This chapter reviews best pesticide regulation practices from Australia, Canada, the United Kingdom and the United States. Reasons for selecting these OECD countries include, but are not limited to, their recent efforts in reforming pesticides regulatory management, certain similarities (e.g. reliance on the import of pesticides), their close co-operation with Mexico on pesticides management (e.g. under the T-MEC Agreement) or their involvement in the preparation of this report.
Regulatory Governance in the Pesticide Sector in Mexico

3. International best practices on pesticide regulation
Abstract
Regulatory management of pesticides is a widely, internationally discussed subject. In part, given the high volume of international trade of agrochemical products and substances. International co-operation can help reducing unnecessary duplication of efforts. For instance, the OECD Pesticide Programme aims to harmonise the testing and assessment of agricultural pesticides and to promote work sharing and risk reduction. It supports OECD countries in co-operation in the review of both chemical and biological pesticides used in agriculture. The OECD Network on Illegal trade of Pesticides continues to monitor and act against illegal trade in pesticides. Sharing scientific evidence of the impact of pesticides on human health and environment can also help regulators across borders.
While pesticide regulators from OECD countries actively share experiences and regulatory best practices, it is also true that each country have its own governance model. Each country has its own take on when to accept applications for new pesticide products, and how to make sure regulation is properly enforced. However, international fora are always helpful in sharing experiences on what practices have proven successful, and what the essential areas of opportunities in the management of pesticides are.
Lessons from Australia, Canada, United Kingdom and the United States on pesticide regulatory management
Evolution to a single, independent regulator that manages the pesticides registration process has brought significant improvements.
Having adequate and predictable resourcing is essential to deliver high-quality regulatory services, and to keep technology infrastructure updated. An updated cost-recovery model has proven successful in Canada and Australia.
International co-operation has allowed countries to access a greater pool of knowledge and resources on pesticides management. Having mandates and explicit criteria on how to consider and adopt international regulatory practice is key to attain benefits from international integration while ensuring domestic independence.
A risk-based approach has to permeate all stages of the regulatory management cycle of pesticides. Regulators benefit also from reflecting pesticides hazards in the registration requirement criteria, and in enforcement strategies.
The availability of guidance resources for potential applicants reduces inefficiencies during the registration process. Australia provides a number of documents including data guidelines, risk assessment manuals, paid consultancies for applicants and self-service queries to inform the type of application needed.
An explicit list of prohibited and restricted use of substances and chemical products improves market transparency and avoids potential legal challenges, by stating what substances constitute unacceptable risks.
To ensure an adequate regulatory compliance, regulators have implemented different approaches, including the facilitation of reporting of illegal trade and incidents, by industry and users.
Systematic stakeholder engagement allows identifying regulatory gaps and increase transparency and accountability.
Case study 1: Canada
Context
Pesticides are key in a number of domestic industries, including agriculture, forestry, mining, and industrial and consumer products. They are also used to protect endangered species from predators, to protect native flora and fauna against invasive alien species, and to control pests carrying human pathogens, such as West Nile Virus. In 2017, 73.4% of the sales were commercial products for use in the agricultural sector and 21.4% were for use in the non-agricultural sector. Sales of pest control products in Canada increased from 92.9 million kilograms of active ingredients (kg a.i.) in 2012 to 120.1 million kg a.i. in 2016 (Pest Management Regulatory Agency, 2020[1]).
Canada is a net importer of pesticides, with limited manufacturing. According to Industry Canada data, average revenue for listed pesticide manufacturers averaged $695,000 in 2018 for roughly 50 institutions (Government of Canada, 2021[2]).
In the last decade, the total number of active ingredients registered for use in Canada has increased from just over 500 at the end of 2009 to 610 at the end of 2019. In the same 10-year period, the number of registered products increased from approximately 5700 to 7600. A number of products were removed from the market, either at the manufacturer’s request or as a result of re-evaluation decisions (Pest Management Regulatory Agency, 2020[1]).
Policy and institutions governing the pesticide management system
Laws, by-laws and technical regulations
The primary federal legislation for regulating pesticides in Canada is the Pest Control Products Act and its regulations. The Pest Control Products Act states that no person shall manufacture, possess, handle, store, transport, import, distribute or use a pest control product that is not registered under the Pest Control Products Act, except as otherwise authorised under the Act or unless specifically exempted by the Pest Control Products Regulations (Pest Management Regulatory Agency, 2017[3]).
However, there are other federal legislation relevant to the regulation of pesticides:
Pest Control Products Fees and Charges Regulations
Pest Control Products Incident Reporting Regulations
Review Panel Regulations
Pest Control Products Sales Information Reporting Regulations
List of Pest Control Product Formulants and Contaminants of Health or Environmental Concern
Pesticide Residue Compensation Act
Pesticide Residue Compensation Regulations
Assessor's Rules of Procedure
Agriculture and Agri-Food Administrative Monetary Penalties Act
Agriculture and Agri-Food Administrative Monetary Penalties Regulations respecting the Pest Control Products Act and Regulations
Food and Drug Regulations
In addition, provincial/territorial and municipal governments may implement further restrictions through the enactment of legislation and by-laws, respectively, depending on local conditions. Nevertheless, provinces, territories and municipalities may not register or otherwise authorise pesticides that the PMRA has not evaluated, registered or authorised.
Institutions involved in pesticide regulation
The Pest Management Regulatory Agency (PMRA), within Health Canada, is the sole federal agency responsible for regulating pesticides throughout their lifecycle. This branch of Health Canada, created in 1995, consolidates the federal resources and responsibilities for pest management regulation.
Pesticides are regulated in Canada to ensure they pose the minimal risk possible to human health and the environment. Under authority of the Pest Control Products Act (PCPA), Health Canada has the following overarching objectives:
Registers pesticides after a stringent, science-based evaluation that ensures any risks are acceptable;
Re-evaluates the pesticides currently on the market on a 15-year cycle to ensure the products meet current scientific standards; and
Promotes sustainable pest management (Health Canada, 2021[4]).
The Pest Management Regulatory Agency must register or authorise pesticides before they can be used or sold in the country. Health Canada also promotes and verifies compliance with the PCPA and takes enforcement action to address situations of non-compliance where warranted. The programs and initiatives look to improve the regulatory process and provide pest control products and strategies that are available in Canada with acceptable risk and value.
Health Canada works with provincial, territorial and federal departments in Canada to help refine and strengthen pesticide regulation across the country. These partnerships seek to ensure that the needs of the citizens are addressed at all levels of government, and that the policies that Health Canada implements meet these needs.
Beyond Canada, Health Canada also works closely with a number of international organisations including: United States Environmental Protection Agency (EPA), the North American Tripartite (NAT) Technical Working Group (formerly the North American Free Trade Agreement Technical Working Group), the Organization for Economic Co-operation and Development (OECD), and the Codex Alimentarius.
Regarding resources and cost recovery mechanisms in place, as mentioned above, Health Canada is the sole federal entity responsible for regulating pesticides throughout their lifecycle. A cost recovery system is in place to recover a portion of the costs (approximately 30%) incurred in the implementation of the federal pesticide program as it relates to work generated by applicants (Health Canada, 2021[5]) including pre-market assessments, amendments to registrations, or specification of maximum residue limits.
As reported in PMRA’s 2019-20 Annual Report (Pest Management Regulatory Agency, 2020[1]), PMRA’s resources were as follows:
Table 3.1. Funding and revenue of the PMRA 2019-2020
Million CAD
|
2019-2020 funding and revenue |
Total |
|---|---|
|
Base Funding |
$26.5 |
|
Revenue - Application Fees ($5.4) and Annual (Charge $9.4) |
$13.5 |
|
Non-base Funding |
$12.8 |
|
Growing forward |
3.3 |
|
Chemicals Management Plan |
5.0 |
|
Departamental pressure funding |
4.5 |
|
Total PMRA Fiscal Year 2019-2020 |
$52.6 |
Source: (Pest Management Regulatory Agency, 2020[1]), Pest Management Regulatory Agency Annual Report 2019-2020, Ottawa.
PMRA received CAD 3.3 million through the Growing Forward initiative to support the registration of minor use products. As a result, newer, more environmentally sustainable, and products that are more modern have been made available.
Through Canada's Chemicals Management Plan, PMRA received CAD 5 million to re-evaluate older pesticides, improve risk management approaches through Incident Reporting and Sales Reporting regulations, and contribute to the development of scientific and regulatory approaches with other jurisdictions on high-priority issues (Additional detail can be found on the Chemicals Management Plan webpage1).
Since the enactment of the Service Fees Act in 2017. In 2019–2020, PMRA completed drafting its Remission Policy for Missed Service Standards. This policy describes the scenarios under which a portion of pre-market application fees will be returned to the applicant when service standards are not met. Originally, this policy was to take effect on April 1, 2020; however, the Treasury Board of Canada Secretariat delayed its implementation for one year until April 1, 2021, due to the COVID-19 pandemic (Pest Management Regulatory Agency, 2020[1]).
There are approximately 385 full-time employees at PMRA, 73% are scientists, including biologists, toxicologists, epidemiologists, environmental scientists, and chemists.
Data collection and analysis, including IT tools in place
PMRA uses Information Technology to manage submissions as follows:
Data Analysis:
Pesticide Product Information Database
APEX (data reporting software) used internally for analysis of submissions by staff.
IT systems:
E-Index Builder for compiling an application dossier.
E-PRS (Electronic Pesticide Regulatory System) Secure Web Portal for submitting the dossier to PMRA and database for storing all data and documents related to a dossier.
In addition to using the information from the Electronic Pesticide Regulatory System (E-PRS), either through the Air Pollutants Exposure model (APEX )reporting or portal access, PMRA also uses the Pest Control Product Sales Reporting database to conduct analysis to support the development of policies and regulations. The Sales reporting database is not accessible publically. The Pesticide Product Information Database is publicly available, which includes information on products, active ingredients, and programs related to pesticides and other pest control products (Open Government Portal, 2020[6]).
PMRA also uses various external data sources to conduct analyses, such as:
The Global Maximum Residual Limits (MRL) database
Pesticide import/export datasets (Statistics Canada, CBSA)
Pesticide Product Information System (PPIS - U.S. EPA)
Kynetec Gfk Sigma CP datasets
Business Register database, National Account Longitudinal Microdata File (NALMF) from Statistics Canada
Pre-registration, registration and post-registration processes
Figure 3.1 shows the regulatory lifecycle of pesticides in Canada, from pre-market initial registration to the post-market re-evaluation, demonstrating that the complexity and extent of available information increases after the initial registration.
