutrients
Note: The EU-level mandatory limit on trans fat, which applies in all EU+2 countries, is excluded. Most voluntary reduction targets apply to a specified range of products. 1 Policy applies to bread or cereal products. 2 Policy applies to a wider specified range of products. 3 Policy is implemented in the Wallonia region (Belgium) only.
Source: This material has been reproduced from the World Cancer Research Fund International NOURISHING policy database https://policydatabase.wcrf.org and nutrition policy index https://www.wcrf.org/policy/nutrition-policy/. Data for Cyprus, Iceland and Luxembourg data are cross-checked with the WHO Global Database on the Implementation of Nutrition Action (GINA), https://extranet.who.int/nutrition/gina/en.
A review suggests that the impact of reformulation policies is greater when they are mandatory, aligned with other regulations, and thoroughly monitored and evaluated to continuously engage the food and drinks industry (Vandevijvere and Vanderlee, 2019[190]). Similarly, studies have found that, to be effective, voluntary agreements may need to include ambitious targets, independent monitoring mechanisms, and disincentives for non-participation or non-compliance (Bryden et al., 2013[191]; Durand et al., 2015[192]). Further supporting the case for stronger agreements, Durand et al. (2015[192]) suggest that voluntary restrictions without appropriate measures to increase compliance may lead to competitive disadvantages for companies that apply them, whereas mandatory restrictions would level the playing field, removing a barrier to establishing a healthier food supply.
Public mass media campaigns promoting healthy nutrition target the whole population, but may fail to be effective across all population groups
While health-promoting mass media campaigns are effective to disseminate messages that help prevent non-communicable diseases – including cancer – they should go hand in hand with ensuring that sufficient healthy options are both financially and geographically accessible for those wishing to take advantage of them (WHO, 2023[193]). A review found that interventions aimed at affecting the individual, including educational campaigns, were most likely to be effective among people with higher levels of education and income, and were thus likely to contribute to widening inequalities, despite benefits at a population level (McGill et al., 2015[194]). Equity-promoting communication campaigns can thus be more effective if they specifically include avenues and measures to target at-risk populations (Box 3.8), and go hand in hand with measures that modify the food environment. Although mass communication campaigns on healthy nutrition have been implemented in most EU+2 countries, few include measures to reach specific population groups. Only seven countries have implemented measures to direct communication at young people. For example, using techniques from social marketing, in 2021 the Danish Veterinary and Food Administration collaborated with an influencer who shared sponsored content on YouTube to inspire young people to adhere to the Danish dietary guidelines (WCRFI, 2023[167]).
Box 3.8. Scotland (United Kingdom) and Oregon (United States) reach out to populations with lower socio‑economic characteristics through communication campaigns
Scotland, United Kingdom
Scotland has developed a resource called “Eat Well Your Way”, using methods derived from behaviour change techniques to help consumers make small manageable changes towards better nutrition. Designed to be accessible, the advice can be adapted to the user’s circumstances, and is aimed at populations with lower incomes who need the most support. The materials developed consider possible increases in financial difficulties due to high inflation, providing advice on affordable ways to improve diets (Food Standards Scotland, 2023[195]; WCRFI, 2023[167]).
Oregon, United States
The state of Oregon in the United States uses a targeted social marketing campaign called “Food Hero” to help people with low incomes increase their consumption of vegetables, fruit and home‑cooked family meals. Developed through a needs assessment using focus groups and phone surveys, Food Hero uses toolkits, a website (with recipes and culturally adapted resources to reach migrant populations), a newsletter, social media, traditional media and grocery store communications – all available in English and Spanish – to reach its target audience (Tobey et al., 2011[196]; Oregon State University, 2023[197]). Evaluations suggest favourable changes in perceptions of healthy food preparation as time‑consuming and of a fruit- and vegetable‑rich diet as expensive (Tobey et al., 2016[198]), and in practices in home‑based meal preparation (Tobey et al., 2017[199]) and teaching about nutrition in schools (Kirk et al., 2020[200]).
Schools can set patterns for healthy nutrition across the life‑course
Children spend a large proportion of their time in school, which provides an opportunity to improve nutritional habits and knowledge. Such efforts include providing healthy school meals and beverages free of charge or at affordable prices, distributing nutrition education materials and setting standards for food products available in or near schools (OECD, 2019[154]; WHO, 2020[165]). A systematic review found that school-based food interventions can result in a significant improvement in targeted dietary behaviours – such as fruit and vegetable intake, total and saturated fat consumption, and sodium consumption – both in and outside the school environment (Micha et al., 2018[201]). Interventions encompassing various strategies, including nutrition education and involvement of parents and teachers in promoting healthy eating habits, have been shown to improve students’ dietary behaviours and knowledge significantly (Evans et al., 2012[202]).
All EU+2 countries with available information apply standards for school meals, and 19 have mandatory standards (see Table 3.3). Nevertheless, a Slovenian study found that although the country has mandatory guidelines for school meals, not all schools had adapted their menus to adhere to these. Better menus were linked to higher socio‑economic status of the municipality and to larger schools, which found it easier to purchase high-quality products within their budgets (Gregorič et al., 2015[203]). This highlights the importance of programme evaluation and emphasises that guidelines alone may not be sufficient to result in changes in practice when financial barriers are not addressed. Additionally, as school meals may present a significant cost to families with lower incomes, it is important to ensure that all children benefit from the measures. Estonia, Finland and Sweden finance school meals from the state budget, making them free of charge in all primary and secondary schools; Hungary and Latvia do so in primary schools only. France and Germany implement universal subsidies, while ten other European countries provide free meals based on specific criteria, such as family income, or to specific target schools (WCRFI, 2023[167]).
Following best practices, Hungary and Romania regulate all food available in schools, including beyond school hours and at school events not held on school premises (WCRFI, 2023[167]). Only one EU+2 country has implemented national standards on food in the immediate vicinity of school (Romania, in 2020), while 12 have implemented voluntary or mandatory measures limiting sugar-sweetened beverage provision in schools (see Table 3.3). Since 2006, Latvia has prohibited distribution of soft drinks, sugar confectionery and salty snacks in schools; in 2012, the country set more in-depth criteria to determine which foods are prohibited, limited or encouraged in public institutions, according to nutrient content. These standards apply to foods and beverages served in schools, hospitals, social care and rehabilitation institutions, pre‑school canteens and cafeterias.
Promoting physical activity in disadvantaged groups requires the use of school, work and leisure settings
Given the relevance of physical activity for cancer prevention – both independently and through associations with body weight and other risks – promotion of physical activity must take place through multiple channels. These include setting-specific programmes in schools, workplaces and the healthcare system; policies to increase access to sports facilities; urban design, environment and transport policies; and communication and information policies (see also discussion of active transport in Section 3.3.4).
Availability of and participation in physical education in school settings has been shown to make children more active in, outside and beyond school, and to contribute to healthy lifestyles that last into adulthood (Dohle and Wansink, 2013[204]; Black et al., 2019[205]). Particularly important given the rise of overweight and obesity among adolescents (see Section 3.2.1), school-based programmes promoting a healthier diet in conjunction with additional physical activity were found to lead to an overall mean reduction in children’s BMI of 0.3 kg/m2 (Wang et al., 2015[206]); this can also have beneficial effects for cancer prevention throughout the life‑course. Physical activity can be promoted in the school setting through a whole‑of-school approach, encouraging inclusion of physical activity lessons in curricula, active recess, active lessons and active transport to and from school – as in Estonia (Box 3.9). Although all EU27 countries mandate inclusion of physical activity classes in school curricula, there is considerable variation in how it is defined, quantified, perceived and assessed in schools (OECD/WHO, 2023[37]). Government-level support for active transport to and from school is available in 12 EU+2 countries (Section 3.3.4).
Given the large proportion of time most adults spend at their jobs, workplaces can similarly be effective settings to influence lifestyles (Proper and van Oostrom, 2019[207]). Workplaces across OECD countries have started to implement structural changes such as introduction of standing desks or incentives to take the stairs, as well as specific wellness programmes entailing health risk assessments, education materials, classes, seminars, group activities and counselling on healthy lifestyles (OECD, 2019[154]). These are particularly important for desk-based work, where employees are sedentary much of their day; for companies, the initiatives can reduce absenteeism and increase productivity at work (OECD, 2022[144]). Government-level support for active transit to and from work is provided in 12 EU+2 countries (Section 3.3.4), and Ireland collaborates with employers to promote active forms of transport (Box 3.9). However, equity impacts of workplace‑based interventions should be considered to ensure that the benefits can be gained by workers with different roles and levels of education. A qualitative study about employees’ interest in workplace programmes found that those with a medium level of education expressed higher levels of interest than those with low levels (Sponselee et al., 2022[208]). This highlights the importance of adapting interventions to specific settings and population groups to ensure equitable benefits, ideally taking into account the views and articulated needs of target groups and co-developing the interventions. High participation is crucial to maximise the effectiveness of interventions (OECD, 2022[144]).
Box 3.9. Initiatives in Estonia and Ireland take a comprehensive approach to engage schools and workplaces in physical activity
Estonia, Schools in Motion Programme
In Estonia, the Schools in Motion Programme takes a whole‑of-school approach, covering physical education, active recess, active lessons and active transport to and from school. Participating schools, comprising 28% of all general education schools in 2021, are supported through seminars, workshops and skills training, and have access to easy-to‑use materials and research – for example, tips on how to make the indoor and outdoor environment more physical activity friendly, and techniques for reducing sedentary time during classes. Using these resources, each school can develop and adapt their own action plan.
Ireland, promotion of sustainable travel to and from workplace
In Ireland, the National Transport Authority manages the Smarter Travel Workplaces and Smarter Travel Campus behavioural change programmes on behalf of the Department of Transport, Tourism and Sport. These initiatives collaborate with major employers and higher education institutions to promote sustainable commuting and travel choices through materials such as promotional posters on the benefits of walking and guidance on setting up initiatives, while larger organisations can qualify for development of specific action plans, using online travel surveys and analyses.
Source: OECD/WHO (2023[37]), Step Up! Tackling the Burden of Insufficient Physical Activity in Europe, https://doi.org/10.1787/500a9601-en.
It is important to note that a comprehensive package of policies is needed to target all drivers of physical activity across daily life, including school, work, transport and leisure. A comprehensive approach must consider the built environment that supports physical activity, drawing on synergies with air pollution policies, such as active transit interventions and infrastructure (see Section 3.3.4). Another important avenue for increasing physical activity is increasing access to sports facilities – particularly for disadvantaged communities or the elderly population (OECD/WHO, 2023[37]). Of the 29 EU+2 countries, 20 have specific physical activity policies that target groups with a particular need (e.g. children, elderly people, those with low socio‑economic status, people with disabilities and ethnic minority groups). For example, in 2018, Hungary implemented an EU-funded project to set up 850 sports programmes nationwide, with a key aim to improve quality of life of the population in less developed regions. Similarly, Italy’s Sport for All Project aims to guarantee access to sport for children and families experiencing economic disadvantage, to encourage children to engage in physical activity, and to support sports clubs and associations (WCRFI, 2023[167]).
The role of primary care and involvement of communities in promoting physical activity and healthy nutrition
Primary healthcare constitutes the first level of contact for most people with the healthcare system and brings healthcare closer to places where people live and work. It is key to improving population health and equity. By providing a wide range of services (including health promotion and disease prevention), estimates suggest that primary healthcare can address more than 80% of people’s health needs, delaying the onset of diseases and reducing mortality rates (OECD, 2020[209]; OECD, 2022[210]). Promising strategies to support behaviour change include promotion of healthy nutrition and physical activity in primary healthcare though counselling or physical activity prescribing.
Physical activity on prescription programmes exist in 10 EU+2 countries, although policy design varies. Key healthcare‑mediated interventions to promote healthy lifestyles include the EU Physical Activity on Prescription (EUPAP) model in Sweden (EUPAP Consortium, 2020[211]). Since the early 2000s, Sweden has been implementing this intervention programme, including person-centred individualised counselling, written evidence‑based physical activity recommendations, follow-up and community support. All healthcare professionals are licensed to prescribe physical activity. The programme is considered a good way to increase levels of physical activity in the target population, reaching individuals from various socio‑economic groups. In Slovenia, the Netherlands and Portugal, various programmes have been designed with the aim of improving lifestyles, including some that specifically do so through integration with community-based services (Box 3.10).
Box 3.10. Healthcare provider- and community-based initiatives are in place in Slovenia, the Netherlands and Portugal
Integration between primary care and community services, Slovenia
Slovenia took action in 2002 to integrate population and individual services by creating health promotion centres in all primary healthcare centres across the country. GPs were tasked with providing preventive check-ups and referring at-risk patients to health promotion centres for free lifestyle interventions against key risk factors. Owing to persistent inequalities between regions, genders and socio‑economic groups, the centres’ capacities were expanded during 2013‑16, as cross-sectoral partnerships with various stakeholders were established, including social services and non-governmental organisations at the local/community level. This led to adoption of local health promotion strategies and action plans to target population groups and reduce health inequalities.
Through the programme, more than half of Slovenia’s population had been screened for lifestyle and risk factors by 2019, while almost 50 000 patients take part in the lifestyle interventions run by the health-promotion centres annually. Rates of preventable mortality have declined at a population level. Slovenia’s approach was enabled by strong public health and governance structures, along with accountability mechanisms that monitored outcomes and took corrective action when necessary. Building on this experience, the services could be used to advance health literacy (see Section 3.3.6), transferring skills and knowledge to patients via mechanisms including the introduction of lay educators, group workshops among patients with chronic diseases (supervised by members of the primary care team) and telemedicine, including webinars (Petric et al., 2019[212]; Susič and Klemenc-Ketiš, 2020[213]).
Combined Lifestyle Intervention, Netherlands
The Combined Lifestyle Intervention Programme is designed to help people living with overweight and obesity, and addresses risk factors such as stress or sleep disorders. Participants are referred by their GP to a local Programme, where they receive dietary advice, physical activity training and counselling on behavioural change over a period of two years. The intervention has been found effective and often cost-effective, with a strong evidence base. It demonstrates effectiveness among diverse socio‑economic backgrounds, although noticeable variations in its implementation exist across regions (OECD, 2022[35]).
Nutrition and physical activity evaluation and promotion through primary care, Portugal
Portugal is leveraging its national healthcare system to deliver brief counselling on nutrition, and counselling and prescription of physical activity. Training is provided to health professionals, including an online course on communication, while the electronic health systems of the national health service are integrated with a brief nutrition counselling intervention to promote its effective implementation in primary care. Based on a brief assessment of levels of physical activity and sedentary lifestyle, the GP can give brief counselling on physical activity tailored to each patient, and a programme is implemented by trained GPs and exercise physiologists to create personalised prescriptions for people with certain chronic conditions. Portugal also participated in the pilot EUPAP project among ten countries aiming to transfer and adapt the Swedish model. The pilot will finish in 2024, after which next steps will be decided from evaluations. A new model for management of obesity in primary healthcare is being developed to complement current approaches of management integrated into hospital care (Ministry of Health, 2020[214]; 2022[215]).
3.3.4. Policies are required to reduce environmental exposure to carcinogens
Recognising its cross-border implications, the EU is co‑operating to reduce particulate matter pollution
Air pollution constitutes an important cancer risk – the vast majority stemming from ambient PM (see Section 3.1.4). The EU has collaborated on reducing PM for decades, including measures such as the 2008 Air Quality Directives, the 2016 National Emissions Commitments and recently the European Green Deal. A range of EU-level source‑specific emissions directives, policies and programmes are under way to help achieve the targets set; thanks to various regional, country and local initiatives, there was an overall reduction in emissions in the EU27 by 30% for PM10 and 32% for PM2.5 between 2005 and 2020 (EEA, 2022[216]), corresponding to a reduction in pollution exposure estimates (see Section 3.2.2). In September 2023, the European Parliament voted to align air quality standards in the EU27 with WHO’s guidelines (5 ug/m3 for PM2.5 and 15 μg/m3 for PM10) to take effect in 2035, if approved by the European Commission and European Council. The new targets would be substantially stricter than the current (2023) standards of 25 ug/m3 for PM2.5 and 40 μg/m3 for PM10. In 2023, countries in the European region also adopted the Budapest Declaration, which emphasises inclusion of equity and sustainability in addressing environmental determinants of disease, and makes a commitment to strengthen inter-linkages between environmental and health policies (WHO, 2023[217]).
Under the 2016 National Emission Ceiling Directive, which sets country-specific PM2.5 reduction targets, only Hungary and Poland failed to meet their requirements by 2021. To meet the country-specific 2030 PM2.5 targets set in the Directive, 20 countries need a reduction in PM2.5 of 10% or more – including Hungary, Poland and Romania, which must cut their PM2.5 emissions by more than half to reach their targets. The main contributors to PM2.5 pollution in Europe are residential heating and cooling, industry, road transport and agriculture.
