This chapter focuses on the state of play in relation to the assessment of pesticides and the process of approval/(re)registration of new and existing active ingredients and pesticide products. In particular, it describes the registration scope, strategy, process, and data requirements, and how Mexico performs the evaluation of pesticides during this process. It also presents information on how Mexico revokes pesticide registrations of pesticides already registered and on the market. This chapter also includes a review of the current approach to how regulators monitor and enforce compliance with regulatory requirements as they relate to pesticide management
Regulatory Governance in the Pesticide Sector in Mexico

2. Central aspects of pesticide regulation: registration, evaluation and enforcement
Abstract
Mexico has a mandatory registration scheme for pesticides in place
Pesticide registration is a scientific, legal, and administrative procedure undertaken before a pesticide product can be sold and used. It aims to ensure that the product is effective for its intended purpose and does not pose an unacceptable risk to human or animal health or the environment (Frezal and Garsous, 2020[1]; FAO & WHO, 2013[2]).
Companies that want to produce a new pesticide or active ingredient must submit a registration dossier to the competent government authority in the country of intended use. A pesticide product will be authorised for sale or export in a specific country only after a complete review and assessment of the submitted pesticide dossier and approval by the responsible authority (Frezal and Garsous, 2020[1]).
Internationally recognised elements of a pesticide registration scheme
Each country remains independent in deciding on its pesticide registration scheme. Nevertheless, the OECD (OECD, n.d.[3]), FAO and WHO have developed guidelines for governments concerning potential elements of such schemes to support countries’ efforts and, where feasible, promote international harmonisation. They call for, among other things, that governments introduce the necessary legislation for the registration of pesticides. This should include establishment of a registration procedure, based on the principle that the sale and use of pesticides that have not been registered are prohibited. A comparison of FAO and WHO guidelines with the situation in Mexico is provided in Table 2.1. As it is observed in Chapter 1, the Mexican pesticide management framework covering the pesticide registration scheme does not currently have one unified objective or aim.
Table 2.1. Comparison of the FAO and WHO guidelines on the regulatory elements of the pesticides registration scheme and Mexico’s requirements
|
FAO and WHO guidelines |
Mexico’s regulatory framework |
|---|---|
|
Application procedure |
Yes |
|
Data requirements |
Yes |
|
Main criteria for decision-making on registration |
Limited to formal aspects (e.g. If an applicant does not provide additional information requested, the application is considered null and void) |
|
Communication of the justification of the decision |
Yes |
|
Validity periods for registrations |
Only for pesticides registered after 2005 |
|
Provisions that a registration can be reviewed at any time (which could lead to cancellation of the registration) |
Registration can be cancelled, but no systematic process of re-evaluation of pesticides registrations in place |
|
Enshrined appeal procedure; |
Yes |
|
Provisions on confidentiality, protection of Intellectual Property Rights |
Yes |
|
Provisions on dissemination of publically available information. |
Yes |
|
Defining the “unacceptable risk” |
No |
Source: Author based on (FAO & WHO, 2013[2]; FAO & WHO, 2015[4]).
In many countries, the evaluation of the biological efficacy of a pesticide is part of the registration procedure. Companies submitting a product for registration must supply data on its efficacy on the crops or for the uses involved. An assessment of the efficacy of a pesticide usually includes data on its direct efficacy, the sustainability (Box 2.1) of its application and (sometimes) the economic impact of registering the product. In relation to agronomic sustainability, key questions include whether registering the pesticide is compatible with or contributes to sustainable production practices or existing integrated pest management (IPM), and whether it may jeopardise the future development of IPM in the crop (FAO, 2006[5]).
Box 2.1. Best practice - crop profiles and crop timelines
Crop profiles and crop timelines, as they are produced for instance in North America, may be a useful tool for sustainability assessments. Crop profiles are descriptions of crop production and pest management practices compiled on a regional or national basis for specific commodities, and crop timelines are descriptions of generalised crop phenology, pest occurrence and human activity for specific crops.
Source: (FAO, 2006[5]).
The environmental risks posed by pesticides have encouraged several countries to include in their registration schemes an environmental‑risk assessment of pesticide products. It is aimed, for instance, to evaluate potential negative consequences to non-target organisms and environmental compartments. (Frezal and Garsous, 2020[1]).
Human health risk assessments are aiming to present the level of risk of a pesticide, under specific use conditions and are recommended to be conducted for pesticides that human health hazards are of concern (FAO, n.d.[6]). Human health risk could be divided into occupational risks and dietary risks (FAO, n.d.[7]). Human risks assessments may concern risks to workers and users at different stages of the product life cycle and risks to public health with special attention to vulnerable groups.
During the registration process, appropriate procedures should be in place to ensure that products submitted for registration comply with specifications or standards for pesticides, that the quality of the product be verified and that the labelling and packaging of approved pesticides comply with set standards (FAO & WHO, 2011[8]).
Further information can be requested for instance on the technical material and/or the formulated pesticide product. Information on authorisations in other countries, refusal of registration or cancellation of registration (including reasons) in other countries, existing pesticides assessments, established residue limits in other countries can also be requested, similarly as the safety data sheets of the products (FAO & WHO, 2013[2]).
Pesticide registration also involves the regular review of already registered pesticides to ensure that they meet the latest health and environmental risk-assessment standards. This re-evaluation process can lead to the removal of some products from the market (i.e. pesticide de-registration) (Frezal and Garsous, 2020[1]).
Furthermore, it is suggested that governments should make provisions for the effective monitoring and enforcement of pesticide regulations, including the establishment of licensing and inspection schemes for importers and retailers (FAO & WHO, 2013[2]; FAO & WHO, 2015[4]).
Legal grounds and scope of pesticides registration in Mexico
Article 376 of the General Law of Health (GLH) (Mexican Congress (Congreso de los Estados Unidos Mexicanos), 1984[9]) states that a registration is required, among others, for pesticides, fertilisers and toxic or hazardous substances. It clarifies that a registration can only be granted by the Secretary of Health. In the case of pesticides, COFEPRIS is acting on its behalf.
The PLAFEST Regulation (Mexican Congress (Congreso de los Estados Unidos Mexicanos), 2014[10]) concerns the registration requirements and procedures (see Figure 2.1) for more information on the institutional framework). In line with Article 7 of this legal instrument, the following chemical and biological pesticide products that are applied in the field or greenhouses on agricultural crops are subject to registration:
chemical pesticides:
Technical pesticide (a pesticide in which the active ingredient is at its maximum concentration, resulting from its synthesis and that of its related compounds, and used exclusively as raw material in pesticides formulation);
Formulated products for agricultural use;
biochemical pesticides for agricultural use (e.g. pheromones);
microbial pesticides for agricultural use (consisting of a microorganism like bacterium or fungus, the active ingredient);
botanical pesticides for agricultural use (made of substances extracted from plants or metabolites obtained from their extracts, and used for pest control purposes);
miscellaneous pesticides for agricultural use (products having no pesticide physico-chemical and toxicological properties, but having characteristics enabling pest control).
Figure 2.1. The pesticides registration process in Mexico is managed jointly by COFEPRIS, SEMARNAT and SENASICA
* Not issuing resolution by COFEPRIS within statutory deadlines is understood as a negative response to the application request.
Source: Author based on the PLAFEST Regulation (Congreso de la Unión, 2004[11]).
Both active ingredients and formulated products have to be registered. Adjuvants and inert ingredients do not need registration, but information on the latter should be provided under data requirements.
Aerial spraying of pesticides
The Official Mexican Standard NOM-052-FITO-1995 regulates the requirements for the start of operation of aerial application of agricultural pesticides. These requirements apply to the civil or legal persons, as well to the owners of the starting/landing runways and the related aircraft.
The Official Mexican Standard NOM-003-STPS, published in 1999, includes a provision related to the safety of the aerial application of pesticides. Persons other than the Signaller (“banderero” in Spanish) should not have access to the application area. The norm also prescribes the list of PPE to be provided to the Signaller. The company is responsible for the demarcation of the treatment area and the buffer zones in a way that it is clearly visible for the pilot (SENASICA, 2019[12]) Proposed updates to NOM-003-STPS (in consultations from 2016) include an important obligation (in particular from the occupational health and safety perspective), for the civil or legal persons using the services of the workers to apply pesticides, to supervise that the Signaller follows the prescribed risk reduction measures and takes a shower and changes clothes after pesticide application.
2.1.3. Data requirements for pesticides registration in Mexico
Each country is independent in determining the scope of data required for pesticides registration, taking into account its national circumstances. However, the OECD guidance and information materials (OECD, 1994[13]) & (OECD, 2005[14]), as well as the FAO and WHO guidelines provide information on certain types of data that can be required for these purposes (FAO & WHO, 2013[2]).
In line with the 1994 OECD survey, most of the OECD governments require information on:
identity (of the active ingredient as well as any inert ingredients in the pesticide product formulation);
physical-chemical properties;
function, mode of action and handling;
manufacturing, quality control and analytical methods (to detect residues in food or water);
residues (the quantity and characteristics of residues likely to occur in food);
efficacy (in controlling the target pest);
toxicity (to man);
ecotoxicity (to wildlife and beneficial insects);
fate and behaviour in the environment (OECD, 1994[13]).
A comparison of the FAO and WHO guidelines for data requirements with Mexico’s requirements is provided in the Annex 4D.
In relation to the data registrants are required to submit, Mexico applies a two-tier approach. According to the PLAFEST Regulation, certain common information is requested for all registration requests and some specific data is required based on the type and use of a pesticide product.
The common information includes:
An application form;
For domestically produced and imported pesticides: a certified letter from the supplier, specifying:
commercial and common name of the product and its composition (percentage);
name and address of supplier;
name and address of the product purchaser, which must be the registrant and
registration number, if the product is already registered (only for domestically produced pesticides).
For pesticides manufactured abroad by the registrant, a letter containing a sworn statement confirming veracity of this situation.
The specific technical data required is described in Table 2.2. For chemical pesticides there are different data requirements for the registration of active ingredients and formulated products. There are also different data requirements for biochemical pesticides, microbial pesticides, botanical pesticides and miscellaneous pesticides for agricultural use. (More detail on the data requirements can be found Annex D).
Table 2.2. Technical data requirements for the registration of pesticides in Mexico vary depending on their type
Data requirements according to Article 12 of the PLAFEST Regulation, as amended in 2014.
|
Chemical pesticide – technical pesticide |
Chemical pesticide – formulated product for agricultural use |
Biochemical pesticides for agricultural use |
Microbial pesticides for agricultural use* |
Botanical pesticides for agricultural use |
Miscellaneous pesticides for agricultural use |
|
|---|---|---|---|---|---|---|
|
Information on identity and composition |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Physico-chemical properties |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Analytical methods/procedures |
Yes |
No |
Yes |
Yes |
No |
No |
|
Toxicological information |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
|
Ecotoxicological and environmental fate information |
Yes |
No |
No |
Yes |
No |
No |
|
Proposed label |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Biological effectiveness opinion |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Hazard category |
Yes |
No |
No |
No |
Yes |
No |
|
Other information |
No |
MRLs for each crop requested Information and documentation required for technical pesticide unless already registered by registrant |
Information on the agent’s biological properties Product stability information |
Storage stability study |
No |
* Certain specificities are applicable to information required for registration of a microbial pesticide based on genetically modified organisms.
Source: Elaboration by author based on the PLAFEST Regulation.
In relation to biochemical pesticides for agricultural use, microbial pesticides for agricultural use, botanical pesticides for agricultural use and miscellaneous pesticides, the PLAFEST Regulation specifies that registrants may provide only a limited amount of information for registration purposes:
if a registrant has already registered a technical pesticide or a formulation based on the same active ingredient, and the product to be registered has the same supplier holding the registration previously granted;
if the pesticide has been identified by COFEPRIS, in consultation with SADER (SENASICA) and SEMARNAT, as a reduced risk pesticide.
Biological efficacy data is regulated separately from the PLAFEST Regulation, in the Mexican Official Standard NOM-032-FITO-1995. SADER (SENASICA) requires, among others, administrative data, product identity and composition (name, IUPAC, CAS), physico-chemical data, toxicological information, or product label. In relation to the biological effectiveness studies, Mexico does not accept studies carried out outside the country (SENASICA, 2020[15]).
The PLAFEST Regulation explicitly states that Mexico accepts studies and methodologies developed in line with the OECD Test Guidelines, FAO guidelines, the US EPA Testing Guidelines and the Analytical Methods developed by the Collaborative International Pesticides Analytical Council. In line with recommendations from the Mexican authorities, studies submitted should be conducted according to the OECD Principles of GLP and the industry is expected to submit proof of GLP certification.
In line with the PLAFEST Regulation,
Physico-chemical, toxicological, ecotoxicological, environmental fate and physical properties studies should be developed under guidelines recognised by the international organisations. If no guidelines exist, the method used should be described, and the corresponding justification should be included. Studies should be conducted by laboratories with quality assurance systems, or by a third party authorised. It is considered as having a quality assurance system, when the laboratory applies national or international guidelines accepted by the ISO, or when following its own good practices guidelines. (…)When conducted outside Mexico, studies should be written in Spanish or in English (Mexican Congress (Congreso de los Estados Unidos Mexicanos), 2014[10]).
Provisions are in place to protect confidentiality and proprietary rights held on test data submitted for pesticides registration. The information submitted under the registration application is considered confidential. The PLAFEST Regulation also requires that registrant provides information on the name of the author of the toxicological or ecotoxicological studies submitted or the name of the institution or laboratory that produced data. It is not clear, however, if registrants should provide certification of the right to use the data by the author or institution/laboratory and if the application for registration is accepted in the absence of such certification.
When filling the administrative part of the registration application, the applicant is informed that data provided under the registration procedure can contain confidential information and the latter is requested to indicate if agrees to make the data public by the authorities (COFEPRIS[16]).
If a registrant has no access to certain data required under the registration, COFEPRIS could allow using the data of the already registered product, provided that registrant obtained authorisation to access these data from the registered product supplier. For the biological effectiveness, an interested party shall provide the letter issued by SADER (SENASICA) acknowledging access to the biological effectiveness information, and the technical opinion of the supplier of the formulated product.
Equivalence registration and registration solely for export
The PLAFEST Regulation envisages a possibility to register a technical pesticide or concentrated technical pesticide that is equivalent to an already registered one. It is also possible to request registration for pesticides solely intended for export, provided that the pesticide product will not be sold or used in Mexico. In this situation, a more limited technical information is required.
Emergency use of pesticides is allowed
In special circumstances, the responsible authority may have to consider allowing the use of pesticides that are unregistered, cancelled or registered for other purposes. The goal is to control an outbreak of vector-borne disease, avert a significant risk to human health or the environment (e.g. a significant risk to endangered or threatened species or beneficial organisms) or to avert significant agricultural losses (FAO & WHO, 2013[2]).
