Cancer mortality rates in the EU declined by 10% in the decade leading to 2020, with substantial reductions across many important cancers. However, without major changes, cancer will become the leading cause of death in Europe by 2035. Indeed, new cancer cases have been increasing, with breast, prostate, colorectal and lung cancers estimated to account for half of all cancers diagnosed in EU countries in 2022. Furthermore, the gaps in cancer outcomes between countries – as well as by region, socio‑economic status and gender within countries – are large, providing opportunities to learn from good practices. Alongside national cancer control plans, the European Commission’s Europe’s Beating Cancer Plan is underpinning efforts to tackle cancer in EU countries. National cancer registries that integrate or can be linked to information on clinical data, screening, genomic and socio‑economic status will be a key instrument in monitoring policies across the spectrum of cancer prevention and care.
Beating Cancer Inequalities in the EU

2. Trends in the cancer burden
Abstract
Key findings
There were an estimated 2.74 million new cancer cases in the 27 EU Member States (EU27) in 2022, representing an age‑standardised incidence rate of 571.5 new cases for every 100 000 people. Between 2010 and 2022, the rate of new cancer cases increased in 14 of 24 of the EU27 plus Norway and Iceland (EU+2 countries).
The most common cancer sites in the EU27 in 2022 were breast, prostate, colorectum and lung, which together represented 50% of all new cancer cases. The same sites, with the addition of pancreatic cancer, were the leading causes of death in 2020, accounting for 52% of all cancer deaths.
Cancer mortality decreased by 10% in the EU27 between 2010 and 2020, although cancer still represented 22.5% of all deaths in 2020. In the decade to 2020, mortality rates fell across a number of cancers, including colorectal (‑15%), cervix uteri (‑16%) and stomach cancers (‑27%).
Estimated cancer survival probabilities improved for most cancers in EU+2 countries between 2005‑09 and 2010‑14, except for cervical cancer and acute lymphoblastic leukaemia. Seven countries (Austria, Croatia, Czechia, Iceland, Latvia, Malta, and Slovenia) had decreases in cervical cancer five‑year net survival.
There is wide variation in cancer mortality across EU+2 countries. In 2020, breast cancer mortality rates varied almost two‑fold across countries, and the mortality rates for colorectal, liver, lung, stomach and prostate cancers varied between more than two‑fold and four‑fold.
For many cancers, lower estimated five‑year survival probabilities are found in Central and Eastern European countries (Bulgaria, Croatia, Czechia, Lithuania, Poland, Romania and the Slovak Republic), while Western European and Nordic countries (Belgium, Norway, Sweden, Iceland, Germany and Portugal, among others) and Cyprus consistently have estimated five‑year survival probabilities in the top quintile.
Within countries, cancer mortality rates can be up to 37% higher across different regions, suggesting scope for targeted interventions to reduce regional disparities.
There are also large differences in cancer outcomes by sex and socio‑economic status within countries. Lung cancer mortality rates were 2.6 times higher among men with lower than higher levels of education, and 1.7 times higher among women with lower than higher levels of education.
Integrated national cancer control plans are found in 25 of the 29 EU+2 countries. The areas most prioritised in the plans are screening, treatment, prevention and quality of cancer care. Cancer in children, adolescents and young adults; cancer networks; digitalisation; and health information are less often prioritised.
A national cancer registry covering the full population exists in 24 of the 29 EU+2 countries. Four countries (Spain, Italy, Romania and France) have regional registries covering varying percentages of the population, while Greece does not have any population-based cancer registries.
Harmonising standards and improving interoperability across databases facilitates integration of cancer registries and national screening data; this leads to better monitoring of the cancer burden and cancer care. It is critical to enable the linkage of data related to socio‑economic status, ethnicity and migration to cancer registries in order to monitor cancer inequalities and inform targeted policy actions.
2.1. The burden of cancer in Europe is large
2.1.1. Cancer is a major public health concern in Europe
It is estimated that almost 2.8 million citizens in Europe were diagnosed with cancer in 2022
In 2022, there were an estimated 2.78 million new cancer cases (across all sites excluding non-melanoma skin cancer) in the 27 European Union Member States (EU27), plus Iceland and Norway (EU+2 countries), including 2.74 million cases in the EU27 and 39 112 cases in Iceland and Norway (ECIS, 2023[1]). This translates to about five people being diagnosed every minute, or one cancer case diagnosed every 11 seconds. Compared to 2.72 million estimated cancer cases in 2020, it represents an increase of around 65 000 cases. Among children, there were an estimated 9 294 new cancer cases in 2022 in the 29 EU+2 countries. It is estimated that by 2040 new cancer diagnoses among all ages will increase by around 18% in the EU27 compared to 2022.