Residential heating and cooling is the largest contributor to premature mortality from PM2.5 pollution in European countries (Khomenko et al., 2023[218]). Thus, changes in the energy sector will be crucial for meeting the emission reduction commitments for PM2.5, as burning of solid biomass and fossil fuels for residential heating constitutes a substantial part of emissions in some countries. The EU is using several tools to address the challenge of residential energy use, including regulating product emissions and requiring standardised energy efficiency labels on consumer appliances. It is also promoting more energy-efficient solutions, such as upgrading heating systems and improving insulation through renovations (European Commission, 2016[219]), and aims to renovate 35 million buildings by 2030 (European Commission, 2023[220]). Several EU-wide measures exist to address emissions and pollution from other sectors, including agriculture, industry, transport, energy and shipping.
Country and local-level policies promoting healthy living and active transport address particulate matter pollution and benefit health via other pathways
The European Commission’s flagship campaign to promote sustainable urban transport and the European Mobility Week consists of a week-long annual event wherein cities and towns engage in initiatives to promote non-car transit. Beyond this critical EU-wide co‑operation, individual countries are implementing their own initiatives to combat air pollution, including moves to make public transit more affordable or free in recent years (Table 3.4). Luxembourg and Malta offer free nationwide public transit, and Austria, Cyprus, the Netherlands and Germany offer affordable nationwide tickets, valid across most modes of transit. Some capitals have gone beyond national-level policies to offer free (Tallinn, Estonia) or very affordable (Prague, Bratislava, Madrid, Rome, Vienna and several others) public transit (Greenpeace, 2023[221]). In addition to reductions in air pollution, investment in public transit contributes to reductions in noise exposure, increases in safety for pedestrians and cyclists, and additional space for communities.
Table 3.4. Affordable public transit networks are in place in some EU+2 countries
|
Free nationwide public transit |
Availability of long-term network tickets valid on all or most transit modes in the country |
Good ticket affordability (less than EUR 3/day) |
Capitals with lowest-cost public transit |
|---|---|---|---|
|
Luxembourg |
Luxembourg |
Luxembourg |
Tallinn, Estonia* |
|
Malta1 |
Austria |
Malta1 |
Luxembourg City, Luxembourg* |
|
– |
Cyprus |
Austria |
Valletta, Malta* |
|
– |
Netherlands |
Germany1 |
Prague, Czechia |
|
– |
Malta1 |
Spain |
Bratislava, Slovak Republic |
|
– |
Germany1 |
– |
Madrid, Spain |
|
– |
– |
– |
Rome, Italy |
|
– |
– |
– |
Vienna, Austria |
Note: Capitals with * represent those with free public transit. Capitals in bold represent those with free or substantially discounted fares specifically for lower-income individuals (discounts for students, elderly people and those with disabilities not shown). Cities scoring at least 50 out of 60 points on Greenpeace’s scale of affordability of long-term tickets are included.
1. Malta’s free nationwide public transit system does not include express buses or the ferry between the two main islands, while Germany’s Deutschlandticket excludes long-distance trains.
Source: Greenpeace (2023[221]), “Climate & Public Transport Tickets in Europe”, Greenpeace, Vienna, https://greenpeace.at/uploads/2023/05/report-climate-and-public-transport-tickets-in-europe.pdf.
Figure 3.11 contains selected categories of results of a comprehensive scan of implemented national-level policies that encourage physical activity, which have synergies with reducing air pollution (see also Section 3.3.3). Present in most EU+2 countries, these initiatives range from national policies on cycling routes, lanes and greenways to public transit campaigns and tax-deductible tickets. Some countries (Bulgaria, Germany, Hungary, Ireland, Lithuania, Portugal, Slovenia and Sweden) have national policies focused on teaching or improving cyclist or pedestrian safety, targeting people as young as pre‑schoolers, schoolchildren and the general public (WCRFI, 2023[167]). Among EU+2 countries, 16 have national policies in place to support walking or cycling infrastructure, 6 of which do so via legislation or regulation, placing a legislative emphasis on their implementation. National governments have mass communication campaigns in place to promote public or active transit in 8 countries, and 17 have national programmes to support active transit to and from school or work, including financial incentives in some. Box 3.11 outlines some of the best practices in OECD countries.
Figure 3.11. Governments are taking action to support public and active transit
Note: Includes nationally implemented initiatives only. Cyprus and Iceland not included.
1. Based on sources from the Luxembourg Government (2023[222]; 2021[223]; n.d.[224]).
Source: Where not otherwise stated, this material has been reproduced from the World Cancer Research Fund International MOVING policy database https://policydatabase.wcrf.org and physical activity policy index https://www.wcrf.org/policy/physical-activity-policy/.
Box 3.11. Lessons learned on active transport from the OECD’s Healthy Cities report
The OECD’s Healthy and Sustainable Cities – Best Practices in Public Health report examines 13 case studies of urban initiatives that promote healthy lifestyles, some of which deliver additional benefits through synergies with air pollution. Associated with the lowest cost of cycling initiatives, the “CycleOn” bike safety education programme in the Netherlands targets the elderly population. Cycling infrastructure changes include Copenhagen’s smart traffic signals that uses real-time information from commuters to prioritise cycling and buses, Île‑de‑France’s bike sharing programme, and Denmark’s Cycle Superhighways that develop safe biking routes between municipalities. Cycle Superhighways, which started in 2012 and currently form the largest network of cycling roads in Europe, have led to a substantial increase in bicycle traffic on specific highways.
Modifications to the urban environment such as Barcelona’s “Superblocks” transform multi-block areas into communities promoting public space and mobility. “The People First Cities” initiative transformed Pontevedera, Spain into a pedestrian city and the Cycling Cities Programme redesigned urban space in Utrecht, the Netherlands to promote active transport. Other places are looking at making car transit more expensive (as Stockholm does with its congestion charge) or alternative transport more affordable, as done via Belgium’s nationwide programme providing financial incentives to cyclists and Portugal’s programme to reduce public transit costs.
The OECD estimates that scaling up of these interventions would result in major health and economic benefits to OECD countries, with much of that impact (between 60% and 99% depending on the intervention) arising from the decrease in air pollution rather than the increase in physical activity. Among these 13 interventions, five have been scaled up across different cities in the same country while two have been scaled up nationwide.
Source: OECD (forthcoming[225]), Healthy and Sustainable Cities: Best Practices in Public Health.
Cities have a major role to play in population health, particularly when it comes to reducing road transport pollution
With about half of the OECD population living in urban areas and the increasing trend towards urbanisation, the OECD has identified three major health-related challenges that cities must address: lower levels of physical activity (see Section 3.3.3), greater exposure to air pollution, and urban heat islands – referring to accumulation of heat in cities (OECD, forthcoming[226]), for which local-level policies are becoming particularly important.
The EU has worked to identify best practices as well as barriers and facilitators to improving air quality in cities. These include the Air Implementation Pilot Study on 12 cities and the Urban Innovative Actions Project, which funded the testing and implementation of new solutions to existing issues in cities. The Green City Accord is an EU initiative wherein cities commit to improving five areas of environmental management, including air pollution, reporting on their progress every three years (European Commission, 2023[227]).
European cities are implementing transport-related interventions, recognising this as a major pollution source. A database of peer-reviewed studies on urban interventions targeting road pollution includes 93 policy interventions studied in EU+2 countries (Khreis et al., 2023[228]). A review of the database shows that regulatory tools (such as speed limit reductions/regulations, low-emission zones, vehicle replacement programmes and vehicle use restrictions) and alternative fuel technology (such as promoting a switch to electric or compressed natural gas) are being considered to address air pollution. Studies that estimated the impact of interventions on either PM2.5 or PM10 exhaust emissions from vehicles or traffic-related air pollution reported reductions.
It is important to select or design air pollution interventions conscientiously to make them equity-promoting
Groups with lower socio‑economic characteristics may be more exposed to air pollution – as they have less access to cleaner fuel options for heating and transportation – and at greater risk of exposure to occupational hazards (see Section 3.2.2). On the other hand, as people with higher incomes often choose to live in city centres in many cities, they may also be more exposed to urban air pollution (OECD/EU, 2020[15]).
Certain air pollution reduction policies, such as subsidies for public transit and provision of school buses, can promote equity by reaching different populations (Public Health England, 2019[229]). School-related interventions can be targeted to reach either broad or specific population groups, given that children spend up to a third of their time in school. Interventions aimed at improving air quality for students during the school day include adding air purifiers in classrooms, implementing heating, ventilation and air conditioning systems with high efficiency filters, and using green infrastructure as physical barriers (Rawat and Kumar, 2023[230]).
Conversely, interventions such as increased taxes or fees, freight bans or subsidies on privately owned electric vehicles – which are generally owned by more affluent groups – can increase inequities. Low-emission zones, wherein more polluting vehicles are not able to enter certain areas, can also increase inequities: vulnerable groups are more likely to own such vehicles, while higher socio‑economic groups may be more likely to live in areas that benefit most from the improvement in air quality. For example, a study on two low-emission zones in Rome showed that more of the benefit in reduced emissions accrued to the better-off population, as they lived closer to the city centre (Cesaroni et al., 2012[231]). In contrast, however, a study assessing equity impacts of low-emission zones in Brussels indicated that the policy had both environmental and equity-promoting benefits because areas with low socio‑economic characteristics that were the most polluted were included inside the low-emission zone, and also had better public transit networks (Verbeek and Hincks, 2022[232]).
When specific policies are put in place that may be beneficial overall in terms of air pollution but have a risk of increasing inequities, proactive planning and adjustments can be made. Poland’s programme to upgrade residential heating systems include subsidies of up to 90% for low-income households. A 2022 updated programme design also includes better outreach to low-income individuals, an easier application process, and greater technical and implementation assistance (Karver, Badiani-Magnusson and Carroll, 2022[233]). Czechia’s new Green Savings Programme provides larger subsidies for low-income households – of up to 95% (as well as an advance) for households upgrading from solid fuel boilers to more sustainable solutions (IEA, 2022[234]).
The EU’s asbestos-free future plan aims to reduce occupational risk via stricter exposure limits, pre‑construction planning and increased awareness
The European Commission issued a communication on working towards an asbestos-free future in September 2022 that sets out a comprehensive, multi-pronged strategy to address risks arising from asbestos, to be implemented alongside Europe’s Green Deal and Europe’s Beating Cancer Plan. The pillars include enhancing cancer screening and diagnostics (Chapter 4) for people who may have been exposed to asbestos; preventing exposure through identification, logging and removal of asbestos in existing buildings; revising regulations around construction waste management; and providing financing to Member States to support these processes (European Commission, 2022[235]).
Furthermore, a major pillar of the Commission’s asbestos strategy relates to reducing occupational exposure. The 2009 Asbestos at Work Directive put in place stringent regulations on employers regarding training, planning and protection against asbestos. In June 2023, the European Parliament and Council reached an agreement on strengthening this regulation, including training and protective equipment, and a transition period during which countries will need to shift to a more modern and sensitive method for counting asbestos fibres (European Commission, 2023[236]). The new regulation also requires work involving demolition or asbestos removal to receive a permit from national authorities, and companies must obtain information on materials that could contain asbestos before beginning work in older premises. Workers who may be exposed must wear appropriate protective equipment and receive specified training, and countries must maintain registries of occupational-related asbestos disease (Council of the EU, 2023[237]). It is estimated that the new occupational exposure limit, alongside accompanying measures, may result in a decrease in excess life‑time cancer risk from 125 cases of cancer per 100 000 exposed workers (based on the current limits) to 12 cases per 100 000 (European Council, 2023[44]).
The European Commission’s communication gives some examples of best practices on asbestos, such as France’s legislative requirement that in cases of potential asbestos exposure, search and identification of materials that contain asbestos has to be undertaken prior to construction. Poland has maintained an asbestos database since 2013, and has a national programme for safe removal of asbestos, including information, training and monitoring. The Flemish Government in Belgium has undertaken various initiatives, including requiring owners of units built prior to 2001 to record any asbestos present, requiring asbestos removal as a precondition for solar panel installation and, from 2022, requiring certification detailing asbestos inventories and their safe management or removal prior to the sale of a building (European Commission, 2022[235]). Some countries have requirements around licensing or accreditation of asbestos-related work; for example, in June 2023, British Columbia became the first Canadian province requiring licensing for asbestos abatement contractors (Canadian Occupational Safety, 2023[238]). In geographical regions with higher asbestos presence (see Section 3.2.2), prioritisation should be given to identification and registration of asbestos stock prior to commencement of renovation work.
The EU Agency for Safety and Health at Work is undertaking a survey of workers to assess exposure to cancer risk factors in order to add to understanding of the burden of occupation-related asbestos diseases. Survey results will also help to inform an awareness-building campaign about safe removal of asbestos targeted at companies, workers, building owners and public administrators engaged in Europe’s renovation wave (European Commission, 2022[235]).
3.3.5. Prevention of cancers caused by human papillomavirus and hepatitis B and C can be strengthened in EU+2 countries
Human papillomavirus vaccination is offered free of charge for both girls and boys in most EU+2 countries
Historically, HPV vaccination recommendations have primarily targeted girls. In recent years, however, for reasons of gender equity and to support population-wide immunity, nearly all EU+2 countries have adapted their recommendations to include boys too. In contrast to issues experienced in the past, current HPV vaccine supplies available to European countries are sufficient to support broader access (WHO, 2022[239]). All EU+2 countries provide HPV vaccinations for adolescents as part of the national programme, generally targeting children around the age of 12‑13 (Table 3.5). Some programmes vaccinate from as early as age 9 (Austria, Germany, Greece, Malta and Poland). Catch-up vaccinations for older individuals are also provided in some countries, but coverage is generally limited by age, as the cost-effectiveness of HPV vaccination above age 26 has been found to be fairly low (Kim et al., 2021[240]). At least 19 of the 29 EU+2 countries have HPV vaccination registries, which support immunisation programme delivery with consistent and high-quality data (Table 3.5).
Although vaccination has been extended to boys in nearly all EU+2 countries, coverage remains lower than for girls owing to its relatively recent inclusion in many countries. In Iceland and Romania, vaccination was extended to boys in late 2023, while Bulgaria and Estonia have not yet extended their coverage to boys, although Estonia plans to do so in 2024. Certain at-risk populations may be targeted for vaccination as well, such as MSM (e.g. in France) (Petit and Epaulard, 2020[241]) or immunocompromised individuals (e.g. in Luxembourg and Spain) and sex workers (e.g. in Spain) (Colzani et al., 2021[21]).
Table 3.5. National HPV vaccination programmes differ across and sometimes within EU+2 countries
|
Country |
All children included in vaccination strategy |
Targeted age (primary vaccination) |
Catch-up |
Vaccine registry |
National school-based vaccination programme |
|
Austria |
✓ |
9‑12 |
Up to age 21 |
✗ |
✓ |
|
Belgium |
✓ |
11‑12 (Flanders) 13‑14 (Wallonia, Brussels) |
12‑18 (in Wallonia and Brussels regions) 1 |
✓ |
✗ (Flanders) ✓ (Wallonia, Brussels) |
|
Bulgaria |
Girls only |
12‑13 |
✗ |
✗ |
|
|
Croatia |
✓ |
14‑15 |
✗ |
✓ |
|
|
Cyprus |
✓ |
11‑12 |
Up to age 13 |
✗ |
✓ |
|
Czechia |
✓ |
13‑14 |
✗ |
✗ |
|
|
Denmark |
✓ |
12 |
Up to age 17 |
✓ |
✗1,2 |
|
Estonia |
Girls only 3 |
12‑14 |
✓ |
✓ |
|
|
Finland |
✓ |
10‑12 |
13‑16 (boys) |
✓ |
✓ |
|
France |
✓ |
11‑14 |
Up to age 19; 26 for MSM |
✗1 |
✓ |
|
Germany |
✓ |
9‑14 |
Up to age 18 |
✗1 |
✗ |
|
Greece |
✓ |
9‑12 |
Up to age 15 |
✗ |
✗ |
|
Hungary |
✓ |
13 |
✓ |
✓ |
|
|
Iceland |
✓ |
12 |
✓ |
✓ |
|
|
Ireland |
✓ |
12 |
Up to age 25 |
✓ |
✓ |
|
Italy |
✓ |
11‑12 |
Differs by region |
✓ |
✗ |
|
Latvia |
✓ |
12‑17 |
✓ |
✗ |
|
|
Lithuania |
✓ |
11‑12 |
✓ 1 |
✗ |
|
|
Luxembourg |
✓ |
9‑14 |
Up to age 20 |
✓ |
✗ |
|
Malta |
✓ |
9‑14 |
✓ |
✗ |
|
|
Netherlands |
✓ |
10 |
Up to age 26 |
✓ |
✗ |
|
Norway |
✓ |
12‑13 |
Up to age 20 |
✓ |
✓ |
|
Poland |
✓ |
12‑13 |
✗ |
✗ |
|
|
Portugal |
✓ |
10 |
Up to age 17 (to initiate schedule) up do age 26 (to finalise schedule)1 |
✓ |
✗ |
|
Romania |
✓ |
11‑18 |
✓ |
✗ |
|
|
Slovak Republic |
✓ |
12‑15 1 |
✗ |
✗ |
|
|
Slovenia |
✓ |
12‑13 |
✓ |
✓ |
|
|
Spain |
✓ |
11‑12 |
✓ |
✓ |
|
|
Sweden |
✓ |
11‑12 |
Up to age 18 (girls) |
✓ |
✓ |
1. Based on comments from the EU Expert Thematic Group on Cancer Inequalities Registry.
2. Some local and regional school-based vaccination programmes are implemented in Denmark.
3. Estonia plans to expand the vaccination programme to boys in 2024.
Source: 2023 OECD Survey on Cancer Care Performance, OECD (2023[131]), EU Country Cancer Profiles, EPF, (2023[242]), European Parliamentary Forum for Sexual and Reproductive Rights, Brussels, https://www.epfweb.org/sites/default/files/2023-06/HPV%20Atlas_EN%202023-JUN19.pdf; Colzani, E. et al. (2021[21]), “Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma”, https://doi.org/10.2807/1560-7917.ES.2021.26.50.2001659; European Cancer Organisation (2022[26]), “Putting HPV on the Map: The state of HPV prevention programmes in the WHO European Region”, https://www.europeancancer.org/resources/256:hpv-prevention-programmes.html; Likumi (2023[243]), Amendments to the Cabinet of Ministers 2000. Regulation No. 26 of September 330 of “Vaccinations regulations”, https://likumi.lv/ta/id/341661; Ministry of Health of Romania (2023[244]), Order no. 3120/2023 for the approval of the population segments that benefit from the prescription, release and settlement under the compensation regime of immunological drugs used to produce active immunity or used to prevent communicable diseases.
