This chapter deals with the biology of apple (Malus domestica). It contains information for use during the risk/safety regulatory assessment of genetically engineered varieties of apple intended to be grown in the environment (biosafety). It includes elements of taxonomy, centres of origin, cultivation, reproductive biology, genetics, hybridisation and introgression, as well as ecology. Annexes present the Malus species, apple’s common diseases and pests, and current biotechnology developments.
Safety Assessment of Transgenic Organisms in the Environment, Volume 9

2. Biology of Apple (Malus domestica)
Abstract
Introduction
This chapter was prepared by the OECD Working Party on the Harmonisation of Regulatory Oversight in Biotechnology, with Belgium and Germany as the co-lead countries. It was initially issued in 2019 as the Consensus Document on the Biology of Apple (Malus Domestica Borkh.). Production data have been updated in this publication, based on FAOSTAT.
Species or taxonomic group
Classification and nomenclature
The genus Malus belongs to the rose family (Rosaceae) which is traditionally divided into four subfamilies on the basis of fruit type. These include: Rosoideae (e.g. Rosa, Fragaria, Potentilla and Rubus; fruit, achene); Prunoideae (e.g. Prunus; fruit, drupe); Spiraeoideae (e.g. Spirea; fruit, follicle or capsule), and Maloideae (e.g. Malus, Pyrus and Cotoneaster; fruit, pome) (Schulze-Menz, 1964). The systematic classification of Rosaceae has changed over the years and molecular analysis has added to the debate on the subfamily groupings (Potter et al., 2007). Using nucleotide sequence data from nuclear and chloroplast regions of 88 genera of Rosaceae, the Rosaceae family was re-classified into three subfamilies: Rosoideae (base chromosome number x = mostly 7), Dryadoideae (e.g. Cercocarpus, Dryas and Purshia; fruit, achene or aggregate of achenes; x = 8 or higher) and Spiraeoideae (mostly x = 8, 9, and rarely x = 15 or 17). All genera previously assigned to Prunoideae (x = 8) and Maloideae (x = 17) were included in the Spiraeoideae (Potter et al., 2007). This subfamily, however, is to be called Amygdaloideae rather than Spiraeoideae under the International Code of Nomenclature (McNeill et al., 2012). The latest classification of the Rosaceae family is thus based on three subfamilies: Rosoideae, Amygdaloideae, including Malus, and Dryadoideae. Although the traditional definition of the four major rosaceous subfamilies may be collapsing from a taxonomic view, this grouping still has great utility from an economic and horticultural standpoint and is still commonly used in literature.
The genus Malus is currently organised into six taxonomic sections, one being Malus to which the species Malus domestica belongs (USDA-ARS, 2018; see Table 2.1). Altogether, 59 species of Malus (also listed as M.) are cited in the taxonomy database of the USDA-ARS Germplasm Resources Information Network (GRIN) and are provided in Annex 2.A. However, the number of species included in the genus is an ongoing subject of debate, revolving around the acceptance of putative hybrids. For example, M. arnoldiana (M. baccata × M. floribunda), which is considered a “secondary species” developed through interspecific hybridisation of “primary species” (Jackson, 2003; Luby, 2003; Rieger, 2006; Hancock et al., 2008).
Table 2.1. Classification and synonyms of Malus domestica
|
Scientific name |
Malus domestica Borkh. |
|
|
Pertinent synonym(s) |
Malus Bork (L.) Britton, nom. inval.; Malus pumila auct.; Malus pumila var. domestica (Borkh.) C. K. Schneider; Malus sylvestris auct.; Malus sylvestris var. domestica (Borkh.) Mansf.; Pyrus malus L. |
|
|
Taxonomic context |
Family |
Rosaceae Juss. |
|
Subfamily |
Amygdaloideae |
|
|
Tribe |
Maleae |
|
|
Subtribe |
Malinae |
|
|
Genus |
Malus Mill. |
|
|
Section |
Malus |
|
|
Species |
Malus domestica Borkh. |
Source: USDA-ARS (2018), Germplasm Resources Information Network (GRIN), https://www.ars-grin.gov (accessed 1 October 2019).
The cultivated apple M. domestica is thought to be the result of interspecific hybridisation (see section on centres of origin and diversity). The binomial M. domestica Borkh. has been generally accepted as the appropriate scientific name replacing the earlier scientific name M. pumila (Korban and Skirvin, 1984; Qian et al., 2010). Throughout its history of cultivation, more than 10 000 cultivars of M. domestica have been developed, although many of those are now lost (Way et al., 1990; Janick et al., 1996; Rieger, 2006). Currently, about 100 cultivars are grown commercially, the most popular worldwide including: ‘Fuji’, ‘Delicious’, ‘Golden Delicious’, ‘Gala’, ‘Granny Smith’, ‘Idared’, ‘Jonagold’, ‘Braeburn’, ‘Cripps Pink’, ‘Jonathan’, ‘Elstar’ and ‘McIntosh’ (Jackson, 2003) see Figure 2.1). Most of the cultivars are diploid, while some of them are triploid (e.g. ‘Jonagold’, ‘Mutsu’, ‘Schöner von Boskoop’) and a few are tetraploid (e.g. ‘Antonovka Ploskaya’, ‘Wealthy Tetraploidnyi’, ‘Papirovka Tetraploidnaya’, ‘McIntosh Tetraploidnyi’).
Figure 2.1. Commercially grown apple cultivars

Note: (a) ‘Fuji’, (b) ‘Delicious’, (c) ‘Golden Delicious’, (d) ‘Gala’, (e) ‘Granny Smith’, (f) ‘Idared’, (g) ‘Jonagold’, (h) ‘Braeburn’, (i) ‘Cripps Pink’, (j) ‘Jonathan’, (k) ‘Elstar’ and (l) ‘McIntosh’.
Source: Courtesy of Bundessortenamt and Julius Kühn-Institute, Germany.
Description
M. domestica is botanically described as follows:
Tree – a small- to medium-sized, branched, deciduous tree with a single trunk and a broadly spreading canopy. The trees are generally 2-10 m tall (in cultivation, tree size and shape are heavily dependent on rootstock and training [planting] system). Young stems and twigs are somewhat tomentose (hairy), while older branches are glabrous (smooth) (Bailey and Bailey, 1976; Webster, 2005a). Spurs (very short shoots) grow very slowly and primarily produce flowers and subsequent fruits. They are formed on one-year-old shoots. Root suckers can emerge from the rootstock.
Leaves – are alternately arranged, dark green, simple oval-shaped with a serrated edge, 4‑13 cm long x 3-7 cm wide, with irregularly saw-toothed margins, and usually hairy beneath (Webster, 2005a; Rieger, 2006).
Buds – are purplish brown, ovoid and densely hairy. Buds either give rise to shoots/leaves (vegetative buds) or flowers (flower buds). Flower buds are larger and plumper than growth buds and have a downy surface.
Flowers – are 3-4 cm in diameter. Each flower blossom has 5 sepals, 5 petals, varying from white to pink, and about 20 stamens with yellow anthers in three whorls (10+5+5). The pistil comprises of a stigma and five styles united at the base (Jackson, 2003; Hancock et al., 2008). The five styles are slightly longer than the stamen. The ovary is inferior, positioned beneath the sepals, petals and stamen. The peduncle and calyx (all sepals) are usually woolly, and the calyx is persistent in the fruit (Webster, 2005a). Flowers are usually terminal on spurs, although they may grow laterally from one-year-old shoots in some cultivars, borne in groups of 4-6, in inflorescences that have variously been described as corymbs, corymbose racemes, cymes, and false cymes (Jackson, 2003; Rieger, 2006).
Fruit – is an ellipsoid to obovoid, globe-like pome indented at the base and the apex (see Figure 2.2). The fruits are usually greater than 5 cm in diameter weighing 200-350 grammes. Fruits vary in colour and can be uniformly red, green or yellow or bi-coloured. Bi-coloured fruit can be striped or blushed red on a yellow or green background (see Figure 2.1). Each fruit contains a cortex of (edible) flesh between the skin and the core line. The central core has a fleshy pith with a papery capsule of five fused carpels. Each carpel typically contains two seeds. Seeds are smooth, shiny, and chestnut brown (Jackson, 2003; Rieger, 2006).
Figure 2.2. Flower and fruit of apple, cut lengthwise, showing the relation of the parts of the flower
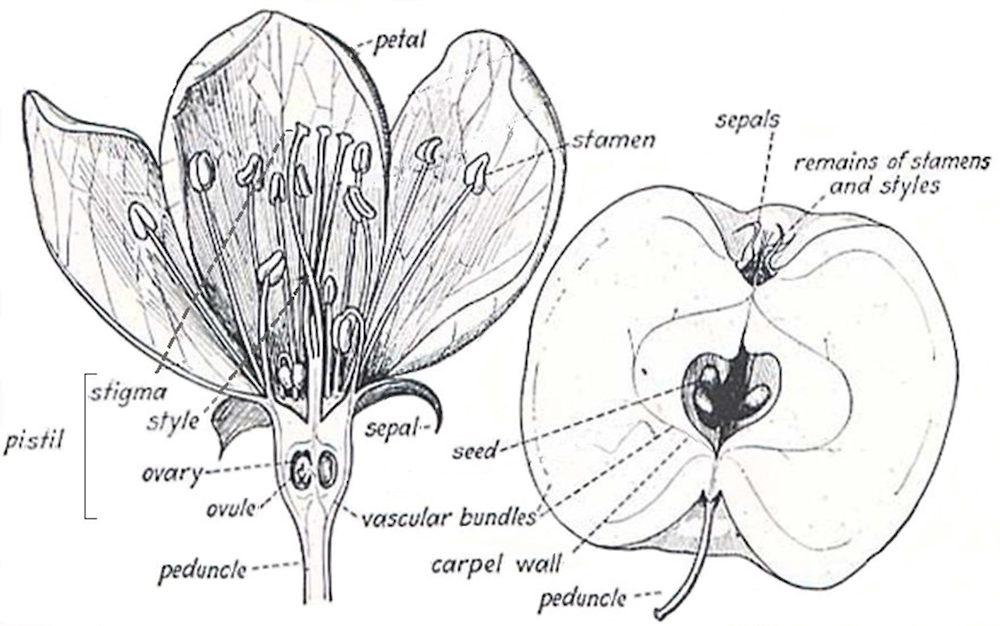
Source: Adapted from Iowa State University (2003), Reproductive Terms.
Roots – consist of a horizontal layer of permanent, thickened, spreading scaffold roots less than 50 cm from the surface, and numerous vertical “sinkers” descending to an impermeable layer or water table (Jackson, 2003).
Centres of origin and diversity, geographic distribution, natural and managed ecosystems and habitats, cultivation and management practices
Centres of origin and diversity
The centre of origin for domesticated apple species lies within the Tian Shan Forest regions of Central Asia, including Kazakhstan, Kyrgyzstan and Tajikistan (Vavilov, 1951; Dzhangaliev, 2003). The wild species Malus sieversii (Ledeb.) M. Roem. occurring in these forest regions has been identified as the initial progenitor to the genome of the cultivated M. domestica on the basis of morphological, historical and molecular evidence (Robinson et al., 2001; Harris, Robinson and Juniper, 2002; Velasco et al., 2010; Duan et al., 2017).
The domestication of Malus occurred around 8 000 to 2 000 BCE in Central Asia, possibly near Almaty, Kazakhstan (Vavilov, 1930, as referred to in Robinson et al., 2001; Zohary, Hopf and Weiss, 2012). Apple seeds and trees from selected forms were then dispersed along the trade routes of the Silk Route from Central Asia, east to the People’s Republic of China (hereafter ‘China’) and west to Europe (Harris, Robinson and Juniper, 2002), resulting in the random establishment of apple germplasm along the Silk Route (see Figure 2.3).
Figure 2.3. Evolutionary history of the cultivated apple
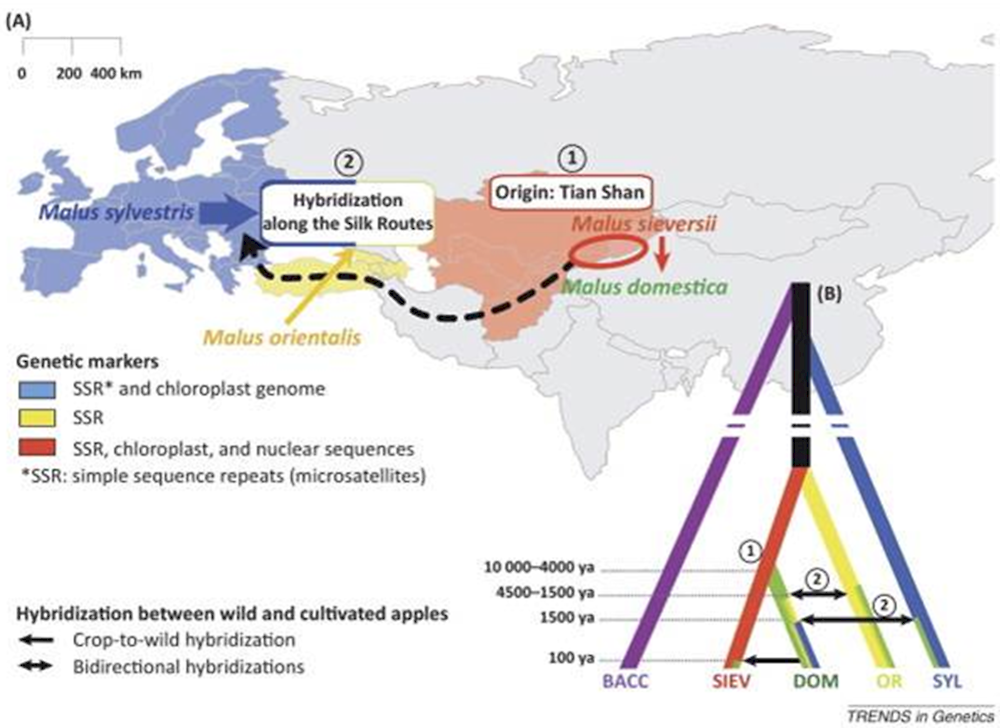
Note:
(A) Origin in the Tian Shan Mountains (1) followed by dispersal (2) from Asia to Europe along the Silk Route. Arrow thickness is proportional to the genetic contribution of various wild species to the genetic makeup of Malus domestica.