Figure 3.1. Pesticide regulatory lifecycle of Canada
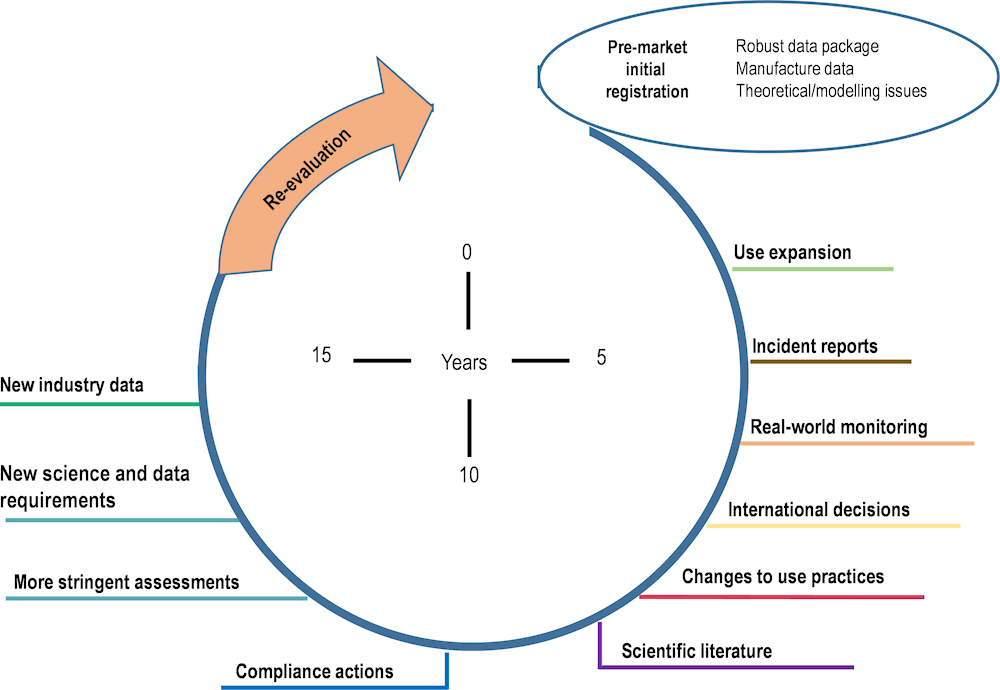
Source: Replicated from (Pest Management Regulatory Agency, 2020[7]), Proposed Integrated Approach to Pesticide Evaluation, Ottawa.
Pre-market
For pre-market submissions of pesticide registration requests, PMRA follows its Management of Submissions Policy (MOSP) (Pest Management Regulatory Agency, 2017[3]). MOSP includes information on the types of submissions, associated timelines, as well as a description of the various steps in the process. Main submission types include Categories A, B, and C, from eight categories in total. Category A is for new active ingredients and major new uses (e.g. a change in use site category such as going from field crops to greenhouse crops). Category B includes changes within the existing use site category (e.g. adding a crop, change in rate, etc.), while Category C is for precedent-based applications.
Under the MOSP, PMRA typically reviews applications in chronological order (i.e., on a first-come, first-served basis) within each MOSP category subdivision. However, timelines can be adjusted under specific circumstances:
If there is a critical need.
When related submissions are grouped to follow the same review timeline when one submission depends upon the success of the other.
Due to processing of a “tailgate” submission which is defined as a submission for a new or existing product for which a current submission is open, past screening and awaiting a regulatory decision. In this situation, tailgate submission cannot be reviewed until the previously submitted application has been accepted or proposed for registration).
While a re-evaluation is underway, and submissions are received to expand, or change use patterns, or to make substantial amendments to the conditions of registration. Thus, in order to reach consistent and timely regulatory decisions, PMRA coordinates the review of these pre-market submissions and the science review component of the re-evaluation. Consequently, PMRA applies any updated science findings to any subsequent (pre-market and post-market) decisions.
A consultation document is published for all major decisions (for example, new active ingredients and major new uses of registered pesticides) as defined in the Pest Control Products Act (Health Canada, 2002[8]). Any comments received during the consultation period are considered before a final decision is made pertaining to registration.
Post-market
Under the Pest Control Products Act, the PMRA may initiate a re-evaluation of a registered pesticide if the information required or the procedures used in evaluating the pesticide’s health or environmental risks or the value have changed. In addition, the Pest Control Products Act requires PMRA to initiate re-evaluations for each registered pesticide on a 15-year cycle, based on the date of the most recent major decision affecting the registration, including its initial registration.
As part of its multi-year re-evaluation planning, PMRA explores opportunities to maximise efficiency by aligning Health Canada’s re-evaluation schedule with that of other international regulatory bodies, or other parts of the Canadian federal government.
PMRA may consider other factors in the scheduling of re-evaluations earlier than the statutory requirement such as clustering similar active ingredients and re-evaluating them as a group. Whenever human health or environmental risk concerns require prompt attention, PMRA will take appropriate regulatory action regardless of the re-evaluation review status.
Any unacceptable risks identified through re-evaluations or special reviews requires the PMRA to initiate action, either by placing additional restrictions on the way the pesticide is allowed to be used or removing it from the market entirely.
Re-evaluation
The PMRA follows the Management of Pesticides Re-evaluation Policy (Pest Management Regulatory Agency, 2016[9]) which outlines the process and timelines from initiation to the publication of a final decision. Following initiation there is a scoping phase where PMRA considers previously conducted assessments to determine if they continue to meet the standards of current science/policy for health and environment in all review areas (that is, health, environment and value). Scoping reviews also include scans of other available information including, but not limited to public literature, incident reports, status of active ingredients in other jurisdictions, and conditions of product use. The scoping exercise identifies whether a re-evaluation will be of a Category 1, Category 2 or Category 3. These designations represent the amount of time and effort required to complete the re-evaluation and do not reflect or imply the level of risk associated with the pest control product or its active ingredient.
PMRA has implemented a risk-based prioritisation for re-evaluations, and considerations for risk prioritisation can be found in the current Re-evaluation and Special review Work Plan 2020-2025 (Pest Management Regulatory Agency, 2020[10]).
Another relevant document regarding the re-evaluation process is the “Policy on Cancellations and Amendments Following Re-evaluation and Special Review (Pest Management Regulatory Agency, 2018[11])”, which aims to clarify expectations, obligations and communications around the implementation of the regulatory decisions.
Special Reviews
The Pest Control Products Act requires the PMRA to initiate a special review of a registered pest control product when there are reasonable grounds to believe that the health or environmental risks of the product are unacceptable. Likewise, when an OECD member country prohibits all uses of an active ingredient for health or environmental reasons. Once any of these is triggered, the evaluation will be targeted to address the aspects of concern related to the pest control product that prompted the special review. PMRA follows the Approach to Special Reviews directive, which describes a systematic approach from preliminary analysis to assessment of the aspects of concern through to final decision (Pest Management Regulatory Agency, 2014[12])
The depth of and length of time to conduct a special review depend on the complexity of the aspects of concern associated with a given pest control product as well as the amount of information requiring assessment.
Risk-based considerations
The PCPA legislation under which pesticides are regulated in Canada requires that regulatory decisions are risk-based as opposed to hazard-based. This legislation dictates that the risks and value of a product, when used according to the conditions of registration, which includes following label directions, must be considered acceptable by the federal regulator for the product to enter and remain on the market in Canada. Assessments of health risk, environmental risk, and value are central to the PMRA's decision-making process. They provide a solid factual and contextual basis for making sound registration decisions that protect human health and the environment from unacceptable risks from pesticides. Each of the three components (health risk, environmental risk and value) must be acceptable before a pesticide can be registered. This means that products that are not effective do not have acceptable value and, therefore, would not be registered even if the health and environmental risks were acceptable. Conversely, if a product is very efficacious and useful to an important commodity, it would not be registered if health and/or environmental risks are not acceptable. The development of the required conditions of use that are feasible, is also a key part in assessing risk and value (Pest Management Regulatory Agency, 2021[13]).
The PMRA Framework for Risk Assessment and Risk Management is designed to protect human health and the environment. The PMRA uses a comprehensive body of scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pest control products (pesticides). This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies. The PMRA's scientific risk-based approach to the regulation of pesticides is consistent with international standards and is similar to Health Canada’s regulatory approach for other types of chemicals. This framework provides predictability and transparency to the process used to protect the health of citizens and their environment and helps ensure risk management decision-making considers all relevant criteria in a comprehensive fashion (Pest Management Regulatory Agency, 2021[13]). It also provides sufficient flexibility to incorporate alternative approaches such as Risk21 methodology and tools developed by the Health and Environmental Sciences Institute (HESI), when applicable
Although the framework is presented as a series of sequential steps leading from a starting point, such as an application to register a new pesticide, to a defined end point such as the decision to register, the underlying process is highly iterative and interactive. This is particularly evident in the development of risk management options. If there is a concern that the use of a product as proposed by the applicant may be associated with an unacceptable level of risk, the PMRA will consider restrictions on use or other conditions to reduce the risk to acceptable levels. The process usually results in a number of possible management options. Each of these options must be described in sufficient detail to allow quantitative re-examination of the potential risks. Typically, this requires several iterations of the assessment of risk and recalculation of risk under the different options considered (Pest Management Regulatory Agency, 2021[13]).
The majority of the registration decisions within the PMRA concern chemical pesticides. Accordingly, this framework is based largely on the processes and approaches used to arrive at decisions about a new chemical pesticide, a major new use, post-market pesticides under re-evaluation, or special review. . It may also be used when considering incident reports examined during these processes. This framework also applies to registration decisions for biopesticides (microbial and pheromone pesticides), non-synthetic pesticides (plant extracts, or other naturally derived substances), and devices, with modifications specific to each situation.
This framework is divided into a number of identifiable decision steps and components, as noted below in Figure 3.2.