Two- or three‑dose regimens are most common. In April 2022, the WHO Strategic Advisory Group of Experts – incorporating evidence on the effectiveness of a one‑dose regimen from an IARC study of over 15 000 vaccinated girls – recommended that for girls under age 20 either one or two doses could be given (WHO, 2022[245]; IARC, 2023[246]). Among OECD countries, Australia, the United Kingdom (England), Ireland and Mexico have already adopted the change to a one‑dose regimen, which greatly simplifies and lowers the cost of vaccination programmes (IARC, 2023[246]). This has potential to increase coverage, as coverage with the last dose of vaccine is typically lower than with the first. According to the 2023 OECD Policy Survey on Cancer Care Performance, other countries actively considering this change include Canada, Costa Rica and Slovenia. The potential transition to a one‑dose regimen will facilitate reaching vulnerable populations for completing HPV vaccination, particularly for more rural populations or people with low socio‑economic status, who have less access to or personal availability to attend preventive health visits.
The PartnErship to Contrast HPV (PERCH), a Joint Action Project funded by the EU, brings together 18 countries with the objective of raising vaccination rates in regions with low coverage by sharing knowledge and experience; improving data and monitoring systems; and improving knowledge, awareness and abilities of both the general public and healthcare professionals about HPV vaccination, aligning with WHO’s strategy to achieve 90% HPV vaccination coverage by the age of 15 (PERCH, 2023[247]). With a strong regulatory commitment and high trust in the healthcare system, Portugal has achieved some of Europe’s highest rates of HPV vaccination (Box 3.12).
Box 3.12. Portugal has achieved very high HPV vaccination coverage among girls
HPV vaccines have been included in Portugal’s National Vaccination Programme since 2008. The Programme has achieved high vaccination rates among girls, reaching 94% coverage of the last dose in 2022 among 15‑year-old girls according to data from the WHO (see Section 3.2.3). It was expanded to include boys in October 2020. Several factors have contributed to its success, including meticulous regulatory oversight and efforts to increase accessibility and affordability, supported by a strong foundation of trust within the healthcare system, as well as favourable perceptions about the importance, effectiveness and safety of the vaccine (see Section 3.2.3).
One of the key ingredients for this success is a high level of public commitment surrounding the National Vaccination Programme. The programme is overseen by the Directorate‑General of Health, which provides technical guidelines, ensuring adaptability to new vaccines, evolving disease epidemiology and societal changes. Accessibility of HPV vaccination is another factor leading to the National Vaccination Programme’s success: all vaccines are free of charge for target populations. National campaigns to disseminate and promote information about HPV vaccination have also helped to improve accessibility among the general population, further fostering a strong immunisation culture.
Source: EU Expert Thematic Group on Cancer Inequalities Registry.
Clinician recommendations and reminders from healthcare providers have an indispensable role in supporting human papillomavirus vaccination
One mode of HPV vaccination delivery is in facilities such as vaccination centres or primary healthcare centres. In all EU+2 countries, doctors and nurses can provide HPV vaccination. The crucial role of healthcare provider recommendations has been recognised as a key lever for parental decisions to vaccinate, as has the importance of provider communication regarding HPV vaccination. Available studies suggested that clinician recommendation is often the top reason parents choose to vaccinate their children (Polonijo, 2020[248]), even in cases of initial hesitancy (Public Health Agency of Canada, 2022[249]). Ultimately, the CDC recommends that healthcare providers are well-informed to advise on and answer questions regarding the HPV vaccine. It also suggests that healthcare providers bundle vaccinations, offering HPV with other relevant adolescent vaccines such as whooping cough and meningitis (CDC, 2021[250]).
Reminders from healthcare providers (including via mail, phone or text messages to parents) regarding upcoming vaccinations and to reach those who have missed any is an effective strategy to support HPV vaccination (Jacobson et al., 2016[52]). Physician reviews of patient vaccination status prior to visits, alerts to physicians at the point of care and feedback to physicians about vaccination levels of their patient panel can also help to increase uptake. A randomised trial covering 22 primary care practices in Pennsylvania and New Jersey, the United States, examined a physician intervention comprising HPV vaccine education, point-of-care reminders and panel feedback. Results suggest that the most effective intervention for completion of all three HPV doses consisted of physician intervention combined with family-focused elements, including phone reminders for scheduled preventive visits. The joint physician/family intervention group had a 13 percentage point higher rate of receiving all recommended HPV vaccine doses than the control groups (Fiks et al., 2013[251]). As Germany considers vaccination to be physically accessible to all population groups, it is considering ways to increase acceptance and awareness of HPV vaccines, evaluating approaches such as patient reminders and training of healthcare professionals regarding the vaccine (Robert Koch Institut, 2023[252]).
School-based vaccination helps to increase human papillomavirus vaccination coverage and reduce socio‑economic and geographical disparities
In addition to healthcare centre delivery, 14 of the 29 EU+2 countries have implemented school-based delivery programmes to increase HPV vaccination coverage, as many children do not attend regular preventive healthcare visits. Most Nordic countries have school-based programmes and some of the highest rates of HPV vaccine coverage. France has recently joined this group of countries: a new school-based vaccination programme targeted at children in grade 5e (around age 12) was introduced in September 2023 (Government of France, 2023[253]). School-based vaccination programmes facilitate wide reach across the entire age cohort and rapid vaccine delivery to a large population of students. They also help to raise awareness of the vaccine among children and parents (Brotherton et al., 2013[254]), increase vaccine uptake in underserved areas (Kaul et al., 2019[255]), and reduce the cost and burden of individual vaccination appointments. School-based vaccination has also been shown to increase parental acceptance of the HPV vaccine, as its inclusion in the national school vaccination programme carries scientific and medical endorsement (Davies et al., 2021[256]). In Sweden, a nationwide cohort study provided evidence that school-based vaccination led to higher uptake than out-of-school strategies, and led to lower inequalities in uptake across education and income groups, and by parents’ country of birth – all key determinants of vaccine uptake (see Section 3.2.3).
The effectiveness of school-based vaccination programmes has also been demonstrated across other OECD countries such as Australia, Canada, New Zealand, and the United States. In 2006, the Australian Government launched a national HPV vaccination programme for girls, including both routine school-based vaccination and a time‑limited catch-up programme: in 2009, 70% of girls aged 12‑17 were fully vaccinated (Brotherton et al., 2013[254]). By 2012, prevalence of the four strains of HPV infections targeted by the vaccines had substantially decreased among sexually active women aged 18‑24 in Australia while evidence of a decrease in men suggested presence of a herd effect even before the inclusion of boys in the programme (Patel et al., 2018[257]). Several organisational factors that facilitate school-based vaccination have been identified in the design of vaccination programmes, such as national and regional policy, programme management and leadership, organisational models and institutional relationships, infrastructure, workforce capacity and activity, programme financing, communication with parents and students, and clinic organisation and delivery (Perman et al., 2017[258]).
Targeted policies to communicate about human papillomavirus vaccination are critical to raise confidence around vaccines
HPV vaccination programmes in some countries have been affected by public distrust and low confidence around the vaccine (see Section 3.2.3). A systematic review of low trust in the HPV vaccine in Europe found that the most common themes entailed concerns about the adequacy of existing information about the vaccine; potential side effects; and general mistrust of new vaccines, healthcare professionals and health authorities (Karafillakis et al., 2019[259]). In Denmark, for example, HPV vaccination coverage rates in girls decreased from around 90% for the birth cohorts of 1998‑2000 to only about 54% for those born in 2003 (Suppli et al., 2018[260]), following negative public and media attention.
In response, countries are making efforts to encourage HPV vaccination through education and information campaigns. At the national level, public health authorities often lead campaigns to promote national vaccine programmes, including HPV vaccination. Denmark, for example, undertook major efforts to address the decline in HPV vaccination following the negative public attention stemming from media stories about perceived side effects (Box 3.13). The information campaign helped to build public trust in HPV vaccination and inform parents that the risk of cervical cancer diagnoses outweighs the risk of adverse events related to vaccination. In the Netherlands, the national information campaign is combined with targeted initiatives to counter vaccine hesitancy. Various localities organise focus groups and discussions with minority groups and host information evenings (Budding-Hennink, 2021[261]). HPV vaccination education campaigns are most effective when used in conjunction with other policy levers to increase uptake, such as home visits, reducing out-of-pocket payments, school-based vaccination programmes and outreach programmes targeting low-income settings (CPSFT, 2019[262]).
Box 3.13. The Stop HPV – Stop Cervical Cancer campaign in Denmark has improved HPV vaccination rates
In May 2017, the Danish Health Authority, Danish Cancer Society and Danish Medical Association partnered on a campaign called “Stop HPV – Stop Cervical Cancer”, which included accessible online and social media information targeted at parents. In addition, the Danish Cancer Society opened a hotline to answer parents’ questions about the HPV vaccine. The campaign combined personal stories from women with cervical cancer and health professionals, and facts and evidence on vaccine safety and efficacy. Following the campaign, the percentage of parents who trusted the vaccination increased, and the number of vaccinated girls in Denmark doubled in 2017 compared to 2016 (HPV World, 2023[263]; Soborg and Jaconsen, 2019[264]).
Innovative delivery approaches help campaigns reach lower socio‑economic groups
As stated in Section 3.2.3, in many countries, groups with lower socio‑economic status or with a migration background tend to have lower HPV vaccination rates than the general population. Adapted delivery approaches have been developed to reach populations that face cultural, geographical or other structural barriers in access to vaccination. These include expanding the scope of practice of some health professionals to improve vaccination rates in remote areas. In Denmark and Iceland, for example, pharmacists are allowed to administer HPV vaccination. Expanding the location of HPV vaccination sites to pharmacies or mobile clinics is another option for consideration. HPV vaccination buses were deployed in 2023 in the Netherlands by the National Institute for Public Health and the Environment (RIVM) and the Municipal Public Health Service. The bus visits several locations designated as HPV stops throughout the country, and pop-up vaccination stops are installed temporarily in locations that many young people pass through, such as educational institutions and train stations. Australia has developed transport services, including HPV bus vaccination teams, to visit remote areas. HPV vaccination vans also exist in the United States, where an extra measure has been proposed to include dental practices in vaccination efforts (Vanderpool, Stradtman and Brandt, 2019[265]). The RIVER-EU Project is also developing interventions to increase HPV vaccine uptake among underserved groups (Box 3.14).
Box 3.14. Countries are learning from best practices in human papillomavirus vaccination for underserved groups in Europe
The EU-funded RIVER-EU Project, running from 2021 to 2026, is developing interventions to increase HPV and measles, mumps and rubella vaccine uptake among underserved groups. For HPV, the Project has assessed barriers to vaccination among five selected target communities: migrants and refugees in Greece, Ukrainian migrants in Poland, adolescent girls of Turkish and Moroccan descent in the Netherlands, and Roma populations in the Slovak Republic. To identify best practices and translatable lessons, the Project has examined selected migrant communities in Europe with particularly high vaccination rates. Using this acquired knowledge, the Project develops and adapts interventions together with the target communities, which are then implemented and evaluated. It has also developed an online system with content for healthcare professionals (RIVER-EU, 2023[266]). Key drivers for increasing uptake of vaccines include ensuring accessibility of vaccines in schools and clinics, framing of vaccination as the norm, and ensuring high trust in local healthcare providers from the same community – those with shared native language, culture and perceived trustworthiness (Essa-Hadad et al., 2023[267]; Schloemer, de Zeeuw and van Enter, 2023[268]).
Preventing liver cancer due to hepatitis requires more targeted policies
To reduce incidence of HBV and HCV, the WHO (2017[269]) Action plan for the health sector response to viral hepatitis in the European region lays out policy targets including childhood vaccination, antenatal screening and syringe distribution. Hepatitis B immunisation coverage among 1‑year-olds is generally high in the EU27, ranging in 2021 from 84% in Estonia and 85% in Austria to 99% in Portugal and Malta (WHO, 2023[270]). Nearly all EU+2 countries have a national policy of universal vaccination against HBV, except Denmark, Finland and Iceland. Hungary has a nationwide school-based vaccination programme that targets adolescents (ECDC, 2022[30]).
Meanwhile, ensuring the 90% screening coverage of pregnant women laid out by WHO Action plan (2017[269]) remains important to prevent chronic cases of HBV as, although perinatal transmission accounts for a small proportion of HBV infections, 90% of these lead to chronic infections (ECDC, 2020[271]). Mother-to-child or vertical transmission accounted for 52% of HBV transmission for chronic cases in 2020 (only reported by Denmark, Greece, the Netherlands and Slovenia). Risks of transmission could be reduced through universal antenatal screening, in place in 25 EU+2 countries. Ten of the 13 countries with data available achieved the antenatal screening target of 90% in 2020 (ECDC, 2022[30]).
Vaccination programmes alone are, however, inadequate to eliminate HBV infections, since they do not prevent transmission through drug injections, sexual activity or others. The ECDC considers distribution of clean syringes and opioid substitution therapy to reduce drug use via injections particularly effective methods to address transmission of HCV, and strengthening of harm-reduction programmes is recommended in most countries to reduce transmission of HBV and HCV (ECDC, 2022[30]). Promotion of safer sex is also important for prevention of HBV and HCV. For MSM, for example, the latest available results from the European MSM Internet Survey 2017 demonstrate that only 41% of those who had had sexual intercourse with non-steady partners over the last 12 months reported that they always used condoms (ECDC, 2020[271]). Sexual health programmes are thus an important means to prevent infections, including with HBV and HCV, for the general population as well as risk groups (ECDC, 2022[28]).
Due to increased risk of exposure to hepatitis viruses in vulnerable groups such as people engaging in high-risk sex, migrant populations and people who inject drugs – as well as estimations of low vaccination coverage among them (see Section 3.2.3) – a targeted approach is worth consideration. For instance, the Netherlands is a low-endemic country with universal childhood vaccination, which maintains a targeted programme to provide complimentary vaccination to MSM and sex workers (RIVM, 2023[272]). Some EU+2 countries also have targeted vaccination programmes for people who inject drugs, MSM, people in prison settings and healthcare workers, although monitoring challenges persist because of significant data gaps across countries (ECDC, 2020[273]). Prevention of liver cancer due to HBV and HCV additionally entails early identification and treatment of acute infection cases. It is important that these reach vulnerable populations, which is more effective if strategies are devised in a targeted manner such as in Greece and France (Box 3.15).
Box 3.15. Countries are employing targeted strategies to test and treat specific vulnerable populations for hepatitis B and C viruses
Screening and linkage to services for people who inject drugs in the Thessaloniki metropolitan area in Greece
The ALEXANDROS Programme in the Thessaloniki metropolitan area is a community-based programme using peer-driven recruitment of people who inject drugs, conducting screening and linking them to healthcare services. The Programme aimed to reach those most in need – i.e. predominantly active injectors who are not linked to harm-reduction programmes, as this population is considered to be at the core of the HCV epidemic (ongoing transmission, high prevalence), and has limited opportunities for HCV testing and care as it is not linked to other services. The Programme achieved high coverage among the target population, finding a high prevalence (63%) of HCV antibodies, indicating exposure. Of those who had HCV antibodies, less than 10% reported any previous treatment with direct acting antivirals. Of those with chronic HCV monoinfection, 97% were entered into the national HCV treatment registry to apply for free treatment, 62% were referred to HCV care and more than half were identified as having initiated treatment at a follow-up point. People who inject drugs that had HCV and HIV co-infection were linked with HIV services.
The Scanvir Programme targets hard-to-reach groups in several regions in France
As part of France’s HCV elimination strategy, the Scanvir Programme is implemented in several regions in France. The intervention entails specific testing days with innovative methods in institutions interacting with vulnerable populations such as people who use drugs, prisoners and migrants. Institutions (addictology departments, risk-reduction centres for drug users, communal centres for social action and detention centres) identify and refer patients for Scanvir sessions on dedicated days for screening for HIV, HCV, HBV and liver stiffness. The method is considered efficient, providing multidisciplinary service while saving human care resources and targeting settings where vulnerable populations can be found. After screening, treatment is offered. Initial results suggest high rates of uptake of screening in referred patients and high rates of treatment initiation (79% of those with HCV detected in bloodstream).