The emergency use of unregistered pesticides is not allowed in Mexico. However, to address phytosanitary, zoosanitary or sanitary emergencies, the PLAFEST Regulation allows for a use of registered pesticides for purposes different than provided for in the registration and to import it, if it is not available or not sufficiently available in the country.
In such case, the holder of the registration has to be notified, and in the case of an imported pesticide, agree to it. The authority declaring the emergency has to notify all other authorities co-operating under the PLAFEST Regulation on the temporary use of a pesticide, location of the use and its estimated duration. In the case of import, the authority has to obtain an import permit from COFEPRIS.
Minor uses of pesticides
Minor uses, including the majority of speciality crops, are the uses of pesticides where the potential use is on a scale not sufficiently large to justify registration of that use from an applicant’s perspective alone. In particular, when the associated costs of generating the data required for obtaining and maintaining regulatory approval and potential liability from those uses once approved are taken into account. This results in a situation where speciality crop industries are either without or are lacking sufficient access to pest control products to adequately protect those crops. OECD has a vision of greater harmonisation of regulatory systems such that data reviews prepared to a common format in one region or country can be used to support regulatory decisions in another country. Towards this objective, OECD has published a number of guidance documents focussing on minor uses (OECD, 2020[17]).
At this time, Mexico does not have regulatory provisions addressing minor uses of pesticides. Addressing this issue would upgrade the regulatory framework in Mexico and would support the harmonisation of regulatory systems with its trade partners. It would also support national stakeholders by providing speciality crop industries with access to pest control products to protect those crops adequately. For instance, the financial support provided by the Australian Government for the minor use grants program is considered as critically important to increase farmers' access to chemical uses (Matthews et al., 2020[18]). Another example of such support, the Canadian Growing Forward initiative, is presented in Chapter 3.
New and non-traditional pesticides
The PLAFEST Regulation includes a category of “miscellaneous” pesticides. They are defined as products having no pesticide physico-chemical and toxicological properties, but having characteristics enabling pest control.
Under this category, SENASICA has evaluated so-called "resistance inducers". However, as this type of product is not clearly defined in the Mexican regulatory framework, the registration and the evaluation for assessing biological effectiveness were considered challenging. The former was based on the qualities declared by the promoter of the product, and the latter on the determination of biological effectiveness is based on parameters adapted to the mode of action (SENASICA, 2020[15]).
Technical modification of the registration
The PLAFEST Regulation contemplates the possibility of technical modification of a pesticide registration. It includes a change or extension of use including crop, pest, dose, animal species and aspects related to the function or use; adjustment of the expiry date; changes in the formulation inert ingredients. In such situation, the following technical information is requested:
for use modification or extension per crop, pest and dose for agricultural pesticides - the biological effectiveness technical opinion issued by SADER (SENASICA) in favour of the company;
maximum residue limit for each crop requested, for use modification or extension per crop or animal species, for agricultural pesticides;
for modifications of the expiration date, information related to the study of storage stability;
proposed label;
for changes in the formulation inert ingredients:
official letter describing the change of inert ingredients in the previously registered formulation, and the modified formulation, and specifying the reasons for such change;
identity and composition of the formulation previously registered and the modified formulation;
type of formulation; and
hazard category.
Current practices in relation to pesticides data sharing and exchange
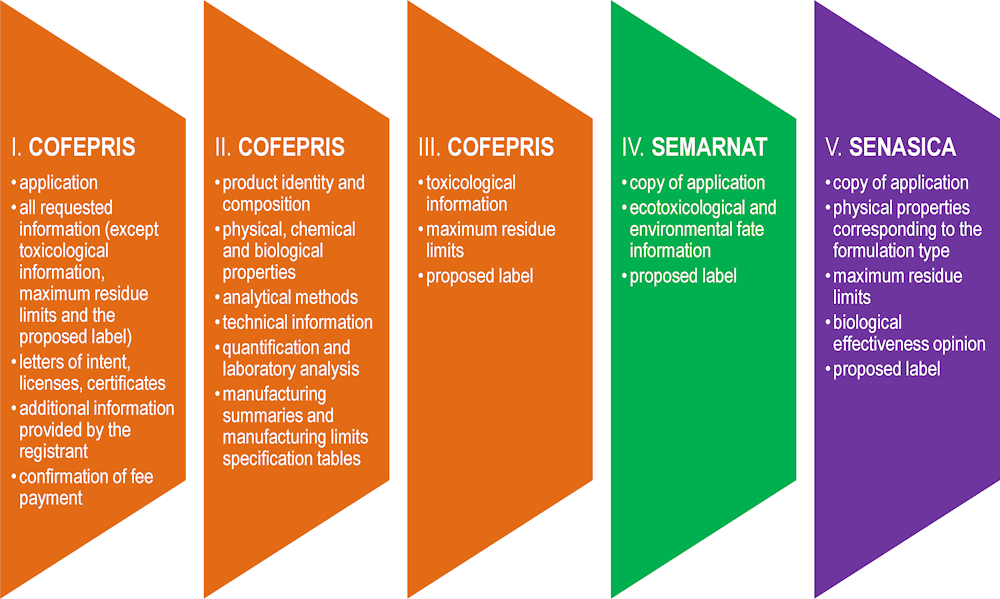
In Mexico, all the documentation relevant for pesticides product registration has to be provided to COFEPRIS in a traditional, paper format (original and copy). A registrant may use an electronic form available from COFEPRIS (the PLAFEST form), but the form only concerns the administrative filing of the registration application and not the data within the data dossier. To support sharing information between COFEPRIS, SEMARNAT and SENASICA (also in the paper format), this information has to be submitted by registrants in five parts (Figure 2.2). This allows COFEPRIS to distribute parts IV and V to SEMARNAT and SENASICA respectively, while maintaining parts I, II and III for its own use.
Figure 2.2. Registration information has to be provided in five parts to support information sharing among authorities
While COFEPRIS receives the full pesticide registration dossier from a registrant, SEMARNAT and SENASICA only receive the parts relevant for their specific disciplines. If the latter need other data to complete their opinion, they have to request access to the remaining part from COFEPRIS. This adds another layer of complexity and there is a risk that information from COFEPRIS arrives too late to allow SEMARNAT and SENASICA to meet their regulatory deadlines.
Moving away from a paper registration process to an on-line registration and exchange of information system (which would include the data in a dossier) for pesticides in Mexico, should provide benefits for authorities. First, it would ensure that regulatory work can continue in every condition and it would allow for a fast and secure sharing of registration information among authorities involved. It would allow access to relevant information by all relevant authorities, from everywhere, and would support the compliance and enforcement activities, particularly in the field. Stakeholders should welcome such an approach, as it will bring tangible benefits to them. It should facilitate not only regulatory work, but also the information submission process for industry and the access to updated information for the public.
This transition requires certain investments at the implementation stage, particularly in the IT-‑infrastructure and equipment. It might also be beneficial to retain a possibility to use “paper” communication in the mid‑term, to support inclusion of all relevant stakeholders. However, the digitalisation of the pesticides registration and evaluation process seems inevitable. It has already happened in many OECD countries, for instance in Canada and the EU.
Mexico already allows for an on-line electronic information provision for one of the aspects of its pesticides management programme. A so‑called PLAFEST form is used to apply for a pesticide import permit via the One-Stop‑Window of the Mexican Foreign Trade Receipt System (VUCEM). An electronic signature is one of the technical requirements to use this option.
The PLAFEST form includes information on the company, the uses of pesticides, product data (e.g. commercial name, CAS number, composition of the product, its classification, toxicological data, country of production/formulation, country of export or import), information on the producer, formulator, provider and final user (Government of Mexico[19]).
The PLAFEST Regulation does not include a mechanism for exchanging confidential information with regulatory authorities in other countries. Explicitly addressing such a possibility in the Mexican regulatory framework would support the future co-operation of pesticides authorities with their counterparts in other countries, for instance for joint evaluations. The OECD Recommendation concerning the Exchange of Confidential Data on Chemicals, OECD/LEGAL/0204, recommends that adherents to this legal instrument take steps to develop the conditions which would allow for the exchange of confidential data (OECD, 1983[20]).
Mexico is encouraged to use OECD electronic tools to facilitate exchanges of pesticide data (e.g. the Globally Harmonised Submission Transport Standard (OECD, n.d.[21]), a standardised set of technical specifications used to assemble electronic files for any pesticide package in a predefined manner), as well as to join the OECD work on facilitating the development and adoption of other electronic tools, such as efforts to identify common global label data requirements to assess the benefits of the use of structured data in IT systems, which receive, maintain, and share label information.
Pesticides registration process in Mexico
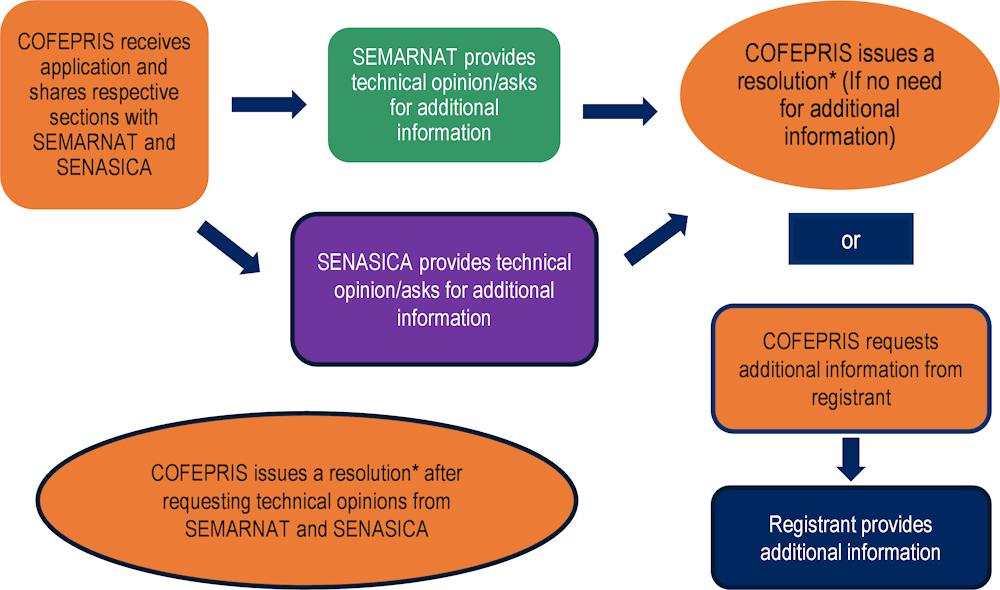
PLAFEST regulates the process for registering pesticides (Figure 2.3). There is no pre-registration phase, so in principle the registration stage starts with the submission of the registration application to COFEPRIS. However, as a pre-requisite for applying for a registration is to obtain SENASICA’s technical opinion on biological efficacy, this pre-registration step in practice should be taken into account.
The PLAFEST regulation describes the data and information a registrant must submit to COFEPRIS as well as the timelines for the activities carried out by the relevant authorities and the applicant during the process. No additional support in terms of clarifying the requirements, such as development of guidelines, is provided to applicants.
After receiving the application, COFEPRIS provides SEMARNAT and SADER (SENASICA) with information relevant for their technical evaluation. Both authorities are able to ask COFEPRIS to request additional information or clarification from the applicant. If the authorities do not request additional information or clarification, it is understood as a positive opinion towards the registration request.
If neither COFEPRIS, SEMARNAT nor SENASICA request additional information or clarifications, COFEPRIS requests technical opinions from SEMARNAT and SENASICA. If any of these authorities abstains from providing its opinion, it is considered positive for the applicant.
If an applicant is requested to provide additional information or clarifications, the process is put on hold until the information is provided (for a maximum of 60 days, in line with the PLAFEST Regulation). The authorities can only request information once during the registration process. After receiving input from the applicant, SEMARNAT and SENASICA are requested to provide their technical opinion. If an applicant does not provide the requested information, the application is considered null and void.
COFEPRIS issues a resolution that could either be positive, which results in granting the registration, or negative, which results in rejecting the registration application.
The PLAFEST Regulation also contains a provision that if COFEPRIS does not issue a resolution (i.e. a decision) within the statutory deadlines it is understood as a negative response to the application request. Some Mexican stakeholders have raised concerns that statutory deadlines linked to the pesticides registration are not always met.
The registration procedure described above does not apply to products, whose registration would be requested via the Joint Evaluation Programme conducted together with the authorities responsible for pesticides registration in Mexico’s “commercial partners”, as it is indicated in the PLAFEST Regulation. In such a case, a separate procedure is to be established among the Mexican authorities, authorities from commercial partner(s) and the applicant. This provision, introduced in 2014 to the PLAFEST Regulation, is aimed in particular towards the Mexican counterparts in T-MEC Agreement (i.e. Canada and the United States), has not been applied in practice yet in Mexico. On the other hand, a joint evaluation of pesticides has been put in practice by Canada and the United States (Box 2.3).
The PLAFEST Regulation establishes a timeline for a review of a pesticide registration application (Figure 2.3). Taking into account the maximum allowed time, COFEPRIS should deliver its resolution within 180 working days after receiving the registration application.
Unfortunately, detailed information on the actual average duration of the pesticide assessment period is unavailable. Nevertheless, it is possible to compare the statutory duration of the process as described in the PLAFEST Regulation with the assessment timelines in other jurisdictions in the OECD area (Box 2.2). It shows, in general, that in Mexico the timeline allocated for an evaluation of a new pesticide is much shorter than elsewhere.
Figure 2.3. Timeline of the review of a pesticide application in Mexico
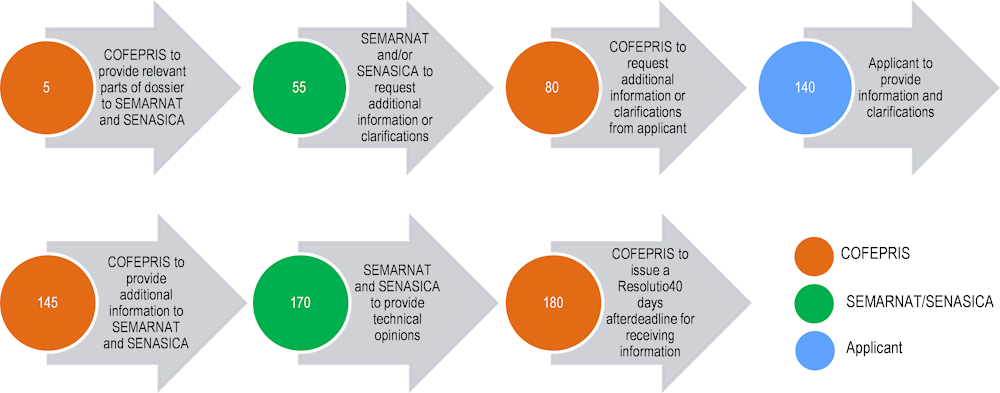
Note: The numbers indicate maximum allowed working days for a given action counting from the submission of the pesticide registration application.
Source: Author based on the PLAFEST Regulation.