(B) Genealogical relationships between wild and cultivated apples. Approximate dates of the domestication and hybridisation events between wild and cultivated species are detailed in the legend. Abbreviations: BACC, M. baccata; DOM, M. domestica; OR, M. orientalis; SIEV, M. sieversii; SYL, M. sylvestris; ya, years ago.
Source: Cornille, A. et al. (2014), “The domestication and evolutionary ecology of apples”, Trends in Genetics, Vol. 30, p. 57-65.
Hybridisations occurred between the apples coming from Central Asia and closely related species present along the Silk Route. This gave rise to diverse forms of hybrids from which the present-day cultivated apple might have been selected and propagated by vegetative means. Several species contributed to the genetic background of the current apple populations: some of the ones that are considered to have contributed are the Siberian crab apple M. baccata L. (Borkh.), the Caucasian crab apple M. orientalis Uglitzk. and the European crab apple M. sylvestris L. Mill. (Cornille et al., 2012). The wild European crab apple M. sylvestris, in particular, is considered to be a major contributor to M. domestica in Western Europe, as it is genetically more closely related to this species than to its Central Asian progenitor, M. sieversii (Cornille et al., 2012, 2014; Duan et al., 2017).
Geographic distribution
M. domestica is cultivated throughout temperate areas of the world. In general, M. domestica is well adapted to a range of climates but its ideal growing conditions are the cool-temperate zone between about 35-50° latitude with high light intensity, warm days and cool nights (Webster, 2005b; Rieger, 2006). Its range is farther north than most other fruit crops due to its relatively late blooming and cold hardiness (Rieger, 2006). It is also cultivated in less suitable climates (i.e. semi-arid, subtropical and tropical) where irrigation, altitude and various farming practices are used to overcome climatic limitations (Westwood, 1993; Hampson and Kemp, 2003). An example of the range of temperatures over which apples are successfully produced is provided by Jackson (2003). At the extreme cold end of the range, Poland, with winter monthly minimum temperatures of -17°C and summer monthly maximum temperatures of 30°C, has successful apple cultivation. Egypt with winter minimums of 1°C and summer maximums of 43°C reflects the extreme warm end of the range (Jackson, 2003). Global climate change is likely to affect the current geographic distribution of M. domestica.
Ecosystems and habitats where the species occurs natively and where it has naturalised
M. domestica is a product of selection by human intervention and hybridisations over thousands of years in many parts of the world (see the section describing the intensively managed ecosystems where the species is grown or occurs on its own). Outside of its cultivation areas, M. domestica has naturalised in different parts of the world, where it grows in abandoned pastures, clearings, roadsides and borders of woods (Randall, 2017).
Wild apple populations, also known as “crab apples”, are native throughout the northern hemisphere in temperate areas (Luby, 2003). They are mainly found on the edge of woods and areas of scrub, in moist or coastal regions (e.g. Routson et al., 2012). In Europe, there are five endemic Malus species: the crab apple from Sicily M. crescimannoi, the Florentine (or Hawthorn-leaf) crab apple M. florentina, the Paradise apple M. pumila, the Lebanese (or three-lobed) crab apple M. trilobata and the European crab apple M. sylvestris (IUCN, 2019). There are four Malus species native to North America – the Southern crab apple M. angustifolia (United States [US]), the Sweet crab apple M. coronaria (Canada, US), the Oregon crab apple (also termed Pacific crab apple) M. fusca (Canada, US) and the prairie crab apple M. ioensis (US) (VASCAN, 2018; USDA-NRCS, 2018). M. sieversii is native to western China and Central Asia (Richards et al., 2008; Gharghani et al., 2009; Gross et al., 2012; Nikiforova et al., 2013; Volk et al., 2013) but has become a rare and threatened plant in China (Yan et al., 2008; IUCN, 2019).
Crab apple accessions of different Malus species may be grown as ornamentals in landscaping, parks or gardens (Fiala, 1994). Many of these accessions offer prolific spring bloom and a wide range of decorative fruits differing in size, shape and colour (Fiala, 1994). Further, crab apples may be used as pollinisers in apple orchards.
Intensively managed ecosystems where the species is grown or occurs on its own, including management practices
The apple M. domestica is one of the most widely cultivated tree fruits (Table 2.2). Cultivation started as early as 4 000 BCE in the Near East (Figure 2.3) and apples reached the eastern Mediterranean region by 2 000 BCE and Greece and Italy by 900 and 800 BCE.
Table 2.2. Overview of continents, territories and countries where apple is cultivated
|
Continent |
Territories and countries |
|---|---|
|
Africa |
Algeria, Egypt, Kenya, Libya, Madagascar, Malawi, Morocco, South Africa, Tunisia, Zimbabwe. |
|
Americas |
Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Ecuador, El Salvador, Grenada, Guatemala, Honduras, Mexico, Paraguay, Peru, Saint Vincent and the Grenadines, United States, Uruguay. |
|
Asia |
Afghanistan, Armenia, Azerbaijan, Bhutan, People's Republic of China, Cyprus, Democratic People’s Republic of Korea, Georgia, India, Iran, Iraq, Israel, Japan, Jordan, Kazakhstan, Korea, Kyrgyzstan, Lebanon, Nepal, Pakistan, Syria, Tajikistan, Turkey, Turkmenistan, Uzbekistan, Yemen. |
|
Europe |
Albania, Austria, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Montenegro, Moldova, Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, Russian Federation, Serbia, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Ukraine, United Kingdom. |
|
Oceania |
Australia, New Zealand. |
Source: FAO (2022), FAOSTAT, www.fao.org/corp/statistics/en (accessed 16 February 2022).
The Roman armies carried apples across Europe, planting seeds wherever they settled. By the 1200s, cultivated apples were becoming increasingly popular in the Nile Delta and throughout Europe, where they appeared in the gardens of both royalty and commoners. By the 1600s, there were at least 120 cultivars of apple described (Luby, 2003). European colonists introduced M. domestica to the Americas (1500‑1600s), South Africa (1650s), Australia (1788) and New Zealand (1814). By the late 1800s, M. domestica had been introduced into southern and eastern Asia, where it supplanted the Chinese soft apple M. x asiatica Nakai, the primary cultivated apple in that region for over 2 000 years (Luby, 2003; Hancock et al., 2008). Today, the global apple market is dominated by European and North American M. domestica varieties.
M. domestica is a labour-intensive, highly managed crop, especially by the time an orchard has reached maturity and is ready for commercial production. Although barely any fruit is produced during the first year(s) after plantation, there is still a number of agronomic issues that must be properly managed to ensure good tree growth. These include nutrient amendments (through fertigation or direct soil application), disease and pest control, irrigation, weed control and pruning. Table 2.3 highlights a typical management schedule for apple production. Trees and fruit are prone to a number of fungal, bacterial and pest problems (see section on pests and diseases), which can be controlled by a number of non-organic and organic means. Many commercial orchards pursue a programme of chemical sprays but a trend in orchard management is the use of biological control methods, which include, for instance, the introduction of a natural predator to reduce the population of a particular pest (see section on biological control organisms).
M. domestica is not regarded as a weed of agriculture (Randall, 2017). Volunteer plants originating from seed in apple orchards are rare due to the perennial nature of the crop and orchard management practices that include herbicide treatment of the tree row and mowing of the alley between rows (Stover and Marks, 1998).
Table 2.3. Highlights of a typical management schedule for apple production
|
Time of year – Phase |
Action |
|---|---|
|
Winter – Dormancy |
- Prune dormant trees - Remove root suckers - Perform root pruning - Analyse soil composition - Apply soil fertiliser and lime - Apply herbicides |
|
Spring – Fruit set |
- Perform pruning (mechanical) - Apply mulching - Perform irrigation - Apply frost protection - Place bees in orchards when blossom begins - Apply chemical and/or mechanical thinning - Monitor and control pests (insects and diseases) - Monitor and control weeds |
|
Summer – Fruit growth |
- Perform irrigation - Apply hand thinning - Perform summer pruning - Remove root suckers - Apply mulching - Apply foliar fertiliser (calcium to prevent bitter pit) - Monitor and control pests (insects and diseases) - Monitor and control weeds - Prepare soil for planting |
|
Fall – Harvest period |
- Harvest apples - Store apples in cold or controlled atmosphere storage - Apply leaf fall spraying - Build the trellis (support) system for new trees - Perform tree planting |
Source: Adapted from AAFC (2013), Crop Profile for Apple in Canada, 2013, Pesticide Risk Reduction Program, Pest Management Centre, Agriculture and Agri-Food Canada, Ottawa.
Reproductive biology
Generation time and duration under natural circumstances, and where grown or managed
The whole sexual reproduction cycle (fertilisation, seed formation and seedling growth) only occurs naturally in the wild (see “Life cycle of apple under natural conditions”), in the production of some rootstocks and in apple breeding programmes (for the latter two, see section on breeding approaches). The life cycle of apple in managed ecosystems starts from the adult phase (see “Life cycle of apple in managed ecosystems”).
The life cycle of apple under natural conditions
Under natural circumstances, the life cycle of wild apple starts from a seed, released by frugivorous animals or by the rotting of fruit during winter on the ground. After germination, the juvenile growing period follows. During this period morphological and physiological characteristics of the plant differ relative to the adult stage. The inability to flower and produce fruit and seeds is one of the main characteristics of the juvenile period. The plant enters the adult stage once flower bud differentiation occurs. During the adult stage, the plant is fertile and will flower almost every year. The juvenile period of wild apple plants under natural conditions usually takes between 6-12 years and is influenced by environmental as well as genetic factors. Years with a high crop load can be followed by years with low to no crop load, a phenomenon known as biennial bearing (Tromp, Webster and Wertheim, 2005).
The life cycle of apple in managed ecosystems
In managed commercial orchards, one- or two-year-old trees are planted that were propagated in fruit tree nurseries by budding or grafting on rootstocks. The buds or grafts are collected from mature commercial apple cultivars. This means that the resulting trees are no longer juvenile. Nevertheless, it is unlikely that one-year-old trees will bear fruit. In commercial orchards, the first yield will be obtained from the second year on and will progressively increase until a maximum yield is reached after 5-7 years.
The life span of a commercial high-density apple orchard is about 15 years, although many orchards do maintain a sufficient production beyond this age. A number of factors, including tree health, pests, apple cultivar, soil quality, environmental factors (i.e. heat units, winter injury), market opportunities, etc., play a role in the life span of a commercial orchard.
Generally, apple fruits reach maturity about 120-150 days after flowering but some cultivars mature in as little as 70 days, others in as long as 180 days (Rieger, 2006). Time to maturity varies with temperature (e.g. warmer temperatures reduce time to maturity) and is therefore geographically dependent. However, rankings of “early” or “late” maturing varieties relative to each other are fairly consistent (Jackson, 2003). Short-season cultivars tend to have a wide climatic tolerance; they do well in colder, northerly apple-producing regions such as Canada (e.g. ‘McIntosh’) and may also be grown as early-season crops in countries like France and New Zealand (e.g. ‘Cox’) (Jackson, 2003). Long-season cultivars like ‘Braeburn’, ‘Fuji’, ‘Cripps Pink’ and ‘Granny Smith’ generally cannot be grown successfully in northern areas and do best in the milder climates, mainly in the southern hemisphere (Hampson and Kemp, 2003; Jackson, 2003).
Reproduction (production of flowers, fruits, seeds and root suckers)
Floral biology
Flower development
In apple, flower development lasts between nine and ten months, starting with the formation of floral primordia in the mixed flower buds (i.e. buds producing flowers in addition to leaves and shoots) in summer and early autumn. At leaf fall, flower and leaf primordia are present in a large portion of the flower buds (Kotoda et al., 2000; Dennis, 2003; Jackson, 2003; Koutinas, Pepelyankov and Lichev, 2010). During winter, development slows due to bud inhibition by (endo)dormancy. Bud break follows after winter-chilling and heat-unit requirements are met. The king (apical) flower of the apple inflorescence opens first, followed by the lateral flowers (Jackson, 2003). Flowering occurs in spring when white to deep pink flowers develop in a cyme-like inflorescence of 4‑6 flowers (Figure 2.4). Apple flowers can be borne in both terminal and lateral flower buds on both spurs and shoots. Flower clusters on lateral buds open later than do those on terminal buds and generally produce smaller fruit (Jackson, 2003). The flowering period takes 1-4 weeks, depending on the weather conditions, beginning with the (king flower of the) terminal flower buds and ending with the (lateral flowers of the) lateral flower buds.
Flowering is affected by many biotic (endogenous phytohormones, previous year’s crop load, pathogens and pests) and abiotic (light, water stress, nutrients, temperature and exogenously applied chemicals) factors. Also, cultivation practices drive flowering and include grafting, pruning, scoring and/or ringing the base of the tree (Jackson, 2003). The flowering period can significantly differ between cultivars. The difference in flowering time between commercial cultivars and local cultivars can be more than one month in colder temperate regions (e.g. Northwestern Europe) and about 2-3 weeks in warmer regions (e.g. Southern Europe).
Figure 2.4. Open king flower and closed lateral flowers

Source: Courtesy of A. De Schrijver.
Effective pollination period
The length of the flowering period during which viable pollen is produced varies depending on weather conditions and generally lasts from 7 to 30 days (Jackson, 2003). During flowering, the stigma produces extracellular secretions which provide a moist environment for pollen deposition and germination (Jackson, 2003). Once the pollen grains have germinated, the pollen tubes grow down the style into an ovule where fertilisation of the egg cell (to form a zygote) and polar nuclei of the egg sac (to form the endosperm) occur (Dennis, 2003). Successful fertilisation depends on the pollen grains reaching the ovule before it degenerates.
The time during which the flower can be fertilised, if pollination is not limited, is assessed by the effective pollination period (EPP) (Williams, 1966). The EPP is defined as the number of days during which pollination is effective in producing fruit and is determined under orchard conditions by recording the initial fruit set (fruit set after pollination) and final (mature) fruit set in hand-pollinated flowers. Variation in EPP values has been claimed to be due both to environmental effects, mainly temperature (Tromp and Borsboom, 1994), and to flower quality (Jackson, 2003). The EPP values are highly variable among cultivars, years and sites. Typically, EPP values vary between 2 and 9 days (Sanzol and Herrero, 2001).