Source: Submissions to the European Commission Best Practice portal; Debette‑Gratien, M. et al. (2023[274]), “Towards hepatitis C elimination in France: Scanvir, an effective model to test and treat drug users on dedicated days”, https://doi.org/10.1111/jvh.13798.
3.3.6. Promoting health literacy in individuals and organisations can promote control over cancer risk factors
Efforts to improve population health literacy have historically focused on the abilities of individuals to find, understand and use information in health decision making. In recent decades, health literacy has increasingly been viewed as an interaction between the individual and their environment. It is considered to be content- and context-specific, and related importantly to whether systems facilitate the task of accessing and taking action on health information (Sørensen et al., 2012[275]). As such, there has been increased interest in health literacy on an organisation level, leveraging health systems to make health-related tasks less demanding. Health literacy-responsive organisations compensate for gaps in individual health literacy through organisational structures, policies and processes that make it easier to find, understand, appraise and use information and services to improve and maintain good health (M-POHL, 2023[276]). Investing in health literacy contributes to effectiveness of care and quality of healthcare received by the population, which is particularly important for those with low socio‑economic characteristics who may face more barriers to care and experience more health risk factors (see Section 3.2). It can act as a cost-effective mechanism to ensure provision of people‑centred care and the competencies required to navigate it.
Measuring the state of health literacy in the population guides development of an overarching strategy
National surveys on health literacy allow countries to understand challenges and needs among their populations. Across the 29 EU+2 countries, 18 were identified as having launched a national or subnational survey to assess population health literacy levels (Figure 3.12). In addition to the use of survey results as a supporting argument for taking action, these efforts build awareness of health literacy, identify at-risk populations and share best practices. The WHO Action Network on Measuring Population and Organisational Health Literacy (M-POHL) was founded in 2018 with the aim of collaborating to measure, understand and improve health literacy across European countries (M-POHL, 2023[277]). M-POHL launched the European Health Literacy Population Survey 2019‑21 (HLS19), which resulted in data from 14 EU+2 countries on health literacy, identifying vulnerable groups and aspects of health literacy that were most challenging.
Among the 26 respondents to the 2023 OECD Policy Survey on Cancer Care Performance, 13 countries reported that they have adopted a health literacy strategy that addresses cancer risk factors, the majority of which reported that the strategy includes cancer awareness and self-efficacy to address cancer risk factors. Some – such as Austria, Norway, Portugal and the United Kingdom (Scotland) – have adopted nationwide health literacy action plans. The Norwegian strategy aims to increase health literacy in the population during 2019‑23, incorporating it into all planning, development, implementation and evaluation of health and care services, and public health work, and at all service and administrative levels (Council of Europe, 2023[278]). Other countries – such as Belgium and Germany – include health literacy in their national cancer control plans (Sørensen, 2020[279]). Germany’s National Cancer Plan includes a goal of ensuring access to high-quality information, counselling and support for cancer patients, strengthening patient literacy, and improving provider communication and patient-centred discussion (Federal Ministry of Health, 2023[280]). Similarly, in Poland, the Ministry of Health finances information, education and promotion activities under its National Oncology Strategy, such as the information and educational campaign “I am planning a long life”, dedicated to lung, colorectal, malignant skin, prostate, breast and cervical cancer prevention and screening (Ministry of Health, 2023[281]). In Luxembourg, the National Cancer Plan includes a key objective to disseminate information about risk factors. Events are also organised to increase awareness and improve health literacy – for example, “Octobre rose” and “Broschtkriibslaf” for breast cancer, “Mars bleu” for colorectal cancer, “Relais pour la vie” for all cancers and “Lëtz Go Gold” for paediatric cancer.
Figure 3.12. National and subnational surveys on health literacy have been conducted in 18 EU+2 countries
Note: 26 EU+2 countries responded to the 2023 OECD Policy Survey on Cancer Care Performance. Information for Belgium on health literacy strategies is not available.
1. Data derived from country participation in the HLS19.
2. Data derived from country responses to the 2023 OECD Policy Survey on Cancer Care Performance.
Source: International Report on the Methodology, Results, and Recommendations of the European Health Literacy Population Survey 2019‑21 (HLS19) of M-POHL, Austrian National Public Health Institute, https://m-pohl.net/sites/m-pohl.net/files/inline-files/HLS19%20International%20Report.pdf; 2023 OECD Policy Survey on Cancer Care Performance.
Interventions to improve health literacy should be multi-faceted and adapted to the needs of the population
To improve health literacy, personal competences need to be strengthened and situational demands reduced. Some individual health literacy interventions such as cell phone‑based health education messages, animation or informative videos, use of audio or illustrations alongside text, small-group education, and use of simplified language can improve health literacy and lead to changes in health behaviours (Walters et al., 2020[282]). Abilities to assess the validity of health-related information can be developed from a young age through the school curriculum, helping to narrow disparities emerging later in life (Council of Europe, 2023[278]). Europe’s Beating Cancer Plan includes improving health literacy on cancer risk by updating the European Code Against Cancer (ECAC) as a flagship initiative on prevention, promoting co‑operation between health and social services to give people the necessary information and tools to make healthier choices (Schüz et al., 2015[283]). The ECAC is a health education tool aimed at raising awareness about evidence‑based cancer prevention actions among EU citizens. It is currently being updated, co‑ordinated by the IARC/WHO, to provide a 5th edition, following recommendations issued under the last EU Joint Action on Cancer (Espina et al., 2021[284]). In relation to this, an EU mobile application for cancer prevention is being developed under the EU4Health Programme to support dissemination of the messages from the ECAC. A health literacy for cancer prevention and care programme will also be launched to develop and share best practices to strengthen health literacy in cancer prevention and care programmes, with a focus on disadvantaged groups (European Commission, 2022[285]).
Among adults, health literacy is often lower among those from groups with low socio‑economic status (see Section 3.2.4), while efforts focused on individual behaviour change may be more effective in higher socio‑economic groups. This highlights a need for complementary policies that make it easier for people of all socio‑economic groups to access, comprehend, appraise and apply health information (Gibney et al., 2020[286]). Vulnerable populations such as people with low levels of education or migrants may particularly benefit from organisational measures that create health literacy-enabling environments. Putting essential information first, using videos alongside written materials, and using pictographs alongside numerical information improved understanding among people with low health literacy (Housten et al., 2020[287]).
Healthcare organisations can create environments that make navigating choices within health and healthcare easier for people with low health literacy. To improve organisational health literacy, strong leadership is necessary in healthcare organisations to integrate it into planning, structure and operations; prepare the workforce; monitor progress; and ensure co-creation by including populations served in the design, implementation and evaluation of health information and services (Brach et al., 2012[288]). Multi-level interventions – with a mix of elements such as patient education and mobilisation, communication training for clinicians, and support with navigation within the healthcare system – have been found to be most effective (Housten et al., 2020[287]). An assessment tool recently developed in Switzerland for health-literate primary care settings includes a range of indicators such as training staff on health literacy and good communication techniques; dedicating sufficient time to patient communications; providing translation where necessary; using plain language and clear visual materials; and providing assistance to patients in completing forms and evaluating health information (De Gani et al., 2020[289]). The United States-based Agency for Healthcare Research and Quality (2020[290]) highlights good practices such as training clinicians in communication techniques including teach-back (wherein patients explain in their own words what they need to know or do), show-me (wherein patients demonstrate an action to the clinician, such as how to use an inhaler) and chunk-and-check (wherein clinicians break down information into smaller pieces and then confirm patient understanding) methods.
Collaboration between government stakeholders, healthcare providers, organisations and civil society is necessary to develop comprehensive and effective strategies
A health literacy alliance launched by the German Ministry of Health in 2017 includes 14 partners, such as the German Hospital Association, the German Medical Association, the Association of Private Health Insurance and government stakeholders, who each committed to action to improve health literacy within their areas of responsibility (Federal Ministry of Health, 2017[291]). Germany has also created a unified national health portal where people can access reliable, high-quality and easily understandable information on all areas of health and healthcare (Federal Ministry of Health, 2020[292]). The Austrian Health Literacy Alliance has undertaken activities such as establishing a working group on organisational health literacy and developing health literacy assessment tools for various types of organisations (The Austrian Health Literacy Alliance, 2023[293]).
System-level changes require partnerships across all sectors, involving various levels and departments within governments, the private sector and the populations affected, while meaningful engagement with civil society is needed to ensure co-creation of solutions that adapt to local needs (Sørensen et al., 2021[294]). Box 3.16 highlights selected actions on health literacy in Portugal, Slovenia and the Netherlands. It is important to note that in addition to activities focusing on prevention of cancer, health literacy has further implications for cancer screening (Chapter 4) and treatment (Chapter 5).
Box 3.16. A number of actions related to health literacy have been implemented in EU Member States
Portugal
The Portuguese Health Literacy and Behavioural Sciences Plan 2023‑30 aims to contribute to the creation and implementation of ecosystems that lead to recognition of the benefits of adopting a healthy lifestyle, appropriate use of the National Health Service and the importance of disease management. The Plan focuses on individuals, communities, health systems and policies, supporting a lifecycle approach aimed at promoting well-being at school and in retirement and an active lifestyle. Training courses to address the needs of migrant populations have been developed for health professionals, and local communities (for example, in Lisbon) work with migrant groups and refugees to design fit-for-purpose solutions and create enabling environments (WHO, 2022[295]; Ministry of Health, 2023[296]).
Slovenia
In 2019, Slovenia carried out a health literacy survey of the population, held interviews with patients and professionals, and reviewed government and health organisation websites to assess whether information is understandable and useful for individuals. The country also developed a health literacy plan which contains seven strategies to: 1) improve access to health information; 2) improve individuals’ understanding of health information; 3) promote patient-centred care; 4) reduce healthcare inequalities through targeted interventions; 5) strengthen the ability of individuals to navigate the health system; 6) promote health literacy as a public health strategy; and 7) measure and evaluate impact (Kolnik, Ljubič and Kmetič, 2023[297]; Kolnik and Ljubič, 2023[298]).
The Netherlands
The Netherlands is yet to launch a comprehensive plan on health literacy. Pharos, the Dutch Centre of Expertise on Health Disparities, works with local governments to adapt and implement actions to improve health literacy. It develops specific tools to ensure that the healthcare system is understandable for everyone, through accessible information materials; training programmes; and guidance for GPs, pharmacies and municipalities in dealing with reduced health literacy. Several courses are available for health professionals – for example, on culturally sensitive communication with migrants – and education materials are developed and tested among target population groups (Pharos, 2023[299]). Additionally, the Dutch Health Literacy Alliance involves 130 organisations engaged in promoting health literacy, and in prioritising and developing both quantitative and qualitative measures.
3.4. Conclusion
Preventing cancer by addressing preventable risk factors is one of the most cost-effective and efficient ways to reduce the burden of cancer in the population (WHO, 2023[300]). Given the high burden of cancer attributed to risk factors in the 29 EU+2 countries, all countries have scope to prioritise prevention policies and learn from best practices in other countries. Spending on prevention is generally considered insufficient across EU+2 countries, as it made up an average of 2.5% of health expenditure in 2019. Estimates from 2021 amount to 5.1%, marking a significant increase, however, this is mostly due to increased spending related to infection prevention and control of the COVID‑19 virus (Chapter 1). Countering alarming trends in cancer burden and inequalities requires key prevention policies to address cancer risk factors, but no policy is sufficient to prevent cancer on its own. A comprehensive package of prevention policies is necessary to tackle different risk factors and target at-risk population groups – including fiscal and regulatory measures; improving availability and accessibility of information in the community; involving primary healthcare, schools and workplaces; and promoting awareness of risks across population groups, among others.
References
[145] Abidi, L. et al. (2020), “Conversations about alcohol in healthcare – cross-sectional surveys in the Netherlands and Sweden”, BMC Public Health, Vol. 20/1, p. 283, https://doi.org/10.1186/s12889-020-8367-8.
[151] Afshin, A. et al. (2017), “The prospective impact of food pricing on improving dietary consumption: A systematic review and meta-analysis”, PloS One, Vol. 12/3, p. e0172277, https://doi.org/10.1371/journal.pone.0172277.
[150] Agabio, R. et al. (2015), A Systematic Review of School-Based Alcohol and other Drug Prevention Programs, https://doi.org/10.2174/1745017901511010102.
[290] Agency for Healthcare Research and Quality (2020), Health Literacy Universal Precautions Toolkit, 2nd Edition, Agency for Healthcare Research and Quality, Rockville, MD, https://www.ahrq.gov/health-literacy/improve/precautions/index.html (accessed on 9 August 2023).
[147] Angus, C. et al. (2017), “Estimating the cost-effectiveness of brief interventions for heavy drinking in primary health care across Europe”, European Journal of Public Health, Vol. 27/2, pp. 345-351, https://doi.org/10.1093/eurpub/ckw122.
[71] Been, J. et al. (2021), “European progress in working towards a tobacco-free generation”, European Journal of Pediatrics, Vol. 180/12, pp. 3423-3431, https://doi.org/10.1007/s00431-021-04116-w.
[156] Bíró, A. (2015), “Did the junk food tax make the Hungarians eat healthier?”, Food Policy, Vol. 54, pp. 107-115, https://doi.org/10.1016/j.foodpol.2015.05.003.
[205] Black, N. et al. (2019), “The effect of school sports facilities on physical activity, health and socioeconomic status in adulthood”, Social Science & Medicine, Vol. 220, pp. 120-128, https://doi.org/10.1016/j.socscimed.2018.10.025.
[56] Bollerup, S. et al. (2017), “Socioeconomic predictors of human papillomavirus vaccination in Danish men: A nationwide study”, Papillomavirus Research, Vol. 3, pp. 18-23, https://doi.org/10.1016/j.pvr.2016.11.004.
[41] Bolte, G., G. Tamburlini and M. Kohlhuber (2009), “Environmental inequalities among children in Europe – evaluation of scientific evidence and policy implications”, European Journal of Public Health, Vol. 20/1, pp. 14-20, https://doi.org/10.1093/eurpub/ckp213.
[141] Boß, L. et al. (2018), “Efficacy of a web-based intervention with and without guidance for employees with risky drinking: Results of a three-arm randomized controlled trial”, Addiction, Vol. 113, pp. 635-646, https://doi.org/10.1111/add.14085.
[288] Brach, C. et al. (2012), Ten attributes of health literate health care organizations, National Academy of Medicine, Washington, DC.
[142] Brendryen, H. et al. (2017), “A pilot randomized controlled trial of an internet-based alcohol intervention in a workplace setting”, International Journal of Behavioral Medicine, Vol. 24/5, pp. 768–777, https://doi.org/10.1007/s12529-017-9665-0.
[254] Brotherton, J. et al. (2013), “Human papillomavirus vaccine coverage among female Australian adolescents: Success of the school‐based approach”, Medical Journal of Australia, Vol. 199/9, pp. 614-617, https://doi.org/10.5694/mja13.10272.
[109] Brown, T., S. Platt and A. Amos (2014), “Equity impact of population-level interventions and policies to reduce smoking in adults: A systematic review”, Drug and Alcohol Dependence, Vol. 138, pp. 7-16, https://doi.org/10.1016/j.drugalcdep.2014.03.001.
[135] Brunborg, G., J. Skogen and J. Burdzovic Andreas (2022), “Time spent on social media and alcohol use among adolescents: A longitudinal study”, Addictive Behaviors, Vol. 130, p. 107294, https://doi.org/10.1016/j.addbeh.2022.107294.
[191] Bryden, A. et al. (2013), “Voluntary agreements between government and business—a scoping review of the literature with specific reference to the Public Health Responsibility Deal”, Health Policy, Vol. 110/2-3, pp. 186-197.
[261] Budding-Hennink, A. (2021), Grote Vaccinatie-Verschillen Tussen Kinderen Met en Zonder Migratieachtergrond [Large Vaccination Differences Between Children With and Without a Migration Background], https://www.medicalfacts.nl/2021/04/28/grote-vaccinatie-verschillen-tussen-kinderen-met-en-zonder-migratieachtergrond/ (accessed on 3 August 2023).
[99] Cahill, K. and T. Lancaster (2014), “Workplace interventions for smoking cessation”, Cochrane Database of Systematic Reviews, Vol. 2, p. CD003440, https://doi.org/10.1002/14651858.CD003440.pub4.
[92] Campaign for Tobacco-Free Kids (2023), Tobacco Control Laws, https://www.tobaccocontrollaws.org/ (accessed on 19 September 2023).
[80] Campaign for Tobacco-Free Kids (2021), Strategic Investment of Tobacco Tax Revenue, Campaign for Tobacco-Free Kids, Washington, DC, https://assets.tobaccofreekids.org/global/pdfs/en/strategic_investment_tobacco_tax_revenue.pdf.
[238] Canadian Occupational Safety (2023), BC mandates licensing requirement for asbestos abatement contractors, https://www.thesafetymag.com/ca/topics/ohs-law-and-legislation/bc-mandates-licensing-requirement-for-asbestos-abatement-contractors/449035 (accessed on 20 September 2023).