In 2020, 1.17 million cancer-related deaths occurred in the EU27 (Eurostat, 2023[2]). The proportion of deaths attributable to cancer in the EU27 has slowly been decreasing – from 25.5% of all deaths in 2012 to 25.1% in 2019. However, cancer represented 22.5% of all deaths in the EU27 in 2020. This sharp decline can be explained by the COVID‑19 pandemic, which reduced the number of deaths with cancer as the underlying cause, and by changes in the international coding rules for underlying causes of death (Henley et al., 2022[3]).
While the burden of cancer among all causes of death is decreasing, the reduction is less pronounced than that for cardiovascular diseases – the current leading cause of death in the EU27 (Figure 2.1). Between 2012 and 2019 (before the COVID‑19 pandemic), the proportion of cancer deaths declined by only 0.4 percentage points, compared to a decline of 4.1 percentage points (from 39.2% in 2012 to 35.1% in 2019) for deaths attributable to cardiovascular diseases. In line with these trends, it is estimated that, without decisive action, cancer will be the leading cause of death in Europe by 2035 (European Commission, 2022[4]). In 2021, cancer also accounted for 27% of potential years of life lost1 in the EU27 countries with available data, compared to 21% accounted for by cardiovascular diseases. Governments are thus facing pressure to prioritise and improve cancer prevention and treatment. In response, authorities have placed prevention and early detection at the centre of countries’ strategies to reduce the burden of cancer (further explored in Chapters 3 and 4).
Overall, with growing cancer case numbers and decreasing mortality rates, the prevalence of cancer is increasing in EU+2 countries (Box 2.1). In 2020, an estimated 9.5 million people (2.1% of the population) living in EU+2 countries had received a cancer diagnosis in the last five years (IARC, 2023[5]).
Figure 2.1. The percentage of deaths with cancer as the underlying cause is decreasing at a slower pace than the percentage with cardiovascular diseases
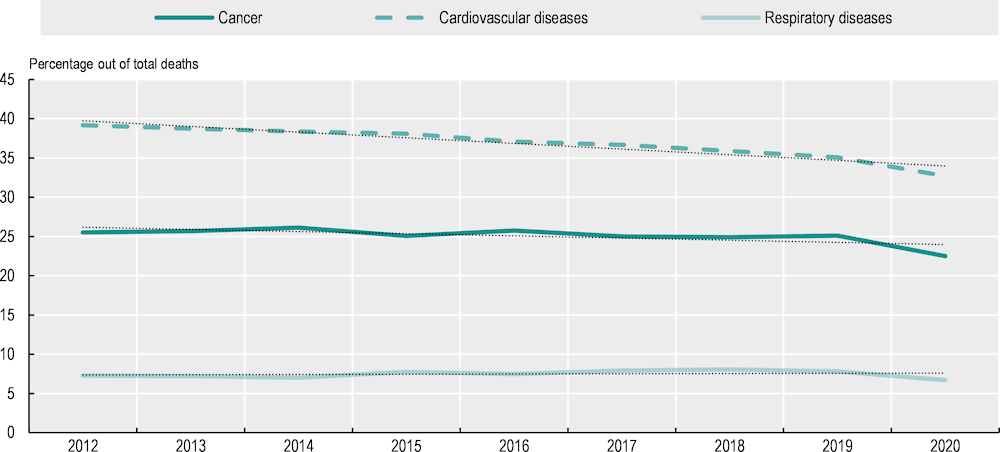
Notes: The graph represents the weighted values for EU27 countries as calculated by Eurostat. A linear model using ordinary least squares was calculated for each series. International Classification of Diseases 10th Revision (ICD‑10) codes used: cancer (C00‑C97), cardiovascular diseases (I00‑I99), respiratory diseases (J00‑J99). COVID‑19 (ICD codes U07.1, U07.2) is not included in any of the disease groups shown in the figure.
Source: Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
Box 2.1. Framework for understanding cancer statistics and data sources
This chapter examines cancer burden relying on three common indicators – incidence, survival and mortality (Figure 2.2). Each of these three indicators provides information on the effectiveness of cancer prevention, detection and treatment, and only together do they provide an accurate picture of cancer care. While prevalence is often referenced in order to provide a snapshot of the living population who currently have or have ever had cancer (including those in remission) in a defined time period, it provides limited insight into the dynamic nature of cancer control and care (Cho et al., 2014[6]; Ellis et al., 2014[7]).