Figure 3.2. Stages of risk assessment and risk management of pest control products in Canada
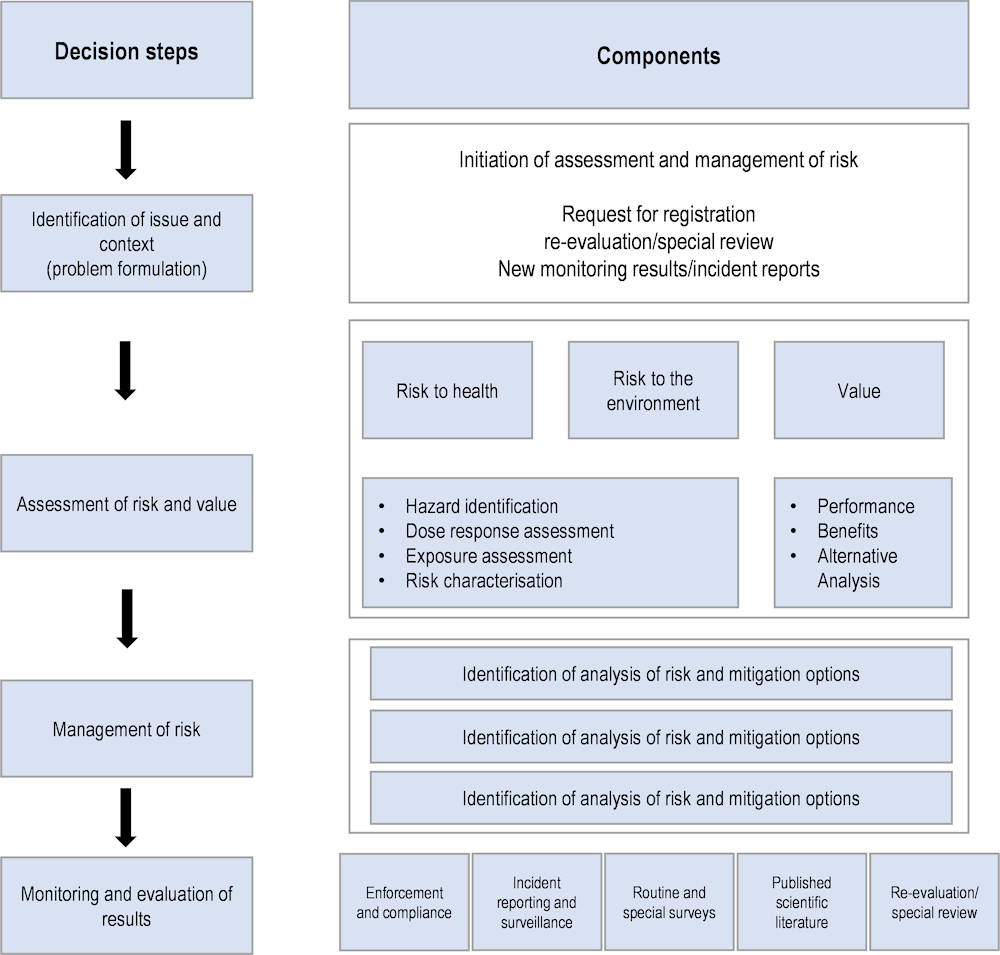
Source: (Pest Management Regulatory Agency, 2021[13]), A decision Framework for Risk Assessment and Risk Management in the Pest Management Regulatory Agency, Health Canada, Ottawa.
Better regulation tools
Monitoring and Enforcement
Health Canada promotes and monitors compliance through the Pest Control Products Act (PCPA) and the Pest Control Products Regulations (PCPR). Health Canada also responds to incidents, complaints, and situations of non-compliance. Health Canada’s recently created Regulatory Operations and Enforcement Branch (ROEB) has compliance officers across Canada whose responsibilities include achieving compliance with the PCPA and PCPR.Compliance officers prioritise and deliver compliance and enforcement activities, as well as develop compliance guidance documents, strategies, and procedures.
The ISO-accredited laboratory in Ottawa provides analytical services for detecting and reporting on pesticide misuse. Pesticides are also analysed to determine whether they meet the specifications upon which registration was granted.
To carry out pesticides compliance and enforcement, PMRA Health Canada delivers promotion, monitoring, and enforcement activities in accordance with the National Pesticides Compliance Program (NPCP), administered jointly by Health Canada’s PMRA and Health Canada’s ROEB Compliance promotion actively prevents non-compliance by informing the public, industry, and associations about the regulatory requirements. Stakeholders and the public are invited to participate in consultations to provide feedback on regulatory decisions and issues.
Health Canada inspectors carry out compliance monitoring, who conduct regular, planned inspections for oversight of activities regulated under the PCPA. The regulated parties and regulated activities targeted by inspections may differ from year to year. Examples include inspections to determine compliance with label directions, and inspections targeting distribution of unregistered products.
Regarding enforcement, violations of the PCPA or PCPR are responded to with appropriate compliance and enforcement measures to encourage compliance. These measures include: education; enforcement letters; voluntary removal; denial of product entry into Canada; amendment, suspenction or cancellation of registration; compliance order; Administrative Monetary Penalty (Warning or Penalty); or prosecution. As part of Health Canada’s regulatory transparency and openness framework, information on inspections and enforcement activities is made publicly available. In addition, persons wishing to report a pesticide incident or suspected contravention of the PCPA have the option of contacting the Pest Management Information Service.2
As an example, during the 2017-2018 fiscal year, Health Canada’s National Pesticide Compliance Program (NPCP) delivered 253 compliance outreach activities to promote compliance with the PCPA; conducted 933 inspections; analysed 428 samples (e.g. soil, plant tissues, animal tissues, liquids, surface wipes) in PMRA’s laboratory to verify compliance; and reported on rates of compliance by subsector and the most common violation types (i.e., sale (34%), possession (25%), and importation (21%)). (Health Canada, 2021[14]).
Regulatory Impact Assessment
The Cabinet Directive on Regulation (Treasury Board of Canada Secretariat, 2020[15]) applies to all regulations that are or will be registered as such under section 6 of the Statutory Instruments Act, and is guided by four principles:
Regulations protect and advance the public interest and support good government;
The regulatory process is modern, open, and transparent;
Regulatory decision-making is evidence-based; and
Regulations support a fair and competitive economy.
The regulatory life cycle approach requires departments and agencies to examine and analyse regulations through all stages of their life cycle, including (Treasury Board of Canada Secretariat, 2020[15]):
Development of regulations: this includes a requirement that departments and agencies conduct a Regulatory Impact Analysis (RIA) on all regulatory proposals, to support stakeholder engagement and evidence-based decision-making.
Regulatory management: departments and agencies are responsible for the ongoing management of regulations and their associated programs and activities. Regulatory program activities may include: compliance and enforcement; inspections and licensing; compliance promotion activities and outreach; data gathering; measuring performance; and providing clear and transparent information and service to citizens on regulations and regulatory and legal responsibilities.
Review and results: this includes a requirement that departments and agencies undertake a regular review of their existing regulatory stock, which should include technical guidance and other associated policies to ensure that the regulations continue to be appropriate and effective, and achieve their intended policy objectives.
Regarding RIA, departments and agencies must conduct a triage of a regulatory proposal to determine its expected impact level and the appropriate mix of analytical requirements of the elements (Treasury Board of Canada Secretariat, 2020[15]). Table 3.2 shows the various types of impacts that the department and agencies have to consider on each of the stakeholder groups:
Table 3.2. Impacts considered for each type of stakeholder in Canada
|
Stakeholders |
Examples of impacts considered |
|---|---|
|
Citizens |
Impacts on well-being (health, safety and security; ability to make informed choices) Impacts on consumers (cost of living, prices, quality and variety of goods available) Income Employment opportunities |
|
Businesses |
Costs of complying with regulatory requirements (including administrative burden) Changes in profit and revenue Business opportunities, growth and innovation Business sustainability |
|
Government |
Costs to implement and administer regulatory programs (compliance and enforcement, outreach, data management, responding to events) Costs and benefits for other levels of government (provincial, territorial, Indigenous, municipal) Impacts on government revenue |
Source: (Treasury Board of Canada Secretariat, 2020[15]), Cabinet Directive on Regulation, Ottawa.
During all stages of the regulatory life cycle, regulators must seek opportunities to engage stakeholders, including Indigenous peoples; pursue regulatory co-operation and regulatory alignment, where appropriate; and co-ordinate with all levels of government to mininize cumulative and unintended impacts of regulations.
Stakeholder Engagement (including public consultation)
In delivering its mandate in registering pesticides, the PMRA is required to fulfil its consultation obligations as stipulated in the PCPA. The PMRA has to perform a public consultation process with stakeholders including federal and provincial government departments and agencies whose interests and concerns are affected by the federal regulatory system before making a decision. 1. To grant or deny an application; 2. about the registration of a pest control product on completion of a re-evaluation or special review; or 3. about any other matter if the Ministry of Health considers it in the public interest to do so.
Under the consultation obligations, the PMRA has to consult the public as to policies, guidelines and codes of practice relating to the regulation of pest control products. In addition, any person may request a special review of the registration of a pest control product, as well as may file a notice of objection to a registration, re-evaluation or special review decision within 60 days after the decision statement is made public.
PMRA uses various methods of consultation including publications, webinars, and in-person meetings. PMRA also provides information to and consults with a variety of stakeholders through various means, including: 1. Seeking advice from external participants involved in the Agency’s work through the Minister of Health’s Pest Management Advisory Council (PMAC) whose members include representatives from pesticide manufacturers, user groups, health and environment non-government organizations, and academia/research institutes; 2. working with other federal government departments and provincial and territorial governments to harmonise pesticides and pest management regulatory and education activities across Canada through to the Federal/Provincial/Territorial Committee on Pest Management and Pesticides3 (Government of Canada, 2015[16]); 3. Hosting regular stakeholder webinars to provide a diverse group of stakeholders with updates on pesticide regulation, as well as opportunities to ask questions.
Regarding all major pesticide registration decisions as defined in the Pest Control Products Act (Health Canada, 2002[8]), such as new registrations or major new uses of a pesticide, re-evaluations or special reviews, a consultation document is published. The consultation document outlines major findings of the evaluations and the proposed decisions, and are made available to the public. The PMRA also solicits comments on regulatory policies, regulatory directives, and guidance documents. Any comments received during the consultation period are considered before a final decision is made.
Transparency and dissemination of information on pesticides
The PCPA requires that a “Register”4 of the Pest Control Products be established. The Register must contain information regarding the applications, registrations, re-evaluations and special reviews.