Box 2.2. Selected timelines for registration and evaluation of pesticides in OECD countries and the European Union
Australia
In Australia, the assessment period required for the approval of an active constituent contained in a chemical product, registration of the associated chemical product and approval of the product label requiring a full assessment of the active constituent and product is 18 months.
Canada
In Canada, the review timeline range for submissions to register new active ingredients and their companion end-use product(s) is between 285 and 655 days.
European Union
In the European Union, it takes between 2.5 and 3.5 years from the date of admissibility of the application to the publication of a Regulation approving a new active substance. For a pesticide product, it takes up to 1.5 years from the date of application to the granting of on authorisation.
United States
In the United States, it takes 24 months to evaluate a new registration of a conventional active ingredient for food use (18 months in the case of a reduced risk pesticide).
Note: As countries vary in the number of options for the registration of active ingredients and pesticide product, the intention was to present timelines for the most “conventional” situations, to support comparison with the standard situations described in the Mexican regulatory framework.
While the added complexity of the EU procedures across all its member states, the European Food Safety Authority and the European Commission (e.g. with peer reviews of Rapporteur Member State Risk Assessments) explains a longer timeline in comparison with Mexico, the significant difference with Canada and the United States – the other T-MEC Agreement countries – is concerning. It suggests that certain aspects of the evaluation process could be less developed in Mexico and therefore addressing them would be essential to support the harmonisation of approach to pesticides evaluation in all three T-MEC partners and related co-operation, for instance in relation to the joint assessments of pesticides.
Policy documents (e.g. 2019 Elements for the Development of an Integral Strategy for Responsible Pesticides Management in Mexico) and feedback from the Mexican stakeholders (e.g. Proposals of the 2018 Mexican National Forum on Pesticides) suggest that the biggest room for improvement is linked to the environmental aspects of the pesticides evaluation process in Mexico
The PLAFEST Regulation also provides regulatory timelines for other activities linked to the modification and extension of a pesticide registration, as well as granting export/import permits (Table 2.3).
Table 2.3. Selected deadlines for other regulatory actions under the PLAFEST Regulation
|
Type of action |
Timeline |
|---|---|
|
Pesticide registration exclusively for export |
150 working days |
|
Technical modification of registration |
150 working days |
|
Extension of registration |
32 working days |
|
Pesticides import permit |
16 working days |
|
Pesticides import authorisation (SEMARNAT) |
20 working days |
Source: Author based on the PLAFEST Regulation.
COFEPRIS is obliged to publish on its website a database with granted registrations as well as with pending requests. The database should be updated at least every 30 days, supporting among other things, the transparency of the registration process. However, this obligation has not always been fulfilled.1 Increasing efforts to publish this information within the regulatory deadline would support transparency of the registration process. Moreover, including information in the database on the starting date of the registration process, as well as of the date of registration itself, could support authorities in collecting timeframe performance statistics and analysing how they implement regulatory deadlines from the PLAFEST Regulation in general.
The database run by COFEPRIS includes the following information on registered pesticides: registration number, company name, active ingredient, commercial name, toxicology category, uses and validity. It also includes MRLs for active ingredients. Search is possible by registry number, company name or active ingredient. No information on the studies behind a given pesticides registration (e.g. study summaries) is available. Moreover, the information is not updated regularly. Information on the year each pesticide was registered would also be very useful for the users.
The Coordinated Sanitary Registry (Registro Sanitario Coordinado, RSCO) registration number (obligatory on the label) includes information on the type of pesticide (e.g. insecticide, herbicide), a number of active ingredient registration, type of product (e.g. liquid or suspension) and a percentage share of active ingredient (SENASICA, 2019[12]).
In February 2021, this registry database included over 7 950 entries, including some 340 cancelled registrations (COFEPRIS[27]). The number of cancellations increased significantly since 2019 (by some 90 registry cancellations), largely in response to the recent actions undertaken by authorities described previously. of this chapter. It is estimated that the validity period is indefinite for about 4 000 entires (NHRC, 2018[28])(see Box 2.5).
In 2018, 530 pesticides in Mexico were considered highly hazardous, 1 137 were classified as having high acute toxicity (according to the WHO classification), 850 were considered as probably carcinogenic to humans (according to the US EPA), 642 being endocrine disruptors (according to the GHS) and 2 464 toxic to bees. COFEPRIS calculated also that there are 90 active ingredients registered in Mexico that are prohibited or not authorised in other countries (NHRC, 2018[28]).
Many active ingredients that are registered and authorised for use in Mexico are banned or severely restricted in its main trade partners, 16 in the United States and 45 in the EU in 2017 (NHRC, 2018[28]).It can affect the Mexican population and the environment and trigger potential trade-related problems.
In the case of applications for registration of pesticide products destined exclusively for export and applications for a technical modification of existing registration, the procedure is similar as in a standard registration. However, only COFEPRIS and SEMARNAT are involved in issuing the resolution on the export registration, while only COFEPRIS and SADER (SENASICA) are involved in the technical modification of the registration.
The decision-making process on evaluating pesticides in Mexico
In order to determine whether the use of a pesticide proposed for registration poses an unacceptable risk to human health or the environment, countries consider matters such as the toxicity of pesticide, their metabolites or degradants, and potential exposure during or after application. FAO and WHO recommend risks from potential exposure pathways to be evaluated, including workers’ exposure, exposure to food residues in food or the exposure of non-target organisms (FAO & WHO, 2013[2]).
As indicated earlier, a pre-requisite for pesticide registration and evaluation in Mexico is to obtain SENASICA’s technical opinion on biological efficacy. In line with the Mexican Official Standard NOM-032-FITO-1995, in order for SENASICA to grant a technical opinion, a company has to provide SENASICA with the product data, including the results of the field studies conducted by an approved test laboratory.
PLAFEST Regulation establishes the regulatory framework for evaluation of pesticides and the division of responsibilities. While three authorities (COFEPRIS, SEMARNAT and SENASICA) are involved in the evaluation process, PLAFEST assigns the leading role to COFEPRIS.
SENASICA is responsible for biological efficacy and phytosanitary aspects of MRLs and SEMARNAT for environmental evaluation, COFEPRIS is responsible for the health evaluation of the pesticide registration application, and more importantly it is the only institution that is entitled to grant (and to cancel) the registration. SEMARNAT and SENASICA may not provide any explicit opinion in the registration and evaluation process. In such case, this is considered for the benefit of the applicant. However, in practice, COFEPRIS is bound in the registration process by the veto power of SEMARNAT and SENASICA as it relates to the technical opinion of the respective components of the registration application. If any of these institutions uses its veto power, it has to be respected, and cannot be overruled by COFEPRIS.
This limits the drive to work together to identify solutions and reach a consensus among all three regulators and to harmonise approaches (e.g. risk management vs. hazard based) so that they can effectively work together. Additionally, as described in Chapter 1, the three main authorities involved in the process work in the context of different framework laws. They may have competing policy objectives, goals and priorities and this can impact the extent of the co-operation between them.
In line with the PLAFEST Regulation, pesticides evaluation in Mexico under the registration process is either:
based on local evaluation (use of mainly local data and locally specific assessments); or
equivalence (based on the determination of the equivalence or near equivalence between the submitted product and a registered product).
For the registration of an active ingredient or a microbial pesticide in Mexico, a registrant is required to submit a study of the impacts on populations of beneficial and pollinizer insects as part of the eco-toxicological information data set. In the case of a microbial pesticide, if there is scientific evidence showing that application of the pesticide does not lead to exposure or damages to non-target organisms, and does not cause environmental pollution, the applicant can be exempted from the requirement to provide a study, upon provision of the justification.
In line with the PLAFEST Regulation, studies on physico-chemical, toxicological, ecotoxicological, environmental fate and physical properties conducted in other countries are accepted.
The General Law of Ecological Balance and Environmental Protection includes a provision that pesticides prohibited in other jurisdictions cannot be legally authorised in Mexico. However, the data requirements specified in the PLAFEST Regulation do not clearly require such information. In general, it is considered that this restriction has not been effectively implemented (Bejarano, 2018[29]). Formalising the process of providing information on pesticides prohibited or restricted in other jurisdictions, for instance during the registration, modification and extension of a registration, as well as when requesting an import permit for pesticides, would support the authorities’ decision-making on registration and cancellation of registrations. Chapter 3 includes information on the approach in Canada, which regulatory framework has specific provisions for a review of a registered pesticide, when an OECD country prohibits all uses of an active ingredient for health or environmental reasons.
It is unclear, to what extent Mexico uses the pesticides assessments performed in other countries and by international organisations and whether its procedures for decisions on registration reflects the granting or refusal of registrations taken under other jurisdictions. It is not explicitly reflected under the PLAFEST Regulation.
The possibility of a joint assessment of pesticides between Mexico and its trade partners is addressed in the PLAFEST Regulation as of 2014. However, it has not materialised yet and one could expect that increased harmonisation of the evaluation process in Mexico might be needed for this, as the two other T-MEC countries are already co-operating in this area (Box 2.3).
Box 2.3. Best practice – Canada and United States co-operation on the joint evaluation of pesticides
In May 2015, Health Canada's Pest Management Regulatory Agency (PMRA) and the United States Environmental Protection Agency's Office of Pesticide Programs (US EPA OPP) announced that they would be collaborating on a bilateral pesticide re-evaluation process for the pollinator assessment of three neonicotinoid pesticides (clothianidin, imidacloprid, and thiamethoxam). The initiative is part of the co-operation under the Regulatory Cooperation Council and the evaluation based on the jointly developed harmonised Guidance for Assessing Pesticide Risks to Bees.
These pesticides are nitroguanidine neonicotinoids, a group of insecticides that have been approved for use in the United States and Canada for a number of years. In recent years, there have been reports in scientific literature suggesting that exposure to neonicotinoids may affect pollinator health; however, these studies have generally been conducted under laboratory situations, or in the field with exposure to doses that are higher than would normally be encountered in the environment.
A summary information on joint reviews is available for instance in Canada’s Pest Management Regulatory Agency annual reports.
While the PLAFEST Regulation contains detailed information on the information requested by the authorities to evaluate a pesticide registration application and the timelines of the evaluation, in principle it does not provide scientific nor technical criteria to support relevant decision-making in relation to the registration of active ingredients and pesticides products. In fact, the only explicitly mentioned common criterion is the procedural one - lack of response from the applicant to the request to provide additional information or clarifications results in no further processing of the application, if the authorities made request within statutory deadlines.
The FAO Pesticide Registration Toolkit includes information on pesticides registration criteria applied by various national registration authorities (FAO, n.d.[31]). Pesticide regulators in other countries prepare guidance documents that include scientific or technical criteria supporting relevant decision-making. Such documents can support the evidence-based decision-making as well as the interpretation of evaluation performed in other jurisdictions, including their potential adaptation to the Mexican conditions. The availability of guidance resources for potential applicants can also reduce inefficiencies during the registration process (examples of guidelines available in Australia and Canada are available in Chapter 3). Development and adoption of international guidelines would also benefit international work-sharing and potentially faster access to new pesticides.
An exception to this rule concerns a possibility to register a technical pesticide or concentrated technical pesticide in equivalence to already registered one. The PLAFEST Regulation contains a set of criteria to decide if a pesticide is chemically equivalent and its toxicological profile is equivalent to a reference profile, including:
A maximum manufacturing level of each non-relevant impurity is not significantly higher than a maximum manufacturing level of the reference profile;
No new relevant impurities are found;
The maximum manufacturing level of relevant impurities is not increasing as related to the maximum manufacturing level of the reference profile;
LD50 results for oral and dermal acute toxicity studies and LC50 for the inhalation toxicity study delivered by the interested party shall not differ by more than a factor of two times, as compared to the reference profile;
The product to be registered that, based on toxicological studies delivered, proving to be less toxic up to a factor of ten, as compared to the profile used, may also be considered as equivalent;
Results of dermal and eye irritability tests must prove that the product is equally or less toxic.
The PLAFEST Regulation contains no directives that would address the undertaking of a risk- benefit analysis in the decision-making on the registration of pesticides. However, it is unclear that, at the moment, a registration decision contemplates comprehensively the economic and agronomic value of introducing a pesticide (SENASICA, 2020[15]), as well as balances it with its risks to human health and the environment.
Some countries, including New Zealand and the United States have incorporated more comprehensive considerations. They address agronomic, economic, social, health and environmental benefits as well as likely consequences of the public not having access to specific pesticides. By applying a benefits test, products can be approved where the overall benefits outweigh the risks posed by their use. A risk/benefit or cost/benefit consideration is a well-established principle of good regulation in wider government regulatory decisions. It enables the balance of interests to be taken into account in rational decision- making. Despite the additional work for the regulator and increased cost for industry, a benefits test could deliver access to more chemical uses and improved safety outcomes (Matthews et al., 2020[18]).
A consultation process is enshrined in the decision-making on pesticide registration in Mexico. Moreover, a decision on registration communicated to the applicant should include a justification. Nevertheless, calls for more transparency and consistency of conclusions, have been voiced (PROCCYT, 2020[32]). It is linked to the fact that, apart from the text of the PLAFEST Regulation, stakeholders in Mexico do not have at their disposal additional information that would allow them to better understand how the Mexican authorities reach their decisions. It is of particular relevance for some types of products (e.g. biopesticides) which might need to be regulated more on a case-by-case basis.
The Federal Law of Responsibilities of Public Servants includes a conflict-of-interest policy and guidelines for public officials. This is a common procedure in many OECD countries. In line with the requirements, the onus remains on public officials to proactively report and resolve real, potential and apparent conflict-of-interest situations as they arise in conjunction with their management (OECD, 2017[33]).
Maximum Residue Limits
A Maximum Residue Limit (MRL) is defined by FAO as:
the maximum concentration of a pesticide residue (expressed as mg/kg), to be legally permitted in or in food commodities and animal feeds. MRLs are based on Good Agricultural Practice (GAP) data and foods derived from commodities that comply with the respective MRLs are intended to be toxicologically acceptable (FAO[34]).
In most of the OECD members MRLs are established at the same time or before a pesticide product is approved for use. In general, most data generated in support of MRLs are developed by the pesticide manufacturer (OECD, 2010[35]). In principle, the applicant should provide the necessary residue data generated in accordance with the Codex Alimentarius and guidelines published by the OECD on Good Laboratory Practice and by FAO guidelines on crop residues for assessment by the responsible authority (FAO & WHO, 2013[2]).
The MRLs are based on field trials and toxicological data. Reference doses and acceptable daily intake are compared with food consumption patterns, residue data and monitoring data (Handford, Elliott and Campbell, 2015[36]). The MRLs are essential in ensuring safe consumer exposure to and protecting vulnerable groups from products containing pesticide residues, MRLs can also be used as a compliance tool to investigate if the pesticide was misapplied.
MRLs are also relevant in the context of the international trade in food. For instance, foods imported to the EU countries are sampled to ensure that they do not contain pesticides above the set MRLs. In 2016, 53.1% of the Mexican samples analysed had quantified residues below or at the MRLs and only 4.5% of samples analysed had quantified residues above the MRLs (EFSA, 2018[37]).