Gametophytic self-incompatibility, cross-incompatibility and semi-incompatibility
Fruit set in most apple cultivars is less than 10% after self-pollination (Komori et al., 1999). Self-fertilisation is inhibited due to the presence of a multi-allelic S locus, which contains pistil S and pollen S genes, that is responsible for S-RNase-mediated gametophytic self-incompatibility (GSI) (see Sassa, 2016 and reference therein). Cultivars with the same S alleles cannot fertilise each other. When an S haplotype in the haploid pollen matches one of the two S haplotypes in the pistil, then the pollen is recognised as “self” and the pollen tube formation is blocked at the upper part of the style, whereas the “non-self” pollen tubes can grow along the style and reach the ovary. Because of this self-incompatibility, outcrossing is promoted, resulting in the majority of cultivars displaying high levels of allelic heterozygosity. However, self‑incompatibility in Rosaceae is broken down by polyploidisation and has been demonstrated in tetraploids of apple (Adachi et al., 2009).
Although many species are believed to be strongly self-incompatible, variations between species in self‑incompatibility strength have been observed. The increase in self‑compatibility in species with a functional self-incompatibility mechanism, like apple, can be caused by a variety of environmental variables such as temperature and flower age (Ferrer et al., 2009). Such species are called partial or pseudo-self-compatible, partially self‑compatible or pseudo-self-fertile (Levin, 1996). Self-incompatibility in apple can also vary in strength depending on the apple cultivar. De Witte et al. (1996) found ‘Fuji’ and ‘Golden Delicious’ to give only 1% and 1.8% set respectively, following self-pollination under conditions, where pollination of these cultivars with the ornamental apple ‘Baskatong’ gave 24% and 25% set respectively. Much higher levels of self-fertility were found in ‘Idared’ (12.3%) and ‘Elstar’ (7%) although very few of the resulting fruits contained seeds (De Witte et al., 1996).
Instances of cross-incompatibility and semi-compatibility exist (Janick and Moore, 1975; Ramírez and Davenport, 2013). These are also controlled by the S locus that plays a role in self-incompatibility. Depending on their S loci, pairs of apple cultivars can be incompatible when both loci are identical and semi-compatible when they carry one different and one similar S locus. Pairs of apple cultivars are fully compatible when they differ in their S loci.
Pollination, pollen dispersal and pollen viability
Pollen dispersal and pollination depend on the pollinator (pollen vector), weather conditions, surrounding habitat and polliniser (pollen donor tree).
Pollinator
Insects, most notably honeybees, but also bumblebees, other wild bees and to a lesser extent some flies, are the primary vectors for pollination in apple. Apple pollen is relatively heavy and not easily carried by the wind (Dennis, 2003; Jackson, 2003).
Apple growers typically rent honeybee hives during the bloom period and it is recommended that they are placed at a density of four or five strong colonies per hectare in a mature orchard (Dennis, 2003). Although honeybees are the insects most used in orchards, they are not as efficient pollinators as some solitary bees and bumble bees. A lot of honeybees take nectar from the flower without even touching the anthers (Delaplane and Mayer, 2000) and do not contribute to pollination. Moreover, Sapir et al. (2017) found that adding bumblebees to honeybees increased cross-pollination. Adding bumblebees had multiple effects on pollination: not only the number of pollinating insects increased but these were now also working in adverse weather conditions and even the foraging behaviour of the honeybees was enhanced.
Weather
Rain during the flowering period may have a negative impact on the pollination of apples, by lowering the foraging activities of pollinators which reduces pollen transfer (Abrol, 2012). Another possibility is that rain inhibits the germination and growth of pollen on the stigma (AHDB, 2017). Similarly, wind can also have negative influences on apple pollination. Strong winds will make it harder for pollinators to transfer pollen from flower to flower (Jackson, 2003). At wind speeds of 15 km/h or more, honeybees do not fly and the limited amount of pollen that would have been transferred will rapidly desiccate (Tromp, Webster and Wertheim, 2005).
During flowering, a minimum temperature of 10°C is needed for effective pollen germination. When the temperature increases to 20°C, pollen germination rate (Yoder et al., 2009; Abrol, 2012) and hence pollen tube growth (Jefferies and Brain, 1984) increases with higher chances of successful fertilisation. Spring frosts can cause severe damage especially for early flowering cultivars and the damage is not always visible immediately after frost (Jackson, 2003; Tromp, Webster and Wertheim, 2005).
Surrounding habitat
Small habitats in the environment support wild pollinators with food and housing and encourage the diversity of wild bees (Sheffield, Ngo and Azzu, 2016). The surrounding habitat needs to be diverse in plant species and provide the bees with adequate nectar and pollen while also offering suitable nesting opportunities (Garibaldi et al., 2013; Sheffield et al., 2013).
Polliniser
The polliniser (i.e. pollen donor tree) used in the orchards is also an important factor for fertilisation. There are two main aspects in choosing the right polliniser: bloom overlap and compatibility with the acceptor tree.
Bloom overlap takes place when two apple cultivars flower synchronously, promoting effective pollen transfer. When pollen is deposited before the receptive period, the pollen should remain viable and fertile for a long enough period to allow successful pollination. Differences in pollen viability have been reported for different cultivars (Petrisor et al., 2012; Moshtagh et al., 2015). Pollen fertility of most apple cultivars is close to 100% but is reduced in some cultivars by unknown factors or triploidy.
The second important aspect is compatibility. In the case of cross-incompatibility, not all cross-pollination will result in fertilisation and seed formation. Semi-compatibility, however, is not a problem if there are enough pollinators, blossom abundance and pollen supply is high and conditions for pollination are good.
Pollen dispersal and dispersal studies
Many apple species are self-incompatible and, hence, fruit production only occurs with pollen transfer between cultivars. Most orchards consist of a limited number of cultivars, which are arranged in monotypic blocks or rows. The economic costs due to fruit yield loss as a result of inadequate pollination highlights the need to more accurately predict patterns of pollen movement in orchards (Kron et al., 2001). Pollen dispersal in apples has received considerable attention. However, most studies are based on apparent rather than realised pollen dispersal (Free and Spencer-Booth, 1964; Wertheim, 1991) and only a few studies have attempted to relate factors other than the distance to pollination success. In apple orchards, the majority of honeybee foraging flights are between flowers on the same tree and secondarily between adjacent trees in the same row and to a lesser extent across rows (Free and Spencer-Booth, 1964; Free, 1966). However, in a study by Kron et al. (2001) in which they examined pollen dispersal by molecular markers, they found the same number of seeds sired by the polliniser along and across the row.
Early research on pollen dispersal was conducted using a pollen donor carrying a dominant gene for red leaf colour, such as ‘Baskatong’. Researchers monitored gene flow in apple orchards by observing the percentages of red-leafed seedlings borne from trees at increasing distances from the ‘Baskatong’ polliniser. A study using this approach found that 69% and 91% of the fertilised seeds occurred within the first 10 m and 60 m of the pollen donor respectively (Reim et al., 2006). Kron et al. (2001) used allozyme markers to determine the parentage of seeds to track pollen flow in orchards where ‘Idared’ was planted as a polliniser. Pollen dispersal generally declined with increasing distance, as 50% of total seeds sired by ‘Idared’ occur within the first four rows, or approximately 20 m (Kron et al., 2001). In a survey of a wild population of the crab apple M. sylvestris, Larsen and Kjær (2009) used microsatellite loci data in conjunction with spatial distances of the individual trees in the population to monitor pollen movement in a natural environment. These authors found that successful pollination occurred mostly between nearby trees, with a median distance of about 23 m. The data suggest that the majority of cross-pollination occurs between receptive flowers and proximate pollen sources. A significant factor affecting pollination intensity is the distance from the pollen source. Other important factors include weather, pollinator presence, cultivar compatibility and flowering synchrony (Kron et al., 2001). Maximum pollen dispersal distances in orchard settings were reported up to 40 m (Wertheim, 1991), 86 m (Kron et al., 2001), 104 m (Reim et al., 2006), 137 m (Tyson, Wilson and Lane, 2011) and 150 m (Soejima, 2007). In-hive transfer of viable pollen between bees foraging in geographically distant areas may explain long-distance pollen flow (Degrandi-Hoffman, Hoopingarner and Klomparens, 1986).
To predict bee-vectored pollen transfer, Tyson, Wilson and Lane (2011) developed a mechanistic model based on monitoring beta-glucuronidase (GUS) activity in seeds borne from trees located at increasing distances from a row of transgenic GUS-expressing ‘Gala’ trees. The authors use the model to examine the effect of buffer rows and isolation distances on outcrossing rates. The model demonstrates that the level of outcrossing is affected by the relative sizes of the nearby orchards. As the size of the orchard with conventional trees becomes smaller relative to the orchard with transgenic trees, the isolation distance required to limit the frequency of outcrossing is increased. Furthermore, the incorporation of buffer rows between the two orchard types generally reduces the isolation distance required in order to limit outcrossing frequency (Tyson, Wilson and Lane, 2011).
Seed production and natural dispersal of fruits and seeds
Seed production
Seed production is the result of fertilisation of the egg cell present in the apple flower and the male gamete present in the pollen. Seeds develop and reside in the central core of the fruit, in the carpels. Each carpel has two ovules that develop into seeds following fertilisation. In general, the seed number of fruit varies between two and seven, depending on the fertilisation intensity.
Natural dispersal of fruits and seeds
The primary means of movement and dispersal of apple seeds in natural settings is through frugivorous mammals, such as bears, foxes and deer (Willson, 1993; Myers et al., 2004) as well as birds (Witmer, 1996 and references therein). For example, white-tailed deer travel a range of many hectares on a daily basis and are considered dispersers of low numbers of apple seeds (Myers et al., 2004). Seeds may accidentally be attached to vertebrates or fruits could be ingested and digested, thereby deliberating the seeds (Galetti, 2002; Herrera, 2002; Myers et al., 2004). Seeds pass through the digestive tract of animals and can germinate and generate a new apple tree. It is also possible that apple seeds are dispersed by humans during the transport of fruit or after eating the fruit.
Seed viability, germination, seedling viability and establishment
In apple, it is accepted that there is no seed bank formed in nature, although this has not been studied in detail. During the winter, cold temperatures (< 5°C) and high humidity break the dormancy of mature seed. This process is known as stratification. The stratification time depends on the genotype. Seeds will either germinate during spring or die. Seed germination rate is very high and can reach up to 95% under controlled conditions of stratification and sowing.
The survival of seedlings in nature is rather low but precise data are not available. Seedling growth in the first year is minimal compared to the later years. Seedlings can grow in very different soil and climate conditions of temperate regions with enough precipitation, although they can grow also in semi-arid regions.
Asexual propagation
Apomixis
Apomixis, a form of asexual reproduction in flowering plants, is common in agriculturally important crops, except for apple and citrus fruits. Apomixis sensu stricto refers to asexual seeds that are genetically identical to the mother plant. Apples are facultative apomicts which means that they contain both sexually and asexually produced seeds in their fruits. The process of apomixis is very similar to sexual reproduction and also leads to viable seeds. However, in contrast to sexual reproduction, there is no fusion of male and female gametes and embryo sac formation occurs without meiosis. The embryo is thus only derived from maternal somatic tissue and is, therefore, a clone of the mother plant (Koltunow, 1993).
Vegetative reproduction
Another form of asexual reproduction in apple trees is the formation of root suckers. Root suckers are sprouts that arise from the roots. Because commercial apple trees are planted on rootstocks, these root suckers are originating from the rootstock and not from the commercial cultivar itself. Several commercial rootstocks, including M.7, M.9 and G.202, tend to produce a lot of root suckers which need to be removed regularly because they compete for water and nutrients with the commercial tree and because they form a potential entry point for fire blight (Miller and Racsko, 2011). These root suckers will not have the chance to flower in commercial orchards. However, after felling, the apple tree’s remaining roots can produce root suckers that flower after several years when the land is not further managed.
Parthenocarpy
Apples can develop fruits without fertilisation, known as parthenocarpic fruits. Parthenocarpic fruits are seedless. There are two distinct types of parthenocarpy: vegetative and stimulative. Vegetative parthenocarpy is spontaneous and arises without pollination or any other externally applied stimulus. Several apple cultivars are intrinsically parthenocarpic, e.g. ‘Spencer Seedless’, but they are rarely of economic value (Jackson, 2003). Stimulative parthenocarpy can be induced by pollination without fertilisation (e.g. with irradiated pollen) or by plant hormones, primarily gibberellins. Although parthenocarpy can be induced with growth regulators in apple, the response is limited and such treatments are not used commercially (Jackson, 2003).
Genetics
Detailed genetic information
Whereas the haploid (x) chromosome numbers of most species in the family Rosaceae are 7, 8 or 9, the Maleae tribe, including Malus domestica, is distinct in having a haploid chromosome number of 17 (x = 17). Two hypotheses are still under investigation to explain the chromosome number of Maleae. The first hypothesis postulates that Maleae originated from ancient hybridisations between species in the Prunoideae (x = 8) and the Spiraeoideae (x = 9), followed by chromosome doubling. The original hybrids would have been sterile and only after chromosome doubling would they have formed fertile allopolyploids (Way et al., 1990; Luby, 2003; Webster, 2005a). The second hypothesis postulates that the genome originated more than 50 million years ago from a genome-wide duplication event of the x = 9 ancestral (most probably) Gillenia chromosomes followed by loss of 1 chromosome (Velasco et al., 2010). As a genome-wide duplication pattern was found for all chromosomes (Velasco et al., 2010; Daccord et al., 2017), this hypothesis likely explains the 17 chromosomes in M. domestica.
Most Malus species, including M. domestica, are diploid with 2n = 34 chromosomes; however, different levels of ploidy (tri-, tetra-, penta- and hexaploidy) are also known (Höfer and Meister, 2010). The mean value for the 2C nuclear deoxyribonucleic acid (DNA) content of M. domestica is 1.514 picograms (pg) per nucleus (Höfer and Meister, 2010).
The nuclear genome of M. domestica cultivar ‘Golden Delicious’ has been sequenced and represents the apple reference genome (Velasco et al., 2010; Daccord et al., 2017). Using the ‘Golden Delicious’ reference, nearly 200 apple genotypes have been re‑sequenced. In addition to the nuclear DNA, whole organelle genomes (chloroplast and mitochondrial DNA) of apple were also sequenced (Goremykin et al., 2012; Nikiforova et al., 2013; Duan et al., 2017).