For each application, the Register must have (among other requirements):
the active ingredient of the product, proposed new uses for it or any uses proposed to be withdrawn; how the application was deposed of or whether it was withdrawn;
the conditions of registration, registration number and registration validity period for each registered product.
information that is provided by applicants and registrants in respect of each registered pest control product both in support of an application for registration or for the amendment of a registration OR for the purposes of a re-evaluation or special review
information provided by applicants and registrants that is used to specify maximum residue limits;
any reports of the evaluation of the health and environmental risks and the value of registered pest control products (Health Canada, 2002[8])
The PCPA also requires that an electronic public registry be established, (refer to the Pesticide Product Information Database5), which includes all information in the Register that is not confidential business information (CBI) and not confidential test data (CTD).
The PMRA publishes and/or posts on the Government of Canada website, the PMRA Annual Report (which details PMRA’s accomplishments and activities over the last fiscal year); the Pest Control Product Sales Report (where registrants report the quantity of pesticides sold in best Canada for a calendar year); and Guidance and Policy documents.
Other regulation regarding transparency:
The Pest Control Products Sales Information Reporting Regulations require registrants of pesticides to report the quantity of every product they make available for sales each year.
The Pest Control Products Incident Reporting Regulations require pesticide registrants and applicants to report to the PMRA all incidents associated with their products.
The PCPA provides the public with the opportunity to inspect the scientific test data supporting pesticide registration decisions.
International regulatory co-operation on pesticides
Canada is recognised internationally for its regulatory model, which has allowed Canada to form partnerships with other regulators, and to play a significant role in developing collaborative approaches to joint pesticide reviews, promoting international regulatory alignment, and addressing barriers to agricultural innovation and trade (Pest Management Regulatory Agency, 2020[1]).
PMRA is involved with several main four international co-operation initiatives including the Stockholm Convention the Rotterdam Convention, the WHO/FAO Codex Committee, and several OECD committees.
Regarding the Stockholm Convention, the PMRA is the federal authority responsible for meeting the obligations and for ongoing participation as it pertains to pesticides. PMRA collaborates with other federal partners by providing scientific experts to work with the Persistent Organic Pollutants Review Committee (POPRC) and the Conference of the Parties (COP) of the Stockholm Convention. At POPRC, PMRA participates in the review for identifying substances as persistent organic pollutants (POP) and making recommendations on the global management. At the COP, PMRA provides experts to negotiate international decisions on the restrictions and the elimination of each POP at the global level.
Regarding the Rotterdam Convention, in collaboration with other federal partners, the PMRA provides scientific experts to work with the Chemical Review Committee (CRC) and the COP of the Rotterdam Convention, and in the development of Canadian positions and submissions. For CRC, PMRA reviews submissions to the Rotterdam Conventionagainst established Convention criteria. At the COP, PMRA provides experts to negotiate international decisions for each substance at the global level.
In the WHO/FAO Codex Committee on Pesticide Residues, the PMRA’s participation seeks to:
Enhance Canada’s influence on Codex deliberations and outcomes.
Promote the development of science-based standards that result in fair practices in food trade (establishment of MRLs).
Promote more effective committee’s work planning.
Promote the timely development of standards.
PMRA is also involved with several OECD initiatives, including various OECD task forces and expert group projects. PMRA participates in meetings of both the OECD Working Party on Pesticides (WPP) as well as the OECD Working Party on Biocides (WPB). Both working parties function as vehicles for global co-operation and facilitate information exchange and alignment of approaches with respect to pesticides assessment.
PMRA also contributes input (via the Canadian Delegation) to the OECD Meeting of the Chemicals and Biotechnology Committee as required. PMRA also provides experts to participate in the OECD WPP Expert Groups on Residue Chemistry, Pollinator Safety, Bio-pesticides, and Electronic Exchange of Pesticide Data. Some examples of OECD WPP initiatives include: development of a common approach to regulating novel pest control products, such as RNAi-pesticides; implementation of technical guidelines (for example, those that provide guidance on alternative approaches to animal testing); identification of residues, metabolites and degradation products; identification of relevant data requirements for regulating bacteriophages; ongoing dialogue related to integrated pest management/pollinator protection; aligning risk assessment of new digital and mechanical technologies for applying pesticides such as innovative drone technology.
PMRA also plays a lead role on the OECD WPP e-label project to identify commonalities in pesticide labels that would support development of e-label solutions. PMRA also actively contributes to the WGB’s Expert Group on Claims Development for Treated Articles.
In support of the OECD WPP’s objectives, PMRA has led discussions with global manufacturers of pesticides regarding new chemistries to broaden collaboration and promote global joint reviews and alignment between international regulatory partners. PMRA has also initiated discussion with OECD partners on post-market review challenges and the potential benefit of having a greater collaboration in this area.
Highlights: best regulatory practices
The PMRA continuously conducts periodical examination of its programs by leveraging internal and external audits and comprehensive reviews to find inefficiencies and eliminate duplication. PMRA launched a multi-year programme renewal project in order to build a stronger and sustainable pesticide regulatory programme that strengthens health and environmental protection and leads to improved quality of scientific decisions. This addresses the increased workload, increasing complexity of the scientific assessments and availability of key data when undertaking assessments. These efforts are guided by consultations for a new risk-based continuous oversight programme delivery model. The PMRA has planned for 2021-22 to (Pest Management Regulatory Agency, 2020[17]):
Develop a risk-based framework for continuous oversight of registered pest control products over the course of the product’s lifecycle by identifying and addressing risk sooner with ongoing risk determination information.
Develop new and leverage existing processes to improve the timing for the identification, collection and analysis of data, and engagement on the subsequent assessments in order to better define areas of risk thereby resulting in smaller and more focused reviews.
Advance and implement new risk management tools to prioritise pest control products for scientific review and risk mitigation.
Recent and on-going reforms
Certain broad trends in pesticide regulation in Canada that led to recent reforms include: the increased number of registered products and workload; ; the increasingly complexity of pesticide evaluations due to rapid technological advances, evolving science, etc.; multilateral collaboration and international obligations; high level of stakeholder engagement and media attention; heightened importance on food and water security; increased stakeholder expectations to balance cost of innovation and product availability).
There were also a number of challenges specific to the pesticide re-evaluation programme (Pest Management Regulatory Agency, 2020[17]). As a result, in 2018 the PMRA, through a fulsome review including consultation with stakeholders, identified some areas of opportunity in the re-evaluation process (Pest Management Regulatory Agency, 2018[18]), as follows:
Health and environmental risks are not being addressed in a timely manner.
Risk issues are pushed to re-evaluation resulting in substantial assessment updates required.
Key information is not available to support re-evaluation assessments.
Inefficient information gathering often leads to duplicative effort from redoing assessments.
Limited engagement and transparency early in the process.
No risk-based prioritisation of workload (Pest Management Regulatory Agency, 2020[17]).
In support of the 2018 Review of the Pesticide Re-Evaluation Program, the PMRA has:
Conducted international comparison of post-market pesticide programmes.
Engaged with other government departments and international partners, as well as with PMRA staff.
Engaged broadly across Canada with stakeholder groups.
Conducted detailed costing analysis of the current pesticide program (Pest Management Regulatory Agency, 2020[17]).
As a result of the re-evaluation programme review, PMRA’s Integrated Pesticide Program will have the following elements moving forward:
Continuous evaluation approach that proactively addresses emerging health and environmental risks and considers pesticide value throughout the pesticide lifecycle.
An integrated approach that increases the protection of health and environment while being more efficient and timely, i.e. a) Risks are identified and addressed sooner; b) Increased oversight and risk characterization of all pesticides and c) Re-evaluations are less complex.
Case study 2: Australia
Context
The pesticide industry is relevant in Australia both in of economic and employment terms. Agricultural pesticides sales in Australia had a market value of AUS 2.7 billion (~USD 2.13 billion)6 during the 2018-2019 financial year (APVMA, 2020[19]). According to a report by Deloitte, the agricultural chemicals contributed to over 9 200 full time equivalent jobs (Deloitte, 2019[20]).
International trade plays a big role in the Australian pesticide Industry. The same report indicates that imports represent 59% of the market for agricultural chemicals, share that has increased from 33% a decade ago.
Australia has a comprehensive pesticides regulatory framework. Its regulatory management also includes systematic efforts of international co-operation with major trade partners and international organisations. In recent years, Australia has conducted regulatory reviews of its management system for pesticides, and has reformed its legislative and regulatory practices in accordance. This case study aims to summarise some of its best practices and draw lessons from its reform efforts.
Policies and institutions governing the pesticide management system
Legislative and regulatory instruments
Australia has a regulatory framework in place that covers both agricultural and veterinary chemicals (commonly referred as agvet chemicals). This framework referred to as the National Registration Scheme for Agricultural and Veterinary Chemicals (NRS) came into full operation in 1995 and is a partnership between the Commonwealth (central) Government and the states and territories. Prior to 1995, state and territory governments were each individually responsible for the registration and control of use of all agvet chemicals. The NRS established a single national framework for the assessment and registration of agvet chemicals, with the states and territories retaining responsibilities for controlling their use once they are sold or supplied to the end-user (APVMA, n.d.[21]).
The NRS is an umbrella for legislative and regulatory instruments that govern the pesticide industry. The Australian Pesticides and Veterinary Medicines Authority (APVMA) administers the NRS in collaboration with other Commonwealth agencies, as well as state and local governments, law enforcement and the judiciary. Institutions involved in pesticide regulation.
The key pieces of legislation are the Agricultural and Veterinary Chemicals (Administration) Act 1992 that establishes a national authority for the registration of agvet chemicals and sets out the functions and powers of that authority. It contains provisions controlling the import and export of chemicals and for enforcement and inspectors. The other key piece of legislation is the Agricultural and Veterinary Chemicals Code Act 1994 (Agvet Code) that sets out the operational provisions for the registration of agvet chemicals, for regulating the supply of those chemicals and for compliance with, and enforcement of, the Agvet Code.