[153] Capacci, S. et al. (2019), “The impact of the French soda tax on prices and purchases: An ex post evaluation”, PLoS One, Vol. 14/10, p. e0223196, https://doi.org/10.1371/journal.pone.0223196.
[82] Carton, T. et al. (2016), “Comprehensive indoor smoking bans and smoking prevalence: Evidence from the BRFSS”, American Journal of Health Economics, Vol. 2/4, pp. 535-556, https://doi.org/10.1162/ajhe_a_00061.
[88] Cathelineau, F. et al. (2021), TABADO, un programme pertinent d’accompagnement des lycéens professionnels et apprentis à l’arrêt du tabac développé en milieu scolaire, https://beh.santepubliquefrance.fr/beh/2021/8/pdf/2021_8_3.pdf.
[250] CDC (2021), 5 Ways to Boost Your HPV Vaccination Rates, https://www.cdc.gov/hpv/hcp/boosting-vacc-rates.html (accessed on 2 August 2023).
[53] CDC (2021), Vaccination Coverage among Adolescents (13-17 Years), https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/index.html (accessed on 3 August 2023).
[231] Cesaroni, G. et al. (2012), “Health benefits of traffic-related air pollution reduction in different socioeconomic groups: The effect of low-emission zoning in Rome”, Occupational and Environmental Medicine, Vol. 69/2, pp. 133-139, https://doi.org/10.1136/oem.2010.063750.
[20] Chesson, H. et al. (2014), “The estimated lifetime probability of acquiring human papillomavirus in the United States”, Sexually Transmitted Diseases, Vol. 41/11, pp. 660-664, https://doi.org/10.1097/OLQ.0000000000000193.
[62] Chu, J. et al. (2013), “Changing epidemiology of hepatitis B and migration – a comparison of six Northern and North-Western European countries”, European Journal of Public Health, Vol. 23/4, pp. 642-647, https://doi.org/10.1093/eurpub/cks067.
[169] Clark, H. et al. (2020), “A future for the world’s children? A WHO–UNICEF–Lancet Commission”, The Lancet, Vol. 395/10224, pp. 605-658, https://doi.org/10.1016/S0140-6736(19)32540-1.
[120] Clements, K. et al. (2022), “How elastic is alcohol consumption?”, Economic Analysis and Policy, Vol. 76, pp. 568-581, https://doi.org/10.1016/j.eap.2022.09.003.
[7] Clinton, S., E. Giovannucci and S. Hursting (2020), “The World Cancer Research Fund/American Institute For Cancer Research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions”, The Journal of Nutrition, Vol. 150/4, pp. 663-671, https://doi.org/10.1093/jn/nxz268.
[34] Collins, S. (2016), “Associations between socioeconomic factors and alcohol outcomes”, Alcohol Research: Current Reviews, Vol. 38/1, pp. 83-94.
[21] Colzani, E. et al. (2021), “Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma”, Euro Surveillance, Vol. 26/50, p. 2001659, https://doi.org/10.2807/1560-7917.ES.2021.26.50.2001659.
[27] Cortesi, P. et al. (2023), “Hepatitis B and C in Europe: An update from the Global Burden of Disease Study 2019”, The Lancet Public Health, Vol. 8/9, pp. e701-e716, https://doi.org/10.1016/S2468-2667(23)00149-4.
[278] Council of Europe (2023), Guide to Health Literacy: Contributing to trust building and equitable access to healthcare, Council of Europe, Strasbourg, https://rm.coe.int/inf-2022-17-guide-health-literacy/1680a9cb75.
[237] Council of the EU (2023), Asbestos: Council and Parliament strike deal on new rules protecting workers, https://www.consilium.europa.eu/en/press/press-releases/2023/06/27/asbestos-council-and-parliament-strike-deal-on-new-rules-protecting-workers/ (accessed on 19 September 2023).
[262] CPSFT (2019), The Community Guide: CPSTF findings for increasing vaccination, https://www.thecommunityguide.org/pages/task-force-findings-increasing-vaccination.html (accessed on 3 August 2023).
[118] Cyprus Bar Association (n.d.), Law on the Sale of Alcoholic Beverages of Cyprus, https://www.cylaw.org/nomoi/enop/non-ind/0_144/full.html.
[256] Davies, C. et al. (2021), “School-based HPV vaccination positively impacts parents’ attitudes toward adolescent vaccination”, Vaccine, Vol. 39/30, pp. 4190-4198, https://doi.org/10.1016/j.vaccine.2021.05.051.
[51] de Figueiredo, A. et al. (2022), State of Vaccine Confidence in the European Union, 2022, Publications Office of the European Union, Luxembourg, https://health.ec.europa.eu/system/files/2023-02/2022_confidence_rep_en.pdf.
[289] De Gani, S. et al. (2020), “Self-assessment tool to promote organizational health literacy in primary care settings in Switzerland”, International Journal of Environmental Research and Public Health, Vol. 17/24, p. 9497, https://doi.org/10.3390/ijerph17249497.
[54] de Munter, A. et al. (2021), “Determinants of HPV-vaccination uptake and subgroups with a lower uptake in the Netherlands”, BMC public health, Vol. 21/1, pp. 1-13.
[274] Debette Gratien, M. (2023), Towards hepatitis C elimination in France: Scanvir, an effective model to test and treat drug users on dedicated days, https://doi.org/10.1111/jvh.13798.
[162] Delgado, J. et al. (2022), “Unhealthy food advertising. A position paper by the AEP Committee on Nutrition and Breastfeeding”, Anales de Pediatría (English Edition), Vol. 97/3, pp. 206.e1-206.e9, https://doi.org/10.1016/j.anpede.2022.07.003.
[110] Demjén, T., Z. Kimmel and J. Kiss (2018), Dohányzás visszaszorítása Magyarországon [Reducing Smoking in Hungary], Hungarian Focal Point for Tobacco Control, Budapest.
[77] Devaux, M. et al. (2023), “Évaluation du programme national de lutte contre le tabagisme en France”, OECD Health Working Papers, No. 155, OECD Publishing, Paris, https://doi.org/10.1787/b656e9ac-fr.
[204] Dohle, S. and B. Wansink (2013), “Fit in 50 years: Participation in high school sports best predicts one’s physical activity after age 70”, BMC Public Health, Vol. 13, pp. 1-6, https://doi.org/10.1186/1471-2458-13-1100.
[24] Drolet, M. et al. (2019), “Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis”, The Lancet, Vol. 394/10197, pp. 497-509, https://doi.org/10.1016/S0140-6736(19)30298-3.
[192] Durand, M. et al. (2015), “An evaluation of the Public Health Responsibility Deal: Informants’ experiences and views of the development, implementation and achievements of a pledge-based, public–private partnership to improve population health in England”, Health Policy, Vol. 119/11, pp. 1506-1514, https://doi.org/10.1016/j.healthpol.2015.08.013.
[28] ECDC (2022), Hepatitis B – Annual Epidemiological Report for 2021, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/en/publications-data/hepatitis-b-annual-epidemiological-report-2021.
[29] ECDC (2022), Hepatitis C: Annual epidemiological report for 2021, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/sites/default/files/documents/AER-HEP-C-2021.pdf.
[30] ECDC (2022), Prevention of Hepatitis B and C in the EU/EEA, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/en/publications-data/prevention-hepatitis-b-and-c-eueea (accessed on 1 August 2023).
[25] ECDC (2020), Guidance on HPV Vaccination in EU Countries: Focus on boys, people living with HIV and 9-valent HPV vaccine introduction, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/sites/default/files/documents/Guidance-on-HPV-vaccination-in-EU-countries2020-03-30.pdf.
[271] ECDC (2020), Monitoring the Responses to Hepatitis B and C Epidemics in the EU/EEA Member States, 2019, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/en/publications-data/monitoring-responses-hepatitis-b-and-c-epidemics-eueea-member-states-2019.
[273] ECDC (2020), Prevention of hepatitis B and C in the EU/EEA and the UK, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/en/publications-data/prevention-hepatitis-b-and-c-eueea-and-uk.
[39] EEA (2023), Europe’s Air Quality Status 2023, https://www.eea.europa.eu/publications/europes-air-quality-status-2023 (accessed on 6 December 2023).
[16] EEA (2022), Air pollution, https://www.eea.europa.eu/publications/environmental-burden-of-cancer/air-pollution (accessed on 18 December 2023).
[216] EEA (2022), Sources and emissions of air pollutants in Europe, European Environment Agency, Copenhagen, https://www.eea.europa.eu/publications/air-quality-in-europe-2022/sources-and-emissions-of-air.
[185] Egnell, M. et al. (2020), “Objective understanding of the Nutri-Score front-of-pack label by European consumers and its effect on food choices: An online experimental study”, International Journal of Behavioral Nutrition and Physical Activity, Vol. 17/1, p. 146, https://doi.org/10.1186/s12966-020-01053-z.
[180] EHPHA (2023), Statement on Front-of-pack Nutrition Labelling in the European Union, European Public Health Association, Utrecht, https://eupha.org/repository/advocacy/2023/EUPHA%20Statement%20on%20FoPNL%20FINAL.pdf.
[94] El Asmar, M. et al. (2022), “How do Europeans quit using tobacco, e-cigarettes and heated tobacco products? A cross-sectional analysis in 28 European countries”, BMJ Open, Vol. 12/4, p. e059068, https://doi.org/10.1136/bmjopen-2021-059068.
[79] Enache, C. (2022), Cigarette Taxes in Europe, Tax Foundation, Washington, DC, https://taxfoundation.org/cigarette-tax-europe-2022/ (accessed on 14 December 2023).
[242] EPF (2023), HPV Prevention Policy Atlas, European Parliamentary Forum for Sexual and Reproductive Rights, Brussels, https://www.epfweb.org/sites/default/files/2023-06/HPV%20Atlas_EN%202023-JUN19.pdf (accessed on 2 August 2023).
[33] ESPAD Group (2019), ESPAD Report 2019: Results from the European School Survey Project on Alcohol and Other Drugs, Publications Office of the European Union, Luxembourg, http://espad.org/espad-report-2019.
[284] Espina, C. et al. (2021), “Sustainability and monitoring of the European Code Against Cancer: Recommendations”, Cancer Epidemiology, Vol. 72/101933, https://doi.org/10.1016/j.canep.2021.101933.
[267] Essa-Hadad, J. et al. (2023), Health system enablers to child vaccination in Israel: The empowering example of the Arab Minority in Israel, RIVER-EU, Groningen, https://river-eu.org/wp-content/uploads/2023/05/river-eu-poster-enablers-israel-final.pdf (accessed on 20 September 2023).
[211] EUPAP Consortium (2020), EUPAP Feasibility Study: Final report, EUPAP Consortium, Lleida, https://www.eupap.org/storage/app/media/WP4%20Feasibility%20Study.pdf.
[186] Eureporter (2023), Facing Mounting Tensions, EU Must Find Quick Answers to Divisive Agri-food Issues, https://www.eureporter.co/health/food-2/2023/09/18/facing-mounting-tensions-eu-must-find-quick-answers-to-divisive-agri-food-issues/ (accessed on 8 December 2023).
[42] Eurogip (2006), Asbestos-related Occupational Diseases in Europe, European Forum of the Insurance against Accidents at Work and Occupational Diseases, Brussels, https://www.eurogip.fr/images/publications/EUROGIP-24E-AsbestosOccDiseases.pdf.
[26] European Cancer Organisation (2022), Putting HPV on the Map: The state of HPV prevention programmes in the WHO European Region, European Cancer Organisation, Brussels, https://www.europeancancer.org/resources/256:hpv-prevention-programmes.html.
[19] European Cancer Organisation (2020), Viral Protection: Achieving the possible. A four step plan for eliminating HPV cancers in Europe, European Cancer Organisation, Brussels, https://www.europeancancer.org/resources/159:viral-protection-achieving-the-possible-a-four-step-plan-for-eliminating-hpv-cancers-in-europe.html.
[220] European Commission (2023), A European Green Deal, https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 9 December 2023).
[236] European Commission (2023), Better Protection of Workers from Asbestos: Commission welcomes political agreement to revise EU rules, https://ec.europa.eu/commission/presscorner/detail/en/ip_23_3557 (accessed on 19 September 2023).
[227] European Commission (2023), Green City Accord, https://environment.ec.europa.eu/topics/urban-environment/green-city-accord_en (accessed on 9 December 2023).
[111] European Commission (2023), Mechanisms of early detection and signposting of young people facing health risks, https://national-policies.eacea.ec.europa.eu/youthwiki/chapters/hungary/76-mechanisms-of-early-detection-and-signposting-of-young-people-facing-health-risks (accessed on 16 August 2023).
[178] European Commission (2023), Nutrition labelling, https://food.ec.europa.eu/safety/labelling-and-nutrition/food-information-consumers-legislation/nutrition-labelling_en (accessed on 8 December 2023).
[87] European Commission (2023), Tobacco Overview, https://health.ec.europa.eu/tobacco/overview_en (accessed on 11 July 2023).
[18] European Commission (2022), Commission acts to better protect people from asbestos and ensure an asbestos-free future, https://ec.europa.eu/commission/presscorner/detail/en/ip_22_5679 (accessed on 5 December 2023).
[285] European Commission (2022), Europe’s Beating Cancer Plan: Communication from the commission to the European Parliament and the Council, https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf.
[235] European Commission (2022), On working towards an asbestos-free future: A European approach to addressing the health risks of asbestos, European Commission, Brussels, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2022%3A488%3AFIN.
[67] European Commission (2021), Europe’s Beating Cancer Plan, European Commission, Brussels, https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf (accessed on 5 July 2023).
[117] European Commission (2021), Excise Duty tables: Part 1 - Alcoholic beverages, European Commission, Brussels, https://taxation-customs.ec.europa.eu/system/files/2021-09/excise_duties-part_i_alcohol_en.pdf.
[81] European Commission (2020), Commission Staff Working Document Evaluation of the 2011/64/EU of Council Directive 21 June 2011 on the structure and rates of excise duty applied to manufactured tobacco, European Commission, Brussels, https://taxation-customs.ec.europa.eu/system/files/2020-02/10-02-2020-tobacco-taxation-report.pdf (accessed on 6 July 2023).
[219] European Commission (2016), An EU Strategy on Heating and Cooling, European Commission, Brussels, https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1575551754568&uri=CELEX:52016DC0051 (accessed on 26 July).
[44] European Council (2023), Infographic – The impact of asbestos on workers’ health, European Council, Brussels, https://www.consilium.europa.eu/en/infographics/impact-asbestos-workers-health/ (accessed on 19 September 2023).
[188] European Health and Digital Executive Agency (2022), EU REformulation MOnitoring (EUREMO): Feasibility study for a monitoring system on reformulation initiatives for salt, sugars and fat : final report, Publications Office of the European Union, Luxembourg, https://op.europa.eu/en/publication-detail/-/publication/28108ba7-6646-11ed-b14f-01aa75ed71a1/language-en.
[149] European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2023), Unplugged - a Comprehensive Social Influence programme for schools: life skills training with correction of normative beliefs, http://www.emcdda.europa.eu/best-practice/xchange/unplugged_en.
[78] European Parliament (2023), Regulation of the European Parliament and of the Council concerning batteries and waste batteries, https://data.consilium.europa.eu/doc/document/PE-2-2023-INIT/en/pdf.
[202] Evans, C. et al. (2012), “Systematic review and meta-analysis of school-based interventions to improve daily fruit and vegetable intake in children aged 5 to 12 y”, The American Journal of Clinical Nutrition, Vol. 96/4, pp. 889-901, https://doi.org/10.3945/ajcn.111.030270.
[40] Fairburn, J. et al. (2019), “Social inequalities in exposure to ambient air pollution: A systematic review in the WHO European Region”, International Journal of Environmental Research and Public Health, Vol. 16/17, p. 3127, https://doi.org/10.3390/ijerph16173127.
[280] Federal Ministry of Health (2023), Handlungsfeld 4: Stärkung der Patientenorientierung [Field of Action 4: Strengthening patient orientation], Federal Ministry of Health, Bonn, https://www.bundesgesundheitsministerium.de/themen/praevention/nationaler-krebsplan/handlungsfelder/handlungsfeld-4.html (accessed on 18 August 2023).
[292] Federal Ministry of Health (2020), Health Portal Goes Online, https://www.bundesgesundheitsministerium.de/presse/pressemitteilungen/2020/3-quartal/gesundheitsportal.html (accessed on 18 August 2023).
[291] Federal Ministry of Health (2017), Foundation of the “Alliance for Health Literacy”, https://www.bundesgesundheitsministerium.de/ministerium/meldungen/2017/juni/allianz-fuer-gesundheitskompetenz.html (accessed on 18 August 2023).
[140] Fellbaum, L. et al. (2023), “The effectiveness of workplace interventions for the prevention of alcohol use: A meta-analysis”, Addiction, Vol. 118/11, pp. 2043-2061, https://doi.org/10.1111/add.16276.
[251] Fiks, A. et al. (2013), “Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt”, Pediatrics, Vol. 131/6, pp. 1114-1124, https://doi.org/10.1542/peds.2012-3122.
[195] Food Standards Scotland (2023), Eat Well Your Way, http://eatwellyourway.scot (accessed on 8 December 2023).