Genetic linkage mapping has contributed significantly to our current understanding of the structure of the Malus genome. First genetic linkage studies on apple were done in the late 1980s (Chevreau and Laurens, 1987; Manganaris and Alston, 1987; 1988a,b) and were followed by international apple genome mapping projects in Europe (King et al., 1991) and New Zealand (Gardiner et al., 1996). The first linkage map of the apple genome was published by Hemmat et al. (1994). With the establishment of simple sequence repeat (SSR) and single nucleotide polymorphism (SNP) markers, the number of linkage studies increased rapidly. Today, there are more than 60 different apple linkage maps available in the Genome Database for Rosaceae (https://www.rosaceae.org/). These maps were established using different parental genotypes, with different types of DNA markers, and were established for traits ranging from disease resistance to fruit quality, aroma, flower and fruit development, harvesting time and tree growth parameters, etc. The biggest advance in this field of research is the development of a multi-parental, high-density, integrated Genetic Linkage Map (iGLMap) of apple, comprising 15 417 SNP markers (Di Pierro et al., 2016).
The first apple reference transcriptome was published by Velasco et al. (2010) and significant improvements were made by Bai, Dougherty and Xu (2014). The use of high‑throughput proteomics and metabolomics approaches in apple research has been increasing exponentially during the last few years (for reviews see Liu et al. (2017)).
Breeding approaches
The commercial apple tree, as in the case of other fruit trees, is a composite of a rootstock and a scion. The rootstock constitutes the root system and a small proportion of the lower trunk whereas the scion grafted or budded onto the rootstock forms the upper fruiting part (Webster and Wertheim, 2003). Rootstocks influence the performance of the grafted scion and can affect traits like drought and disease resistance of the tree, and vigour, precocity and fruiting of the scion (Webster and Wertheim, 2003). Various compounds, such as minerals, proteins, ribonucleoproteins, ribonucleic acid (RNA) and small RNAs are transferred between the rootstock and scion, but the molecular mechanisms of how the rootstock influences scion phenotypes is not yet clarified in detail (Kumari et al., 2015).
The scion is the cultivar or the part of the tree that has name recognition, such as ‘Jonagold’. Once a commercial cultivar is obtained, they are always propagated asexually (Webster and Wertheim, 2003). Sexual propagation is not preferred as each seed is genetically unique with considerably different properties of the parental genotypes, which would result in a plant that is not “true-to-type”. Additionally, cultivars propagated from seeds generally bear fruits of poor size, appearance and quality. Although rootstocks can be propagated via seeds, the propagation of rootstocks is becoming increasingly asexual (Tromp, Webster and Wertheim, 2005). Rootstocks and scions are generally Malus species or interspecific Malus hybrids.
Breeding in apple is focused on the development of better rootstock and scion cultivars. The main accomplishment in apple rootstock breeding was the development of dwarfing apple rootstocks, which started in the early 1920s, by the East Malling Research Station in Kent, England (United Kingdom) (Tukey, 1964; Mudge et al., 2009). The M.9 rootstock, released by this research station, and its improved selections belong until now to the most used rootstocks worldwide. Other efforts in rootstock breeding resulted, for example, in the selection of dwarfing rootstocks tolerant to winter cold or in resistance to fire blight, collar rot and other diseases (Cummins and Aldwinckle, 1994). In apple scion breeding, obtaining a combination of high fruit quality (fruit size, inner fruit quality, fruit colour, flavour and aroma) with disease and pest resistance is the main objective. Furthermore, a range of other traits is considered important in the selection of new (scion) cultivars, like high and regular yielding, the percentage of marketable fruits (Peil et al., 2011), adaptation to climatic conditions and storability of fruits. Low allergenic or high flavonoid content, red fruit flesh or low chilling are minor objectives.
There are several characteristics of M. domestica that inhibit rapid genetic improvement of cultivars, most notably: a long juvenile period, self-incompatibility, the high level of heterozygosity resulting from the necessary cross-pollination and inbreeding depression (Brown and Maloney, 2003). The breeding programmes follow, in general, a common strategy, which establishes a series of back-crossings between hybrids exhibiting a trait of interest and susceptible commercial cultivars. Usually, the source of resistance genes comes from sexually compatible wild apple relatives. The traditional breeding method that is most commonly used is a modified backcross, where a different recurrent parent is used in each generation of backcrossing to circumvent self‑incompatibility. This process is laborious and time-consuming because it demands several generations to recover near-isogenic lines of high commercial value expressing the desired trait. Typically, the process takes more than 20 years. For example, it took several decades to successfully introgress a scab resistance trait from crab apple into a commercial cultivar (Gessler and Pertot, 2012).
Recent advances in genomic technologies allow for the acceleration of the development of cultivars of apple. Modern breeding programmes using molecular tools have been shown to allow for early screening (typically at the seedling stage) for certain inherited traits, like scab resistance (e.g. Bus et al., 2000; Patocchi et al., 2009; Baumgartner et al., 2015) without the need to phenotype the plants themselves. Two important international research programmes, RosBREED (www.rosbreed.org) in the United States and FruitBreedomics (www.fruitbreedomics.com) (Laurens et al., 2012) in Europe, have provided such molecular tools for screening and developed pre-breeding material. Although simple sequence repeats (SSR) are still used for marker-assisted selection of traits, the development of single nucleotide polymorphism (SNP) markers (Jänsch et al., 2015) and the establishment of an apple 480k SNP genotyping array (Bianco et al., 2016) can allow for genomic selection of traits, such as fruit quality traits as demonstrated by Kumar et al. (2012).
Recombinant DNA technology developments can also address some of the breeding bottlenecks in the development of elite cultivars and rootstocks of apple (for more information, see Annex 2.B.). Successful reduction of the juvenile period to one single year was achieved via constitutive expression of the BpMADS4 gene from silver birch in early flowering apple lines (Flachowsky et al., 2007, 2009, 2011, 2012). Further, it is a promising tool as it can be used for the direct introduction of specific genes (traits) into a particular cultivar while retaining all other desirable qualities. For example, scab‑resistant transgenic apple cultivars were obtained via Agrobacterium-mediated transfer and further attempts resulted in the first successful report of a cisgenic apple (Vanblaere et al., 2011).
Hybridisation and introgression
The natural facility of interspecific crossing (extent, sterility and fertility)
Most species in the genus Malus can be readily hybridised after artificial cross‑pollination (Luby, 2003; Hancock et al., 2008) but interspecific crossing can also occur under natural conditions. The latter is the case in forests in Flanders where 7% of the sampled Malus sylvestris trees were hybrid forms of M. sylvestris and M. domestica (Keulemans, Roldan-Ruiz and Lateur, 2007). The capacity for inter-species hybridisation within the genus Malus is evident by the numerous hybrids among Malus sp. (e.g. Korban, 1986; Schuster and Büttner, 1995; USDA-ARS, 2018). The majority of Malus sp. are diploid and inter-fertile, as there are no apparent physiological or genetic barriers (Korban, 1986; Vanwynsberghe, 2006). Triploid and tetraploid commercial apple cultivars exist but introgression of their genes into wild species seems unlikely since triploids are considered sterile and tetraploid introgression in diploid wild species will give sterile triploid progeny.
Outside of the genus Malus, the potential for natural hybridisation with other genera appears to be limited. While there has been extensive intergeneric hybridisation reported among closely related taxa (e.g. in the former subfamily Maloideae), a summary presented by Robertson et al. (1991) indicates no intergeneric crosses involving Malus sp. outside of breeding programmes. A reported Malus × Chaenomeles hybrid was subsequently discounted by Rudenko (1976, cited in Robertson et al., 1991) and a proposal that the species M. florentina (Zuccagni) C. K. Schneid. was the product of hybridisation between Malus and Sorbus sect. Torminaria (called × Malosorbus) was also subsequently challenged by several authors who considered it a relictual species of Malus (e.g. Huckins, 1972, cited in Robertson et al., 1991). This has been further supported by more recent taxonomic work (Qian et al., 2008).
Experimental crosses
Artificial interspecific hybrids are easily produced (Luby, 2003). M. domestica, which is thought to be of hybrid origin (Korban, 1986), can be readily hybridised with its congeners in the genus Malus (Korban, 1986; Vanwynsberghe, 2006; Kron and Husband, 2009). Interest in controlled hybridisation for the improvement of cultivated apples dates back to the 1700s and reports of successful experimental interspecific hybridisations began in the late 1800s (Korban, 1986). Since then, interspecific hybridisation has played a major role in genetic improvement and a large number of crosses have been made among Malus sp. in research and breeding programmes throughout the world, primarily to improve the cultivated apple or to develop new hybrid species with distinctive characteristics (Korban, 1986). A list of experimental interspecific hybrids that have been documented in the genus Malus is provided by Korban (1986) and includes about 60 different species combinations. Some new interspecific combinations with M. domestica have been added to this list (Vanwynsberghe, 2006).
Breeding programmes have produced intergeneric hybrids between apple and pear (Malus × Pyrus), and apple and hawthorn (Malus × Crataegus) as reported in Robertson et al. (1991); however, these relied on techniques such as embryo rescue (Banno et al., 2003). Forced intergeneric hybridisation of Cydonia (quince) with Malus resulted in fertile genotypes which have been identified as an artificial hybrid genus × Cydomalus. However, the seedlings produced are generally weak and of low viability and germinability (Bell and Leitão, 2011). F2 progeny from F1 genus × Cydomalus hybrids seem to originate from apomictic seeds (Information communicated to the authors by W. Keulemans). Hybridisation barriers between Malus and related species are complex and involve several processes: reduced pollen germination, although pollen adhesion on the stigma seems not affected, reduced pollen tube growth in the style and the ovary, and incongruity of pollen and egg cell. Almost no seeds are formed after an intergeneric cross and seeds are in most cases not viable (Vanwynsberghe, 2006).
Information and data on introgression
A number of studies show that there is a potential for gene introgression from M. domestica into native Malus species. Coart et al. (2003, 2006) evaluated hybridisation between M. domestica and the European wild crab apple M. sylvestris in Belgium using nuclear microsatellites and found that 11% of the sampled M. sylvestris trees were of hybrid origin. Larsen et al. (2006) on the other hand, did not find M. domestica x M. sylvestris hybrid individuals in natural M. sylvestris populations in Denmark despite the overlap in geographical distribution and flowering time of M. domestica and M. sylvestris. The fact that handmade interspecific crosses between these two species yield viable seeds which exhibited normal growth and development up to young seedlings suggests there is some other, still unknown, reproductive barrier operating to maintain genetically distinct populations (Larsen, Jensen and Kjær, 2008). A study conducted by Kron and Husband (2009) in southern Ontario examined populations of the introduced M. domestica and the native tetraploid crab apple M. coronaria and found that their geographic ranges and flowering times overlapped sufficiently for cross-pollination to occur. The study found that 27.7% of seeds from open-pollinated fruit was of hybrid origin. However, the ability of the resulting hybrid plants to survive and backcross with M. coronaria is unknown at this time, and the adult trees within the populations were all identified to be distinct species (either M. domestica or M. coronaria). Successful backcrosses seem unlikely since the hybrids are expected to be triploids, which are normally not fertile.
General interactions with other organisms and ecology
Interaction with natural and other ecosystems where the species is cultivated or managed
Interactions between cultivated apple Malus domestica and other organisms include those with agricultural diseases and pests, beneficial organisms, soil organisms, as well as with other Malus species. For the latter, please refer to the section “Natural facility of interspecific crossing”.
Pests and diseases
M. domestica is susceptible to a number of fungal and bacterial plant diseases (see Annex 2.C.). The most economically important disease of apples worldwide is apple scab caused by the fungal pathogen Venturia inaequalis (Cooke) Wint. Another significant disease reported in all major apple production regions with a widespread yearly occurrence and high pest pressure is fire blight caused by the bacterium Erwinia amylovora (Burrill) Winslow et al. Fire blight can, under the right conditions, wipe out entire orchards within a growing season (AAFC, 2013). Other significant fungal diseases reported as having localised yearly occurrence with high pest pressure or widespread sporadic occurrence with high pest pressure include, respectively, black rot (in Ontario), caused by the Botryosphaeria obtusa (Schwein.) Shoemaker and powdery mildew, caused by the Podosphaera leucotricha (Ellis & Everh.) E.S. Salmon (AAFC, 2013). Viruses and viroids can occur in apple orchards but many of them are symptomless in most commercial cultivars. The apple mosaic virus (ApMV) is one of the most widespread apple viruses (Reddy, 2010).
A variety of pests can threaten apple trees and fruits (Carlson, 2008; Sherwani, Mukhtar and Wani, 2016). One of the most common pests of apples is the codling moth (Cydia pomonella). This insect eats holes and burrows the core of the apple fruit. Different scale insects, including the San Jose scale (Quadraspidiotus perniciosus) and many bugs, like the brown marmorated stink bug (Halyomorpha halys), living on apple, impact fruit quality. A group of insects that damages fruit trees are mites. The European red mite (Panonynchus ulmi), the twospotted spider mite (Panonynchus urticae) and the apple rust mite (Aculus schlechtendali) are major mite pests in apple orchards. Leaf rollers can damage both fruits and leaves, while the woolly apple aphid (Eriosoma lanigerum) affects leaves, bark and rootstocks. An overview of pests is presented in Annex 2.D.
Some vertebrates can also be considered pests to apple growers. Birds, such as pigeons and crows, can peck holes in the fruit or wood (Tromp, Webster and Wertheim, 2005; AAFC, 2013).
Beneficial insects
Pollinators
The interaction between honey bees, bumble bees and apple trees are mutually beneficial: the bees aid in fertilisation (see section on flower development) and receive nectar and pollen in return.