Institutions involved in pesticide regulation
Pesticide regulation, up to the point of retail sale, is implemented by an independent regulatory agency located within the Department of Agriculture, Water and Environment. The Australian Pesticides and Veterinary Medicines Authority (APVMA) is the independent statutory authority responsible for the assessment, registration and regulation of agricultural and veterinary chemicals in Australia (APVMA, n.d.[22]). The APVMA was created under the Agricultural and Veterinary Chemicals (Administration) Act 1992. This Act defines APVMA’s functions, including the following:
to assess the suitability for sale in Australia of active constituents for proposed or existing chemical products, chemical products and labels for containers for chemical products;
to provide information to the Governments and authorities of the Commonwealth, the States and the participating Territories about approved active constituents for proposed or existing chemical products, registered chemical products, reserved chemical products and approved labels for containers for chemical products and to co-operate with those Governments and authorities on matters relating to the management and control of chemical products;
to keep records and statistics of approvals and registrations granted, and permits and licences issued, by it under the Agvet Codes;
to evaluate the effects of the use of chemical products in the States and participating Territories
to co-operate with Governments and authorities of the Commonwealth, the States and the participating Territories for the purpose of facilitating a consistent approach to the assessment and control of chemicals;
in co-operation with Governments and authorities of the Commonwealth, the States and the participating Territories, to develop codes of practice, standards and guidelines for, and to recommend precautions to be taken in connection with, the manufacture, export, import, sale, handling, possession, storage, disposal and use of chemical products in the States and participating Territories
to collect, interpret, disseminate and publish information relating to chemical products and their use;
to encourage and facilitate the application and use of results of evaluation and testing of chemical products;
to exchange information relating to chemical products and their use with overseas and international bodies having functions similar to the APVMA’s functions;
From this extensive list of activities, it is clear that APVMA does not only focus on technical evaluations required during the registration process. APVMA also covers a wide range of activities related to the governance cycle of pesticides. This includes international co-operation, regulatory enforcement, and stakeholder engagement. Having a one-stop-shop regulator for assessment, approval and registration and control of supply of pesticides has proven effective in Australia, since its inception more than two decades ago.
APVMA manages a wide list of regulatory and legislative instruments, from the NRS, that affect the manufacture, trade and evaluation of chemicals.7 This includes key legislative instruments for pesticides including standards for Maximum Residue Limits (MRLs), efficacy criteria that products have to meet to be considered effective, and the application requirements for the registration of a pesticide product.
Financing
Most of APVMA’s funding comes from a cost-recovery scheme that includes both fees8 and levies.9 A recovery levy is a tax and is imposed via a separate taxation act. The difference is that the revenue from the levy is earmarked to fund activities provided to the group that pays the levy (APVMA, 2020[21]). This way, the fees charged to the regulated industry are entrusted directly to APVMA, rather than being handed to the country’s treasury. Having an independent regulator funded directly by its activities, rather than from the centralised annual budget, is a practice commonly adopted by OECD countries. This has key advantages, including budget predictability, and the fact that the size of the regulator directly responds to the demand an industry has of regulatory services.
In accordance with Australian Government cost recovery policy, cost-recovered agencies must conduct a review of fees and charges at lest every five years to ensure that fees and charges remain in line with government policy as well as the cost of the activities they relate to. The APVMA implemented its most recent cost-recovery arrangements on 1 July 2020. This reform was implemented from a recommendation of an independent review. This review found that APVMA’s activities were not being covered by the current fee structure, and that the finance gap would only increase in the future if no further action takes place. The review also found that APVMA’s IT system was not sufficient to support the management required (PWC, 2017[24]).
Data Collection and Use of ICTs
APVMA has made digitalisation a core strategy to improve its regulatory performance. In 2018, APVMA published a four-year digitalisation strategy that identifies the main problems of its ICT infrastructure and the objectives for improvement. APVMA’s infrastructure had a series of drawbacks that were hampering its service delivery.10 As it was, APVMA’s digital infrastructure was not able to meet digital end-to-end self-service processes for staff and clients. The lack of sufficient storage infrastructure was also a barrier to digitalise more than 200,000 analogue records. These records on average have 300 double-sided pages, including both text and images. Regarding data, APVMA was also worried about losing information stored in obsolete platforms and keeping up to date with cyber security measures to minimise potential attacks (APVMA, 2018[22]).
The Australian Government provided AUS 10.1 million to the APVMA in its 2018-19 budget to fund three years’ worth of its digital strategy, through its Enabling Technology Program. This programme aims to improve the efficiency of the registration process. This programme has three overarching goals: digitalising pesticide records, enhancing data analytics and business intelligence capabilities and enabling a single view for clients for on-line registration processes.
Pre-registration, registration and post-registration processes
Resources for pre-Registration
The pesticide registration process is regulated under the Agricultural and Veterinary Chemicals Code Act 1994 supported by regulations and other legislative instruments. Applications are required for a number of services, including registration of new products with both existent and new actives, for variations of products, and certificates for import and exports.
APVMA has a series of resources to help applicants from the start of the process. Before registration applicants have access to a tool that helps them understand whether an agricultural product needs registration. This electronic questionnaire guides the potential applicant to several questions that after responding gives you an answer on whether your intended product needs a certification. Applicants can also call for a pre-application assistance (PAA).11 APVMA charges a fee-for-service basis, which conducts either by a written response, a face-to-face meeting or a teleconference. PAA can come in three different tiers.
Tier one assistance has the objective of guiding applicants during the early stages of an application. In this tier, APVMA provides advice on 1) planning an application; 2) types of regulatory assessment needed for an application; 3) relevancy of efficiency criteria; 4) assessment modules, fees and timeframes; 5) clarification on guidance documents published by APVMA.
Tier two assistance focuses on technical advice, and usually last two months, including a meeting if necessary. As part of the assistance of tier two, applicants may receive advice on the following: 1) types of supporting data or information appropriate to the application; 2) relevance or suitability of overseas data and/or assessment reports; 3) the types of trials needed to generate appropriate data; 4) a scientific matter relevant to an application; 5) the development of an agreed project plan for a time shift application; and 6) specific aspects of the design of a study or trial.
Tier three assistance provides advice on: 1) appraisal for trial protocols before commencement on studies; 2) assistance on a proposed new methodology or variations of existing data guidelines for generating data; and 3) finalisation of project plans for Global Joint Reviews.
Australia has a relevant amount of demand of the assistance service. In 2020, APVMA received a total of 247 requests of assistance of all three tiers combined. As seen in Table 3.3, the most sought after assistance is Tier 2, which focuses on technical advice. APVMA received 161 requests of this service in 2020. Having an optional pre-application consultation helps to improve efficiency of the registration process itself, by reducing the probability of having misunderstandings on the assessments or lack of information submitted in the application.
Table 3.3. Requests for pre-application APVMA’s assistance in 2020
|
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
|---|---|---|---|---|
|
Tier 1 |
16 |
15 |
17 |
17 |
|
Tier 2 |
31 |
39 |
62 |
29 |
|
Tier 3 |
4 |
3 |
8 |
6 |
Source: APVMA’s Quarterly Performance Reports in 2020, extracted from: https://apvma.gov.au/node/79656, https://apvma.gov.au/node/75211, https://apvma.gov.au/node/71481, & https://apvma.gov.au/node/66806.
As part of the data guidelines to build a dossier, APVMA also provides risk assessment manuals on chemistry and manufacture, environment human health, residues and trade, and spray drift risk.12
Registration process
The registration process is completely managed by APVMA, and varies, in terms of requirements, fees and timeframes, depending on the type of application. This follows a risk-based approach, and considerations such as whether an active ingredient has been previously approved. For a list of criteria that define the timeframe and fees for applications for registration for new products see Table 3.5. Assessment periods are defined by legislation and vary depending on the complexity of the type of application.
In 2020, APVMA received 2,118 applications, across different categories including new product applications, application for variations to existing registrations and application for permits. Within product applications, item 7 is the most common, which considers: chemical product containing an approved active constituent, and approval of the product label, if the product is closely similar to a registered chemical product and efficacy and safety data are not required to demonstrate the similarity of the product to the registered chemical product and chemistry and manufacture data are not required.
Table 3.4 shows the number of applications in each quarter of 2020. Of all these applications, APVMA finalised the application process on time for 97% of pesticide applications in 2020 (APVMA, n.d.[23]).
Table 3.4. Pesticide Applications Received in Australia, 2020
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Total 2020 |
|
|---|---|---|---|---|---|
|
Products1 |
278 |
345 |
447 |
341 |
1 411 |
|
Permits |
336 |
146 |
91 |
123 |
696 |
|
Other |
0 |
0 |
0 |
0 |
0 |
|
Time-shift |
1 |
4 |
6 |
0 |
11 |
|
Ingredient determination |
0 |
0 |
0 |
0 |
0 |
1 Product applications include: new product/ active (new active), new product (existing active), and variations.
Source: APVMA’s Quarterly Performance Reports in 2020, extracted from: https://apvma.gov.au/node/79656, https://apvma.gov.au/node/75211, https://apvma.gov.au/node/71481, & https://apvma.gov.au/node/66806.
The criteria for each type of application can be found in the Agricultural and Veterinary Code Regulations 1995. The APVMA provides tailored guidance on specific requirements for each type of application on its website. For applications that do not require a full assessment, a modular assess may may apply (see Items 2 and 10 described in the table below). For a modular assessment relevant module(s) required for an individual application assessment are applied on a case-by-case basis with corresponding data requirements, fee and timeframes. This provides for an effective allocation of effort and resources in the line with the risks to be assessed.13
Table 3.5. Types of pesticide registration applications for new products
|
Criteria |
Timeframe |
Fee |
|
|---|---|---|---|
|
Item 1 |
Approval of an active constituent contained in a chemical product, registration of the associated chemical product and approval of the product label requiring a full assessment of the active constituent and product. |
18 months |
AUD 116 501 |
|
Item 2 |
Approval of an active constituent contained in a chemical product, registration of the associated chemical product and approval of the product label requiring less than full assessment of the active constituent and product. |
Modular assessment |
Modular fee |
|
Item 3 |
Registration of a chemical product containing an approved active constituent, and approval of the product label, if there is no registered chemical product containing the active constituent and a full assessment of the product is required. |
18 months |
AUD 83 511 |
|
Item 4 |
Registration of a chemical product containing an approved active constituent, and approval of the product label, if there is a registered chemical product containing the active constituent and a full assessment of the product is required and there are no relevant maximum residue limits and poison schedule classification is required. |
18 months |
AUD 44 644 |
|
Item 5 |
a) Registration of a chemical product containing an approved active constituent and approval of the product label; or b) Registration of a chemical product, approval of the active constituent in the chemical product and approval of the product label; or c) Registration of a chemical product and approval of the product label |
8 months |
AUD 7 566 |
|
Item 6 |
a) Registration of a chemical product containing an approved active constituent and approval of the product label; or b) Registration of a chemical product and approval of the active constituent in the chemical product and approval of the product label; or c) Registration of a chemical product and approval of the product label |
8 months |
AUD 6 406 |
|
Item 7 |
Registration of a chemical product containing an approved active constituent, and approval of the product label, if the product is closely similar to a registered chemical product and efficacy and safety data are not required to demonstrate the similarity of the product to the registered chemical product and chemistry and manufacture data are not required. |
3 months |
AUD 2 632 |
|
Item 8 |
Registration of a chemical product containing an approved active constituent, and approval of the product label, if the chemical product is the same as a registered chemical product and the product is to be registered with a different name. |
3 months |
AUD 2 632 |
|
Item 9 |
Registration of a listed chemical product and approval of a product label where the product and label comply with an established standard that has been approved in accordance with section 8U of the code. |
2 months |
AUD 2 632 |
|
Item 10 |
For all situations other than those described in items 1–9: a) Registration of a chemical product containing an approved active constituent and approval of the product label; or b) Registration of a chemical product and approval of the active constituent in the chemical product; or c) Registration of a chemical product and approval of the product label (but only if a separate application for the approval of the active constituent in the chemical product has been lodged). |
Modular assessment |
Modular fee |
Note: The fees included in the table are those to be implemented in 1 July 2020.