[170] Galbraith‐Emami, S. and T. Lobstein (2013), “The impact of initiatives to limit the advertising of food and beverage products to children: A systematic review”, Obesity Reviews, Vol. 14/12, pp. 960-974, https://doi.org/10.1111/obr.12060.
[12] Gallagher, E. and D. LeRoith (2015), “Obesity and diabetes: The increased risk of cancer and cancer-related mortality”, Physiological Reviews, Vol. 95/3, pp. 727-748, https://doi.org/10.1152/physrev.00030.2014.
[1] GBD 2019 Cancer Risk Factors Collaborators (2022), “The global burden of cancer attributable to risk factors, 2010–19: A systematic analysis for the Global Burden of Disease Study 2019”, The Lancet, Vol. 400/10352, pp. 563-591, https://doi.org/10.1016/S0140-6736(22)01438-6.
[69] GECP (2022), Tobacco-free Generation by 2040, https://www.gecp.pt/en/geracao-sem-tabaco-ate-2040/ (accessed on 14 August 2023).
[286] Gibney, S. et al. (2020), “Increasing health literacy may reduce health inequalities: Evidence from a national population survey in Ireland”, International Journal of Environmental Research and Public Health, Vol. 17/16, p. 5891, https://doi.org/10.3390/ijerph17165891.
[100] Gorlova, O. (ed.) (2016), “Cost effectiveness of free access to smoking cessation treatment in France considering the economic burden of smoking-related diseases”, PLOS One, Vol. 11/2, p. e0148750, https://doi.org/10.1371/journal.pone.0148750.
[93] Government of Canada (2023), Canada to Become First Country in the World to Require Health Warnings on Individual Cigarettes, https://www.canada.ca/en/health-canada/news/2023/05/canada-to-become-first-country-in-the-world-to-require-health-warnings-on-individual-cigarettes.html (accessed on 18 September 2023).
[253] Government of France (2023), Campagne de vaccination gratuite contre les papillomavirus dans les collèges, https://www.gouvernement.fr/actualite/campagne-de-vaccination-gratuite-contre-le-papillomavirus-dans-les-ecoles (accessed on 2 August 2023).
[90] Government of Iceland (2022), Frumvarp um Nikótínvörur Orðið að Lögum [Nicotine Products Bill Becomes Law], Government of Iceland, Reykjavik, https://www.stjornarradid.is/efst-a-baugi/frettir/stok-frett/2022/06/16/Frumvarp-um-nikotinvorur-ordid-ad-logum/ (accessed on 13 September 2023).
[138] Government of Ireland (2023), Public Health (Alcohol) (Labelling) Regulations 2023, https://www.irishstatutebook.ie/eli/2023/si/249/made/en/pdf (accessed on 8 December 2023).
[70] Government of Portugal (2023), Geração sem tabaco até 2040 [Tobacco-free Generation by 2040], Government of Portugal, Lisbon, https://www.portugal.gov.pt/pt/gc23/comunicacao/noticia?i=geracao-sem-tabaco-ate-2040 (accessed on 27 October 2023).
[68] Government of the Netherlands (2019), National Prevention Agreement, Government of the Netherlands, The Hague, https://www.government.nl/topics/smoking/documents/reports/2019/06/30/the-national-prevention-agreement (accessed on 6 July 2023).
[172] Grammatikaki, E. et al. (2019), Marketing of Food, Non-alcoholic, and Alcoholic Beverages, Publications Office of the European Union, Luxembourg, https://publications.jrc.ec.europa.eu/repository/handle/JRC118874.
[104] Greenhalgh, E., M. Scollo and M. Winstanley (2022), “Tailored and targeted interventions for low socioeconomic groups”, in Greenhalgh, E. and M. Scollo (eds.), Tobacco in Australia: Facts and issues, Cancer Council Victoria, Melbourne, https://www.tobaccoinaustralia.org.au/chapter-9-disadvantage/9-6-tailored-and-targeted-interventions-for-low-socioeconomic-groups (accessed on 9 August 2023).
[221] Greenpeace (2023), Climate & Public Transport Tickets in Europe, Greenpeace, Vienna, https://greenpeace.at/uploads/2023/05/report-climate-and-public-transport-tickets-in-europe.pdf.
[203] Gregorič, M. et al. (2015), “School nutrition guidelines: Overview of the implementation and evaluation”, Public Health Nutrition, Vol. 18/9, pp. 1582-1592, https://doi.org/10.1017/S1368980014003310.
[133] Gupta, H. et al. (2018), “The association between exposure to social media alcohol marketing and youth alcohol use behaviors in India and Australia”, BMC Public Health, Vol. 18/1, p. 726, https://doi.org/10.1186/s12889-018-5645-9.
[57] Guthmann, J. et al. (2017), “Socioeconomic inequalities to accessing vaccination against human papillomavirus in France: Results of the Health, Health Care and Insurance Survey, 2012”, Rev Epidemiol Sante Publique, Vol. 65/2, pp. 109-117, https://doi.org/10.1016/j.respe.2017.01.100.
[31] HBSC (2023), HBSC Study Data Browser, Health Behaviour in School-aged Children study, https://data-browser.hbsc.org (accessed on 5 December 2023).
[126] Herttua, K., P. Makela and P. Martikainen (2015), “Minimum prices for alcohol and educational disparities in alcohol-related mortality”, Epidemiology, Vol. 26/3, pp. 337-343, https://doi.org/10.1097/EDE.0000000000000260.
[66] Holden, C. et al. (2021), “The role of health literacy in cancer care: A mixed studies systematic review”, PLoS One, Vol. 16/11, p. e0259815, https://doi.org/10.1371/journal.pone.0259815.
[287] Housten, A. et al. (2020), “Health literacy interventions in cancer: A systematic review”, Journal of Cancer Education, Vol. 36/2, pp. 240-252, https://doi.org/10.1007/s13187-020-01915-x.
[263] HPV World (2023), HPV vaccination Crisis and Recovery: The Danish case, https://www.hpvworld.com/articles/hpv-vaccination-crisis-and-recovery-the-danish-case/ (accessed on 2 August 2023).
[246] IARC (2023), Protection from a Single Dose of HPV Vaccine: A major public health impact from IARC studies of vaccine efficacy, International Agency for Research on Cancer, Lyon, https://www.iarc.who.int/wp-content/uploads/2023/04/IARC_Evidence_Summary_Brief_4.pdf (accessed on 2 August 2023).
[181] IARC (2021), The Nutri-Score: A Science-Based Front-of-Pack Nutrition Label: Helping consumers make healthier food choices, International Agency for Research on Cancer, Lyon, https://www.iarc.who.int/wp-content/uploads/2021/09/IARC_Evidence_Summary_Brief_2.pdf.
[9] IARC (2018), Red Meat and Processed Meat, International Agency for Research on Cancer, Lyon, https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono114.pdf.
[234] IEA (2022), New Green Savings program: Boiler subsidies for low income households, https://www.iea.org/policies/16386-new-green-savings-program-boiler-subsidies-for-low-income-households (accessed on 28 July 2023).
[301] IHME (2023), GBD Compare Data Visualisation, http://vizhub.healthdata.org/gbd-compare.
[2] IHME (2019), GBD Compare, Institute for Health Metrics and Evaluation, University of Washington, http://vizhub.healthdata.org/gbd-compare (accessed on 27 July 2023).
[17] ILO (2021), Exposure to Hazardous Chemicals at Work and Resulting Health Impacts: A global review, International Labour Organization, Geneva, https://www.ilo.org/wcmsp5/groups/public/---ed_dialogue/---lab_admin/documents/publication/wcms_811455.pdf.
[52] Jacobson, R. et al. (2016), “The most effective and promising population health strategies to advance human papillomavirus vaccination”, Expert Review of Vaccines, Vol. 15/2, pp. 257-269, https://doi.org/10.1586/14760584.2016.1116947.
[75] Joossens, L. and M. Raw (2006), “The Tobacco Control Scale: A new scale to measure country activity”, Tobacco Control, Vol. 15/3, pp. 247-253, https://doi.org/10.1136/TC.2005.015347.
[45] Kakoulaki, G. et al. (2023), Identification of Vulnerable EU Regions Considering Asbestos Presence and Seismic Risk, Publications Office of the European Union, Luxembourg, https://publications.jrc.ec.europa.eu/repository/handle/JRC133139.
[43] Kameda, T. et al. (2014), “Asbestos: Use, bans and disease burden in Europe”, Bulletin of the World Health Organization, Vol. 92/11, pp. 790-797, https://doi.org/10.2471/blt.13.132118.
[22] Kamolratanakul, S. and P. Pitisuttithum (2021), “Human papillomavirus vaccine efficacy and effectiveness against cancer”, Vaccines, Vol. 9/12, p. 1413, https://doi.org/10.3390/vaccines9121413.
[259] Karafillakis, E. et al. (2019), “HPV vaccination in a context of public mistrust and uncertainty: A systematic literature review of determinants of HPV vaccine hesitancy in Europe”, Human Vaccines & Immunotherapeutics, Vol. 15/7-8, pp. 1615-1627, https://doi.org/10.1080/21645515.2018.1564436.
[233] Karver, J., R. Badiani-Magnusson and J. Carroll (2022), Clean air and heating choices: How to change homeowners’ behavior in Poland, https://blogs.worldbank.org/climatechange/clean-air-and-heating-choices-how-change-homeowners-behavior-poland (accessed on 28 July 2023).
[255] Kaul, S. et al. (2019), “School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas”, Papillomavirus Research, Vol. 8, p. 100189, https://doi.org/10.1016/j.pvr.2019.100189.
[171] Kelly, B., R. Bosward and B. Freeman (2021), “Australian children’s exposure to, and engagement with, web-based marketing of food and drink brands: Cross-sectional observational study”, Journal of Medical Internet Research, Vol. 23/7, p. e28144, https://doi.org/10.2196/28144.
[8] Kerschbaum, E. and V. Nüssler (2019), “Cancer prevention with nutrition and lifestyle”, Visceral Medicine, Vol. 35/4, pp. 204-209, https://doi.org/10.1159/000501776.
[218] Khomenko, S. et al. (2023), “Spatial and sector-specific contributions of emissions to ambient air pollution and mortality in European cities: A health impact assessment”, The Lancet Public Health, Vol. 8/7, pp. e546-e558, https://doi.org/10.1016/s2468-2667(23)00106-8.
[228] Khreis, H. et al. (2023), Urban policy interventions to reduce traffic-related emissions and air pollution: Database for a systematic evidence map., https://carteehdata.org/library/dataset/urban-policy-intervention-f08c (accessed on 1 August 2023).
[124] Kilian, C. et al. (2023), “Reducing alcohol use through alcohol control policies in the general population and population subgroups: A systematic review and meta-analysis”, EClinicalMedicine, Vol. 59, p. 101996, https://doi.org/10.1016/j.eclinm.2023.101996.
[240] Kim, J. et al. (2021), “Human papillomavirus vaccination for adults aged 30 to 45 years in the United States: A cost-effectiveness analysis”, PLoS Medicine, Vol. 18/3, p. e1003534, https://doi.org/10.1371/journal.pmed.1003534.
[200] Kirk, C. et al. (2020), “P46 Can a Healthy School Celebrations Teacher Toolkit change classroom food options? The Food Hero campaign experience”, Journal of Nutrition Education and Behavior, Vol. 52/7, p. S37, https://doi.org/10.1016/j.jneb.2020.04.091.
[298] Kolnik, T. and A. Ljubič (2023), Nacionalna Strategija za Spremljanje in Dvig Zdravstvene Pismenosti [National Strategy for Monitoring and Raising Health Literacy], Ministry of Health, Ljubljana, https://zdravstvena-pismenost.si/wp-content/uploads/2023/06/10-Nacionalna-strategija-za-spremljanje-in-dvig-zdravstvene-pismenosti.pdf.
[297] Kolnik, T., A. Ljubič and V. Kmetič (2023), Projekt “Dvig Zdravstvene Pismenosti v Sloveniji” [Project: “Raising Health Literacy in Slovenia”], Ministry of Health, Ljubljana, https://zdravstvena-pismenost.si/for-professional-public/ (accessed on 11 December 2023).
[161] Lavriša, Ž. and I. Pravst (2019), “Marketing of foods to children through food packaging is almost exclusively linked to unhealthy foods”, Nutrients, Vol. 11/5, p. 1128, https://doi.org/10.3390/nu11051128.
[119] Le service public fédéral (SPF) Santé publique, Sécurité de la Chaîne alimentaire et Environnement (2016), Alcool, https://www.health.belgium.be/fr/sante/prenez-soin-de-vous/alcool-et-tabac/alcool.
[65] Le, C. et al. (2021), Health Literacy in Five Immigrant Populations in Norway: Pakistan, Poland, Somalia, Turkey, and Vietnam. English Summary., Norwegian Directorate of Health., https://www.helsedirektoratet.no/rapporter/befolkningens-helsekompetanse (accessed on 5 July 2023).
[148] Lee, N. et al. (2016), “What works in school-based alcohol education: A systematic review”, Health Education Journal, Vol. 75, pp. 780-798, https://doi.org/10.1177/0017896915612227.
[187] Lehmann, U. et al. (2017), “Nutrient profiling for product reformulation: Public health impact and benefits for the consumer”, Proceedings of the Nutrition Society, Vol. 76/3, pp. 255-264, https://doi.org/10.1017/S0029665117000301.
[243] Likumi (2023), Amendments to the Cabinet of Ministers 2000. Regulation No. 26 of September 330 of “Vaccinations regulations”, Likumi, Riga, https://likumi.lv/ta/id/341661 (accessed on 23 August 2023).
[164] Lobstein, T. (2023), Will food marketing restrictions, food reformulation, or food procurement standards have an impact on health inequities? A Best-ReMaP literature review, with guidance for undertaking, Best-ReMaP: Healthy Food for a Healthy Future, Ljubljana, https://bestremap.eu/wp-content/uploads/2023/05/Health-equity-impact-literature-review_TLobstein_v3.pdf.
[182] Løvhaug, A., S. Granheim and S. Djojosoeparto (2022), “The potential of food environment policies to reduce socioeconomic inequalities in diets and to improve healthy diets among lower socioeconomic groups: An umbrella review”, BMC Public Health, Vol. 22/433, p. 433, https://doi.org/10.1186/s12889-022-12827-4.
[222] Luxembourg Government (2023), Launch of the 15th edition of the campaign “Mam Vëlo op d’Schaff oder an d’Schoul”, https://gouvernement.lu/fr/actualites/toutes_actualites/communiques/2023/05-mai/15-action-mam-velo-op-schaff.html.
[223] Luxembourg Government (2021), “Dry mam Vëlo” campaign and presentation of the new version of the Police’s mobile application, https://gouvernement.lu/fr/actualites/toutes_actualites/articles/2021/05-mai/04-kox-velo.html.
[224] Luxembourg Government (n.d.), mobiliteit.lu, https://www.mobiliteit.lu/en/.
[163] Magnus, A. et al. (2009), “The cost-effectiveness of removing television advertising of high-fat and/or high-sugar food and beverages to Australian children”, International Journal of Obesity, Vol. 33/10, pp. 1094-1102, https://doi.org/10.1038/ijo.2009.156.
[125] Manthey, J., D. Jasilionis and H. Jiang (2023), “The impact of alcohol taxation increase on all-cause mortality inequalities in Lithuania: An interrupted time series analysis”, BMC Medicine, Vol. 21/1, p. 22, https://doi.org/10.1186/s12916-022-02721-6.
[146] Manthey, J. et al. (2021), “Can alcohol consumption in Germany be reduced by alcohol screening, brief intervention and referral to treatment in primary health care? Results of a simulation study”, PLoS One, Vol. 16/8, p. e0255843, https://doi.org/10.1371/journal.pone.0255843.
[176] Mantilla Herrera, A. et al. (2018), “Cost-effectiveness of product reformulation in response to the health star rating food labelling system in Australia”, Nutrients, Vol. 10/5, p. 614, https://doi.org/10.3390/nu10050614.
[194] McGill, R. et al. (2015), “Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact”, BMC public health, Vol. 15/1, pp. 1-15.
[10] McTiernan, A. (2008), “Mechanisms linking physical activity with cancer”, Nature Reviews Cancer, Vol. 8/3, pp. 205-211, https://doi.org/10.1038/nrc2325.
[168] Mediano Stoltze, F. et al. (2019), “Prevalence of child-directed marketing on breakfast cereal packages before and after Chile’s Food Marketing Law: A pre-and post-quantitative content analysis”, International Journal of Environmental Research and Public Health, Vol. 16/22, p. 4501, https://doi.org/10.3390/ijerph16224501.
[23] Meites, E. et al. (2019), “Human papillomavirus vaccination for adults: Updated recommendations of the Advisory Committee on Immunization Practices”, Morbidity and Mortality Weekly Report, Vol. 58/32, pp. 698-702, https://doi.org/10.15585/mmwr.mm6832a3.
[201] Micha, R. et al. (2018), “Effectiveness of school food environment policies on children’s dietary behaviors: A systematic review and meta-analysis”, PLoS One, Vol. 13/3, p. e0194555, https://doi.org/10.1371/journal.pone.0194555.