Biological control organisms
In managed systems, one can exploit the mutual interactions between organisms living on apple and the apple host. In some specific cases, it is possible to manage and control pests and diseases with biological control organisms (BCOs). The application of BCOs in cultivated apple has been investigated in several parts of the growth cycle, from flowering (Pusey, Stockwell and Mazzola, 2009) to post-harvest storage (Jamalizadeh et al., 2011). The use of arthropods against pests which damage the leaves and fruits of the apple plants, such as aphids and mites, is common (Asante, 1997; Nicholas, Spooner-Hart and Vickers, 2005; Brown and Mathews, 2007; Zhou et al., 2014; Walker, Suckling and Wearing, 2017). Even birds can be deployed as BCOs for the predation of caterpillars (Mols and Visser, 2002) but this measure is rarely applied in commercial orchards. This is also the case for bacteria that have been used as BCOs to suppress pathogens like blue mould (Etebarian et al., 2005) and grey mould (Jamalizadeh et al., 2008), or for the fungus Trichoderma to control Phytophthora in apple seedlings (Roiger and Jeffers, 1991).
Alternative strategies are under investigation to use BCOs as antagonists against E. amylovora. One approach for instance is the use of bumblebees as a vector to bring the BCOs to the flowers during bloom (entomovectoring) (Remy et al., 2016, 2017).
Animals
Various mammals, including rodents, rabbits, hares and deer feed on tree tissues, including girdling of bark and feeding on roots, young branches, leaves and buds (AAFC, 2013). Various birds, such as crows and woodpeckers, and some mammals, including bears, feed on fruits.
Apple seeds contain small amounts of amygdalin, a cyanogenic glycoside, which is a naturally occurring plant pre-toxin considered to play a role in plant defence against herbivores due to bitter taste and release of toxic hydrogen cyanide upon tissue disruption (Dar et al., 2016). The presence of small amounts of toxicants and other metabolites (organic acids, phenolic compounds) in apple and products derived from apple and the effect this may have on humans are addressed in the OECD Consensus Document on Compositional Considerations for New Cultivars of Apple (OECD, 2019a).
Soil micro-organisms
Soil micro-organisms interacting with apple are poorly studied. In most cases, rhizosphere micro-organisms are studied in relation to replant diseases in commercial apple plantations (e.g. Čatská et al., 1982; Jiang et al., 2017) or specific treatments on the crop like triazole fungicides (Sułowicz and Piotrowska-Seget, 2016). In some cases, rhizosphere micro-organisms can be used for biological control of soil-borne diseases in apple (Sindhu, Rakshiya and Sahu, 2009) or to improve growth and nutrient uptake (Rengel and Marschner, 2005; Rumberger, Merwin and Thies, 2007).
Annex 2.A. Malus species
Annex Table 2.A.1. Species and hybrid species in the genus Malus
|
Scientific name |
Common name (English) |
|
|---|---|---|
|
1 |
M. × adstringens Zabel |
|
|
2 |
M. angustifolia (Aiton) Michx |
Southern crab apple |
|
3 |
M. × arnoldiana (Rehder) Sarg. ex Rehder |
|
|
4 |
M. × asiatica Nakai |
|
|
5 |
M. × astracanica (hort. ex Dum) Cours |
|
|
6 |
M. × atrosanguinea (hort. ex Späth) C. K. Schneid. |
|
|
7 |
M. baccata (L.) Borkh. |
Siberian crab apple |
|
8 |
M. baoshanensis G. T. Deng |
|
|
9 |
M. brevipes (Rehder) Rehder |
|
|
10 |
M. chitralensis Vassilcz. |
|
|
11 |
M. coronaria (L.) Mill. |
Sweet crab apple |
|
12 |
M. crescimannoi Raimondo |
|
|
13 |
M. × dawsoniana Rehder |
|
|
14 |
M. domestica Borkh. |
Apple |
|
15 |
M. doumeri (Bois) A. Chev. |
|
|
16 |
M. florentina (Zuccagni) C. K. Schneid. |
Hawthorn-leaf crab apple |
|
17 |
M. floribunda Sieb. ex Van Houtte |
Japanese crab apple |
|
18 |
M. fusca (Raf.) C. K. Schneid. |
Oregon crab apple |
|
19 |
M. × gloriosa Lemoine |
|
|
20 |
M. halliana Koehne |
Hall crab apple |
|
21 |
M. × hartwigii Koehne |
|
|
22 |
M. honanensis Rehder |
|
|
23 |
M. hupehensis (Pamp.) Rehder |
Chinese crab apple, Hupeh crab |
|
24 |
M. ioensis (Alph. Wood) Britton |
Iowa crab apple, prairie crab apple |
|
25 |
M. kansuensis (Batalin) C. K. Schneid. |
|
|
26 |
M. komarovii (Sarg.) Rehder |
|
|
27 |
M. leiocalyca S. Z. Huang |
|
|
28 |
M. × magdeburgensis Hartwig |
|
|
29 |
M. mandshurica (Maxim.) Kom. ex Skvortsov |
Manchurian crab apple |
|
30 |
M. × micromalus Makino |
Kaido crab apple |
|
31 |
M. × moerlandsii Door. |
|
|
32 |
M. muliensis T. C. Ku |
|
|
33 |
M. ombrophila Hand.-Mazz. |
|
|
34 |
M. orientalis Uglitzk. |
|
|
35 |
M. orthocarpa Lavallee ex anon. |
|
|
36 |
M. × platycarpa Rehder |
|
|
37 |
M. prattii (Hemsl.) C. K. Schneid. |
|
|
38 |
M. prunifolia (Willd.) Borkh. |
Chinese crab apple, plum-leaf crab apple |
|
39 |
M. pumila Mill. |
Paradise apple |
|
40 |
M. × purpurea (A. Barbier) Rehder |
|
|
41 |
M. × robusta (Carrière) Rehder |
Siberian crab apple |
|
42 |
M. sargentii Rehder |
Sargent’s crab apple |
|
43 |
M. scheiderckeri (L. H. Bailey) Späth ex Zabel |
|
|
44 |
M. sieversii (Ledeb.) M. Roem. |
|
|
45 |
M. sikkimensis (Wenz.) Koehne ex C. K. Schneid. |
|
|
46 |
M. × soulardii (L. H. Bailey) Britton |
Soulard crab apple |
|
47 |
M. spectabilis (Aiton) Borkh. |
Asiatic apple, Chinese crab apple |
|
48 |
M. spontanea (Makino) Makino |
|
|
49 |
M. × sublobata (Dippel) Rehder |
|
|
50 |
M. sylvestris (L.)Mill. |
European crab apple |
|
51 |
M. toringo (Siebold) de Vriese |
Toringo crab apple |
|
52 |
M. toringoides (Rehder) Hughes |
|
|
53 |
M. transitoria (Batalin) C. K. Schneid. |
|
|
54 |
M. trilobata (Poir.) C. K. Schneid |
|
|
55 |
M. tschonoskii (Maxim.) C. K. Schneid. |
|
|
56 |
M. × xiaojinensis M. H. Cheng & N. G. Jiang |
|
|
57 |
M. yunnanensis (Franch.) C. K. Schneid. |
Yunnan crab apple |
|
58 |
M. zhaojiaoensis N. G. Jiang |
|
|
59 |
M. zumi (Matsum.) Rehder |
Source: USDA-ARS (2018), Germplasm Resources Information Network (GRIN), https://www.ars-grin.gov (accessed 1 October 2019).
Annex 2.B. Biotechnological developments
Apple has become a model species for Rosaceae genetic and genomic research. James et al. published in 1989 the first Agrobacterium tumefaciens-mediated leaf disc transformation in apple. In the subsequent years, the main objective in several laboratories around the world was to improve the methodology and to create a “clean vector technology” for marker-free transgenic apples. Due to the lack of availability of “apple own” genes giving a commercially interesting advantage (e.g. disease resistance), the first achievements in genetic engineering of apple (from the early 1990s on) mainly relied on genes from other species and have been reviewed by Gessler and Patocchi (2007), Hanke and Flachowsky (2010) and Rai and Shekhawat (2014). The main target traits in apple transformation are disease and pest resistance, in particular fungal resistance to scab and powdery mildew and resistance to the bacterial fire blight disease. Other traits in apple on which research has been conducted include: i) stress tolerance; ii) herbicide resistance; iii) self-incompatibility; iv) fruit ripening and other fruit characteristics; v) allergens; vi) precocity and flower induction; and vii) dwarfing and rooting ability in rootstock genotypes.
To date, only a few apple genes of potential interest have been mapped. Four disease resistance genes of apple have become available for transformation: two genes (Rvi6, Belfanti et al., 2004; Rvi15, Schouten et al., 2014) providing resistance to apple scab, one gene (Fb-Mr5, Broggini et al., 2014) leading to fire blight resistance and one gene (Pl2, Rikkerink et al., 2016) mediating resistance to powdery mildew. Other candidate resistance genes have been identified but map-based cloning of these genes is still in progress (Gessler and Pertot, 2012; Broggini et al., 2014; Schouten et al., 2014). Although most efforts have been done on the cloning of R genes, a few genes encoding for other traits in apple have also been identified, like the Ma genes controlling the content of malic acid in apple fruits and the Co gene leading to a columnar like growth habit of the tree (Xu et al., 2012; Okada et al., 2016). New research initiatives, such as TranscrApple, may increase the number of genes that become available for transformation.
Transgenic apples on the market and transgenic apples for which biosafety research is ongoing are summarised below:
Disease resistance: Scab-resistant transgenic apple cultivars were obtained via Agrobacterium-mediated transfer of scab resistance genes Rvi6 from M. floribunda, a species of crab apple that shows natural resistance to some strains of the apple scab fungus, to cultivar ‘Gala’ (Barbieri et al., 2003; Belfanti et al., 2004; Silfverberg-Dilworth et al., 2005; Malnoy et al., 2008). Further research resulted in the first intragenic and cisgenic apple lines of the cultivar ‘Gala’ with scab resistance (Joshi et al., 2011; Vanblaere et al., 2011, 2014; Krens et al., 2015), containing the Rvi6 gene from M. floribunda under the control of the promoter from the apple Rubisco gene or the native Rvi6-promoter respectively. These plants were planted in a field trial to study scab resistance in an orchard situation (Krens et al., 2015). In 2015, the development of the first cisgenic apple with increased resistance to fire blight was reported (Kost et al., 2015). A cisgenic apple line C44.4.146 of the susceptible apple cultivar ‘Gala Galaxy’ was regenerated using the FB_MR5 gene from the wild apple M. robusta.
Breeding cycle acceleration: Fruit trees typically have long breeding cycles. Successful reduction of the juvenile period of apple to one single year was achieved via constitutive expression of the MADS4 transcription factor gene from silver birch (Betula pendula Roth.) in early flowering apple cultivars ‘Pinova’ (Flachowsky et al., 2007, 2009, 2011, 2012), ‘Gala’, ‘Mitchgl Gala’ and ‘Santana’ (Weigl et al., 2015). The BpMADS4-based breeding technology allows for more rapid introgression of agronomically relevant traits (e.g. disease resistances) from wild apples into domestic apple cultivars (e.g. Schlathölter et al., 2018).
Fruit quality: Apples have been engineered using a gene-silencing technique called ribonucleic acid interference (RNAi), in order to reduce the production of polyphenol oxidases (PPO), which causes the apple’s flesh to brown when sliced or bitten (Xu, 2013). Transgenic versions of the varieties ‘Golden Delicious’, ‘Granny Smith’ and ‘Fuji’ have been approved for marketing (OECD, 2019b).
Annex 2.C. Apple diseases
The following lists include the most relevant diseases in terms of economic losses.
Annex Table 2.C.1. Bacteria (including phytoplasma)
|
Scientific name |
Common name (English) |
|---|---|
|
Agrobacterium rhizogenes |
Hairy root |
|
Agrobacterium tumefaciens |
Crown gall |
|
Erwinia amylovora |
Fire blight |
|
Phytoplasma |
Apple chat fruit |
|
Pseudomonas syringae |
Blister spot |
|
Apple-proliferation phytoplasma |
Annex Table 2.C.2. Fungi
|
Scientific name |
Common name (English) |
Occurrence |
|---|---|---|
|
Alternaria alternata |
Alternaria rot |
W |
|
Alternaria mali |
Alternaria blotch |
A, AF, NA |
|
Armillaria mellea |
Root rot |
W |
|
Athelia rolfsii |
Southern blight |
AF, NA, SA |
|
Biscogniauxia marginata |
Blister canker |
NA |
|
Botryosphaeria berengeriana |
Apple ring rot and canker |
A |
|
Botryosphaeria dothidea |
Apple ring rot |
AF, NA, SA, O |
|
Botryosphaeria stevensii |
Black rot, frog eye leaf spot, canker |
AF, E, NA, SA, O |
|
Botrytis cinerea |
Grey mould rot |
W |
|
Butlerelfia eustacei |
Fish-eye rot |
A, E, NA |
|
Cadophora malorum |
Side rot |
A, NA |
|
Cladosporium spp. |
Mouldy core, core rot |
W |
|
Colletotrichum spp. |
Bitter rot |
E |
|
Corticium stevensii |
Thread blight |
NA |
|
Cytospora ceratosperma |
A |
|
|
Diplocarpon mali |
Marssonina blotch |
A, E, NA |
|
Epicoccum spp. |
Mouldy core, core rot |
W |
|
Fusarium spp. |
E |
|
|
Geastrumia polystigmatis |
Sooty-blotch complex |
W |
|
Gloeopeniophorella sacrata |
Peniophora root canker |
O |
|
Grovesinia moricola |
A |
|
|
Gymnosporangium clavipes |
Quince rust |
NA |
|
Gymnosporangium globosum |
American hawthorn rust |
NA |
|
Gymnosporangium juniperi-virginianae |
Cedar apple rust |
NA |
|
Gymnosporangium libocedri |
Pacific Coast pear rust |
NA |
|
Gymnosporangium yamadae |
Japanese apple rust |
A |
|
Helicobasidium longisporum |
O |
|
|
Helminthosporium papulosum |
Black pox |
NA |
|
Lepteutypa cupressi |
Monochaetia twig canker |
NA |
|
Leptodontidium trabinellum |
Sooty-blotch complex |
|
|
Leucostoma cinctum |
Leucostoma canker, dieback |
E, NA |
|
Monilinia fructigena |
European brown rot |
A, AF, E |
|
Monilinia laxa |
European brown rot |
A, AF, E |
|
Monilinia mali |
Monilinia leaf blight |
A |
|
Mucor spp. |
Mucor rot |
|
|
Mycosphaerella pomi |
Brooks fruit spot |
NA |
|
Nectria cinnabarina |
Nectria twig blight |
E, NA, O |
|
Nectria ditissima |
Nectria canker |
A, AF, NA, SA, O |
|
Neofabraea malicorticis |
Anthracnose canker, bull’s eye rot |
E, NA, O |
|
Paraconiothyrium fuckelii |
Blossom-end rot, Leptosphaeria canker, fruit rot |
W |
|
Peltaster fructicola |
Sooty-blotch complex |
W |
|
Penicillium spp. |
Blue mould |
|
|
Peyronellaea obtuse |
Black rot, frog eye leaf spot, canker |
AF, E, NA, SA, O |
|
Phacidiopycnis malorum |
E |
|
|
Phomopsis prunorum |
Phomopsis canker, fruit decay, rough bark |
A, E, NA |
|
Phyllosticta solitaria (Blotch) |
NA |
|
|
Podosphaera leucotricha |
Powdery mildew |
W |
|
Rosellinia necatrix |
Rosellinia root rot |
W |
|
Schizothyrium pomi |
Fly-speck |
W |
|
Scytinostroma galactinum |
White rot |
NA |
|
Sphaeropsis spp. |
E |
|
|
Stemphilium spp. |
E |
|
|
Trichothecium roseum |
Pink mould rot |
W |
|
Venturia inaequalis |
Apple scab |
W |
|
Xylaria mali |
Black root rot |
NA |
|
Xylaria polymorpha |
Black root rot |
W |
Note: A: Asia, AF: Africa, E: Europe, NA: North America, O: Oceania, SA: South America, W: worldwide.