In line with the PLAFEST Regulation, COFEPRIS and SENASICA share the responsibility for developing and implementing MRLs in Mexico. COFEPRIS is responsible for conducting risk assessments to set MRLs, while SENASICA issues a technical opinion on the phytosanitary aspects of MRLs of pesticides.
In 2014-17, SADER (SENASICA) and COFEPRIS worked on the Official Mexican Standard for MRLs. NOM-082-FITO/SSA1-2017 on Maximum Residue Limits, Technical Guidelines and Authorisation and Review Process was published in October 2017.
In line with NOM-082-FITO/SSA1-2017, an authorisation can be granted for an MRL generated during field studies conducted in Mexico or based on:
MRLs in Codex Alimentarius (as long as they are valid and correspond to the same pesticide/crop combination or pesticide/group of crops combination);
MRLs established by US EPA; Canadian PMRA; members of the European Union, members of the OECD, as well as Argentina and Brazil (as long as the use of pesticide is comparable, they are valid and correspond to the same pesticide/crop combination or pesticide/group of crops combination);
MRLs generated in Mexico and based on field studies conducted in the countries indicated in the previous bullet (as long as the use of pesticide is comparable).
Prior to the adoption of NOM-082-FITO/SSA1-2017, Mexico used the MRLs from the US EPA. Its legal provisions required also consideration of Codex MRLs and Mexico would accept Codex MRLs in the absence of a national MRL (OECD, 2010[35]). The 2014 update of the PLAFEST Regulation included also a temporary provision (until NOM was published) allowing using MRLs established in the countries abovementioned, on condition that COFEPRIS conducts a relevant risk assessment.
An MRL can be revised if the status of the international source of the MRL has changed (e.g. the MRL has been modified or cancelled), based on new dietary risk analysis conducted by COFEPRIS or based on the results of the National Residues Monitoring Programme. Import only MRLs, for pesticides not used domestically, are not covered by this NOM-082-FITO/SSA1-2017.
NOM-082-FITO/SSA1-2017 describes what information related to MRLs has to be provided by the applicant during the pesticides registration process. It also recognised the use of the OECD MRL calculator in the process (Box 2.4).
Box 2.4. Best practice - OECD MRL calculator
The OECD has developed an MRL calculator (OECD[38]) to harmonise pesticide MRLs across OECD countries.
The use of this calculator has been officially recognised in Mexico in NOM-082-SAG-FITO-SSAI-2017.
The applicant is recommended to use the calculator to calculate the MRL value for MRLs generated during field studies conducted in Mexico and MRLs generated in Mexico and based on field studies conducted in the countries specified in NOM-082-SAG-FITO-SSAI-2017.
Therefore, the country has harmonised its approach with the other T-MEC countries, Canada and the United States.
NOM-082-FITO/SSA1-2017 states that the authorised MRLs shall be in the public domain and applicable to any application for registration of the same pesticide/crop combination, provided that the pattern of use of the registrant is comparable to the pattern of use of the source taken as reference. The authorised MRLs, whose reference source is CODEX Alimentarius, are exempt from demonstrating comparability of the use pattern. COFEPRIS and SADER (SENASICA) are responsible for monitoring compliance with this NOM.
In the context of the need to provide an equal level of health (and the environment) protection for imported food products and ensuring a level-playing field for farmers in Mexico, it could be noted that import MRLs are not covered by NOM-082-FITO/SSA1-2017.
Under the OECD Pesticides Programme, members and partners as well as other stakeholders work to develop harmonised Test Guidelines and Guidance Documents on pesticide residue chemistry to support the assessment of pesticide exposure by identifying these residues in food or animal feedstuffs for purposes of dietary risk assessment and setting MRLs. Such guidance also supports the mutual understanding of such assessments. For instance, the Expert Group on Residue Chemistry is working on developing guidance on the definition of a residue, based on a common approach to residue identification of the pesticide and its metabolites and degradation products. Mexico would be encouraged to participate in this work.
Work to complete the implementation of NOM-082-FITO/SSA1-2017 is still in progress, in particular as it relates to the relevant regulatory procedures and guidelines. For example, guidelines for accrediting laboratories that could undertake field studies in Mexico necessary to establish national MRLs are needed. There is a need for capacity building activities related to the implementation of NOM-082-SAG-FITO/SSA1-2017, including providing guidelines to the industry on the MRLs evaluation criteria and approval. In this context it is also important to note the need to ensure that information on established MRLs in Mexican public available databases is up-to-date and systematically updated.
Labelling of pesticides
The International Code of Conduct on Pesticide Management defines a pesticide label as:
written, printed or graphic matter on, or attached to, the pesticide or the immediate container thereof and also to the outside container or wrapper of the retail package of the pesticide (FAO & WHO, 2015[39]).
Labels convey essential information from the product manufacturer to the user of pesticides about the product and the relevant safety and use recommendations. Labels may also contain information on hazards of the pesticide product. It is an important tool to protect human health and the environment. For labelling purposes, the pesticide formulation or end-use provides basis for classification, not the active ingredient (FAO & WHO, 2015[39]).
According to FAO and WHO guidelines, proposed labels should be subject to approval by the registration authority during the registration process. The sale of pesticides that are not properly labelled should be prohibited. Requirements for labels should be based on relevant international standards and recommendations on pesticide labelling (FAO & WHO, 2015[40]).
In line with the PLAFEST Regulation, the proposed label has to be included as part of the registration application. It has to be approved by the authorities. All pesticide products in Mexico have to have a label. Labelling is regulated in NOM-232-SSA1-2009 that takes into account international standards and recommendations on pesticide labelling: the FAO\WHO Guidelines on Good Labelling Practice for Pesticides and the Globally Harmonised System of Classification and Labelling of Chemicals.
The label is composed of three sections: safety information (including use and management precautions and recommendations, PPE, first aid and emergency numbers), technical information (including information on the active ingredient, formulation, target pest, validity, hazard statement and warning) and use (including use instructions, calibration of equipment, dose or re‑entry time) (SENASICA, 2019[12]).
The digitalisation of the registration process in Mexico would enable better access to and dissemination of information contained on the labels of pesticides products. It allowed, for instance, the Canadian authorities to run a publicly available label transcript service, that can present information included in the pesticides labels on the market (Health Canada[41]).
Re-registration and re-evaluation of pesticides in Mexico - addressing legacy issues and supporting harmonisation with main trade partners
According to the General Law of Health, a registration can be renewed at the request of the registrant. If it is not requested, or the registrant changes or modifies the product or raw material without prior authorisation from the health authority, said authority (COFEPRIS in the case of pesticides) will cancel or revoke the corresponding registration.
The 2005 modification of the General Law of Health established a 5-year validity period for sanitary registrations (including pesticides), but only obliged holders of the indefinite registrations of pharmaceuticals and health inputs to undergo a revision of their registration. Therefore, holders of pesticides registrations granted before 2005 retained their indefinite registrations (Mexican Congress (Congreso de los Estados Unidos Mexicanos), 1984[9]).
A characteristic of the current Mexican system is that in the case of definite registrations (granted after 2005), in practice no new information is needed for the renewal of existing registration (Bejarano, 2018[29]). The procedure is simplified and short (it lasts maximum 32 working days) and, contrary to the registration process, a lack of response from COFEPRIS is considered as favourable for the applicant: “afirmativa ficta” or “silent-is-consent” rule, although this is not implemented automatically. Information required to renew registration include:
statement from the applicant that the registered product continues to comply with conditions of the granted registration (request will not be processed if non-authorised administrative or technical modifications are indicated);
confirmation of the payment;
certificate of the quality control analysis;
information on inert ingredients, density or weight;
proposed label;
information on MRLs for each requested crop;
common name; and
information on the hazard category.
According to estimations, most pesticides were registered in Mexico before 2005 and therefore has indefinite registration validity. Only few registrations have been cancelled since the 1990s (Bejarano, 2018[29]).
The period of time for which a registration is valid varies across OECD countries. An example of some validity period is provided in (Box 2.5).
Box 2.5. Validity of pesticides registration in the OECD countries
In Australia, approval of an active constituent continues to be in force unless it is cancelled. The registration of a chemical product ends on the day entered in the Register as the date the registration ends.
In Canada, the period of registration may be either finite or indefinite; re-evaluation and special review mechanism are in place (more details available in Chapter 3).
In Chile, the term of validity of the registration is 10 years.
In the European Union, active substances are approved for a maximum period of 10 years.
In Korea, the term of the registration is 10 years.
In New Zealand, a registration is normally valid for 5 years.
In the United States, all pesticides registered for use on food or feed must be reviewed at least once every 15 years.
An unlimited registration period (i.e. for those pesticides in Mexico that were on the market before 2005) means that it is very difficult to address recent developments and new information on the safety of those pesticides. Further, unlimited registration periods for existing pesticides could conceivably create a disincentive to develop new and more environmentally friendly pesticides, as those new pesticides would have to undergo a new evaluation.
A largely administrative character of information provided during the extension of registration of pesticides in Mexico does not provide authorities with updated information on the safety of the registered pesticide. Requesting more information at this stage would provide tangible benefits for the Mexican authorities. For instance, demanding updated data on the safe use of registered pesticides would support the Mexican efforts to timely address human and environmental pressures from pesticides and support removing the most hazardous ones from the list of registered pesticides in the country.
Moreover, in practice there is no systematic process of re-evaluation of pesticides in place in Mexico aside from the possibility to cancel the registration. Other countries have recently recognised the benefits of a technical review programme for pesticides. For example Japan (Box 2.6) is currently reforming its system in this direction. Examples of the pesticides review programmes in other OECD countries are provided in Chapter 3. It is also worth noting in this context that the EU applies a risk proportionate approach to its scheme for the renewal of approval of active substances by applying different renewal timeframes depending on the risk of pesticides (shorter timeframes for higher risk pesticides, longer for low risk pesticides) (European Parliament, n.d.[49]).Such approach supports prioritisation and better allocation of resource.
Box 2.6. Revision of the Pesticide Registration System in Japan
In 2018, Japan announced that it is modifying its Agricultural Chemicals Control Act (Act No. 82 of 1948) that sets out the process of the pesticide registration in Japan. The main changes include:
A periodic re-evaluation (every 15 years) of all registered pesticides. Under the previous system, registrants renew registration of their pesticides every three years but it did not include a scientific review of new findings;
The data requirements for re-evaluation are the same as those required for new registration;
The GAP may be changed or the registration is revoked based on the re-evaluation;
Specifications for technical grade active ingredients shall be established at the time of first registration and re-evaluation;
The registrants shall report, to authorities, newly available information on the safe use of their registered pesticides once a year, e.g. information on pesticide use accidents, revocation or changes of registration in countries outside Japan, and scientific papers concerning the safe use of the pesticides.
The implementation of the reform is taking place in 2018-21.
Source: (Sato, 2018[50]; Japan[51]).
The possibility to cancel pesticide registration is enshrined in the regulatory framework
In line with the COFEPRIS Rules of Procedure and the General Law of Health (Article 380) COFEPRIS is authorised to revoke sanitary authorisation if it becomes known that authorised products constitute a risk to human health. The possibility of revoking a pesticide due to a lack of biological efficacy, is not currently contemplated in the regulatory framework (SENASICA, 2020[15]).
A pesticide registration is considered an acquired right and cannot be revoked without the registrant consent. A potential risk presented by a pesticide is not enough to cancel registration. Scientific evidence (e.g. thorough studies) is needed to demonstrate a risk (NHRC, 2018[28]). This affects the process of cancellations of pesticides registrations in Mexico. For instance, in 2017, COFEPRIS informed the National Human Rights Commission that the use of six active ingredients, including DDT, endosulfan and lindane, was prohibited in 2015 and that it led to the cancellations of 146 sanitary registers. However, when the NHRC verified the information available in the COFEPRIS registry, only one of the six active ingredients in question had no valid (undetermined) registrations2 (NHRC, 2018[28]).
Moreover, if a company holding a pesticide registration goes out of business, legally, authorities cannot cancel the registration unless they first inform the company – even if it no longer exists. This could be a potential explanation why some registrations are still in the registry in Mexico, even if a pesticide is banned. For instance, in February 2021, there were still three endosulfan entries in the COFERPRIS registry, all with an indefinite registration.
Above-mentioned factors have made it difficult for the Mexican authorities to restrict or prohibit pesticides in Mexico. They have hampered their efforts to ensure that the database on pesticides permitted on the market is correct and impacted their compliance with the Multilateral Environmental Agreements dealing with pesticides. Mexican authorities have recently employed alternative methods, by using custom tariff codes, to overcome this obstacle and restrict import of certain pesticides to Mexico.
Requirements for import/export certificates
In line with the FAO and WHO guidelines, import and export requirements should include an explicit prohibition of the import of unregistered, counterfeit, substandard or obsolete pesticides, and regulation of export or transit of non-registered pesticides. It should also establish a licencing system for the import of pesticides. These requirements should also reflect the provisions of the Rotterdam Convention, the Stockholm Convention on persistent organic pollutions (POPs) and the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal (FAO & WHO, 2015[40]).
Responsible use of pesticides
FAO Guidance on pest and pesticide management policy development recognises three steps in pesticide risk reduction:
1. Reducing reliance on pesticides;
2. Selecting pesticides with the lowest risk to human health and the environment from the available registered products that are effective against the pest or disease;
3. Ensuring correct use of the selected products for approved applications and in compliance with international standards (FAO, 2010[52]).
Considering the above-mentioned step 1, the 2006 Mexican Law on Organic Products includes in Article 1.V a goal to promote organic production systems, especially in regions where environmental and socioeconomic conditions are supporting such activity or restructuring production systems is necessary to contribute to the recovery and/or preservation of ecosystems and to achieve compliance with sustainability criteria. As it was presented in Chapter 1, organic production is on the rise in Mexico.
Integrated Pest Management (IPM) means the careful consideration of all available pest control techniques and subsequent integration of appropriate measures that discourage the development of pest populations and keep pesticides and other interventions to levels that are economically justified and reduce or minimise risks to human and animal health and/or the environment. IPM emphasises the growth of a healthy crop with the least possible disruption to agro-ecosystems and encourages natural pest control mechanisms (FAO & WHO, 2016[53]).
In Mexico, IPM supporting campaigns have been undertaken in relation to, for example, avocado tree, citrus, coffee plants cotton pests or fruit flies (SENASICA, 2020[15]). IPM programmes have been adopted for tomatoes, pecan trees, broccoli or chili peppers. IPM components, such as biological control agents, have been identified for maize pests. Implementing further IPM programmes for important Mexican crops is considered beneficial in reducing the use of pesticides in Mexico, a country with – the highest quantity of pesticides per arable land in North America. A large number of growers in Mexico is considered as one of the obstacles for the greater implementation of IPM programmes (e.g. about 2 million growers of maize, working under different conditions) (Blanco et al, 2014[54]).