Source: Agricultural and Veterinary Chemicals Code Regulations 1995.
Better regulation tools
Ex ante evaluation of regulation
In Australia, all new or modified regulations are subject to conduct a Regulatory Impact Assessment (RIA). The limited waivers have to be approved by the Prime Minister. This ex ante assessment of regulation has to be prepared by the office responsible for the management of the specific regulation. That is, the Australian Government Department of Agriculture, Water and the Environment (DAWE) , responsible for the governance and oversight of the NRS, has to conduct ex ante evaluation to all the changes to its regulatory inventory or of new proposed regulation. In fact, Australia’s ex ante system is one of the OECD’s highest ranked in a composite indicator that includes the quality of the methodology, systematic adoption of RIA, transparency, and oversight and quality control (OECD, 2018[24]).
As part of the ex ante assessment process, DAWE has to answer a series of questions, including the costs and benefits of regulations, and to weigh different alternatives to solve a previously identified policy problem. All of the process is defined by a guide prepared by the Department of Prime Minister and Cabinet (Commonwealth of Australia, 2020[25]). These questions are further explained in the guide, and summarised in Box 3.1.
Box 3.1. Regulatory Impact Analysis elements in Australia
What is the problem you are trying to solve?
Why is government action needed?
What policy options are you considering?
What is the likely net benefit of each option?
Who did you consult and how did you incorporate their feedback?
What is the best option from those you have considered?
How will you implement and evaluate your chosen option?
Source: Commonwealth of Australia (2020), The Australian Government Guide to Regulatory Impact Analysis, (Commonwealth of Australia, 2020[25]).
Regulatory enforcement
APVMA has an active role to ensure that the industry complies with the Agvet code. APVMA conducts traditional enforcement activities, such as inspections. However, to improve communication access on non-compliance, APVMA has an on-line portal to report a suspected non-compliance and adverse experience with chemicals. The adverse experience tools allow both registrants and users file a form with information on unexpected negative consequences of produce use. The party filing the report may inform details of the product, incident, crop/plant, animal, human, and chemical use. This information may be directly sent to the manufacturer of the pesticide and to APVMA. The adverse experience reports are assessed by the APVMA and annual summaries of adverse experience reports are published registration and approval holders are required, by law, to advise the APVMA and provide to the APVMA any new information that they become aware of that shows that the agvet chemical may not meet the safety, efficacy or trade criteria.
The platform to report suspected non-compliance covers three possible scenarios: advertising and supply of unregistered agricultural and veterinary chemicals, inappropriate manufacture of agvet chemical products, and importation of agvet chemical products. APVMA ensures confidentiality in treating the reports, and considers a risk-based approach on the potential harm for people, animals, the environment and international trade, when prioritising the follow-up of incidents.
Public consultation
The APVMA seeks input from interested stakeholders throughout the agvet chemical registration and review processes as well as during the development of APVMA operational and regulatory processes. The Agvet Code requires that the APVMA seeks input before it registers an agvet chemical containing a brand new active constituent. The existing chemical review process also involves extensive stakeholder consultation.
International regulatory co-operation on pesticides
Australia has a strong programme on international co-operation for pesticide regulatory assessments including work share arrangements. APVMA works both with peers from other countries and with international organisations. APVMA allows data generated in according to international guidelines including OECD, FAO, the United States, Canada, European Food Safety Authority, EU Biocidal Products Regulation, European and Mediterranean Plant Protection Organisation.
Australia also accepts assessments form the World Health Organization, FAO, United States, Canada, New Zealand, the National Industrial Chemicals Notification and Assessment Scheme and the Office of the Gene Technology Regulators. However, Australia has specific criteria defined to accept international assessment. Please see Box 3.2 for a complete list of these criteria.
Box 3.2. APVMA’s criteria for accepting international assessments
The assessment has to be written in English;
It has to contain a full reference list of all the studies cited in the report;
Be an un-redacted report with an adequate level of reporting detail so that a regulatory scientist can peer review the assessment and fully understand the basis for any interpretations, conclusions, recommendations or decisions.
Be submitted in an electronic format that is searchable, and ideally, editable. It does not however need to be specifically formatted for the APVMA.
Be the most recent comprehensive assessment where there are multiple assessments arriving at similar conclusions. If the reports have differing conclusions then all must be submitted to the APVMA.
Include all underlying data and studies relevant to the application, including published and unpublished studies.
Include all original studies cited or evaluated in the international assessment including those not owned by the applicant.
Source: APVMA, Guidance for applicants – submissions of international data, standards and assessments (Last updated on 16 April 2020). Extracted from: https://apvma.gov.au/node/14186 on 17 February 2021.
Highlights: best pesticide management practices
Empty container and obsolete pesticide management schemes
Similar to Mexico, Australia has an industry-led recollection programme to reduce negative impacts of empty pesticide containers. Agsafe is the Australian, industry-led non-profit organisation that runs several programs including drumMUSTER, a collection and recycling of empty pesticide containers, Chem Clear, a collection of unwanted chemicals as well as accreditation and training programs.
drumMUSTER started in 1999 with the goal of installing a paradigm shift on the importance of minding environmental impacts on land management. Its main goal is to recycle and transform empty containers into a range of products. Since its inception, as part of drumMUSTER, Australia has collected more than 36 million containers, which avoided the equivalent of 41 thousand tons of material diverted from landfill. The programme has 837 collection sites, and has trained 1 748 inspectors over the last three years.14 These inspectors have to conduct a re-training every three years.
Regulatory performance framework
In 2015, as part of an effort to improve regulatory delivery, Australia installed an assessment tool called Regulatory Performance Framework (RPF). The RPF has the main goal of reducing unnecessary burden that hampers competitiveness (Commonwealth of Australia, 2014[26]). All regulators have to conduct a self-assessment every year, using the RPF methodology, which has to be comprehensive, timely, externally validated, and publicly available. As part of the RPF implementation, the Department of the Prime Minister and Cabinet (PM&C) issue guidance on how to conduct the assessment. For this, PM&C provides examples inputs, outputs supporting the assessment, shares a selection of case studies of better regulatory practice, and advice on implementation timeframes, among others.
The content of RPF is based in six broad Key-Performance Indicators:
Unnecessary impediments to the efficient operation of regulated entities are removed.
Communication with regulated entities is clear, targeted and efficient.
Actions undertaken by regulators are proportionate to the regulatory risk being managed.
Compliance and monitoring approaches are streamlined and co-ordinated.
Regulators are open and transparent in their dealings with regulated entities.
Regulators actively contribute to the continuous improvement of regulator frameworks.
Each regulator has the task of breaking down each indicator into sub-indicators that reflect their portfolio of work. For example, to assess the first indicator listed above, APVMA includes a sub-indicator that measures the status of International data guidelines, standards and assessment adopted to reduce effort to register agvet chemicals. Then, each sub-indicator is measured with a list of evidence and concrete results.
Table 3.6. APVMA Performance Framework Regulator indicators
|
Performance indicator |
Evidence |
|---|---|
|
1. Unnecessary impediments to the efficient operation of regulated entities are removed. |
Demonstrated understanding of the operating environment for the regulated entities. International data guidelines, standards and assessments adopted to reduce effort to register agvet chemicals. Efficient and effective APVMA business processes. |
|
2. Communication with regulated entities is clear, targeted and efficient. |
Level of satisfaction with information and guidance materials. Level of satisfaction with the quality and timeliness of advice on decisions. Extent and satisfaction with APVMA consultative processes |
|
3. Actions undertaken by regulators are proportionate to the regulatory risk being managed. |
Risk management frameworks and policies are in place and regularly reassessed. Lower regulatory effort is applied to activities of lower regulatory risk. Compliance and enforcement strategies are consistent with agreed risk management policies. |
|
4. Compliance and monitoring approaches are streamlined and co-ordinated. |
Monitoring and enforcement strategies allow for a range of regulatory responses. Compliance activities are responsive to business needs of regulated entities, where relevant. Information requested from regulated entities is necessary and acted upon. |
|
5. Regulators are open and transparent in their dealings with regulated entities. |
Performance information is published. Feedback mechanisms are in place and used to improve service to regulated entities. |
|
6. Regulators actively contribute to the continuous improvement of regulator frameworks. |
Level of stakeholder engagement in implementing regulatory frameworks. Feedback is provided to inform the development or amendment of regulatory frameworks. |
Comprehensive and transparent listing of prohibited and restricted of substances
The Agricultural and Veterinary Chemicals (Administration) Regulations 1995 provides a list of active constituents and chemicals that are prohibited to import, export, manufacture or use, or have restrictions with specific conditions. This helps to increase market transparency, and to communicate what products have unacceptable risks. The annex divides the list in single active constituent and chemicals products defined in two or more active constituents.
Table 3.7 provides an example of a technical sheet of a single active constituent in the annex of this regulatory code. Every item in the annex provides the information stated in the table below, including common name, IUPAC name, CAS number, the precision if the item is a prescribed active constituent or a chemical product, relevant international agreement and the condition or restriction. In this case, Australia has the prohibition of manufacturing and using Aldrin, and export/imports are prohibited with exceptions that warrant a written permit. This substance is part of the Stockholm Convention.