Annex Table 2.C.3. Protista; Oomycota
|
Scientific name |
Common name (English) |
|---|---|
|
Phytophthora cactorum |
Phytophthora crown, collar, root and fruit rot |
|
Phytophthora syringae |
Phytophthora crown, collar, root and fruit rot |
Annex Table 2.C.4. Viruses and viroids
|
Common name (English) |
Acronym |
|---|---|
|
Apple chlorotic leaf-spot virus |
ACLSV |
|
Apple stem-grooving virus |
ASGV |
|
Apple mosaic virus |
APMV |
|
Tulare apple mosaic ilarvirus |
TAMV |
|
Apple stem-pitting foveavirus |
ASPV |
|
Tomato ringspot nepovirus |
TomRSV |
|
Apple fruit-crinkle viroid |
AFCVd |
|
Apple dimple-fruit viroid |
ADFVd |
|
Apple scar-skin viroid |
ASSVd |
Source (annex): Ogawa, J.M. and H. English (1991), Diseases of Temperate Zone Tree Fruit and Nut Crops, University of California, Division of Agriculture and Natural Resources, Publication 3345; Grove, G. (2003), “Diseases of apple”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CAB International, Wallingford, UK, pp. 459-488; Hadidi, A. et al. (2003), Viroids, CSIRO Publishing, Collingwood, Australia; Betere Bomen (2019), Malus; VIDE (2018), Virus Identification Database Exchange - Known susceptibilities of Rosaceae, Malus.
Annex 2.D. Apple pests
Annex Table 2.D.1. Arthropoda (ranked by order)
|
Scientific name |
Common name (English) |
|---|---|
|
Coleoptera (beetles, weevils) |
|
|
Anthonomus piri |
Apple bud weevil |
|
Anthonomus pomorum |
Apple blossom weevil |
|
Curculionidae nenuphar |
|
|
Polydrusus |
|
|
Sitona lineatus |
Pea leaf weevil |
|
Xyleborus dispar |
Pear blight beetle |
|
Diptera (flies) |
|
|
Anastrepha fraterculus |
South American fruit fly |
|
Ceratitis capitata |
Mediterranean fruit fly |
|
Dasineura mali |
Apple leaf curling midge |
|
Rhagoletis pomonella |
Apple maggot |
|
Tephritidae |
Tephritid fruit flies |
|
Hemiptera |
|
|
Anuraphis farfarae |
|
|
Aphis pomi |
Apple aphid |
|
Campylomma verbasci |
Mullein plant bug |
|
Dysaphis plantaginea |
Rosy apple aphid |
|
Eriosoma lanigerum |
Woolly apple aphid |
|
Edwardsiana crataegi |
|
|
Halyomorpha halys |
The brown marmorated stink bug |
|
Lepidosaphes ulmi |
Apple mussel scale |
|
Lygocoris pabulinus |
Common green capsid |
|
Lygus lineolaris |
Tarnished plant bug |
|
Parthenolecanium corni |
European fruit lecanium |
|
Psylla mali |
Apple sucker |
|
Quadraspidiotus ostreaeformis |
European fruit scale |
|
Rhopalosiphum insertum |
Apple-grass aphid |
|
Hymenoptera (sawflies, wasps ants, bees) |
|
|
Ametastegia glabrata |
Dock sawfly |
|
Hoplocampa testudinea |
Apple sawfly |
|
Isopoda (woodlouse) |
|
|
Oniscidea spp. |
|
|
Lepidoptera (moths) |
|
|
Adoxophyes orana |
Summer fruit tortrix |
|
Adoxophyes reticulana |
|
|
Amphipyra pyrimadoides |
Humped green fruitworm |
|
Archips rosana |
Rose tortrix |
|
Archips podana |
Large fruit-tree tortrix |
|
Archips breviplicanus |
Asiatic leafroller |
|
Archips argyrospila |
Fruittree leafroller |
|
Clepsis spectrana |
Cabbage leafroller |
|
Coccus cossus |
Goat moth |
|
Coleophora hemerobiella |
Fruit tree case moth |
|
Cydia pomonella |
Codling moth |
|
Epiphyas postvittana |
Light brown apple moth |
|
Grapholita molesta |
Oriental fruit moth |
|
Lithophane antennata |
Widestriped green fruitworm |
|
Lacanobia subjuncta |
Lacanobia fruitworm |
|
Malacosoma Neustria |
Lackey moth |
|
Operophtera brumata |
Winter moth |
|
Orthosia incerta |
|
|
Orthosia spp. |
|
|
Phlogophora meticulosa |
Angle shades |
|
Phyllonorycter blancardella |
Spotted tentiform leafminer |
|
Phyllonorycter crataegella |
Apple blotch leafminer |
|
Phyllonorycter elmaella |
Western tentiform leafminer |
|
Spilonota ocellana |
Bud moth |
|
Stigmella malella |
Banded apple pigmy |
|
Stigmella incognitella |
Grey apple pigmy |
|
Synanthedon myopaeformis |
Red-belted clearwing |
|
Yponomeuta malinellus |
Apple ermine |
|
Zeuzera pyrina |
Leopard moth |
|
Thysanoptera (thrips) |
|
|
Thripidae spp. |
Thrips |
|
Trombidiformes (mites) |
|
|
Aculus schlechtendali |
Apple rust mite |
|
Eriophyes pyri |
Pearleaf blister mite |
|
Epitrimerus pyri |
Pear rust mite |
|
Panonychus ulmi |
European red mite |
|
Phyllocoptes malinus |
|
|
Tetranychus mcdanieli |
McDaniel spider mite |
|
Tetranychus urticae |
Twospotted spider mite |
|
Tetranychus viennensis |
Fruit tree spider mite |
Annex Table 2.D.2. Nematodes
|
Scientific name |
Common name (English) |
|---|---|
|
Meloidogyne arenaria |
|
|
Meloidogyne hapla |
|
|
Meloidogyne incognita |
|
|
Meloidogyne javanica |
|
|
Pratylenchus penetrans |
|
|
Pratylenchus vulnus |
|
|
Xiphinema americanum |
American dagger nematode |
|
Xiphinema rivesi |
Sources (annex): Grove, G. (2003), “Diseases of apple”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CAB International, Wallingford, UK, pp. 459-488; Betere Bomen (2019), Malus; UCANR (2018), How to Manage Pests: Apple, University of California Agriculture and Natural Resources, http://ipm.ucanr.edu/PMG/selectnewpest.apples.html (accessed 1 October 2019); Fruit pluktuin (2019), Schadelijke insecten, http://www.fruitpluktuin.nl/fruit/Insecten/schadelijke-insecten# (accessed 1 October 2019); Groenkennisnet (2019), Appel, https://wiki.groenkennisnet.nl/display/BEEL/Appel (accessed 1 October 2019).
References
AAFC (2013), Crop Profile for Apple in Canada, 2013, Pesticide Risk Reduction Program, Pest Management Centre, Agriculture and Agri-Food Canada, Ottawa.
Abrol, D.P. (2012), Pollination Biology–Biodiversity Conservation and Agricultural Production, Springer, New York, United States.
Adachi, Y. et al. (2009), “Characteristics of fruiting and pollen tube growth of apple autotetraploid cultivars showing self-compatibility”, Journal of the Japanese Society for Horticultural Science, Vol. 78/4, pp. 402–409.
AHDB (2017), Apple Best Practice Guide, Agriculture and Horticulture Development Board, UK, https://apples.ahdb.org.uk/agronomy-pollination-and-fruit-set.asp (accessed 1 October 2019).
Asante, S.K. (1997), “Natural enemies of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae): A review of the world literature”, Plant Protection Quarterly, Vol. 12/4, pp. 166‑172.
Bai, Y., L. Dougherty and K. Xu (2014), “Towards an improved apple reference transcriptome using RNA-seq”, Molecular Genetics and Genomics, Vol. 289, pp. 427-438.
Bailey, L.G. and E.Z. Bailey (1976), Hortus Third: A Concise Dictionary of Plants Cultivated in the United States and Canada, McMillan Publishing Co., New York, US.
Banno, K. et al. (2003), “Breeding and characteristics of symmetric intergeneric hybrids between apple and pear”, Acta Horticulturae, Vol. 622, pp. 265-276.
Barbieri, M. et al. (2003), “Progress of map-based cloning of the Vf-resistance gene and functional verification: Preliminary results from expression studies in transformed apple”, Horticultural Science, Vol. 38, pp. 329–331.
Baumgartner, I.O. et al. (2015), “Breeding elite lines of apple carrying pyramided homozygous resistance genes against apple scab and resistance against powdery mildew and fire blight”, Plant Molecular Biology Reporter, Vol. 33, pp. 1573-1583.
Belfanti, E. et al. (2004), “The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety”, Proceedings of the National Academic of Sciences of the USA, Vol. 101, pp. 886‑890.
Bell, R.L. and J.M. Leitão (2011), “Cydonia”, in C. Kole (ed.), Wild Crop Relatives: Genomic and Breeding Resources, Springer-Verlag, Berlin Heidelberg, pp. 1-16.
Betere Bomen (2019), Malus.
Bianco, L. et al. (2016), “Development and validation of the Axiom®Apple480K SNP genotyping array”, The Plant Journal, Vol. 86, pp. 62-74.
Broggini, G.A.L. et al. (2014), “Engineering fire blight resistance into the apple cultivar ‘Gala’ using the FB_MR5 CC‑NBS-LRR resistance gene of Malus x robusta 5”, Plant Biotechnology Journal, Vol. 12/6, p. 728-733.
Brown, M.W. and C.R. Mathews (2007), “Conservation biological control of rosy apple aphid, Dysaphis plantaginea (Passerini), in eastern North America”, Environmental entomology, Vol. 36/5, pp. 1131‑1139.
Brown, S.K. and K.E. Maloney (2003), “Genetic improvement of apple: Breeding, markers, mapping and biotechnology”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CAB International, Wallingford, UK, pp. 31-60.
Bus, V.G.M. et al. (2000), “Marker assisted selection for pest and disease resistance in the New Zealand apple breeding programme”, Acta Horticulturae, Vol. 538, pp. 541-547.
Carlson, E. (2008), Major Insect Pests of Apple in Georgia, https://wiki.bugwood.org/Major_insect_pests_of_apple/Georgia (accessed 1 October 2019).
Čatská, V. et al. (1982), “Rhizosphere micro-organisms in relation to the apple replant problem”, Plant and Soil, Vol. 69, pp. 187-197.
Chevreau, E. and F. Laurens (1987), “The pattern of inheritance in apple: Further results from leaf isozyme analysis”, Theoretical and Applied Genetics, Vol. 75, pp. 90-95.
Coart, E. et al. (2006), “Chloroplast diversity in the genus Malus: New insights into the relationship between the European wild apple (Malus sylvestris (L.) Mill.) and the domesticated apple (Malus domestica Borkh.)”, Molecular Ecology, Vol. 15, pp. 2171-2182.
Coart, E. et al. (2003), “Genetic variation in the endangered wild apple (Malus sylvestris (L.) Mill.) in Belgium as revealed by amplified fragment length polymorphism and microsatellite markers”, Molecular Ecology, Vol. 12, pp. 845–857.
Cornille, A. et al. (2014), “The domestication and evolutionary ecology of apples”, Trends in Genetics, Vol. 30, p. 57‑65.
Cornille, A. et al. (2012), “New insight into the history of domesticated apple: Secondary contribution of the European wild apple to the genome of cultivated varieties”, Public Library of Science Genetics, Vol. 8/.5 e1002703.
Cummins, J. and H. Aldwinckle (1994), “New resistant rootstocks from Geneva”, Fruit Varieties Journal (USA), Vol. 48, p. 39.
Daccord, N. et al. (2017), “High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development”, Nature Genetics, Vol. 49/7, pp. 1099-1108.
Dar, S.A. et al. (2016), Plant-Insect Interactions – Cyanogenic Glucosides, Imperial Journal of Interdisciplinary Research, Vol. 2, pp. 1107-1118.
De Witte, K. et al. (1996), “Fruit set, seed set and fruit weight in apple as influenced by emasculation and cross-pollination”, Acta Horticulturae, Vol. 423(December), pp. 177-184.
Degrandi-Hoffman, G., R. Hoopingarner and K. Klomparens (1986), “Influence of honey bee (Hymenoptera: Apidae) in-hive pollen transfer on cross-pollination and fruit set in apple”, Environmental Entomology, Vol. 15/3, pp. 723‑725.
Delaplane, K.S. and D.F. Mayer (2000), “Crop pollination by bees”, Zoosystematics and Evolution, Vol. 78/1, pp. 192-192.
Dennis, F. (2003), “Flowering, pollination and fruit set, and development”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CAB International, Wallingford, UK, pp. 153-166.
Di Pierro, E.A. et al. (2016), “A high-density, multi-parental SNP genetic map on apple validates a new mapping approach for outcrossing species”, Horticulture Research, Vol. 3, p. 16057.