The OECD Pesticides Programme has an IPM Hub that provides information on IPM policies, programmes, production guidelines and IPM case studies in OECD countries and serves as a platform for information sharing and co-operation between all stakeholders (OECD, n.d.[55]).
In relation to step 2, the issue of substituting in Mexico pesticides with less hazardous ones has encountered certain problems in the past, as it has been for instance the case of the significant number of registrations of pesticides restricted or prohibited under the Multilateral Environmental Agreements ratified by Mexico or prohibited in other jurisdictions. The recent Recommendation 82 of 2018 issued by the National Human Rights Commission may be a key element to improve the substitution of pesticides in Mexico.
In relation to step 3, as it will be described in this Section, Mexico has in place guidelines, initiatives and regulatory framework to promote the safe use of pesticides. However, while the application of pesticides requires training in Mexico, there is no certification scheme in place. Such scheme would allow the Mexican authorities evaluating pesticides to better account for the occupational health and safety risks of workers, who may have different conditions, when it comes to exposure or risk profiles, than the public. Moreover, the application of pesticides and any emerging pesticide resistance is, in general, not monitored by authorities (SENASICA, 2020[15]).
The current regulatory framework in Mexico does not differentiate between the professional uses of pesticides and the use by the general public. In practice, there are no restrictions on buying pesticides, although technical advice is needed to purchase pesticides for agriculture use (to identify the pest and select the appropriate product). Such restrictions are being applied in other countries (see Box 2.7.) to minimise unreasonable adverse effects to the environment and poisoning with pesticides (OECD, 2017[56]). Regulatory options used by authorities in the OECD countries to mitigate the risks for non-professionals include authorising only some types of formulations, requiring specific packaging or only allowing uses in certain conditions. Further information can be found in the 2017 Report of the OECD Seminar on Risk Reduction and Pesticide Non-professional uses.
Box 2.7. Best practice on restricting availability of certain pesticides to the general public
In the United States, US EPA classifies pesticides as either general use (unclassified) pesticides or restricted use pesticides (RUPs). RUPs are not available for purchase or use by the general public, as they have the potential to cause unreasonable adverse effects to the environment and injury to applicators or bystanders without added restrictions.
Source: (EPA, n.d.[57]).
In Mexico, persons who apply a pesticide in a given area are required to post warning signs, but it is only recommended that such postings take place before the pesticide is applied. If it is done after the application, a date and hour of application should be provided (SENASICA, 2019[12]).
On the other hand, authorities recommend to the purchaser to verify that a pesticide is in the original packaging, in good quality, and has a guarantee seal in place, as well as to check the validity of the product and that it has a registration number when purchasing the product. Moreover, pesticides should be purchased only at sellers certified by SENASICA, which runs the public on-line register (SENASICA, 2019[12]). As described in Chapter 3, Australia has in place an online portal to improve communication between the users of pesticides and the authorities and to support reporting on non-compliance and adverse experience with pesticides.
While the Mexican regulatory framework does not prohibit advertising unregistered, illegal, or counterfeit pesticides, or misleading advertising of pesticides, as recommended by FAO and WHO (FAO & WHO, 2015[40]), the General Law of Health includes a provision that the Secretary of Health shall authorise pesticide advertising.
In general, Mexico does not require buffer zones for the application of pesticides, with the exception of aerial applications where the landing track must be located at least 500 meters away from cities, water bodies, channels or drains (SENASICA, 2020[15]).
In this context, is worth noting that the OECD has developed a website about the regulatory approaches used by governments to address the issue of pesticide spray drift. It also provides links to peer-reviewed scientific papers that are in the public domain, validated spray drift models, spray drift field study results and other information important to spray drift risk assessment and risk management. (OECD[58]). Mexico might benefit from the on‑going OECD work in this regard in the context of the planned update of its Official Standard addressing aerial spraying.
There seems to exist a significant regional disparity on the efficacy and prudential use of pesticide technologies throughout Mexico. Export- oriented large-scale farmers seem also to have the best pesticide practices in place. New OECD work on responding to the use of new digital and mechanical technologies for pest management, in particular the application of pesticides by drones, may also be of interest to Mexico.
Obsolete pesticides
Stocks of obsolete, unwanted and banned pesticides continue to represent a serious public health and environmental threat (FAO, 2009[59]). FAO has a dedicated Programme on the Prevention and Disposal of Obsolete Pesticides. FAO collaborates with countries to prevent more obsolete pesticides from accumulating and assists them to dispose of their existing stockpiles (FAO[60]). According to FAO data, the stocks of obsolete pesticides in Mexico are estimated to amount to 1 151 185 tonnes (FAO[61]).
The General Law on the Prevention and Integral Management of Waste (LGPGIR) regulates obsolete pesticides in Mexico. Mexico has in place an inventory of obsolete pesticides and contaminated sites (updated in 2016), but it is descriptive and generic, and thus has limited information on the holders of small amounts of obsolete pesticides. Management plans for obsolete pesticides involving all stakeholders are needed, as well as a comprehensive plan to stop the accumulation of obsolete pesticides. The existing inventories of contaminated sites in general only provide information about the type of contaminants (e.g. pesticides) (SEMARNAT, 2017[62]).
Empty pesticide containers
In line with the FAO guidelines, empty pesticide containers should be managed to minimise risk to human health and the environment. For instance, the containers should be decontaminated and it should be possible for users to return them when empty (FAO & WHO, 2008[63]).
Empty pesticide containers are treated as hazardous waste in Mexico, in line with the General Law on Prevention and Integral Management of Waste, and information on triple rinsing of the empty containers should be included on the label. Primary Collection Centres (CAP) are places where farmers can deposit empty containers, after triple rinsing, drying and perforation. Collected containers are then sent to Temporary Collection Centres (CAT) that prepare the containers for their final disposal at the authorised recycling centres (SENASICA, 2019[13]). In 2015, there were 959 CAP and 66 CAT in Mexico. Industry associations have supported efforts by disseminating information on triple rinsing, collection of empty containers and their final disposition among the Mexican stakeholders (SAGARPA, 2015[64]).
In Mexico, approximately 50 million empty pesticide containers (in total approximately 6 700 tonnes) are disposed of each year. However, many containers are abandoned in the fields, which leads to environmental problems. The Mexican authorities, together with stakeholders involved in the production, distribution, management and disposal of the containers have implemented a national programme for the collection of empty pesticides containers “We keep a Clean Field” (Conservemos un Campo Limpio) (SAGARPA, 2015[64]). However, the participation in the programme is currently not mandatory and the programme does not cover certain types of pesticides, such as biopesticides.
In 2015, there were 29 formal Management and Collection of Empty Containers Plans, registered at state level (SAGARPA, 2015[64]). The establishment of container management plans has been effective in increasing the recovery of empty containers in Mexico (OECD, 2012[65]); however additional resources are needed to support better implementation. It is estimated that only 10% of the funding needed is provided to the Mexican authorities to cover the annual costs of the collection of empty containers (SENASICA, 2020[15]).
Long standing tradition of co-operation among authorities, industry and other stakeholders to promote safe use of pesticides and address emerging issues
The promotion of safe use of pesticides is an area of shared responsibility among all stakeholders (government, pesticide industry, suppliers and users). Industry-led awareness campaigns on the correct and safe use of pesticides have a long tradition in Mexico.
Since 1983, the Mexican crop protection industry has implemented an awareness campaign on the correct and safe use of pesticides. It is called Good Use and Management of Agrochemicals (CUIDAGRO-BUMA, acronym in Spanish). It is intended for final users as well as students, academia, medical personnel and the public and builds on the FAO guidelines (SAGARPA, 2015[64]).
The topics addressed in CUIDAGRO-BUMA include the risks associated with misuse of pesticides in the field, prevention of poisoning and first aid, understanding pesticide labels, transport and storage and application of pesticides and the use of PPE. CUIDAGRO-BUMA activities are co-ordinated with local and federal authorities (in particular SENASICA), UNDP and academia (SENASICA, 2020[15]).
Mexican authorities disseminate information to the public including guidance material on good practices and the safe use of pesticides, leaflets on the purchase, management and application of pesticides and the protection of pollinators.
Mexican authorities have published a catalogue of registered pesticides products in Mexico and their authorised uses for many years. The catalogue contains a list of prohibited and restricted products in Mexico. The products are listed per crop, the pesticides approved for control of plant health problems, safety intervals (days after the application before to harvest), and authorised maximum residues limits for each product (Pérez-Olvera, Navarro-Garza and Miranda-Cruz, 2011[66]). In 1991, Mexico published a list of prohibited and restricted pesticides, which included 20 and 11 entries respectively (NHRC, 2018[28]).
COFEPRIS published the latest update of this catalogue in 2016. More regular updates would support better availability of relevant information on pesticides in the market. This is particularly the case as not all of the information in the Catalogue is included in the COFEPRIS on-line database on pesticide registrations. A potential merger of the Catalogue content with COFEPRIS database would support better dissemination of the relevant information. Also in 2016, COFEPRIS published a Catalogue of Pesticides with Reduced Risk,
In 2018, SENASICA published “General directives for the operation, certification and recognition of the Contamination Risk Reduction Systems, Good Use and Management of Pesticides and Good Agricultural Practice in the Harvesting Activities during the primary production of plants”. This document sets requirements for growers to be certified, and such certification is valid for 2 years but could be suspended or cancelled in case of any infractions (NHRC, 2018[28]). Technical requirements relevant for obtaining such certificates were published by SENASICA in 2019 (SENASICA, 2019[67]).
In 2019, SENASICA published a Manual of Good Use and Management of Pesticides in the Field (see Box 2.8). The document was developed in co‑operation with SEMARNAT and academia. This manual consists of two Parts. Part I describes the FAO concept of the Integrated Pesticides Management (IPM), while Part II addresses several relevant topics of Good Use and Management of Pesticides (SENASICA, 2019[12]). The document contains practical and user friendly information. When referring to international standards or recommendations at the national level only, it also indirectly points to areas of potential improvement of the Mexican regulatory framework on pesticides (e.g. lack of obligatory inventories of pesticides in companies or lack of obligatory signalling of pesticides application, except for aerial spraying).
Box 2.8. Dissemination of information on pesticides and their safe use
2019 Manual of good use and management of pesticides in the field
The manual addresses the following information linked to pesticides management:
classifications and labelling of pesticides;
purchasing pesticides
transport and storage of pesticides;
intoxications and first aid;
how to select PPE and application equipment;
how to behave during and after application of pesticides, including personal hygiene and cleaning of the equipment;
empty pesticides containers and their management; and
illegal trade of pesticides.
Source: (SENASICA, 2019[12]).
Presentations from a three-day course on Regulation and Surveillance of Agricultural Pesticides in Mexico, held in the City of Mexico in March 2019 are publicly available on the website of SENASICA. They provide general information on pesticide management in Mexico covering a number of subjects, such as:
regulatory framework on pesticides in Mexico;
formulation, commercialisation, storage and application of pesticides;
Maximum Residues Limits;
import and export of pesticides;
environmental requirements for pesticides registration; or
pesticides waste management (SENASICA, 2019[68]).
The use of personal protective equipment is regulated by the Mexican Official Standard dealing with work and safety conditions in the workplace: NOM-017-STPS-2008 on the use and management of Personal Protective Equipment in the workplace and the Official Mexican Standard on Safety and hygiene conditions in agricultural activities. The latter is currently in the process of revision to focus solely on pesticides. The 1999 version of this standard required the civil or legal persons using the services of the workers to apply pesticides to use only registered pesticides, not expired, in recommended dose; provide its personnel with PPE and have a list of trained personnel.
The project of the updated standard, NOM-003-STPS-2016, includes, among other things, additional obligations to verify that all containers include the original label; have Safety Data Sheets for all hazardous pesticides and use only certified personnel for aerial spraying of pesticides. It also requires to signal areas where pesticides are mixed, filled or stored and where the use of PPE is obligatory, as well as to signal containers and area of storage of pesticides.
Nevertheless, despite the awareness-raising, training and educational efforts mentioned above, there continue to exist a significant disparity in the real-life use of pesticides, which can be observed in the results of the enforcement activities. Many factors can contribute to this. The size of the country and the number of farmers that can affect reaching out to all relevant stakeholders with relevant information is one of the possible ones. Further strengthening and broadening of the joint activities of the authorities and industry, possibly merged with policy instruments (for instance taxation mentioned in Chapter 1 or completion and implementation of NOM-003-STPS-2016) could support addressing this issue in Mexico.
Regulatory compliance and enforcement of pesticides
The 2018 OECD Regulatory Enforcement and Inspections Toolkit (Box 2.9) provides guidance on practical ways that enforcement agencies can improve their practices to achieve better regulatory compliance. These principles take into account the fact that governments usually face budget limitations and suggest ways to improve enforcement under these circumstances.
Box 2.9. OECD Regulatory Enforcement and Inspections Toolkit
1. Evidence-based enforcement: deciding what to inspect and how should be grounded on data and evidence, and results should be evaluated regularly.
2. Selectivity: inspections and enforcement cannot be everywhere and address everything, and there are many other ways to achieve regulations’ objectives.
3. Risk focus and proportionality: the frequency of inspections and the resources employed should be proportional to the level of risk and enforcement actions should be aiming at reducing the actual risk posed by infractions.
4. Responsive regulation: inspection enforcement actions should be modulated depending on the profile and behaviour of specific businesses.
5. Long-term vision: clear objectives should be set and institutional mechanisms set up with clear objectives and a long-term road-map.
6. Co-ordination and consolidation: less duplication and overlaps will ensure better use of public resources, minimise burden on regulated subjects, and maximise effectiveness.
7. Transparent governance: Governance structures and human resources policies for regulatory enforcement should support transparency, professionalism, and results-oriented management. Execution of regulatory enforcement should be independent from political influence, and compliance promotion efforts should be rewarded.
8. Information integration: Information and communication technologies should be used to maximise risk-focus, co-ordination and information-sharing – as well as optimal use of resources.
9. Clear and fair process: coherent legislation to organise inspections and enforcement needs to be adopted and published, and clearly articulate rights and obligations of officials and of businesses.
10. Compliance promotion: Transparency and compliance should be promoted through the use of appropriate instruments such as guidance, toolkits and checklists.
11. Professionalism: Inspectors should be trained and managed to ensure professionalism, integrity, consistency and transparency.
12. Reality check: Institutions in charge of inspection and enforcement should deliver the performance that is expected from them – in terms of stakeholders satisfaction, of efficiency (benefits/costs), and of total effectiveness (safety, health, environmental protection etc.).
Source: (OECD, 2018[69]).
Throughout the pesticide life cycle, regulated parties must comply with established requirements to minimise risks to human health and the environment. In line with the OECD Guidance on Pesticide Compliance and Enforcement Best Practice, compliance and enforcement activities can be divided into three main groups: compliance promotion, compliance monitoring and responding to non-compliance (enforcement) (Table 2.4).