Table 3.7. Example of a technical sheet
|
Category |
Information |
|---|---|
|
Common name |
Aldrin (HHDN) |
|
IUPAC name |
(1R,4S,4aS,5S,8R,8aR)-1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a-hexahydro-1,4:5,8- dimethano-naphthalene |
|
CAS number |
309-00-2 |
|
Prescribed active constituent/chemical product |
Prescribed active constituent for the purposes of subsection 69CA(2) of the Act |
|
Relevant international agreement or arrangement |
Stockholm Convention |
|
Conditions or restrictions |
Import prohibited except with written permission under a stipulated clause* Manufacture prohibited in all cases Use prohibited in all cases Export prohibited except with written permission under a stipulated clause |
Source: Agricultural and Veterinary Chemicals (Administration) Regulations 1995.
Recent and on-going reforms
Streamlining the regulatory framework for pesticides
As part of an effort to reduce bureaucracy, the Australian Parliament is currently considering proposed amendments to legislation through the Agricultural and Veterinary Chemicals Legislation Amendment (Australian Pesticides and Veterinary Medicines Board and Other Improvements) Bill 2019. The Bill has passed the House of Representatives and is under revision by the Senate (Department of Agriculture, n.d.[138]). The proposed bill includes some aspects that will make the regulatory framework simpler and more flexible. For instance, the bill will amend the requirement legislation, so that APVMA can deal with new information provided in the application with more flexibility, and chemicals with low regulatory concern will have simpler regulatory processes. Other measures include increased computerised decision-making by APVMA, simplification of corporate requirements to APVMA and simplification of reporting requirements for annual returns.
However, while proposing system simplification, there are also additional measures on regulatory enforcement. The bill includes the establishment of a civil penalty for providing false or misleading information to APVMA, and providing suspension or cancellation when false or misleading information is presented in an application for a variation or label approval of agvet products.
On-going review of the pesticides management framework
Australia is conducted a comprehensive, independent review of its pesticide regulatory framework. The Australian government commissioned a panel of four independent experts to conduct this review, which has included stakeholder consultations. The panel published an Issues paper, through the Department of Agriculture, Water and Environment, which includes the main findings and recommendations for reform. DAWE has subjected the report to an on-going public consultation before the panel presents the final reform proposals. To enhance the discussion, the Issues paper poses a range of discussion questions about their findings. The subjects covered in this review cover broad issues such as the optimal objectives of a future regulatory system for pesticides as well as more specific issues such as waste disposal and management.
The review highlights positive features of Australia’s regulatory arrangements that should be preserved. Some of these highlights might be beneficial for an eventual reform in Mexico. The report considers that having an independent regulator, with no political interference in its scientific decision-making has been essential for the success of achieving pesticide policy objectives. Moreover, stakeholders who participated in this review consider that having a centralised regulator has brought significant improvement. Previously, Australia had a separate state-based registration system. Mexico has a registration process that relies only on federal agencies, local and state governments are no involved in the process. However, the registration process includes three different agencies during the product registration process, in contrast with Australia, where APVMA conducts all the required assessments. Another essential practice that the panel encourages APVMA to keep is its risk-based approach to the assessment of product registration (Matthews et al., 2020[27]).
There are however, diverse concerns on the design of the system governance, as well as specific practices. Box 3.3 presents the main challenges summarised in the Issues Paper. To advance the regulatory system, the review presents the following main proposals:
Increasing national consistency of control of use
Removing consumer and non-primary production products from the system
Introducing a benefits test
Changing the way chemical product efficacy is managed
Introducing a registration by reference approach
Introducing an accredited assessor scheme.
This paper also proposes a new vision for the future system, and suggests key principles to govern the design of the future regulatory system together with a simplified hierarchy of system objectives (Matthews et al., 2020[27]).
Box 3.3. Main concerns presented in the on-going review of Australia’s pesticide regulatory framework
1. Australian farmers do not currently have access to the same chemicals, in the same timeframes, as their overseas competitors. Many new products are registered overseas well before they are registered in Australia.
2. The scope of agricultural and veterinary chemicals in the Australian system is extremely broad. This leaves room for duplication/cross-over with other regulatory schemes particularly with consumer and industrial goods. This has the potential to divert government resources from regulating agricultural and veterinary chemicals used by primary producers, veterinarians and non-urban land managers as well as chemicals posing a high health risk.
3. The regulatory system being obscure and difficult to understand. Many stakeholders see it as complex, costly and slow. Governance and consultation arrangements for the system as a whole are unclear and have been argued by stakeholders to be ineffective. Commonwealth–state relationships has been identified as one of the many challenges.
4. The Australian regulatory system dedicates a disproportionate share of resources to pre-market assessment of agvet chemicals, rather than post-market compliance (regardless of the risk).
5. There is significant cross-subsidisation among chemical registration holders in respect of fees and levies paid.
Note: The concerns listed in this box are only those highlighted as the main priorities. An extensive list of all concerns may be found in the report. The list has been slightly edited for contextual clarity.
Source: (Matthews et al., 2020[27]).
Best practice highlights from the United States and the United Kingdom
Highlights from the United Kingdom
UK’s Pesticide Forum
The Pesticides Forum was set up in 1996 in the United Kingdom with the objective to bring together a range of organisations with an interest in pesticides and the impacts of their use. The Forum represents stakeholders with differing views about pesticides and how the impacts of their use should be addressed.
Since 2013, the Forum has played an important role in supporting the UK’s National Action Plan for the Sustainable Use of Pesticides. It provides a space for stakeholder interaction, discussing important issues. The Forum’s main task is to maintain stakeholder oversight of the UK pesticides National Action Plan and provide advice, which allows monitoring progress of the Plan and developing it further. The Pesticides Forum Report combines stakeholder engagement, stakeholder activities and measuring progress. This also gives information that can be used to measure progress on initiatives (Pesticides Forum, 2019[28]).
The Forum is conformed from representatives of organisations covering the farming (conventional and organic production), farming equipment and pesticide industries; environmental and conservation groups; education and training; consumer interests and trades unions who provide a wide range of experience and views on this important sector. In addition, representatives from all the Government Departments responsible for –or those who have an interest in –pesticides in the UK also participate in the meetings.
The forum issues an annual report, which helps to monitor the delivery of the plan using a number of indicators. Indicators typically come in one of three forms:
Indicators measuring impacts directly (e.g. residues detected in water bodies or foodstuffs);
Indicators measuring impacts indirectly (e.g. the numbers of key farmland birds); or
Indicators measuring the behaviour of pesticide users (e.g. users’ participation in continuing professional development).
UK’s Expert Committee on Pesticides
The UK Expert Committee on Pesticides (ECP) provides independent scientific advice to Ministers and Governments on the authorisation of pesticides in the UK and on other matters related to the control of pests (Government of United Kingdom, 2021[29]). ECP business involves consideration of a small proportion of the Health and Safety Executives work, generally the more difficult and novel cases. Each year it supplements this activity by undertaking an exercise to quality assure the HSE casework through a structured audit of finalised cases.
The ECP consists of 15 experts within the field with a mix of expertise. Some of the members are academics working in specialist areas of study relevant to assessing the risks and benefits of pest control. Others are members appointed to consider issues from a public perspective. In addition, it includes members with practical experience of pesticide use and regulation in the farming and amenity sectors. The members are appointed following open public recruitment. The HSE provides the Committee’s Secretariat. Members of the ECP are not salaried staff but do receive a fee for attendance at meetings (Health and Safety Executive, 2020[30]).
Meetings are generally closed to the public due to the commercially sensitive information. However, the Committee aims to hold an open meeting each year. The Committee publishes an annual report, which summarises the work done throughout the year.
United Kingdom’s National Action Plan
The National Action Plan (NAP) seeks to ensure that pesticides are used sustainably, by encouraging the development and introduction of integrated pest management and of alternative approaches. The NAP covers training amongst pesticide users, sales of pesticides, information and awareness raising, inspection of application equipment, aerial applications, handling and storage of pesticides and treatment of their packaging and remnants, Integrated Pest Management (IPM), and indicators. Figure 3.3 shows how the cycle of use controls, evidence, reviews and compliance fit together to underpin the National Action Plan.
Figure 3.3. UK National Plan Cycle
Source: replicated from (Department for Environment FOod and Rural Affairs, 2013[31]), UK National Action Plan for the sustainable use of pesticides, Defra, London.
Progress is in the priority areas are assessed every five years and in the light of any relevant information resulting from the calculation of harmonised risk indicators. Indicators for these and other areas are examined annually in the Pesticides Forum report to provide the quantitative measure of progress. This is also considered alongside achievement of targets set in other related areas such as in implementation of water protection legislation and uptake of measures in agri-environment schemes, which also contribute to minimisation of the use of pesticides. Recently, the UK held a consultation exercise and is now considering responses as part of the process developing a new National Action Plan.
Highlights from the United States
Stakeholder engagement approach from the EPA
EPA often makes policies and procedures available to the public through Pesticide Registration Notices (PRN), website links, or other electronic means. Other transparency mechanisms include (but are not limited to) the following:
EPA invites the public to provide comments on registration applications received for new pesticide active ingredients, new uses, and tolerance petitions.
EPA publishes a notice of these applications in the Federal Register (FR), which directs the public to submit comments to an electronic docket via the website Regulations.gov. Most comments are received electronically, but the FR also provides information on how written comments can be submitted by mail.
EPA also invites the public to submit comments on proposed registration decisions for new active ingredients, significant new uses (e.g. first food use, first residential use, other significant new uses), and proposed decisions for registration review of existing pesticide active ingredients. Various communication methods are used, ranging from email listserv and website updates to Federal Register notices.
Finally, test guidelines and other significant guidance may be shared with the public in draft form before being finalized. EPA engages expert peer reviewers, such as the FIFRA Scientific Advisory Panel, to provide technical advice on emerging methods and topics of scientific controversy. The Pesticide Program Dialogue Committee discusses a variety of pesticide regulatory and implementation issues. These committees include diverse representation from multiple sectors of the stakeholder community and are governed by the Federal Advisory Committee Act (FACA).
The United States has the following peer-review and stakeholder groups:
FIFRA Scientific Advisory Panel (SAP): this panel is composed of biologists, statisticians, toxicologists and other experts who provide independent scientific advice to the EPA on a wide-range of health and safety issues related to pesticides (EPA, n.d.[32]).
Pesticide Program Dialogue Committee (PPDC): is a forum for a diverse group of stakeholders to provide feedback to EPA on various pesticide regulatory, policy, and program implementation issues (EPA, n.d.[33]).