Duan, N. et al. (2017), “Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement”, Nature Communications, Vol. 8, p. 249.
Dzhangaliev, A.D. (2003), “The wild apple tree of Kazakhstan”, Horticultural Reviews, Vol. 29, pp. 63‑303.
Etebarian, H.R. et al. (2005), “Biological control of apple blue mold with Pseudomonas fluorescens”, Canadian Journal of Microbiology, Vol. 51/7, pp. 591-598.
FAO (2022), FAOSTAT, Food and Agriculture Organization of the United Nations Statistics Division, www.fao.org/corp/statistics/en (accessed 16 February 2022).
Ferrer, M.M. et al. (2009), “Effect of variation in self-incompatibility on pollen limitation and inbreeding depression in Flourensia cernua (Asteraceae) scrubs of contrasting density”, Annals of Botany, Vol. 103/7, pp. 1077-1089.
Fiala, L. (1994), Flowering Crabapples: The Genus Malus, Timber Press Inc., Portland, US.
Flachowsky, H. et al. (2012), “Genetic control of flower development in apple and the utilisation of transgenic early flowering apple plants in breeding”, Acta Horticulturae, Vol. 967, pp. 29‑34.
Flachowsky, H. et al. (2011), “Application of a high-speed breeding technology to apple (Malus x domestica) based on transgenic early flowering plants and marker-assisted selection”, New Phytologist, Vol. 192, pp. 364-377.
Flachowsky, H. et al. (2009), “A review on transgenic approaches to accelerate breeding of woody plants”, Plant Breeding, Vol. 128, pp. 217-226.
Flachowsky, H. et al. (2007), “Overexpression of the BpMADS4 gene from silver birch (Betula pendula Roth.) induces early-flowering in apple (Malus x domestica Borkh.)”, Plant Breeding, Vol. 126, pp. 137-145.
Free, J.B. (1966), “The foraging areas of honeybees in an orchard of standard apple trees”, The Journal of Applied Ecology, Vol. 3/2, pp. 261.
Free, J.B. and Y. Spencer-Booth (1964), “The foraging behaviour of honey bees in an orchard of dwarf apple trees”, Journal of Horticultural Science, Vol. 39/2, pp. 78–83.
Fruit pluktuin (2019), Schadelijke insecten, http://www.fruitpluktuin.nl/fruit/Insecten/schadelijke-insecten# (accessed 1 October 2019).
Galetti, M. (2002), “Seed dispersal of mimetic fruits: Parasitism, mutualism, aposematism or exaptation?”, in Seed Dispersal and Frugivory: Ecology, Evolution and Conservation, Third International Symposium-Workshop on Frugivores and Seed Dispersal, São Pedro, Brazil, pp. 6-11.
Gardiner, S.E. et al. (1996), “A detailed linkage map around an apple scab resistance gene demonstrates that two disease resistance classes both carry the Vf gene”, Theoretical and Applied Genetics, Vol. 93, pp. 485-493.
Garibaldi, L.A. et al. (2013), “Wild pollinators enhance fruit set of crops regardless of honey bee abundance”, Science, Vol. 339/March, pp. 1608-1611.
Gessler, C. and A. Patocchi (2007), “Recombinant DNA technology in apple”, Advances in Biochemical Engineering/Biotechnology, Vol. 107, pp. 113-132.
Gessler, C. and I. Pertot (2012), “Vf scab resistance of Malus”, Trees, Vol. 26, pp. 95-108.
Gharghani, A. et al. (2009), “Genetic identity and relationships of Iranian apple (Malus × domestica Borkh.) cultivars and landraces, wild Malus species and representative old apple cultivars based on simple sequence repeat (SSR) marker analysis”, Genetic Resources Crop Evolution, Vol. 56, pp. 829‑842.
Goremykin, V.V. et al. (2012), “The mitochondrial genome of Malus domestica and the import-driven hypothesis of mitochondrial genome expansion in seed plants”, The Plant Journal, Vol. 71/4, pp. 615‑626.
Groenkennisnet (2019), Appel, https://wiki.groenkennisnet.nl/display/BEEL/Appel (accessed 1 October 2019).
Gross, B.L. et al. (2012), “Identification of interspecific hybrids among domesticated apple and its wild relatives”, Tree Genetics and Genomes, Vol. 8, pp. 1223-1235.
Grove, G. (2003), “Diseases of apple”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CAB International, Wallingford, UK, pp. 459-488.
Hadidi, A. et al. (2003), Viroids, CSIRO Publishing, Collingwood, Australia.
Hampson, C.R. and H. Kemp (2003), “Characteristics of important commercial apple cultivars”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CABI International, Wallingford, UK, pp. 61-90.
Hancock, J.F. et al. (2008), “Apples”, in J.F. Hancock (ed.), Temperate Fruit Crop Breeding: Germplasm to Genomics, Springer Media B.V., New York, US.
Hanke, M.V. and H. Flachowsky (2010), “Fruit Crops”, in F. Kempken and C. Jung (eds.), Genetic Modification of Plants, Biotechnology in Agriculture and Forestry, Vol. 64, Springer, Berlin Heidelberg, Germany.
Harris, S.A., J.P. Robinson and B.E. Juniper (2002), “Genetic clues to the origin of the apple”, Trends in Genetics, Vol. 18, pp. 426-430.
Hemmat, M. et al. (1994), “Molecular marker linkage map for apple”, Journal of Heredity, Vol. 85/1, pp. 4‑11.
Herrera, M.C. (2002), “Seed dispersal by vertebrates”, in C.M. Herrera and O. Pellmyr (eds.) Plant-Animal Interactions: An Evolutionary Approach, Blackwell Science, Oxford, UK, pp. 328.
Höfer, M. and A. Meister (2010), “Genome size variation in Malus species”, Journal of Botany, Vol. 2010, Article ID 480873, http://dx.doi.org/10.1155/2010/480873 (accessed 1 October 2019).
Iowa State University (2003), Reproductive Terms,
IUCN (2019), The IUCN Red List of Threatened Species, http://www.iucnredlist.org (accessed 1 October 2019).
Jackson, J.E. (2003), Biology of Apple and Pears, Cambridge University Press, Cambridge, UK.
Jamalizadeh, M. et al. (2011), “A review of mechanisms of action of biological control organisms against post‐harvest fruit spoilage”, EPPO Bulletin, Vol. 41/1, pp. 65-71.
Jamalizadeh, M. et al. (2008), “Biological control of gray mold on apple fruits by Bacillus licheniformis (EN74-1)”, Phytoparasitica, Vol. 36/1, pp. 23.
James, D.J. et al. (1989), “Genetic transformation of apple (Malus pumila Mill.) using a disarmed Ti‑binary vector”, Plant Cell Report, Vol. 7, pp. 658-661.
Janick, J. and J.N. Moore (1975), Advances in Fruit Breeding, Purdue University Press, Indiana, US.
Janick, J. et al. (1996), “Apples”, in J. Janick and J. Moore (eds.), Fruit Breeding Vol. II, Tree and Tropical Fruits, Wiley, New York, US.
Jänsch, M. et al. (2015), “Identification of SNPs linked to eight apple disease resistance loci”, Molecular Breeding, Vol. 35, pp. 1-21.
Jefferies, C.J. and P. Brain (1984), “A mathematical model of pollen-tube penetration in apple styles”, Planta, Vol. 160/1, pp. 52-58.
Jiang, J. et al. (2017), “Microbial community analysis of apple rhizosphere around Bohai Gulf”, Nature Scientific Reports, Vol. 7, e8918.
Joshi, S.G. et al. (2011), " Functional analysis and expression profiling of HcrVf1 and HcrVf2 for development of scab resistant cisgenic and intragenic apples", Plant Molecular Biology, Vol. 75, pp 579-591.
Keulemans, W., I. Roldan-Ruiz and M. Lateur (2007), Studying Apple Biodiversity: Opportunities for Conservation and Sustainable Use of Genetic Resources (apple), SPSDII, Belgian Science Policy, Brussels, Belgium.
King, G.J. et al. (1991), “The ‘European Apple Genome Project’ – Developing a strategy for mapping genes coding for agronomic characters in tree species”, Euphytica, Vol. 56, pp. 89-94.
Koltunow, A.M. (1993), “Apomixis: Embryo sacs and embryos formed without meiosis or fertilisation in ovules”, The Plant Cell, Vol. 5/10, pp. 1425-1437.
Komori, S. et al. (1999), “Discrimination of cross incompatibility by number of seeds per fruit and fruit set percentage in apples”, Bulletin of the National Institute of Fruit Tree Science, Vol. 33, pp. 97-112.
Korban, S.S. (1986), “Interspecific hybridization in Malus”, Horticultural Science, Vol. 21/1, pp. 41-48.
Korban, S.S. and R.M. Skirvin (1984), “Nomenclature of the cultivated apple”, Horticultural Science, Vol. 19, pp. 177‑180.
Kost, T.D. et al. (2015), “Development of first cisgenic apple with increased resistance to fire blight”, Public Library of Science One, Vol. 10/12: e0143980.
Kotoda, N. et al. (2000), “Expression pattern of homologues of floral meristem identity genes LFY and AP1 during flower development in apple”, Journal of the American Society for Horticultural Science, Vol. 125/4, pp. 398-403.
Koutinas, N., G. Pepelyankov and V. Lichev (2010), “Flower induction and flower bud development in apple and sweet cherry”, Biotechnology and Biotechnological Equipment, Vol. 24/1, pp. 1549-1558.
Krens, F.A. et al. (2015), “Cisgenic apple trees; Development, characterisation, and performance”, Frontiers Plant Science, Vol. 6, p. 286.
Kron, P. and B.C. Husband (2009), “Hybridization and the reproductive pathways mediating gene flow between native Malus coronaria and domestic apple, M. domestica”, Botany, Vol. 87/9, pp. 864-874.
Kron, P. et al. (2001), “Across- and along-row pollen dispersal in high-density apple orchards: Insights from allozyme markers”, The Journal of Horticultural Science and Biotechnology, Vol. 76/3, pp. 286‑294.
Kumar, S. et al. (2012), “Genomic selection for fruit quality traits in apple (Malus x domestica Borkh.)”, Public Library of Science One, Vol. 7/5: e36674.
Kumari, A. et al. (2015), “Grafting triggers differential responses between scion and rootstock”, Public Library of Science One, Vol. 10/4: e0124438.
Larsen, A.S. and E.D. Kjær (2009), “Pollen mediated gene flow in a native population of Malus sylvestris and its implications for contemporary gene conservation management”, Conservation Genetics, Vol. 10/6, pp. 1637‑1646.
Larsen, A.S., M. Jensen and E.D. Kjær (2008), “Crossability between wild (Malus sylvestris) and cultivated (M.x domestica) apples–implications for genetic resource management”, Silvae Genetica, Vol. 57/3, pp. 127-130.
Larsen, A.S. et al. (2006), “Hybridization and genetic variation in Danish populations of European crab apple (Malus sylvestris)”, Tree Genetics and Genomes, Vol. 2/2, pp. 86-97.
Laurens, F. et al. (2012), “Review of fruit genetics and breeding programmes and a new European initiative to increase fruit breeding efficiency”, Acta Horticulturae, Vol. 929, pp. 95-102.
Levin, D.A. (1996), “The evolutionary significance of pseudo-self-fertility”, The American Naturalist, Vol. 148/2, pp 321-332.
Liu, L. et al. (2017), “Apple, from omics to systemic function”, Plant Growth Regulation, Vol. 83/1, pp. 1‑11.
Luby, J.J. (2003), “Taxonomic classification and brief history”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CABI International, Cambridge, UK, p. 1-14.
Malnoy, M. et al. (2008), “Two receptor-like genes, Vfa1 and Vfa2, confer resistance to the fungal pathogen Venturia inaequalis inciting apple scab disease”, Molecular Plant-Microbe Interactions, Vol. 21/4, pp. 448-458.
Manganaris, A.G. and F.H. Alston (1988a), “Inheritance and linkage relationships of glutamate oxaloacetate transaminase in apple: 2. The genes GOT-2 and GOT-4”, Theoretical and Applied Genetics, Vol. 76/3, pp. 449‑454.
Manganaris, A.G. and F.H. Alston (1988b), “The acid phosphatase gene ACP-1 and its linkage with the endopeptidase gene ENP-1 and the pale green lethal gene 1 in apple”, Acta Horticulturae, Vol. 224, pp. 177-184.
Manganaris, A.G. and F.H. Alston (1987), “Inheritance and linkage relationships of glutamate oxaloacetate transaminase isoenzymes in apple: 1. The gene GOT-l, a marker for the S incompatibility locus”, Theoretical and Applied Genetics, Vol. 74/1, pp. 154-161.
McNeill, J. et al. (2012), International Code of Nomenclature for Algae, Fungi and Plants (Melbourne Code) Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 (Regnum Vegetabile 154), Koeltz Scientific Books, Germany.
Miller, D.D. and J. Racsko (2011), “Rootstock effects on fruit drop and quality of ‘Gala Galaxy’ and ‘Golden Delicious Reinders’ apples”, Acta Horticulturae, Vol. 903/August, pp. 397‑404.
Mols, C.M. and M.E. Visser (2002), “Great tits can reduce caterpillar damage in apple orchards”, Journal of Applied Ecology, Vol. 39/6, pp. 888-899.
Moshtagh, F. et al. (2015), “Investigation on pollen viability, germination and tube growth in some apple cultivars in climate conditions in Shirvan”, Journal of Applied Environmental and Biological Sciences, Vol. 4/12, pp. 295-302.
Mudge, K. et al. (2009), “A history of grafting”, Horticultural Reviews, Vol. 35, pp. 437-493.
Myers, J. et al. (2004), “Seed dispersal by white-tailed deer: Implications for long-distance dispersal, invasion, and migration of plants in eastern North America”, Oecologia, Vol. 139, pp. 35‑44.
Nicholas, A.H., R.N. Spooner-Hart and R.A. Vickers (2005), “Abundance and natural control of the woolly aphid Eriosoma lanigerum in an Australian apple orchard IPM program”, BioControl, Vol. 50/2, pp. 271-291.
Nikiforova, S.V. et al. (2013), “Phylogenetic analysis of 47 chloroplast genomes clarifies the contribution of wild species to the domesticated apple maternal line”, Molecular Biology Evolution, Vol. 30, pp. 1751-1760.