Table 2.4. Compliance and enforcement activities
|
Compliance and enforcement activity |
Compliance promotion |
Compliance monitoring |
Responding to non-compliance |
|---|---|---|---|
|
Intent |
Improve regulated parties’ awareness of regulatory requirements |
Verify that regulatory requirements are being met |
Bring a known or potential non-compliance situation into compliance |
|
Examples |
Risk communication |
Inspections |
Letters |
|
Reports |
Market surveys |
Meetings |
|
|
Information bulletins |
Samplings |
Orders |
|
|
Seminars |
Recalls |
||
|
Trade shows |
Administrative penalties |
||
|
Websites |
Prosecutions |
||
|
Stakeholder engagement and partnerships |
Source: (OECD, 2012[65]).
In line with FAO/WHO recommendations, compliance monitoring and enforcement should:
ensure monitoring and data collection with respect to pesticides;
set out powers and responsibilities of authorities to impose reporting requirements on manufacturers, importers, distributors and sellers of pesticides;
establish a mechanism for the reporting of pesticide-related incidents by all relevant authorities and parties;
define the powers of inspectors and their qualifications;
provide procedures and criteria for inspections and sample taking, as well as provisions for the designation of official laboratories for analysis of samples; and
define the actions that will be considered as offences as well as determine proportional and deterrent fines (FAO & WHO, 2015[40]).
Authorities should ensure that their inspection and enforcement activities include evaluating for compliance of the label with national regulations and develop ways to identify non-compliant, illegal and counterfeit pesticides through the careful examination of the label (FAO & WHO, 2015[39]).
While an effective registration system is essential, post-registration activities such as surveillance, education and enforcement are equally important (FAO & WHO, 2011[8]). For instance, monitoring residues on food allows governments to assess consumer safety, detect residues from improper use, and protect the credibility of exporters with their customers, while training on the use of pesticides is needed to ensure that safety information reaches the individual users.
In line with the OECD and FAO/WHO guidance, good collaboration on enforcement between the pesticide authorities and other relevant agencies such as the customs department, police department and ministry of trade is crucial for the implementation of the regulatory framework. A system for co‑ordination of enforcement should be formally established, as well as training for enforcement officials, on substandard and illegal products. Close collaboration between authorities and industry is key (FAO & WHO, 2011[8]).
Provisions and co-operation on compliance and enforcement in place in Mexico
Mexico has in place a regulatory framework that includes most of compliance and monitoring elements. For example, in accordance with the Mexican Federal Law of Administrative Procedures, in order to perform inspections, government inspectors have to present a signed order by the authority within the jurisdiction. Such an order has to include a precise location, reason for the inspection, the inspection scope and the legal grounds for the inspection. The inspected entities can provide feedback and evidence in relation to the inspection scope.
Following the inspection, the company will need to confirm corrective actions implemented in writing. If the authority is satisfied that the company is now in compliance, the authority issues a formal document closing the inspection procedures. If the company does not comply, the case is sent to the legal department.
Moreover, the General Law of Health and Plant Health Law oblige federal authorities to establish co-ordination mechanisms to implement these laws. This takes place in practice, for instance if SENASICA finds a violation that belongs to the competence of COFEPRIS or SEMARNAT, it informs them accordingly and these authorities undertake further actions to address the violations (e.g. for non-registered products COFEPRIS is informed, for expired products PROFEPA under SEMARNAT is informed). However, the co-operation is not formalised (e.g. in the form of a Memorandum of Understanding) and there is no common enforcement strategy, as the enforcement activities are decided by each of the authorities separately.
For example, SENASICA verifies compliance with applicable Official Mexican Standards and prioritises under its enforcement activities the good use and management of pesticides. Annual inspection plans focus on a number of companies to inspect. Selection of the companies to inspect is done based or complaints received or randomly taking into account the following criteria:
Mexican States that do not have certified companies in the Phytosanitary Directory;
Mexican States that have not been visited recently; and
Mexican States with a high agriculture activity (SENASICA, 2020[15]).
In general, data on SENASICA’s inspections for 2012-18 show an increase in the proportion of companies found to be non-complaint with certain aspects of pesticides management, while at the same time the overall number of inspections is decreasing since 2015 (see Table 2.5). The latter is linked to the decreased budget allocation. Only four financial fines were applied by SENASICA in this period of time (SENASICA, 2020[15]).
Table 2.5. The share of the follow-up to SENASICA’s inspections is increasing
Official inspections in the establishments dealing with pesticides (manufacturers, importers, formulators, distributors and users) and their result, 2012-18
|
Year |
Number of companies inspected |
Companies with legal follow-up |
Notification to Profepa |
Notification to COFEPRIS |
|---|---|---|---|---|
|
2012 |
96 |
0 |
0 |
0 |
|
2013 |
97 |
0 |
0 |
0 |
|
2014 |
120 |
21 |
0 |
0 |
|
2015 |
146 |
25 |
0 |
0 |
|
2016 |
128 |
53 |
17 |
5 |
|
2017 |
61 |
45 |
15 |
4 |
|
2018 |
76 |
n/a |
n/a |
n/a |
|
Total |
710 |
143 |
32 |
9 |
Source: (SENASICA, 2020[15]; SENASICA, 2018[70]).
The findings point out to key areas of non-compliance of importance for Mexico and could direct authorities in the need for follow-up actions:
Lack of valid certificate to commercialise pesticides (distributors and retailers);
Lack of inventory of pesticides commercialised (distributors and retailers);
Commercialisation of not registered or expired pesticides or in bulk form (distributors and retailers);
Lack of evidence of the capacitation of the personnel (distributors, retailers and pesticide applicators);
No technical advice provided to the distributors and retailers (producers/importers/formulators);
No control of imported, manufactured or formulated pesticides (producers/importers/formulators);
Application of unauthorised pesticides (pesticide applicators) (SENASICA, 2020[15]; SENASICA, 2018[70]).
During 2014-17, COFEPRIS held 893 visits to the formulators and retailers of pesticides and fertilisers and, in consequence, suspended the activity of 123 establishments. It also confiscated over 68 000 tonnes of irregular pesticides and fertilisers in the same period of time (COFEPRIS, 2017[71]).
Increased co-ordination efforts might lead to staff and budget capacity benefits for all authorities involved, but it might require formalisation of co-operation, for instance via Memoranda of Understandings. Scheduled joined inspections could allow for a comprehensive and co‑ordinated approach to the regulated entities, at the same time reducing their administrative burden. Moreover, it might be also feasible to evaluate the effectiveness of the inspection efforts, which is currently challenging for the Mexican authorities.
In line with the PLAFEST Regulation, acts or resolutions issued by authorities implementing this regulation can be appealed in line with the procedure established in Article 83 of the Federal Law of Administrative Procedure.
In summary, there is co-operation on enforcement between the main authorities for pesticides, for example in relation to the notification of infringements. However, compliance and enforcement activities in Mexico are complex and fragmented and there is room for improvement, for example through a more centralised approach – joint inspections or establishing Memoranda of Understanding among the authorities. Moreover, as elsewhere in the world, the enforcement activities are impacted by decreasing resources available. Challenges in this area also derive from data gaps in pesticide monitoring efforts as well as in relation to the uses and application of pesticides in Mexico, as described in other parts of this chapter and in Chapter 1.
Illegal trade of pesticides
International shipments of illegal pesticides3 (e.g. counterfeit, unregistered, illicit or otherwise unauthorised active ingredients and finished products) are a significant challenge for pesticide regulators and custom offices, and is a growing concern for governments. Illegal trade can have significant impacts on human health, food chain safety, and the environment, and it undermines national registration and governments’ risk reduction schemes, and public confidence in such schemes. It also distorts pesticide markets by replacing legitimate products with cheaper and possibly more hazardous products.
The share of illegal pesticides in the global market is estimated to be between 10 and 25%. The European Union Intellectual Property Office (EUIPO) estimates that both direct and indirect effects of counterfeiting in the pesticide sector cause approximately EUR 2.8 billion of lost sales to the EU economy (EUR 1.3 billion for the EU pesticides industry). Illegal pesticides are a major concern in several Latin America countries, such as Argentina, Brazil, Paraguay and Uruguay (Frezal and Garsous, 2020[1]). It is estimated that illegal pesticides constitute 13.8% of the regular EU market (OECD, 2019[72]).
The 2019 OECD Recommendation on Countering the Illegal Trade of Pesticides, OECD/LEGAL/0446 recommends that Adherents establish or strengthen national procedures aimed at countering the illegal trade of agricultural pesticides in line with the Best Practice Guidance (Box 2.10), taking into account national priorities, policies and programmes by:
ensuring there is an appropriate regulatory framework for the management of agricultural pesticides;
ensuring that there are systems in place to detect and take regulatory action against illegal trade of pesticides; and
co-operating on minimising the illegal trade of pesticides (OECD, 2019[72]).
The Council Act instructs OECD to serve as a forum, using a Rapid Alert System (RAS), for the rapid exchange of reports on suspicious or rejected shipments of pesticides, when such information is deemed relevant and urgent. The RAS is a protected website accessible to regulatory authorities for a rapid exchange of information about suspicious or rejected shipments of pesticides.
Box 2.10. OECD Best Practice Guidance to Identify Illegal Trade of Pesticides
Best Practice Guidance provides a tool-box with over 100 practices throughout the life cycle of a pesticide
The document provides guidance for inspectors and regulatory authorities on best practices for identifying and tackling illegal pesticides throughout the complete lifecycle of a pesticide, that is for the following:
Manufacture (Manufacturing and storage facilities, Inspectors);
Formulation;
Export (List of exporters, Record keeping and templates/forms, Registration in destination country, Export certificates);
Transportation (Pre arrival, In transit);
Import (Importer obligations, Inspectors);
Sale/Retail (Distributors, Record keeping and templates/forms, Inspectors and inspections, Education);
Use (Professional users, Inspectors);
Disposal (Pesticide packaging, Illegal pesticides).
Source: (OECD, 2018[73]).
The General Law of Health prohibits the illegal and unregistered use of pesticide, and includes both a criminal sanction of up to 8 years in prison and a monetary fine of up to two thousand days of minimum salary equivalent. To support the fight against illegal trade, SENASICA certifies authorised pesticide dealers) and disseminates recommendations on how to identify illegal pesticide products. Stakeholders in Mexico are also encouraged to notify the General Prosecutor and COFEPRIS about illegal activities, via free and anonymous hotlines (SENASICA, 2019[12]). Mexico, to date, has not participated in OECD activities on illegal trade of pesticides nor posted (or reviewed) any information on the RAS.
On-going reforms of pesticides management in Mexico
Recent years have witnessed many positive developments in the area of pesticides management in Mexico. Policy development resulting from the Mexican National Commission for Human Rights recommendation on pesticides is of particular relevance, as it could be considered as a decisive moment in the country’s path to upgrade its pesticides management framework, taking into account that certain developments were in progress already before it (e.g. the revision of certain NOMs on pesticides or the adoption of the NOM on MRLs).
In December 2018, NHRC issued a Recommendation 82/2018. In line with its title, this recommendation addresses “the violation of human rights to food, clean water, clean environment and health, due to the breach of the general obligation of due diligence to restrict the use of highly hazardous pesticides, to the detriment of the population in general"(Box 2.11) (NHRC, 2018[28]).
Box 2.11. Recommendation 82/2018 of the National Human Rights Commission
Recommendation 82/2018 was issued in response to a complaint filed by 43 persons in 2017, denouncing that the federal Mexican authorities do not comply with the international treaties to which Mexico is a Party, by failure to act administratively, normatively and via public policies to restrict the use of highly hazardous pesticides.
Following investigation, the Commission issued Recommendation 82/2018 that includes 61 recommendations addressed to the Secretary of Environment, the Secretary of Health, COFEPRIS and SENASICA. A copy of the Recommendation was also given to the Mexican Parliament.
Main recommendations:
Urgently adopt regulatory measures for pesticides to protect water quality, the environment and human health, building for instance on the directives of the FAO Code of Conduct. Adopt the definition of highly hazardous pesticide.
Modify the existing regulatory framework, including NOMs, to better address highly hazardous pesticides in their life cycle.
All authorities should adopt a common strategic action plan addressing clearly responsibilities on monitoring, control and compliance with the regulatory framework and the mechanisms of co-ordination should be strengthened.
Establish a multi-stakeholder Special Committee on the identification and investigation on the adverse effects of highly hazardous pesticides.
Ensure strict implementation of the multilateral international agreements dealing with pesticides of which Mexico is a party.
Undertake necessary actions to be able to cancel or revoke existing pesticides registries. Establish stricter and more restrictive rules on the uses and management for new pesticides registrations, as well for the renovation of the registration and existing registrations.
Identify registrations that authorise the use in Mexico of active ingredients or pesticides prohibited in other jurisdictions, in order to analyse, which could affect the environment or human health in Mexico.
Establish the National Programme of Monitoring Pesticides Residues and make the monitoring, contamination and intoxication information publically available.
Elaborate studies (e.g. water and soil contamination by pesticides, intoxications) and prepare capacity building activities and educational campaigns on the safe use of pesticides for the Mexican population.
Source: (NHRC, 2018[28]).
Of particular importance is that many recommendations from the NHRC Recommendation 82/2018 are addressed jointly to the relevant authorities in Mexico and therefore should support synergy in their actions. All the authorities to which Recommendation 82/2018 was addressed have accepted its conclusions and undertaken efforts to address them.
In May 2019, the establishment of an inter‑institutional working group, consisting of COFEPRIS, SEMARNAT and SENASICA was announced. The objectives of this group are to address issues raised by Recommendation 82/2018 and to modernise and strengthen the regulatory and surveillance framework on pesticides in Mexico. The group was established for an indefinite period and a representative of the NHRC was invited to participate in all its meetings, as well as representatives of academia and NGOs. The agreement on the establishment of the group also obliged COFEPRIS and SENASICA to continue working on the cancellations of registration of the most hazardous pesticides (SEMARNAT, 2019[74]).
Since the publication of Recommendation 82/2018, a “Diagnosis on the pesticide contamination of surface water, groundwater and soil” (INECC, 2019[75]) was published in 2019. The same year saw the publications of the “Elements for the Development of an Integral Strategy for Responsible Pesticides Management in Mexico” (Mexican Technical Working Group on Pesticides, 2019[76])(Box 2.12) and the “Manual of good use and management of pesticides” (SENASICA, 2019[12]).
Box 2.12. 2019 Elements for the Development of an Integral Strategy for Responsible Pesticides Management in Mexico
In 2019, a Technical Working Group on Pesticides prepared an analysis of possible elements of the future Mexican integrated strategy for pesticides management. The group was composed of the governmental stakeholders (Secretary of Health and Secretary of Environment), representatives of international organisations (UNEP and PAHO/WHO) and non-governmental stakeholders (INECC and Mexican Toxicological Network).