[296] Ministry of Health (2023), Plano Nacional de Literacia em Saúde e Ciências do Comportamento 2023-2030 – Plano Estratégico [National Health Literacy and Behavioural Sciences Plan 2023-2030 – Strategic Plan], Ministry of Health, Lisbon, https://www.dgs.pt/ficheiros-de-upload-2013/pnlscc-2023-2030-pdf.aspx.
[281] Ministry of Health (2023), Promocja Zdrowia i Profilaktyka Nowotworowa [Health Promotion and Cancer Prevention], Ministrey of Health, Warsaw, https://planujedlugiezycie.pl/index.php/promocja-zdrowia-i-profilaktyka-nowotworowa/.
[107] Ministry of Health (2023), Smokefree Aotearoa 2025 Action Plan, Ministry of Health, Wellington, https://www.health.govt.nz/system/files/documents/publications/hp7801_-_smoke_free_action_plan_v15_web.pdf (accessed on 11 July 2023).
[215] Ministry of Health (2022), Programa Nacional para a Promoção da Atividade Física [National Programme for the Promotion of Physical Activity], Ministry of Health, Lisbon, https://www.dgs.pt/programa-nacional-para-a-promocao-da-atvidade-fisica/ficheiros-externos-pnpaf/relatorio-anual-20221.aspx.
[214] Ministry of Health (2020), Aconselhamento breve para a alimentação saudável nos cuidados de saúde primários: Modelo de intervenção e ferramentas 2020, Ministry of Health, Lisbon, https://nutrimento.pt/activeapp/wp-content/uploads/2021/01/PNPAS_aconselhamentobreve-.pdf.
[244] Ministry of Health of Romania (2023), Order no. 3120/2023 for the approval of the population segments that benefit from the prescription, release and settlement under the compensation regime of immunological drugs used to produce active immunity or used to prevent communicable diseases.
[91] Moodie, C. et al. (2022), “Plain tobacco packaging: Progress, challenges, learning and opportunities”, Tobacco Control, Vol. 31/2, pp. 263-271, https://doi.org/10.1136/tobaccocontrol-2021-056559.
[276] M-POHL (2023), Measuring Organizational Health Literacy, M-POHL, Vienna, https://m-pohl.net/sites/m-pohl.net/files/inline-files/Factsheet%20OHL_1.pdf (accessed on 9 August 2023).
[277] M-POHL (2023), M-POHL - WHO Action Network on Measuring Population and Organizational Health Literacy, https://m-pohl.net/ (accessed on 10 December 2023).
[64] M-POHL (2021), International Report on the Methodology, Results, and Recommendations of the European Health Literacy Population Survey 2019-2021 (HLS19) of M-POHL, Austrian National Public Health Institute, Vienna, https://m-pohl.net/sites/m-pohl.net/files/inline-files/HLS19%20International%20Report.pdf.
[89] National Cancer Institute (2008), The Role of the Media in Promoting and Reducing Tobacco Use, National Cancer Institute, Bethesda, MD, https://cancercontrol.cancer.gov/brp/tcrb/monographs/monograph-19.
[134] Ng Fat, L., N. Cable and Y. Kelly (2021), “Associations between social media usage and alcohol use among youths and young adults: Findings from Understanding Society”, Addiction, Vol. 116/11, pp. 2995-3005, https://doi.org/10.1111/add.15482.
[174] Ni Mhurchu, C., H. Eyles and Y. Choi (2017), “Effects of a voluntary front-of-pack nutrition labelling system on packaged food reformulation: The health star rating system in New Zealand”, Nutrients, Vol. 9/8, p. 918, https://doi.org/10.3390/nu9080918.
[158] Niebylski, M. et al. (2015), “Healthy food subsidies and unhealthy food taxation: A systematic review of the evidence”, Nutrition, Vol. 31/6, pp. 787-795, https://doi.org/10.1016/j.nut.2014.12.010.
[175] Nohlen, H. et al. (2022), Front-of-pack nutrition labelling schemes: An update of the evidence. Addendum to the JRC Science for Policy report “Front-of-pack nutrition labelling schemes: a comprehensive review,” published in 2020, Publications Office of the European Union, Luxembourg, https://publications.jrc.ec.europa.eu/repository/handle/JRC130125.
[122] O’Donnell, A. et al. (2019), “Immediate impact of minimum unit pricing on alcohol purchases in Scotland: Controlled interrupted time series analysis for 2015-18”, BMJ, Vol. 366, p. l5274, https://doi.org/10.1136/bmj.l5274.
[131] OECD (2023), EU Country Cancer Profiles 2023, https://www.oecd.org/health/eu-cancer-profiles.htm.
[32] OECD (2023), Health at a Glance 2023: OECD indicators, OECD Publishing, Paris, https://doi.org/10.1787/7a7afb35-en.
[116] OECD (2022), Consumption Tax Trends 2022: VAT/GST and excise, core design features and trends, OECD Publishing, Paris, https://doi.org/10.1787/6525a942-en.
[35] OECD (2022), Healthy Eating and Active Lifestyles: Best practices in public health, OECD Publishing, Paris, https://doi.org/10.1787/40f65568-en.
[210] OECD (2022), Primary Health Care for Resilient Health Systems in Latin America, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/743e6228-en.
[144] OECD (2022), Promoting Health and Well-being at Work: Policy and Practices, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/e179b2a5-en.
[115] OECD (2021), Preventing Harmful Alcohol Use, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/6e4b4ffb-en.
[209] OECD (2020), Realising the Potential of Primary Health Care, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/a92adee4-en.
[101] OECD (2019), Health for Everyone? Social Inequalities in Health and Health Systems, OECD Publishing, Paris, https://doi.org/10.1787/3c8385d0-en.
[154] OECD (2019), The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/67450d67-en.
[226] OECD (forthcoming), Healthy and Sustainable Cities, Best Practices in Public Health.
[225] OECD (forthcoming), Healthy and Sustainable Cities: Best Practices in Public Health.
[15] OECD/EU (2020), Health at a Glance: Europe 2020. State of Health in the EU Cycle, OECD Publishing, Paris, https://doi.org/10.1787/82129230-en.
[37] OECD/WHO (2023), Step Up! Tackling the Burden of Insufficient Physical Activity in Europe, OECD Publishing, Paris, https://doi.org/10.1787/500a9601-en.
[197] Oregon State University (2023), Food Hero, https://foodhero.org/ (accessed on 8 December 2023).
[257] Patel, C. et al. (2018), “The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent?”, Eurosurveillance, Vol. 23/41, p. 1700737, https://doi.org/10.2807/1560-7917.ES.2018.23.41.1700737.
[247] PERCH (2023), PartnERship to Contrast HPV, https://www.projectperch.eu/ (accessed on 10 December 2023).
[258] Perman, S. et al. (2017), “School-based vaccination programmes: A systematic review of the evidence on organisation and delivery in high income countries”, BMC Public Health, Vol. 17, pp. 1-11, https://doi.org/10.1186/s12889-017-4168-0.
[241] Petit, B. and O. Epaulard (2020), “Men having sex with men and the HPV vaccine in France: A low vaccine coverage that may be due to its infrequent proposal by physicians”, Vaccine, Vol. 38/9, pp. 2160-2165, https://doi.org/10.1016/j.vaccine.2020.01.049.
[212] Petric, K. et al. (2019), Participatory approaches to reaching the Sustainable Development Goals: Slovenia: Integrating population and individual services to reduce health inequalities at the community level through health-promotion centres, WHO Regional Office for Europe, Copenhagen, https://iris.who.int/bitstream/handle/10665/346156/WHO-EURO-2019-3489-43248-60609-eng.pdf.
[299] Pharos (2023), Low Literacy and Limited Health Literacy, https://www.pharos.nl/thema/laaggeletterdheid-gezondheidsvaardigheden/ (accessed on 11 December 2023).
[106] Pharos (2023), Programma – Een rookvrij leven voor iedereen [Programme – A smoke-free life for all], https://www.pharos.nl/over-pharos/programmas-pharos/programma-een-rookvrij-leven-voor-iedereen/ (accessed on 12 September 2023).
[248] Polonijo, A. (2020), “The impact of school-entry mandates on social inequalities in human papillomavirus vaccination”, SSM Population Health, Vol. 12, p. 100647, https://doi.org/10.1016/j.ssmph.2020.100647.
[207] Proper, K. and S. van Oostrom (2019), “The effectiveness of workplace health promotion interventions on physical and mental health outcomes – a systematic review of reviews”, Scandinavian Journal of Work, Environment & Health, Vol. 45/6, pp. 546-559, https://doi.org/10.5271/sjweh.3833.
[249] Public Health Agency of Canada (2022), Vaccine Hesitancy in Canadian Parents, Public Health Agency of Canada, Ottawa, https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-hesitancy-canadian-parents.html (accessed on 2 August 2023).
[229] Public Health England (2019), Review of Interventions to Improve Outdoor Air Quality and Public Health, Public Health England, London, https://assets.publishing.service.gov.uk/media/5fbf93258fa8f559dbb1add9/Review_of_interventions_to_improve_air_quality_March-2019-2018572.pdf (accessed on 26 July 2023).
[72] Public Health France (2023), Quelles sont les dispositions de lutte contre le tabagisme en France ? [What are the anti-smoking provisions in France?], https://www.santepubliquefrance.fr/determinants-de-sante/tabac/articles/quelles-sont-les-dispositions-de-lutte-contre-le-tabagisme-en-france (accessed on 18 September 2023).
[230] Rawat, N. and P. Kumar (2023), “Interventions for improving indoor and outdoor air quality in and around schools”, Science of The Total Environment, Vol. 858, p. 159813, https://doi.org/10.1016/j.scitotenv.2022.159813.
[266] RIVER-EU (2023), Increase MMR and HPV Vaccine Uptake in Underserved Communities, Thereby Boosting Herd Immunity for all Europe, https://river-eu.org/ (accessed on 3 August 2023).
[272] RIVM (2023), Sexually Transmitted Infections in the Netherlands in 2022, National Institute for Public Health and the Environment, Bilthoven, https://rivm.openrepository.com/handle/10029/626792.
[252] Robert Koch Institut (2023), Intervention Study to Increase HPV Vaccination Rates in Germany - InveSt HPV. Interventionsstudie zur Steigerung der HPV-Impfquoten in Deutschland - InveSt HPV, https://www.rki.de/DE/Content/Infekt/Impfen/Forschungsprojekte/InvestHPV/InvestHPV.html.
[127] Roche, A. et al. (2015), “Addressing inequities in alcohol consumption and related harms”, Health Promotion International, Vol. 30/Suppl 2, pp. ii20-35, https://doi.org/10.1093/heapro/dav030.
[157] Rogers, N. et al. (2023), “Changes in soft drinks purchased by British households associated with the UK soft drinks industry levy: a controlled interrupted time series analysis”, BMJ open, Vol. 13/12, p. e077059.
[128] Room, R. (2021), “Alcohol monopolies”, Scottish Health Action on Alcohol Problems (SHAAP), https://www.shaap.org.uk/blog/332-alcohol-monopolies.html (accessed on 8 December 2023).
[155] Royo-Bordonada, M. et al. (2019), “Impact of an excise tax on the consumption of sugar-sweetened beverages in young people living in poorer neighbourhoods of Catalonia, Spain: A difference in differences study”, BMC Public Health, Vol. 19/1, pp. 1-11, https://doi.org/10.1186/s12889-019-7908-5.
[6] Rumgay, H. et al. (2023), “The cost of premature death from cancer attributable to alcohol: Productivity losses in Europe in 2018”, Cancer Epidemiology, Vol. 84, p. 102365, https://doi.org/10.1016/j.canep.2023.102365.
[166] Safe Food Advocacy Europe (2023), Norway to ban unhealthy food adverts aimed at kids, https://www.safefoodadvocacy.eu/norway-to-ban-unhealthy-food-adverts-aimed-at-kids/ (accessed on 10 August 2023).
[14] Safiri, S. et al. (2022), “Global, regional and national burden of cancers attributable to high fasting plasma glucose in 204 countries and territories, 1990-2019”, Frontiers in Endocrinology, Vol. 13, p. 879890.
[159] Saha, S. et al. (2021), “In search of an appropriate mix of taxes and subsidies on nutrients and food: A modelling study of the effectiveness on health-related consumption and mortality”, Social Science & Medicine, Vol. 287, p. 114388, https://doi.org/10.1016/j.socscimed.2021.114388.
[11] Sami, W. et al. (2017), “Effect of diet on type 2 diabetes mellitus: A review”, International Journal of Health Sciences, Vol. 11/2, pp. 65-71.
[184] Santé Publique France (2021), Le Nutri-Score: un logo bien intégré dans le quotidien des Français, https://www.santepubliquefrance.fr/les-actualites/2021/le-nutri-score-un-logo-bien-integre-dans-le-quotidien-des-francais (accessed on 8 December 2023).
[132] Sargent, J. and T. Babor (2020), “The relationship between exposure to alcohol marketing and underage drinking is causal”, Journal of Studies on Alcohol and Drugs. Supplement, Vol. Suppl 19, pp. 113-124, https://doi.org/10.15288/jsads.2020.s19.113.
[5] SCHEER (2021), Opinion on Electronic Cigarettes, https://health.ec.europa.eu/system/files/2022-08/scheer_o_017.pdf (accessed on 5 December 2023).
[268] Schloemer, T., J. de Zeeuw and B. van Enter (2023), Working Out What Works: A participatory project with Turkish and Moroccan communities in the Netherlands to improve HPV vaccine uptake, RIVER-EU, Groningen, https://river-eu.org/wp-content/uploads/2023/07/Schloemer.T_2023_Transferability_NL.pdf.
[283] Schüz, J. et al. (2015), “European Code against Cancer 4th Edition: 12 ways to reduce your cancer risk”, Cancer Epidemiol, Vol. 39/Supplement 1, pp. S1-S10, https://doi.org/10.1016/j.canep.2015.05.009.
[130] Sherk, A. et al. (2023), “The public-private decision for alcohol retail systems: Examining the economic, health, and social impacts of alternative systems in Finland”, Nordic Studies on Alcohol and Drugs, Vol. 40/3, pp. 218-232, https://doi.org/10.1177/14550725231160335.
[183] Shrestha, A. et al. (2023), “Impact of front-of-pack nutrition labelling in consumer understanding and use across socio-economic status: A systematic review”, Appetite, Vol. 187, p. 106587, https://doi.org/10.1016/j.appet.2023.106587.
[86] Sigfusdottir, I. et al. (2008), “Trends in prevalence of substance use among Icelandic adolescents, 1995–2006”, Substance Abuse Treatment, Prevention, and Policy, Vol. 3/1, https://doi.org/10.1186/1747-597x-3-12.
[55] Slåttelid Schreiber, S. et al. (2015), “Socioeconomic predictors of human papillomavirus vaccination among girls in the Danish Childhood Immunization Program”, Journal of Adolescent Health, Vol. 56/4, pp. 402-407, https://doi.org/10.1016/j.jadohealth.2014.12.008.
[102] Smith, C., S. Hill and A. Amos (2021), “Impact of population tobacco control interventions on socioeconomic inequalities in smoking: A systematic review and appraisal of future research directions”, Tobacco Control, Vol. 30/e2, pp. e87-e95, https://doi.org/10.1136/tobaccocontrol-2020-055874.
[264] Soborg, B. and S. Jaconsen (2019), “Designing and evaluating an information campaign on HPV vaccination in Denmark”, European Journal of Public Health, Vol. 29/Supplement_4, https://doi.org/10.1093/eurpub/ckz185.212.
[279] Sørensen, K. (2020), “Health literacy is an emerging strategic priority in national cancer control plans in the EU”, Journal of Cancer Policy, Vol. 26, p. 100255, https://doi.org/10.1016/j.jcpo.2020.100255.
[294] Sørensen, K. et al. (2021), “Building health literacy system capacity: A framework for health literate systems”, Health Promotion International, Vol. 36/Supplement_1, pp. i13-i23, https://doi.org/10.1093/heapro/daab153.
[275] Sørensen, K. et al. (2012), “Health literacy and public health: A systematic review and integration of definitions and models”, BMC Public Health, Vol. 12/1, pp. 1-13, https://doi.org/10.1186/1471-2458-12-80.
[208] Sponselee, H. et al. (2022), “Perceptions of employees with a low and medium level of education towards workplace health promotion programmes: A mixed-methods study”, BMC Public Health, Vol. 22/1, p. 1617, https://doi.org/10.1186/s12889-022-13976-2.
[129] Stockwell, T. et al. (2018), “Estimating the public health impact of disbanding a government alcohol monopoly: Application of new methods to the case of Sweden”, BMC Public Health, Vol. 18/1, p. 1400, https://doi.org/10.1186/s12889-018-6312-x.
[177] Stoltze, F. et al. (2018), “Prevalence of child-directed and general audience marketing strategies on the front of beverage packaging: The case of Chile”, Public Health Nutrition, Vol. 21/3, pp. 454-464, https://doi.org/10.1017/S1368980017002671.
[83] Strassmann, A. et al. (2023), “Nationwide indoor smoking ban and impact on smoking behaviour and lung function: a two-population natural experiment”, Thorax, Vol. 78/2, pp. 144-150, https://doi.org/10.1136/thoraxjnl-2021-218436.