OECD (2019a), “Consensus document on compositional considerations for new cultivars of apple (Malus x domestica Borkh.): Key food and feed nutrients, allergens, toxicants and other metabolites”, Series on the Safety of Novel Foods and Feeds, No. 31, Organisation for Economic Corporation and Development, Paris, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2019)23%20&doclanguage=en (accessed 1 October 2019).
OECD (2019b), OECD BioTrack Product Database, OECD, Paris, https://biotrackproductdatabase.oecd.org/ (accessed 1 October 2019).
Ogawa, J.M. and H. English (1991), Diseases of Temperate Zone Tree Fruit and Nut Crops, University of California, Division of Agriculture and Natural Resources, Publication 3345.
Okada, K. et al. (2016), “Expression of a putative dioxygenase gene adjacent to an insertion mutation is involved in the short internodes of columnar apples (Malus x domestica)”, Journal of Plant Research, Vol. 129/6, pp. 1109‑1126.
Patocchi, A. et al. (2009), “Towards improvement of marker assisted selection of apple scab resistant cultivars: Venturia inaequalis virulence surveys and standardisation of molecular marker alleles associated with resistance genes”, Molecular Breeding, Vol. 24, pp. 337-347.
Peil, A. et al. (2011), “Apple breeding – From the origin to genetic engineering”, Fruit, Vegetable and Cereal Science and Biotechnology, Vol. 5/1, pp. 118-138.
Petrisor, C. et al. (2012), “The rate of pollen germination and the pollen viability at ten apple cultivars in the climatic conditions of Transylvania”, Bulletin UASVM Horticulture, Vol. 69/1, pp. 417-418.
Potter, D. et al. (2007), “Phylogeny and classification of Rosaceae”, Plant Systematics and Evolution, Vol. 266, pp. 5-43.
Pusey, P.L., V.O. Stockwell and M. Mazzola (2009), “Epiphytic bacteria and yeasts on apple blossoms and their potential as antagonists of Erwinia amylovora”, Phytopathology, Vol. 99, pp. 571-581.
Qian, G.Z. et al. (2010), “Proposal to conserve the name Malus domestica against M. pumila, M. communis, M. frutescens and Pyrus dioica (Rosaceae)”, Taxon, Vol. 59/2, pp. 650‑652.
Qian, G.Z. et al. (2008), “Taxonomic study of Malus section Florentinae (Rosaceae)”, Botanical Journal of the Linnean Society, Vol. 158, pp. 223-227.
Rai, M.K. and N.S. Shekhawat (2014), “Recent advances in genetic engineering for improvement of fruit crops”, Plant Cell, Tissue and Organ Culture, Vol. 116, pp. 1-15.
Ramírez, F. and T.L. Davenport (2013), “Apple pollination: A review”, Scientia Horticulturae, Vol. 162, pp. 188–203.
Randall, R.P. (2017), A Global Compendium of Weeds, 3rd Edition, Department of Agriculture and Food, Western Australia, South Perth, Australia.
Reddy, P.P (2010), Bacterial and Viral Diseases and Their Management in Horticultural Crops, Scientific Publishers, Jodhpur, India.
Reim, S. et al. (2006), “Assessing gene flow in apple using a descendant of Malus sieversii var. sieversii F. Niedzwetzkyana as an identifier for pollen dispersal”, Environmental Biosafety Research, Vol. 5/2, pp. 89-104.
Remy, S. et al. (2017), “Biocontrol to protect apple and pear flowers against fire blight using bumblebees”, Communications in Agricultural and Applied Biological Sciences, Vol. 82, pp. 311–320.
Remy, S. et al. (2016), “Protection of apple and pear flowers against fire blight infections using biocontrol organisms applied via bumble bees”, IOBC-WPRS Bulletin, Vol. 117, p. 113.
Rengel, Z. and P. Marschner (2005), “Nutrient availability and management in the rhizosphere: Exploiting genotypic differences”, New Phytologist, Vol. 168, pp. 305-312.
Richards, C.M. et al. (2008), “Genetic diversity and population structure in Malus sieversii, a wild progenitor species of domesticated apple”, Tree Genetics and Genomes, Vol. 5, pp. 339-347.
Rieger, M. (2006), Introduction to Fruit Crops, Food products Press, Binghamton, US.
Rikkerink, H.A. et al. (2016), Resistance Gene and Uses Thereof, US9512442B2.
Robertson, K.R. et al. (1991), “A synopsis of genera in Maloideae (Rosaceae)”, Systematic Botany, Vol. 16/2, pp. 376-394.
Robinson, J.P. et al. (2001), “Taxonomy of the genus Malus Mill. (Rosaceae) with emphasis on the cultivated apple, Malus domestica Borkh”, Plants Systematics and Evolution, Vol. 226, pp. 35-58.
Roiger, D.J. and S.N. Jeffers (1991), “Evaluation of Trichoderma sp. for biological control of Phytophthora crown and root rot of apple seedlings”, Phytopathology, Vol. 81/8, pp. 910-917.
Routson, K.J. et al. (2012), “Gene variation and distribution of pacific crabapple”, Journal of the American Society for Horticultural Science, Vol. 137/5, pp. 325-332.
Rumberger, A., I.A. Merwin and J.E. Thies (2007), “Microbial community development in the rhizosphere of apple trees at a replant disease site”, Soil Biology and Biochemistry, Vol. 39/9, pp. 1645-1654.
Sanzol, J. and M. Herrero (2001), “The ‘effective pollination period’ in fruit trees”, Scientia Horticulturae, Vol. 90/1-2, pp. 1-17.
Sapir, G. et al. (2017), “Synergistic effects between bumblebees and honey bees in apple orchards increase cross-pollination, seed number and fruit size”, Scientia Horticulturae, Vol. 219, pp. 107-117.
Sassa, H. (2016), “Molecular mechanism of the S-RNase-based gametophytic self-incompatibility in fruit trees of Rosoceae”, Breeding Science, Vol. 66/1, pp. 116-121.
Schlathölter, I. et al. (2018), “Generation of advanced fire blight-resistant apple (Malus x domestica) selections of the fifth generation within 7 years of applying the early flowering approach”, Planta, Vol. 247, pp. 1475-1488.
Schouten, H.J. et al. (2014), “Cloning and functional characterisation of the Rvi15 (Vr2) gene for apple scab resistance”, Tree Genetics and Genomes, Vol. 10, pp. 251-260.
Schulze-Menz, G.K. (1964), “Rosaceae”, in H. Melchior (ed.), Engler’s Syllabus der Pflanzenfamilien II, 12th Edition, Gebrüder Borntraeger, Berlin.
Schuster, M. and R. Büttner (1995), “Chromosome numbers in the Malus wild species collection of the genebank Dresden-Pillnitz”, Genetic Resources and Crop Evolution, Vol. 42, pp. 353‑361.
Sheffield, C.S., H.T. Ngo and N. Azzu (2016), A Manual on Apple Pollination, FAO, Rome.
Sheffield, C.S. et al. (2013), “Bee (Hymenoptera: Apoidea) diversity within apple orchards and old fields in the Annapolis Valley, Nova Scotia, Canada”, The Canadian Entomologist, Vol. 145/1, pp. 94-114.
Sherwani, A., M. Mukhtar and A.A. Wani (2016), “Insect pests of apple and their management”, in A.K. Pandey and P. Mall (eds.), Insect Pest Management of Fruit Crops, Biotech, New Delhi, India, pp. 295-306.
Silfverberg-Dilworth, E. et al. (2005), “Identification of functional apple scab resistance gene promoters”, Theoretical and Applied Genetics, Vol. 110, pp. 1119-1126.
Sindhu, S.S., Y.S. Rakshiya and G. Sahu (2009), “Biological control of soilborne plant pathogens with rhizosphere bacteria”, Pest Technology, Vol. 3/1, pp. 10-21.
Soejima, J. (2007), “Estimation of gene flow via pollen spread for the orchard layout prior to the field release of apple transformants”, Acta Horticulturae, Vol. 738(March), pp. 341-345.
Stover, M.E. and P.L. Marks (1998), “Successional vegetation in cultivated and pasture land in Tomkins County, New York”, Journal of the Torrey Botanical Society, Vol. 125, pp. 150‑164.
Sułowicz, S. and Z. Piotrowska-Seget (2016), “Response of microbial communities from an apple orchard and grassland soils to the first-time application of the fungicide tetraconazole”, Ecotoxicology and Environmental Safety, Vol. 124, pp. 193-201.
Tromp, J. and O. Borsboom (1994), “The effect of autumn and spring temperature on fruit set and on the effective pollination period in apple and pear”, Scientia Horticulturae, Vol. 60/1‑2, pp. 23-30.
Tromp, J., A.D. Webster and S.J. Wertheim (2005), “Fundamentals of temperate zone tree fruit production”, Tree Physiology, Vol. 25/12, pp. 1571-1572.
Tukey, H.B. (1964), Dwarfed Fruit Trees, Macmillan Co., New York, and Collier-Macmillan Ltd, London.
Tyson, R.C., J.B. Wilson and W.D. Lane (2011), “A mechanistic model to predict transgenic seed contamination in bee-pollinated crops validated in an apple orchard”, Ecological Modelling, Vol. 222/13, pp. 2084-2092.
UCANR (2018), How to Manage Pests: Apple, University of California Agriculture and Natural Resources, http://ipm.ucanr.edu/PMG/selectnewpest.apples.html (accessed 1 October 2019).
USDA-ARS (2018), Germplasm Resources Information Network (GRIN), National Plant Germplasm System, United States Department of Agriculture, Agricultural Research Service, https://www.ars-grin.gov (accessed 1 October 2019).
USDA-NRCS (2018), Plants Database, United States Department of Agriculture, Natural Resources Conservation Service, https://plants.usda.gov/java (accessed 1 October 2019).
Vanblaere, T. et al. (2014), “Molecular characterisation of cisgenic lines of apple ‘Gala’ carrying the Rvi6 scab resistance gene”, Plant Biotechnology Journal, Vol. 12, pp. 2-9.
Vanblaere, T. et al. (2011), “The development of a cisgenic apple plant”, Journal Biotechnology, Vol. 154, pp. 304‑311.
Vanwynsberghe, L. (2006), “Description of, and possibilities to increase genetic diversity in modern apple”, Dissertationes de Agricultura, nr. 714, Faculteit Bio-ingenieurswetenschappen KU Leuven, Belgium.
VASCAN (2018), The Database of Vascular Plants of Canada, http://data.canadensys.net/vascan/search (accessed 1 October 2019).
Vavilov, N.I. (1951), The Origin, Variation, Immunity and Breeding of Cultivated Plants, The Chronica Botanica Co., Waltham, Massachusetts, US.
Velasco, R. et al. (2010), “The genome of the domesticated apple (Malus x domestica Borkh.)”, Nature Genetics, Vol. 42, pp. 833 -839.
VIDE (2018), Virus Identification Database Exchange - Known susceptibilities of Rosaceae, Malus.
Volk, G.M. et al. (2013), “Malus sieversii: A diverse central Asian apple species in the USDA-ARS National Plant Germplasm System”, Horticultural Science, Vol. 48, pp. 1440-1444.
Walker, J.T.S, D.M. Suckling and C.H. Wearing (2017), “Past, present, and future of integrated control of apple pests: The New Zealand experience”, Annual Review of Entomology, Vol. 62, pp. 231-248.
Way, R.D. et al. (1990), “Apples (Malus)”, Acta Horticulturae, Vol. 290, pp. 3-62.
Webster, A.D. (2005a), “The origin, distribution and genetic diversity of temperate tree fruits”, in J. Tromp et al. (eds.), Fundamentals of Temperate Zone Tree Fruit Production, Backhuys Publishers, Leiden, The Netherlands.
Webster, A.D. (2005b), “Sites and soils for temperate tree-fruit production: Their selection and amelioration”, in J. Tromp, A.D. Webster and S.J. Wertheim (eds.), Fundamentals of Temperate Zone Tree Fruit Production, Backhuys Publishers, Leiden, The Netherlands.
Webster, A.D. and S. Wertheim (2003), “Apple rootstocks”, in D.C. Ferree and I.J. Warrington (eds.), Apples: Botany, Production and Uses, CAB International, Wallingford, UK, pp. 91‑124.
Weigl, K. et al. (2015), “BpMADS4 on various linkage groups improves the utilization of the rapid cycle breeding system in apple”, Plant Biotechnology Journal, Vol. 13, pp. 246-258.
Wertheim, S.J. (1991), “Malus cv. Baskatong as an indicator of pollen spread in intensive apple orchards”, Journal of Horticultural Science, Vol. 66/5, pp. 635-642.
Westwood, M.N. (1993), Temperate-zone Pomology: Physiology and Culture, Timber Press, Portland, US.
Williams, R.R. (1966), Pollination Studies in Fruit Trees. III. The Effective Pollination Period for Some Apple and Pear Varieties, Report of the Agricultural and Horticultural Research Station, University of Bristol, UK.
Willson, M.F. (1993), “Mammals as seed-dispersal mutualists in North America”, OIKOS, Vol. 67/1, pp. 159-176.
Witmer, M.C. (1996), “Do some bird-dispersed fruits contain natural laxatives? A comment”, Ecology, Vol. 77, pp. 1947-1948.
Xu, K. (2013), “An overview of Artic apples: Basic facts and characteristics”, New York State Horticultural Society, Vol. 21/3, pp. 8-10.
Xu, K. et al. (2012), “Genetic characterisation of the Ma locus with pH and titratable acidity in apple”, Molecular Breeding, Vol. 30, pp. 899-912.
Yan, G. et al. (2008), “Genetic polymorphism of Malus sieversii populations in Xinjiang, China”, Genetic Resources and Crop Evolution, Vol. 55, pp. 171-181.
Yoder, K. et al. (2009), “Effects of temperature and the combination of liquid lime sulfur and fish oil on pollen germination, pollen tube growth and fruit set in apples”, Horticultural Science, Vol. 44/5, pp. 1277-1283.
Zhou, H. et al. (2014), “Biological control of insects pests in apple orchards in China”, Biological Control, Vol. 68/1, pp. 47-56.
Zohary, D., M. Hopf and E. Weiss (2012), Domestication of Plants in the Old World, Oxford University Press, New York.