Proposals included in the document
In relation to the needed changes to the Mexican regulatory framework, the document proposes, among others, to:
Eliminate indefinite validity of pesticides registrations from before 2005 and establish a procedure for the cancellation of the pesticides registrations;
Strengthen procedures for renovation of pesticides registrations;
Update to international standards ecotoxicological and environmental requirements linked to new pesticides registrations;
Strengthen regulatory framework on pesticides in relation to their environmental impacts (e.g. in relation to their uses);
Revise health related aspects of the current regulatory framework;
Enhance the publication of official information on pesticides (e.g. the Official Pesticides Catalogue).
In relation to the control and surveillance of the commercialisation and use of pesticides in Mexico, the document proposes, among others, to:
Strengthen control over and requirements of establishments dealing with pesticides;
Control the sale of pesticides (e.g. by establishing obligatory sale registry);
Control the use of pesticides (e.g. aerial spraying, establishing a register of the uses of pesticides);
Establish a national programme of environmental and health monitoring of pesticides, strengthen monitoring of pesticides residues in agricultural products;
Establish procedures to avoid importing pesticides prohibited in other countries.
A discussion on improvements to the Mexican pesticides management system was also held under the first Mexican National Forum on Pesticides in 2018, and it focused on three aspects: agricultural, environmental and sanitary (Box 2.13).
Box 2.13. Proposals of the 2018 Mexican National Forum on Pesticides
Agricultural aspects
Elimination of prohibited and expired pesticides;
Substitution of highly hazardous pesticide
It could be achieved via, for instance, enhanced compliance and enforcement efforts.
Environmental aspects
Following the analysis of the existing framework, adopt a comprehensive law on hazardous substances that would include pesticides and would regulate their whole life‑cycle. The law should include the definition of a pesticide and a highly hazardous pesticide; specify the obligations of each involved authority; address monitoring of pesticides and contaminated sites;
Establishing a national pesticides monitoring programme and setting maximum levels of pesticides in water and soil and establishing infrastructure that would allow verifying compliance;
Introducing a risk evaluation methodology into the registration process for pesticides;
Establishing a national statistical database of sales and use of pesticides (obligation included in the comprehensive law).
Sanitary aspects
Strengthening compliance and enforcement in the area of pesticide sales;
Strengthening monitoring of the use of highly hazardous pesticides;
Capacity‑building activities for the users of pesticides.
In November 2019, the tariff codes were changed by the creation of 19 new tariff codes, modification of 3 existing and suppression of 15 codes, to better identify hazardous pesticides and to prohibit their export and import (e.g. of endosulfan or alaclor) (Secretaría de Economía, 2019[78]).
Moreover, authorities also established a special committee to co-ordinate activities related to the identification and investigation of highly hazardous pesticides (CEIIEAPAP for its acronym in Spanish).
Possible elements for consideration by Mexico in its reforms
On-going Implementation of the proposals contained in the Recommendation 82/2018, in the 2019 Elements for the Development of an Integral Strategy for Responsible Pesticides Management in Mexico, as well as in the 2018 Mexican National Forum on Pesticides suggest that work is in progress, but a lot still has to be done. Many of the proposals included therein also align with the findings of this report.
One of the options for further actions, raised in the on-going discussions and in Chapter 1, is to support better harmonisation of the regulation of pesticides and their uses with T-MEC partners and other international partners, as well as streamlining the currently dispersed rules at the national level, through the adoption of a comprehensive law dealing with pesticides (Mexican Technical Working Group on Pesticides, 2019[76]). It would address Mexico’s civil society's human health and environmental concerns linked to the use of pesticides. It could also help to address other relevant issues, including, inter alia, minor uses, emergency uses, lifecycle of pesticides, application through new technologies or development of new molecules (Mexican Technical Working Group on Pesticides, 2019[76]). The suggestion for developing such a law seems to be supported by the Mexican authorities (SEMARNAT, 2019[74]). With all its benefits, this option would have one potential challenge - time needed to adopt the law, its regulations and relevant NOMs to implement the new framework and put it into practice.
Now might be the ideal time to streamline efforts to upgrade the Mexican pesticides management system. The renewal of the trilateral co-operation between Mexico, Canada and the United States under the T-MEC Agreement is an opportunity for re-invigorating the co-operation in the environmental area. It also seems that there is a momentum as many stakeholders in Mexico are in favour of upgrading the regulatory framework of pesticides management, albeit sometimes with difference reasoning behind it.
Moreover, many OECD countries have recently undergone or are undergoing revision of their pesticides programmes, for example Australia, Japan or the European Union (under its Regulatory Fitness and Performance programme, REFIT (EC, 2020[79])). This could be a source of inspiration for the Mexican efforts. For instance, the goals of the on-going review of the system in Australia could be applied to the Mexican situation. The reforms there are seeking to create a “future regulatory system that is efficient, predictable, adaptive, nationally consistent, open and accountable, and places at its centre the protection of human, animal, plant and environmental health and safety” (Matthews et al., 2020[18]).
Finally, FAO launched its online toolbox in 2016 that could support the efforts on the ground in Mexico. Mexico has already benefitted from FAO training in 2019 that covered, among others, the pesticides registration and evaluation parts of the Toolbox (FAO[80]). The training was requested by Mexico in the follow-up to the NHRC Recommendation 82/2018.
It may be argued that major reform of the pesticide management framework in Mexico will face a challenge. In many OECD countries current reforms have as one of their main priorities cutting “red tape” due to the fact that their legislation have been developed over many decades with increasing obligations for industry and increasing environmental and health consideration. However, this is not the case in Mexico, particularly with respect to environmental considerations. Therefore Mexican reforms should support both streamlining of the legislation, making it more efficient and effective, but at the same time incorporating missing elements.
Increasing the environmental risk management scope in the registration and evaluation procedure in Mexico could lead to extending the time needed for pesticide registration, but it might be counterbalanced with increased health and environmental benefits in Mexico. If the need to better reflect the environmental risk management is reflected, it would require bigger involvement from SEMARNAT in terms of both human and financial resources. It might also require reflecting this increased obligation in the regulatory framework.
Many elements of the current regulatory framework on pesticides management in Mexico have been in place for over 20 years. Adaptation of the framework to the technological and environmental changes and challenges, as well as meeting the changing needs of industry and civil society would be beneficial. Moreover, some of the changes in the past have been made in a piecemeal fashion. Ideally, a simultaneous comprehensive revision of all relevant laws, regulations and NOMs could be considered to streamline and reduce the complexity of the regulatory framework.
Eventual reforms could be based on the principles suggested under the on-going review of the Australian system:
objectivity - the system should be evidence and risk-based in its decision-making;
independence - decisions of the authorities should be independent;
efficiency - using the most efficient regulation required to achieve the objective;
consistency - one coherent national system;
access - the system should be harmonised as much as possible with international regulatory systems, processes and timeframes;
simplicity – one legislation that is modern, outcomes focused, free from unnecessary prescription and is simpler and easier to understand and implement;
certainty - provide confidence about regulatory processes and timeframes;
shared responsibility - the system should facilitate the sharing of responsibility among government, suppliers and users (Matthews et al., 2020[18]).
In line with the FAO and WHO guidelines, the main reasons for updating pesticide legislation are to:
ensure consistency in the overall regulatory framework with effective connections between pesticide legislation and other relevant legislation with minimal contradiction or overlap;
clarify any issues related to responsibilities, authority or mandate of the institutions involved;
incorporate provisions to address new requirements stemming from recent developments or updated priorities;
facilitate multidisciplinary approaches to pesticide management;
comply with requirements of international agreements and recommendations; and
harmonise requirements with countries within the region (FAO & WHO, 2015[40]).
As described in this report, a majority, if not all, of these reasons apply to Mexico.
References
[22] APVMA (2020), Timeframe and fees (webpage), https://apvma.gov.au/node/1088 (accessed on 3 July 2020).
[29] Bejarano, F. (2018), Highly Hazardous Pesticides in Mexico, Pesticide Action Network in Mexico, Texcoco, https://ipen.org/sites/default/files/documents/HHHP%20in%20Mexico%202018REV.pdf.
[54] Blanco et al (2014), “Maize Pests in Mexico and Challenges for the Adoption of Integrated Pest Management Programs”, Journal of Integrated Pest Management, Vol. 5/4, https://doi.org/10.1603/IPM14006.
[44] Chile’s Agriculture and Livestock Service (n.d.), Evaluación y autorización de plaguicidas (Evaluation and authorisation of pesticides)(webpage), https://www.sag.gob.cl/ambitos-de-accion/evaluacion-y-autorizacion-de-plaguicidas (accessed on 3 July 2020).
[71] COFEPRIS (2017), Asegura COFEPRIS más de 68 mil toneladas de plaguicidas y nutrientes vegetales irregulares (Cofepris confiscated more than 68 thousand tonnes of irregular pesticides and fertilizers), https://www.gob.mx/cofepris/prensa/asegura-cofepris-mas-de-68-mil-toneladas-de-plaguicidas-y-nutrientes-vegetales-irregulares (accessed on 2 March 2020).
[27] COFEPRIS (n.d.), Consulta de Registros Sanitarios de Plaguicidas, Nutrientes Vegetales y LMR, (Query on the Sanitary Registry of Pesticides, Vegetal Fertilizers and MRLs), http://siipris03.cofepris.gob.mx/Resoluciones/Consultas/ConWebRegPlaguicida.asp (accessed on 24 February 2020).
[16] COFEPRIS (n.d.), Instructivo de llenado del formato de PLAFEST, (Instructions on filling the PLAFEST form), https://www.gob.mx/cms/uploads/attachment/file/349281/instructivo_PLAFEST.pdf (accessed on 20 July 2020).
[11] Congreso de la Unión (2004), Reglamento en Materia de Registros, Autorizaciones de Importación y Exportación y Certificados de Exportación de Plaguicidas, Nutrientes Vegetales y Sustancias y Materiales Tóxicos o Peligrosos. [Regulation on Registration, Import and Export Authorizations and Export Certificates of Pesticides, Plant Nutrients and Toxic or Hazardous Substances and Materials], http://www.diputados.gob.mx/LeyesBiblio/norma.htm.
[79] EC (2020), Report from the Commission to the European Parliament and the Council. Evaluation of Regulation (EC) No 1107/2009 on the placing of plant protection products on the market and of Regulation (EC) No 396/2005 on maximum residue levels of pesticides, https://eur-lex.europa.eu/legal-content/en/txt/?uri=celex:52020dc0208 (accessed on 19 August 2020).
[37] EFSA (2018), “The 2016 European Union report on pesticide residues in food”, EFSA Journal, European Food Safety Authority, Parma, http://dx.doi.org/10.2903/j.efsa.2018.5348 (accessed on 25 February 2020).
[57] EPA (n.d.), “Restricted Use Products (RUP) Report”, https://www.epa.gov/pesticide-worker-safety/restricted-use-products-rup-report (accessed on 2 March 2021).
[24] European Commission (n.d.), Approval of active substances (webpage), https://ec.europa.eu/food/plant/pesticides/approval_active_substances_en (accessed on 3 July 2020).
[25] European Commission (n.d.), Procedure to apply for authorisation of a PPP, https://ec.europa.eu/food/plant/pesticides/authorisation_of_ppp/application_procedure_en (accessed on 3 July 2020).
[45] European Commission (n.d.), Renewal of approval (webpage), https://ec.europa.eu/food/plant/pesticides/approval_active_substances/approval_renewal_en (accessed on 3 July 2020).
[49] European Parliament (n.d.), Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC, https://eur-lex.europa.eu/legal-content/en/txt/pdf/?uri=celex:32009r1107&from=en (accessed on 26 March 2021).
[52] FAO (2010), International Code of Conduct on the Distribution and Use of Pesticides Guidance on Pest and Pesticide Management Policy Development, Food and Agriculture Organization of the United Nations, Rome, http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Code/Policy_2010.pdf.
[59] FAO (2009), Environmental Management Tool Kit for Obsolete Pesticides Volume 1, Food and Agriculture Organization of the United Nations, Rome, http://www.fao.org/3/i0473e/i0473e.pdf.
[5] FAO (2006), International Code of Conduct on the Distribution and Use of Pesticides. Guidelines on Efficacy Evaluation for the Registration of Plant Protection., Food and Agriculture Organization of the United Nations , Rome, http://www.fao.org/3/a-bt474e.pdf.
[34] FAO (n.d.), “Maximum Residue Limits” in: Pesticide Registration Toolkit, Food and Agriculture Organization of the United Nations, Rome, http://www.fao.org/pesticide-registration-toolkit/information-sources/maximum-residue-limits/en/ (accessed on 25 February 2020).
[6] FAO (n.d.), Hazard identification & characterization | Pesticide Registration Toolkit | Food and Agriculture Organization of the United Nations, http://www.fao.org/pesticide-registration-toolkit/registration-tools/assessment-methods/method-detail/en/c/1186994/ (accessed on 3 May 2021).
[7] FAO (n.d.), Human health risks | Pesticide Registration Toolkit | Food and Agriculture Organization of the United Nations, http://www.fao.org/pesticide-registration-toolkit/registration-tools/registration-criteria/human-health-risks/en/ (accessed on 3 May 2021).
[80] FAO (n.d.), National workshop on FAO’s Pesticide Registration Toolkit and Rotterdam Convention implementation, http://www.pic.int/Implementation/TechnicalAssistance/Workshops/WorkshopMexicoOct2019/tabid/8174/language/en-US/Default.aspx (accessed on 6 July 2020).
[60] FAO (n.d.), Prevention and Disposal of Obsolete Pesticides (website), http://www.fao.org/agriculture/crops/obsolete-pesticides/prevention-and-disposal-of-obsolete-pesticides/en/ (accessed on 21 August 2020).
[31] FAO (n.d.), Registration criteria | Pesticide Registration Toolkit, http://www.fao.org/pesticide-registration-toolkit/registration-tools/registration-criteria/en/ (accessed on 26 March 2021).
[61] FAO (n.d.), Where are the Stocks? In: Prevention and Disposal of Obsolete Pesticides (website), http://www.fao.org/agriculture/crops/obsolete-pesticides/where-stocks/latin-stocks/en/ (accessed on 21 August 2020).
[53] FAO & WHO (2016), International Code of Conduct on Pesticide Management. Guidelines on Highly Hazardous Pesticides, Food and Agriculture Organization of the United Nations/World Health Organization , Rome and Geneva, https://apps.who.int/iris/bitstream/ha.
[39] FAO & WHO (2015), International Code of Conduct on Pesticide Management Guidelines on Good Labelling Practice for Pesticides (revised), Food and Agriculture Organization of the United Nations/World Health Organization , Rome and Geneva, https://apps.who.int/iris/bitstream/handle/10665/195650/9789241509688_eng.pdf;jsessionid=F8984B86B1C6DF63B4B7E4282AE62E81?sequence=1.
[40] FAO & WHO (2015), International Code of Conduct on Pesticide Management. Guidelines on Pesticide Legislation, Food and Agriculture Organization of the United Nations/World Health Organization , Rome and Geneva, http://www.fao.org/3/a-i5008e.pdf.