[260] Suppli, C. et al. (2018), “Decline in HPV-vaccination uptake in Denmark – the association between HPV-related media coverage and HPV-vaccination”, BMC Public Health, Vol. 18/1, p. 1360, https://doi.org/10.1186/s12889-018-6268-x.
[213] Susič, A. and Z. Klemenc-Ketiš (2020), “Successful implementation of integrated care in Slovenian primary care”, Slovenian Journal of Public Health, Vol. 60/1, pp. 1-3, https://doi.org/10.2478/sjph-2021-0001.
[60] Sypień, P. and T. Zielonka (2022), “Knowledge and awareness of Polish parents on vaccination against human papillomavirus”, Vaccines, Vol. 10/7, p. 1156, https://doi.org/10.3390/vaccines10071156.
[152] Taillie, L. et al. (2017), “Do high vs. low purchasers respond differently to a nonessential energy-dense food tax? Two-year evaluation of Mexico’s 8% nonessential food tax”, Preventive Medicine, Vol. 105, pp. S37-S42, https://doi.org/10.1016/j.ypmed.2017.07.009.
[179] Talati, Z. et al. (2017), “Consumers’ responses to health claims in the context of other on-pack nutrition information: A systematic review”, Nutrition reviews, Vol. 75/4, pp. 260-273, https://doi.org/10.1093/nutrit/nuw070.
[84] TCS (2022), The Tobacco Control Scale 2021 in Europe: Recommendations and References, https://www.tobaccocontrolscale.org/recommendations-and-references-2021/ (accessed on 6 July 2023).
[95] TCS (2021), The Reports, https://www.tobaccocontrolscale.org/the-reports/ (accessed on 6 July 2023).
[293] The Austrian Health Literacy Alliance (2023), Austrial Platform on Health Literacy, https://oepgk.at/ (accessed on 11 December 2023).
[98] Thomas, R., J. McLellan and R. Perera (2013), “School-based programmes for preventing smoking”, Cochrane Database of Systematic Reviews, Vol. 2013/4, p. CD001293, https://doi.org/10.1002/14651858.cd001293.pub3.
[97] Tildy, B. et al. (2023), “Implementation strategies to increase smoking cessation treatment provision in primary care: A systematic review of observational studies”, BMC Primary Care, Vol. 24/1, pp. 1-61, https://doi.org/10.1186/S12875-023-01981-2.
[196] Tobey, L. et al. (2011), “Focus on fruits and vegetables for low-income families: Framework for the food hero social marketing campaign in Oregon”, Journal of the American Dietetic Association, Vol. 9/111, p. A12, https://doi.org/10.1016/j.jada.2011.06.037.
[198] Tobey, L. et al. (2016), “Reaching low-income mothers to improve family fruit and vegetable intake: food hero social marketing campaign—research steps, development and testing”, Nutrients, Vol. 8/9, p. 562, https://doi.org/10.3390/nu8090562.
[199] Tobey, L. et al. (2017), “Can healthy recipes change eating behaviors? The Food Hero social marketing campaign recipe project experience and evaluation”, Journal of Nutrition Education and Behavior, Vol. 49/1, pp. 79-82, https://doi.org/10.1016/j.jneb.2016.09.001.
[189] Trieu, K. et al. (2015), “Salt reduction initiatives around the world – a systematic review of progress towards the global target”, PLoS One, Vol. 10/7, p. e0130247, https://doi.org/10.1371/journal.pone.0130247.
[105] Urban, M. et al. (2015), “Tobacco addiction and smoking cessation in Austrian migrants: A cross-sectional study”, BMJ Open, Vol. 5/6, pp. e006510-e006510, https://doi.org/10.1136/bmjopen-2014-006510.
[4] Valen, H. et al. (2023), “A systematic review of cancer risk among users of smokeless tobacco (Swedish snus) exclusively, compared with no use of tobacco”, International Journal of Cancer, Vol. 153/12, pp. 1942-1953, https://doi.org/10.1002/ijc.34643.
[96] van Westen-Lagerweij, N. et al. (2022), “Mentioning smoking cessation assistance during healthcare consultations matters: Findings from Dutch survey research”, European Journal of Public Health, Vol. 32/5, pp. 747-752, https://doi.org/10.1093/eurpub/ckac106.
[50] Vandermeulen, C. et al. (2017), Studie van de vaccinatiegraad in Vlaanderen 2016, Katholieke Universiteit Leuven, Leuven, https://www.laatjevaccineren.be/sites/default/files/2022-04/Vaccinatiegraadstudie%202016.pdf.
[265] Vanderpool, R., L. Stradtman and H. Brandt (2019), “Policy opportunities to increase HPV vaccination in rural communities”, Human Vaccines & Immunotherapeutics, Vol. 15/7-8, pp. 1527-1532, https://doi.org/10.1080/21645515.2018.1553475.
[190] Vandevijvere, S. and L. Vanderlee (2019), “Effect of formulation, labelling, and taxation policies on the nutritional quality of the food supply”, Current Nutrition Reports, Vol. 8, pp. 240-249, https://doi.org/10.1007/s13668-019-00289-x.
[232] Verbeek, T. and S. Hincks (2022), “The ‘just’ management of urban air pollution? A geospatial analysis of low emission zones in Brussels and London”, Applied Geography, Vol. 140, p. 102642, https://doi.org/10.1016/j.apgeog.2022.102642.
[49] Vermeeren, A. and F. Goffin (2018), Statistique de couverture vaccinale en 2ème secondaire en Fédération Wallonie - Bruxelles en 2016 - 2017, Office de la Naissance et de l’Enfance, Brussels, http://hdl.handle.net/2078.1/216150.
[282] Walters, R. et al. (2020), “Establishing the efficacy of interventions to improve health literacy and health behaviours: A systematic review”, BMC Public Health, Vol. 20/1, p. 1040, https://doi.org/10.1186/s12889-020-08991-0.
[13] Wang, M., Y. Yang and Z. Liao (2020), “Diabetes and cancer: Epidemiological and biological links”, World journal of diabetes, Vol. 11/6, p. 227.
[206] Wang, Y. et al. (2015), “What childhood obesity prevention programmes work? A systematic review and meta‐analysis”, Obesity Reviews, Vol. 16/7, pp. 547-565, https://doi.org/10.1111/obr.12277.
[167] WCRFI (2023), NOURISHING and MOVING policy database, https://policydatabase.wcrf.org/nourishing-moving-search (accessed on 8 December 2023).
[160] WCRFI (2023), NOURISHING policy index: Nutrition policy status in 30 European countries, World Cancer Research Fund International, London, https://www.wcrf.org/wp-content/uploads/2023/05/NOURISHING-Policy-Brief-May-2023.pdf.
[58] Wemrell, M. and L. Gunnarsson (2022), “Attitudes toward HPV vaccination in Sweden: A survey study”, Frontiers in Public Health, Vol. 10, p. 729497, https://doi.org/10.3389/fpubh.2022.729497.
[59] Wemrell, M., R. Perez Vicente and J. Merlo (2023), “Mapping sociodemographic and geographical differences in human papillomavirus non-vaccination among young girls in Sweden”, Scandinavian Journal of Public Health, Vol. 51/2, pp. 288-295, https://doi.org/10.1177/14034948221075410.
[48] WHO (2023), Cervical Cancer Elimination Initiative, https://www.who.int/initiatives/cervical-cancer-elimination-initiative (accessed on 1 August 2023).
[217] WHO (2023), Countries of the WHO European Region Adopt Budapest Declaration, Pushing Action to Enhance Environment and Health, https://www.who.int/europe/news/item/07-07-2023-countries-of-the-who-european-region-adopt-budapest-declaration--pushing-action-to-enhance-environment-and-health (accessed on 9 December 2023).
[270] WHO (2023), “Human Papillomavirus (HPV) vaccination coverage”, Global Health Observatory Database, https://immunizationdata.who.int/pages/coverage/hpv.html (accessed on 30 November 2023).
[36] WHO (2023), Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases, https://www.who.int/tools/elena/interventions/fruit-vegetables-ncds (accessed on 6 December 2023).
[193] WHO (2023), More Ways, to Save More Lives, for Less Money: World Health Assembly adopts more Best Buys to tackle noncommunicable diseases, https://www.who.int/news/item/26-05-2023-more-ways--to-save-more-lives--for-less-money----world-health-assembly-adopts-more-best-buys--to-tackle-noncommunicable-diseases (accessed on 8 December 2023).
[300] WHO (2023), Preventing Cancer, https://www.who.int/activities/preventing-cancer (accessed on 11 December 2023).
[103] WHO (2023), Raising Taxes on Tobacco, World Health Organization, Geneva, https://www.who.int/activities/raising-taxes-on-tobacco (accessed on 6 December 2023).
[143] WHO (2023), Screening and Brief Interventions for Substance Use Problems, https://www.who.int/activities/screening-and-brief-interventions-for-substance-use-problems (accessed on 8 December 2023).
[74] WHO (2023), WHO Framework Convention on Tobacco Control, https://fctc.who.int/who-fctc/overview (accessed on 30 June 2023).
[3] WHO (2023), WHO Report on the Global Tobacco Epidemic, 2023: Protect people from tobacco smoke, World Health Organization, Geneva, https://iris.who.int/handle/10665/372043.
[139] WHO (2022), European Framework for Action on Alcohol, 2022–2025: Information sheet, WHO Regional Office for Europe, Copenhagen, https://www.who.int/europe/publications/m/item/european-framework-for-action--on-alcohol--2022-2025.
[63] WHO (2022), Health Literacy Development for the Prevention and Control of Noncommunicable Diseases: Volume 1. Overview, World Health Organization, Geneva, https://www.who.int/publications/i/item/9789240055339.
[295] WHO (2022), Health Literacy Development Project Series: Co-designing health literacy initiatives in migrant communities in Portugal, https://www.who.int/news/item/04-12-2022-health-literacy-demonstration-project-series--co-designing-health-literacy-initiatives-in-migrant-communities-in-portugal (accessed on 11 December 2023).
[239] WHO (2022), HPV Global Market Study, World Health Organization, Geneva, https://cdn.who.int/media/docs/default-source/immunization/mi4a/who-hpv-vaccine-global-market-study-april-2022.pdf (accessed on 2 August 2023).
[121] WHO (2022), No Place for Cheap Alcohol: The potential value of minimum pricing for protecting lives, World Health Organization, Geneva, https://www.who.int/europe/publications/i/item/9789289058094.
[245] WHO (2022), One-dose Human Papillomavirus (HPV) Vaccine Offers Solid Protection against Cervical Cancer, https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer (accessed on 2 August 2023).
[136] WHO (2021), Health Warning Labels on Alcoholic Beverages: Opportunities for informed and healthier choices, World Health Organization, Geneva, https://www.who.int/publications/i/item/9789240044449.
[113] WHO (2021), Saving Lives, Spending Less: The case for investing in noncommunicable diseases, World Health Organization, Geneva, https://www.who.int/publications/i/item/9789240041059.
[76] WHO (2021), “Tobacco control”, Global Health Observatory Data Repository, https://apps.who.int/gho/data/node.main.Tobacco?lang=en (accessed on 30 June 2023).
[38] WHO (2021), WHO Global Air Quality Guidelines: Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide, World Health Organization, Geneva, https://iris.who.int/bitstream/handle/10665/345329/9789240034228-eng.pdf.
[165] WHO (2020), Spotlight on Adolescent Health and Well-being. Findings from the 2017/2018 Health Behaviour in School-aged Children (HBSC) survey in Europe and Canada. International report: summary, World Health Organization, Geneva, https://iris.who.int/bitstream/handle/10665/357175/WHO-EURO-2020-5747-45512-65149-eng.pdf.
[269] WHO (2017), Action plan for the health sector response to viral hepatitis in the WHO European Region.
[61] WHO (2017), Global Hepatitis Report, 2017, World Health Organization, Geneva, https://iris.who.int/bitstream/handle/10665/255016/9789241565455-eng.pdf?sequence=1&isAllowed=y.
[114] WHO (2017), Tackling NCDs: “Best buys” and other recommended interventions for the prevention and control of noncommunicable diseases, World Health Organization, Geneva, https://www.who.int/publications/i/item/WHO-NMH-NVI-17.9.
[85] WHO (2016), Earmarked Tobacco Taxes: Lessons learnt from nine countries, World Health Organization, Geneva, https://iris.who.int/bitstream/handle/10665/206007/9789241510424_eng.pdf?sequence=1&isAllowed=y.
[173] WHO (2015), European Food and Nutrition Action Plan 2015–2020, WHO Regional Office for Europe, Copenhagen, https://iris.who.int/bitstream/handle/10665/329405/9789289051231-eng.pdf.
[112] WHO (2013), Global Action Plan For the Prevention and Control of Noncommunicable Diseases 2013-2020, World Health Organization, Geneva, https://www.who.int/publications/i/item/9789241506236.
[46] Wilk, E. and M. Krówczyńska (2021), “Malignant mesothelioma and asbestos exposure in Europe: Evidence of spatial clustering”, Geospatial Health, Vol. 16/1, https://doi.org/10.4081/gh.2021.951.
[73] Willemsen, M. and J. Been (2022), “Accelerating tobacco control at the national level with the Smoke-free Generation movement in the Netherlands”, NPJ Primary Care Respiratory Medicine, Vol. 32/1, p. 58, https://doi.org/10.1038/s41533-022-00321-8.
[108] Woodruff, S., G. Talavera and J. Elder (2002), “Evaluation of a culturally appropriate smoking cessation intervention for Latinos”, Tobacco Control, Vol. 11/4, pp. 361-367, https://doi.org/10.1136/tc.11.4.361.
[123] Wyper, G. et al. (2023), “Evaluating the impact of alcohol minimum unit pricing on deaths and hospitalisations in Scotland: A controlled interrupted time series study”, The Lancet, Vol. 401/10385, pp. 1361-1370, https://doi.org/10.1016/S0140-6736(23)00497-X.
[47] Zadnik, V. et al. (2017), “Time trends and spatial patterns in the mesothelioma incidence in Slovenia, 1961-2014”, European Journal of Cancer Prevention, Vol. 26, pp. S191-S196, https://doi.org/10.1097/CEJ.0000000000000384.
[137] Zhao, J. et al. (2020), “The effects of alcohol warning labels on population alcohol consumption: An interrupted time series analysis of alcohol sales in Yukon, Canada”, Journal of Studies on Alcohol and Drugs, Vol. 81/2, pp. 225-237.
Notes
← 1. Where not stated otherwise, the data and information used in Section 3.2 are based on national official statistics provided to Eurostat and the OECD, which were validated to ensure the highest standards of data comparability. The sources and methods underlying these data are available in the Eurostat Database and the OECD Health Database.
← 2. Countries that responded to the 2023 OECD Policy Survey on Cancer Care Performance include: Austria, Belgium, Bulgaria, Croatia, Czechia, Cyprus, Denmark, Estonia, France, Germany, Greece, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, the Slovak Republic, Slovenia, Spain and Sweden. Information from Belgium is not available about risk factors.
← 3. Customs and Excise Department (2023), Excise Duties, www.mof.gov.cy/mof/customs/customs.nsf/All/A2C3593B5465A799422577D6002FEAC4.
European Commission (2006), School Food Policy Country Factsheets: Luxembourg, https://joint-research-centre.ec.europa.eu/system/files/2017-07/jrc-school-food-policy-factsheet-luxembourg_en.pdf.
European Commission (2012), School Food Policy Country Factsheets: Cyprus, https://joint-research-centre.ec.europa.eu/system/files/2017-07/jrc-school-food-policy-factsheet-cyprus_en.pdf.
Hornung, J. and F. Sager (2023), “The non-use of evidence in the adoption of a sugar-sweetened beverage tax in OECD countries”, European Journal of Public Health, Vol. 33/4, pp. 659‑664, https://doi.org/10.1093/eurpub/ckad098.
International Trade Administration (2022), Labeling and Marking Requirements: Republic of Cyprus, www.trade.gov/country-commercial-guides/cyprus-labeling-and-marking-requirements.
Just Drinks (2022), Romania to introduce hike in tax on sugary drinks, www.just-drinks.com/news/romania-to‑introduce‑hike‑in-tax-on-sugary-drinks/.
Le Gouvernement du Grand-Duché de Luxembourg (2021), Règlement grand-ducal du 7 mai 2021 relatif à l’utilisation du logo Nutri-Score, https://legilux.public.lu/eli/etat/leg/rgd/2021/05/07/a396/jo.
Le Gouvernement du Grand-Duché de Luxembourg (2023), Taux des droits d’accise, https://douanes.public.lu/fr/accises/taux-droits-accise.html.
Ministry of Education, Sport and Youth (2023), Primary School Price List, http://archeia.moec.gov.cy/mc/576/promitheftes_de.pdf.
Yle News (2021), Government Report Urges Ban on Social Media Junk Food Adverts for Kids, https://yle.fi/a/3‑12102632.
WHO (2021), Cyprus: Physical Activity Factsheet 2021, https://cdn.who.int/media/docs/librariesprovider2/country-sites/physical-activity-2021-cyprus-eng.pdf
WHO (2023), Global Database on the Implementation of Nutrition action (GINA): Policies by country, https://extranet.who.int/nutrition/gina/en/policies/summary.
Comments from the EU Expert Thematic Group on Cancer Inequalities Registry.