Association of American Pesticide Control Officials (AAPCO): an organization composed by federal and local officials from pesticide regulators of the United States and Canada. The main objective is to provide input to the EPA and states for a successful implementation of pesticide regulation. AAPCO oversees the State FIFRA Issues Research and Evaluation Group (SFIREG) (AAPCO, n.d.[34]).
The IR-4 Project: is a multi-government agencies project that aids growers by facilitating registrations of pesticides and biopesticides on specialty food crops (fruits, vegetables, nuts, herbs, spices) and environmental horticulture crops (trees, shrubs, flowers) (IR-4 Project, n.d.[35]).
Risk management
EPA uses a risk-based approach that considers potential effects of exposure to a pesticide in the context of exposure that is expected to occur as a result of the proposed use. The risk assessment approach is well-established and has been heavily reviewed. EPA uses a tiered assessment approach with refinements available when needed due to complexity of the scenario or other risk management considerations. Internal technical committees routinely review assessment products to ensure consistency and provide advice on new methods and protocols. The EPA has available materials for ecological, human health, and pesticide cumulative risk (EPA, n.d.[36]).
To ensure an objective risk management, the EPA divides internally the risk management and risk assessment functions. This way objective analyses can be used to inform decision making. While distinct functions, open communication between risk assessors and risk managers is encouraged. EPA risk managers consider both potential risks and benefits of each proposed registration, consistent with the standards in FIFRA. If potential risks are identified, risk managers may employ several strategies, sometimes concurrently:
Work with risk assessors to refine the assessment (may request additional data from registrant)
Seek additional information on benefits (for balancing under the FIFRA risk-benefits standard)
Suggest that the registrant (the company applicant for registration) alter the proposed use to reduce or eliminate risks
Require advisory and/or mandatory label language (such as changes to directions for use) to address the risks
If concerns cannot be resolved, the registrant may withdraw the application or EPA may reject it.
References
[34] AAPCO (n.d.), AAPCO – Association of American Pesticide Control Officials, https://aapco.org/2015/07/31/aapco-2/ (accessed on 3 March 2021).
[21] APVMA (2020), Cost Recovery Implementation Statement, https://apvma.gov.au/sites/default/files/publication/67671-cost_recovery_implementation_statement_1_july_2020.pdf.
[19] APVMA (2020), “Gazette Agricultural and Veterinary Chemicals”.
[22] APVMA (2018), Digital Strategy 2018-2022, https://apvma.gov.au/sites/default/files/publication/29521-apvma-digital-strategy-2018-2022_0.pdf.
[23] APVMA (n.d.), Performance report: December 2020, https://apvma.gov.au/node/79686 (accessed on 24 February 2021).
[25] Commonwealth of Australia (2020), The Australian Government Guide to Regulatory Impact Analysis, http://www.pmc.gov.au/regulation.
[26] Commonwealth of Australia (2014), Regulator Performance Framework, http://www.cuttingredtape.gov.au (accessed on 22 February 2021).
[20] Deloitte (2019), Agvet Chemicals-Market Drivers and Barriers, http://www.deloitte.com/about (accessed on 15 February 2021).
[31] Department for Environment FOod and Rural Affairs (2013), UK National Action Plan for the Sustainable Use of Pesticides (Plant Protection Products), http://www.defra.gov.ukwww.defra.gov.uk (accessed on 5 March 2021).
[32] EPA (n.d.), FIFRA Scientific Advisory Panel (SAP) Basic Information | FIFRA Scientific Advisory Panel | US EPA, https://www.epa.gov/sap/fifra-scientific-advisory-panel-sap-basic-information (accessed on 3 March 2021).
[36] EPA (n.d.), Overview of Risk Assessment in the Pesticide Program | Pesticide Science and Assessing Pesticide Risks | US EPA, https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/overview-risk-assessment-pesticide-program (accessed on 4 March 2021).
[33] EPA (n.d.), Pesticide Program Dialogue Committee (PPDC) | Pesticide Advisory Committees and Regulatory Partners | US EPA, https://www.epa.gov/pesticide-advisory-committees-and-regulatory-partners/pesticide-program-dialogue-committee-ppdc (accessed on 3 March 2021).
[2] Government of Canada (2021), Pesticide and Other Agricultural Chemical Manufacturing - 32532 - Summary - Canadian Industry Statistics - Innovation, Science and Economic Development Canada, https://www.ic.gc.ca/app/scr/app/cis/summary-sommaire/32532 (accessed on 15 February 2021).
[16] Government of Canada (2015), Federal, Provincial, Territorial, https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/federal-provincial-territorial.html (accessed on 14 February 2021).
[29] Government of United Kingdom (2021), UK Expert Committee on Pesticides (ECP) - GOV.UK, https://www.gov.uk/government/groups/expert-committee-on-pesticides (accessed on 24 February 2021).
[30] Health and Safety Executive (2020), UK Expert Committee on Pesticides Annual Report 2019, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/893080/expert-committee-pesticides-annual-report-2019.pdf.
[5] Health Canada (2021), Pest Control Products Fees and Charges Regulations, https://laws-lois.justice.gc.ca/eng/regulations/SOR-2017-9/index.html (accessed on 8 February 2021).
[4] Health Canada (2021), Pest Management Regulatory Agency, https://www.canada.ca/en/health-canada/corporate/about-health-canada/branches-agencies/pest-management-regulatory-agency.html (accessed on 13 February 2021).
[14] Health Canada (2021), Pesticide Compliance and Enforcement Annual Reports, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/corporate-plans-reports/pesticide-compliance-enforcement-annual-reports.html (accessed on 14 February 2021).
[8] Health Canada (2002), Pest Control Products Act, https://laws.justice.gc.ca/eng/acts/P-9.01/page-3.html#h-418226 (accessed on 13 February 2021).
[35] IR-4 Project (n.d.), IR-4 Project, https://www.ir4project.org/ (accessed on 3 March 2021).
[27] Matthews, K. et al. (2020), Issues paper—review of the agvet chemicals regulatory system: future reform opportunities, Department of Agriculture, Water and the Environment, https://haveyoursay.agriculture.gov.au/53499/widgets/281250/documents/138791.
[24] OECD (2018), OECD Regulatory Policy Outlook 2018, OECD Publishing, Paris, https://dx.doi.org/10.1787/9789264303072-en.
[6] Open Government Portal (2020), Pesticide Product Information Database, https://open.canada.ca/data/en/dataset/e10b0d6e-04ac-4014-a64a-666c3874bbe0 (accessed on 14 February 2021).
[13] Pest Management Regulatory Agency (2021), A Framework for Risk Assessment and Risk Management of Pest Control Products, Health Canada, Ottawa, https://eur02.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.canada.ca%2Fen%2Fhealth-canada%2Fservices%2Fconsumer-product-safety%2Freports-publications%2Fpesticides-pest-management%2Fpolicies-guidelines%2Frisk-management-pest-control-products.html&data=04%7C01%7CAlberto.MO (accessed on 13 February 2021).
[17] Pest Management Regulatory Agency (2020), Canadian Regulatory Update.
[1] Pest Management Regulatory Agency (2020), Pest Management Regulatory Agency Annual Report 2019–2020, Health Canada, Ottawa, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/corporate-plans-reports/annual-report-2019-2020.html#a54 (accessed on 10 May 2021).
[10] Pest Management Regulatory Agency (2020), Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2020-2025, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-note/2020/special-review-work-plan.html (accessed on 18 February 2021).
[7] Pest Management Regulatory Agency (2020), Proposed Integrated Approach to Pesticide Evaluation, Health Canada, Ottawa.
[11] Pest Management Regulatory Agency (2018), Policy on Cancellations and Amendments Following Re-evaluation and Special Review, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/policies-guidelines/regulatory-directive/2018/dir2018-01-policy-cancellations-amendments.html (accessed on 18 February 2021).
[18] Pest Management Regulatory Agency (2018), Post-Market Pesticide Re-evaluation Review Stakeholder Engagement Sessions.
[3] Pest Management Regulatory Agency (2017), Management of Submissions Policy, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/policies-guidelines/regulatory-directive/2017/dir2017-01-management-submissions-policy.html (accessed on 13 February 2021).
[9] Pest Management Regulatory Agency (2016), Management of Pesticides Re-evaluation Policy, Health Canada, Ottawa.
[12] Pest Management Regulatory Agency (2014), Approach to Special Reviews, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/policies-guidelines/regulatory-directive/2014/dir2014-01-approach-special-reviews-dir2014-01.html (accessed on 18 February 2021).
[28] Pesticides Forum (2019), Pesticides in the UK: The 2019 report on the impacts and sustainable use of pesticides.
[15] Treasury Board of Canada Secretariat (2020), Cabinet Directive on Regulation, https://www.canada.ca/en/government/system/laws/developing-improving-federal-regulations/requirements-developing-managing-reviewing-regulations/guidelines-tools/cabinet-directive-regulation.html (accessed on 14 February 2021).
Notes
← 1. https://www.canada.ca/en/health-canada/services/chemical-substances/chemicals-management-plan.html.
← 2. https://www.canada.ca/en/health-canada/corporate/contact-us/pest-management-information-service.html
← 3. The Federal, Provincial, Territorial (FPT) Committee on Pest Management and Pesticides was established to help strengthen FPT relationships in the area of pest management and pesticides. The Committee also provides advice and direction to FPT governments on programs, policies and issues.
← 4. Subsection 42 of the Pest Control Products Act.
← 5. https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/protecting-your-health-environment/public-registry.html
← 6. The exchange rate was calculated on February 12, 2021. This rate should not be taken as an official OECD statistic, as it sole purpose is to provide an approximation.
← 7. For a complete list of legislative Acts that regulate Australian agrochemicals, please visit: https://apvma.gov.au/node/4131.
← 8. Fees when a good, service or regulation is provided directly to a specific individual or organization.
← 9. Charges imposed when a good service or regulation is provided to a group of individuals or organizations.
← 10. This case study reflects the challenges presented by APVMA when its digitalisation strategy was published, noting that some of the issues may have been solved during the Enabling Technology Program that was launched in 2019.
← 12. APVMA’s assessment manuals can be found in the following link: https://apvma.gov.au/node/45561.
← 13. The following microsite has detailed information on Item 10: https://apvma.gov.au/node/68166.
← 14. All facts and figures were drawn from drumMUSTER website on 18 February 2021. https://www.drummuster.org.au/our-story/results/.