[4] FAO & WHO (2015), International Code of Conduct on Pesticide Management. Guidelines on Pesticide Legislation, Food and Agriculture Organization of the United Nations and World Health Organization , Rome and Geneva, http://www.fao.org/3/a-i5008e.pdf.
[2] FAO & WHO (2013), International Code of Conduct on the Distribution and Use of Pesticides Guidelines on data requirements for the registration of pesticides, Food and Agriculture Organization of the United Nations and World Health Organization, Rome and Geneva, http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Code/DataReq2013.pdf.
[8] FAO & WHO (2011), International Code of Conduct on the Distribution and Use of Pesticides. Guidelines for Quality Control of Pesticides, Food and Agriculture Organization of the United Nations/World Health Organization, Rome and Geneva, https://apps.who.int/iris/bitstream/handle/10665/70574/WHO_HTM_NTD_WHOPES_2011.4_eng.pdf?sequence=1.
[63] FAO & WHO (2008), International Code of Conduct on the Distribution and Use of Pesticides Guidelines on Management Options for Empty Pesticide Containers, http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Code/Containers08.pdf.
[1] Frezal, C. and G. Garsous (2020), New digital technologies to tackle trade in illegal pesticides, Joint Working Party on Trade and Environment, http://hhttp://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=com/tad/env/jwpte(2020)8/final&doclanguage=en.
[42] Government of Australia (n.d.), Agricultural and Veterinary Chemicals Code Act 1994, https://www.legislation.gov.au/Details/C2016C00999 (accessed on 3 July 2020).
[43] Government of Canada (2020), Registration of Pest Control Products (continued)(webpage), https://laws-lois.justice.gc.ca/eng/acts/P-9.01/page-3.html#h-418226 (accessed on 3 July 2020).
[19] Government of Mexico (n.d.), Permiso de importación de plaguicidas y sustancias tóxicas sujetos a control por SEMARNAT (Pesticides and toxic substances import permit subject to control by SEMARNAT_, https://www.gob.mx/tramites/ficha/permiso-de-importacion-de-plaguicidas-y-sustancias-toxicas-sujetos-a-control-por-semarnat/COFEPRIS732 (accessed on 30 June 2020).
[36] Handford, C., C. Elliott and K. Campbell (2015), “A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards”, Integrated Environmental Assessment and Management, Vol. 11/4, pp. 525-536, http://dx.doi.org/10.1002/ieam.1635.
[23] Health Canada (2019), Service Standards for Categories A-L Authorizations under the Pest Control Products Regulations (webpage), https://www.canada.ca/en/health-canada/corporate/about-health-canada/legislation-guidelines/acts-regulations/service-standards-high-volume-regulatory-authorizations/service-standard-categories-authorizations-under-pest-control-products-regulations.html (accessed on 3 July 2020).
[41] Health Canada (n.d.), Search Product Label (online database), https://pr-rp.hc-sc.gc.ca/ls-re/index-eng.php (accessed on 7 July 2020).
[30] Health Canada’s Pest Management Regulatory Agency (2016), Re-evaluation Note REV2016-04, Joint PMRA / USEPA Re-evaluation Update for the Pollinator Risk Assessment of the Neonicotinoid Insecticides (webpage), https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-note/2016/rev2016-04-joint-pmra-usepa-evaluation-update-pollinator-risk-assessment-neonicotinoid-insecti (accessed on 2 July 2020).
[75] INECC (2019), Diagnosis on the pesticide contamination of surface water, groundwater and soil (Diagnóstico sobre la Contaminación por Plaguicidas en Agua Superficial, Agua Subterránea y Suelo), Mexican National Institute of Ecology and Climate Change, City of Mexico, https://www.gob.mx/cms/uploads/attachment/file/495283/Diagno_stico_sobre_la_Contaminacion_por_Plaguicidas_en_Agua_Superficial__Agua_Subterr_nea_y_Suelo_versi_n_final_s-d.pdf.
[51] Japan (n.d.), Summary of the Revision of the Pesticide Registration System, https://members.wto.org/crnattachments/2019/TBT/JPN/19_1637_00_e.pdf.
[46] Korea Law Translation Center (2015), Pesticide Control Act, https://elaw.klri.re.kr/eng_service/lawView.do?hseq=35599&lang=ENG (accessed on 3 July 2020).
[18] Matthews, K. et al. (2020), Issues paper—review of the agvet chemicals regulatory system: future reform opportunities, Department of Agriculture, Water and the Environment, https://haveyoursay.agriculture.gov.au/53499/widgets/281250/documents/138791.
[10] Mexican Congress (Congreso de los Estados Unidos Mexicanos) (2014), Decree reforming the PLAFEST Regulation, http://transparencia.cofepris.gob.mx/index.php/es/marco-juridico/reglamentos (accessed on 17 July 2020).
[9] Mexican Congress (Congreso de los Estados Unidos Mexicanos) (1984), General Health Law (Ley General de Salud), Federal Official Gazette (Diario Oficial de la Federación), City of Mexico, http://www.ordenjuridico.gob.mx/Documentos/Federal/pdf/wo11037.pdf (accessed on 12 March 2020).
[76] Mexican Technical Working Group on Pesticides (2019), Elements for the Development of an Integral Strategy for Responsible Pesticides Management in Mexico (Elementos para Desarollar na Estrategia Integral para la Gestión Responsable de Plaguicidas en México), https://www.gob.mx/cms/uploads/attachment/file/451603/Elementos_para_Desarrollar_una_Estrategia_Integral_de_Manejo_Responsable_de_Plaguicidas_final__3_.pdf.
[47] New Zealand Food Safety (2019), Agricultural Compound and Veterinary Medicine (ACVM) registration process (webpage), https://www.mpi.govt.nz/processing/agricultural-compounds-and-vet-medicines/acvm-overview/authorisation-of-acvm/acvm-registration-process/ (accessed on 3 July 2020).
[28] NHRC (2018), Recommendation 82/2018 (Recomendación No. 82/2018), Mexican National Human Rights Commission, City of Mexico, https://www.cndh.org.mx/sites/all/doc/Recomendaciones/2018/Rec_2018_082.pdf.
[17] OECD (2020), “Guidance Document on the Exchange and Use of International Efficacy and Crop Safety Data for Minor Uses”, OECD Environment, Health and Safety Publications Series on Pesticides, No. No. 101, OECD, Paris, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2020)1&doclanguage=en (accessed on 26 June 2020).
[72] OECD (2019), Recommendation of the Council on Countering the Illegal Trade of Pesticides, OECD/LEGAL/0446, https://legalinstruments.oecd.org/en/instruments/OECD-LEGAL-0446 (accessed on 17 June 2020).
[73] OECD (2018), Best Practice Guidance to Identify Illegal Trade of Pesticides, Series on Pesticides, No. 99, ENV/JM/MONO(2018)35, OECD Publishing, Paris, https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2018)35&doclanguage=en.
[69] OECD (2018), OECD Regulatory Enforcement and Inspections Toolkit, OECD Publishing, Paris, https://dx.doi.org/10.1787/9789264303959-en.
[33] OECD (2017), OECD Integrity Review of Mexico: Taking a Stronger Stance Against Corruption, OECD Public Governance Reviews, OECD Publishing, Paris, https://dx.doi.org/10.1787/9789264273207-en.
[56] OECD (2017), Report of the OECD Seminar on Risk Reduction and Pesticide Non-professional Uses, Series on Pesticides No. 88, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2017)3&doclanguage=en.
[65] OECD (2012), OECD Guidance on Pesticide Compliance and Enforcement Best Practices Series on Pesticides No. 71, Organisation for Economic Cooperation and Development, Paris, https://www.oecd.org/env/ehs/pesticides-biocides/Pesticides_Compliance_Guidance.pdf.
[35] OECD (2010), “OECD Survey On Pesticide Maximum Residue Limit (MRL) Policies: Survey Results”, OECD Environment, Health and Safety Publications. Series on Pesticides No. 51, OECD, Paris, https://www.oecd.org/env/ehs/pesticides-biocides/45275745.pdf.
[14] OECD (2005), Appendix 6. Format for the listing of test and study reports and other documentation. Part 4. OECD, EU, US, Canadian, Japanese and Australian numbering systems for data and information on active substances,.
[13] OECD (1994), Data requirements for pesticide registration in OECD Member countries: survey results, OECD Environment Monographs No. 71, OECD Publishing, Paris, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?doclanguage=en&cote=ocde/gd(94)47 (accessed on 22 February 2021).
[20] OECD (1983), Recommendation of the Council concerning the Protection of Proprietary Rights to Data submitted in Notifications of New Chemicals, OECD/LEGAL/0203, https://legalinstruments.oecd.org/en/instruments/OECD-LEGAL-0203.
[21] OECD (n.d.), Globally Harmonised Submisssion Transport Standard (website), http://www.oecd.org/chemicalsafety/submission-transport-standard/ (accessed on 26 November 2020).
[55] OECD (n.d.), Integrated Pest Management Hub, https://www.oecd.org/chemicalsafety/integrated-pest-management/.
[58] OECD (n.d.), Managing Pesticide Spray Drift (website), http://www.oecd.org/env/spraydrift/ (accessed on 19 August 2020).
[38] OECD (n.d.), OECD Maximum Residue Limit Calculator (website), https://www.oecd.org/env/ehs/pesticides-biocides/oecdmaximumresiduelimitcalculator.htm (accessed on 17 March 2021).
[3] OECD (n.d.), Publications on Registration, https://www.oecd.org/chemicalsafety/pesticides-biocides/publicationsonregistration.htm (accessed on 2 March 2021).
[66] Pérez-Olvera, A., H. Navarro-Garza and E. Miranda-Cruz (2011), “Use of Pesticides for Vegetable Crops in Mexico”, in Pesticides in the Modern World - Pesticides Use and Management, InTech, http://dx.doi.org/10.5772/18510.
[32] PROCCYT (2020), Response to the OECD Questonnaire (unpublished).
[64] SAGARPA (2015), Plan de Manejo de Residuos Generados en Actividades Agrícolas. Primera Etapa: Diagnóstico Nacional (Management Plan for Wastes Generated in Agricultural Activity. First Stage: National Diagnose), Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, https://www.gob.mx/cms/uploads/attachment/file/346978/Manejo_de_residuos_Detallado.pdf.
[50] Sato, S. (2018), Japan Revising Domestic Pesticide Registration System, USDA Foreign Agricultural Service, Tokyo, https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Japan%20Revising%20Domestic%20Pesticide%20Registration%20System_Tokyo_Japan_8-7-2018.pdf (accessed on 26 June 2020).
[78] Secretaría de Economía (2019), “Decreto por el que se modifica la Tarifa de la Ley de los Impuestos Generales de Importación y de Exportación y el Decreto por el que se establece el impuesto general de importación para la región fronteriza y la franja fronteriza norte”, (Decree that modifies the Tariff of the Law on General Import and Export Taxes and the Decree establishing the general import tax for the border region and the northern border strip), Federal Official Gazette, 6 November 2019, City of Mexico, http://www.dof.gob.mx/nota_detalle.php?codigo=5577973&fecha=06/11/2019.
[74] SEMARNAT (2019), Federal Government formalises inter-sectoral Group on pesticides regulation (Formaliza Gobierno Federal integración de Grupo Intersecretarial para Regulación sobre Plaguicidas)(press release), https://www.gob.mx/semarnat/prensa/formaliza-gobierno-federal-integracion-de-grupo-intersecretarial-para-regulacion-sobre-plaguicidas (accessed on 30 July 2020).
[62] SEMARNAT (2017), National Implementation Plan. Mexico 2016 (Plan Nacional de Implementación. México 2016), http://chm.pops.int/Countries/CountryProfiles/tabid/4501/Default.aspx (accessed on 22 July 2020).
[77] SEMARNAT, INECC, UN Environment and PAHO (n.d.), Primer Foro Nacional Sobre Plaguicidas. Actividades y Acuerdos (First National Pesticides Forum. Activities and Agreements), https://www.paho.org/mex/index.php?option=com_docman&view=download&slug=1357-00-resumen-sobe-las-actividades-y-los-acuerdos-del-primer-foro-nacional-sobre-plaguicidas&Itemid=493 (accessed on 10 June 2020).
[15] SENASICA (2020), Response to the OECD Questionnaire (unpublished).
[68] SENASICA (2019), Curso de Regulación y Vigilancia de Plaguicidas Agrícolas (Course on Regulation and Surveillance of Agricultural Pesticides), https://www.gob.mx/senasica/documentos/curso-de-regulacion-y-vigilancia-de-plaguicidas-agricolas?state=published.
[12] SENASICA (2019), Manual of good use and management of pesticides in the field (Manual Para eL Buen Uso y Manejo de Plaguicidas En Campo), Mexican National Service for Agri-Food Health, Safety and Quality, CIty of Mexico, https://www.gob.mx/cms/uploads/attachment/file/452645/MANUAL_PARA_EL_BUEN_USO_Y_MANEJO_DE_PLAGUICIDAS_EN_CAMPO.pdf.
[67] SENASICA (2019), Requisitos generales para la certificación y reconocimiento de sistemas de reducción de riesgo de contaminación (General requirements for certification and recognition of contamination risk reduction systems), https://www.gob.mx/cms/uploads/attachment/file/573822/Anexo_T_cnico_1._V2.1_2019.pdf.
[70] SENASICA (2018), Retos y Oportunidades para Fortalecer la Regulación Agrícola de Plaguicidas (Challenges and Opportunities to Strengthen Agriculture Regulation of Pesticides), https://www.paho.org/mex/index.php?option=com_content&view=article&id=1357:el-uso-sostenible-de-los-plaguicidas-es-fundamental-para-alcanzar-desarrollo-en-las-zonas-agricolas-del-pais&Itemid=499 (accessed on 13 February 2020).
[48] US EPA (n.d.), About Pesticide Registration (webpage), https://www.epa.gov/pesticide-registration/about-pesticide-registration (accessed on 3 July 2020).
[26] US EPA (n.d.), PRIA Fee Category Table - Registration Division - New Active Ingredients (webpage), https://www.epa.gov/pria-fees/pria-fee-category-table-registration-division-new-active-ingredients (accessed on 3 July 2020).
Notes
← 1. For instance, in February 2021, the latest available information on the applications for pesticide registration concerned January-May 2019: https://www.gob.mx/cofepris/documentos/consulta-de-ingreso-de-solicitudes-de-registro-sanitario (accessed on 2 July 2020).
← 2. At the time this review was carried out.
← 3. For the purposes of this report, “illegal trade of agricultural pesticides” is defined as in the 2019 OECD Recommendation on Countering the Illegal Trade of Pesticides, OECD/LEGAL/0446: Any form of trade of an agricultural pesticide that leads to a violation of domestic law, including counterfeiting, fraud and other forms of deception (OECD, 2019[72]).