Figure 2.2. Cancer burden definitions
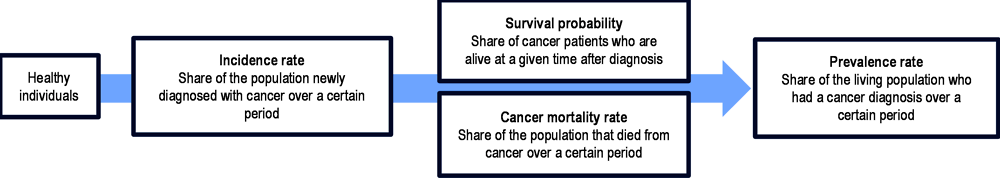
Source: Authors based on Cho, H. et al. (2014[6]), “When do changes in cancer survival mean progress? The insight from population incidence and mortality”, https://doi.org/10.1093/jncimonographs/lgu014; Ellis, L. et al. (2014[7]), “Cancer incidence, survival and mortality: Explaining the concepts”, https://doi.org/10.1002/ijc.28990.
Incidence rates provide an understanding of the rate of new cancer diagnoses within a given period – often over the course of a year. When key cancer risk factors such as tobacco use or unhealthy diets increase, cancer incidence will increase in the following years. In parallel, increases in diagnostic or screening activities will also increase incidence, as more cases are detected at an early stage. This is generally a positive development that will lead to lowered mortality rates and higher probability of survival – a higher share of patients with cancer surviving for a given period. However, it can also result in overdiagnosis of cases that would not have had any clinical significance, or that would have progressed slowly enough to not affect mortality. As such, a higher survival probability – which can represent improved, appropriate early-stage diagnosis or improvement of cancer treatment – could also be artificially inflated due to overdiagnosis. Mortality rates facilitate an understanding of how many people within the population have died from cancer over a period, and are essential to show progress in cancer control and treatment. It is important to remember that an increase in mortality rates can result from a large increase in incidence, despite a parallel improvement in cancer care and probability of survival.
Data sources
Estimates of cancer incidence (2022 and 2010) and of mortality (2022) are obtained from the European Cancer Information System (ECIS) of the European Commission (ECIS, 2023[1]). Observed incidence data are obtained from national sources collected through the 2023 OECD Policy Survey on Cancer Care Performance2, to which 26 EU+2 countries responded. Prevalence data are from the International Agency for Research on Cancer (IARC) (2023[5]). Observed mortality data for 2010‑20 are from Eurostat (2023[2]). Survival estimates are obtained from the CONCORD‑3 study (Allemani et al., 2018[8]), and are age‑standardised using the International Cancer Survival Standard weights. This report uses the European age standardisation 2013 edition (Eurostat, 2013[9]) when reporting incidence and mortality rates. Further information is taken from the EU Country Cancer Profiles (OECD, 2023[10]), complemented by the 2023 OECD Policy Survey on Cancer Care Performance.
Cancer incidence rates vary near 2‑fold across EU+2 countries
The estimated cancer incidence rates for 2022 are shown in Figure 2.3. After adjusting for different population age structures, overall cancer incidence rates were highest in Norway and Denmark, at close to 28% higher than the EU27 average. Ireland, the Netherlands, Croatia, and Hungary were also among the 20% of countries with the highest incidence (the highest quintile) among EU+2 countries, with incidence rates above 622 per 100 000 population. In Bulgaria and Austria, overall estimated cancer incidence was the lowest, with rates more than 14% lower than the EU27 average. Low incidence was also seen in Romania, Spain, Greece and Lithuania (all with estimated incidence below 542 per 100 000 – the lowest quintile). In the EU27, cancer incidence rates are estimated to vary near 2‑fold across countries.
Between 2010 and 2022, estimated cancer incidence increased in 14 of the 24 countries with available data. The largest estimated increases were in Romania and Poland – two countries that experienced an improvement in detection capabilities (OECD, 2023[11]; OECD, 2023[12]). Over the same period, estimated cancer incidence decreased in Czechia (7%), Iceland (‑6%), the Slovak Republic (‑3%), Lithuania (‑3%) and Belgium3 (‑2%).
In 2020, the highest mortality rates (for both men and women combined) occurred in Hungary (321 per 100 000 population, which is 32% higher than the EU27 average), and high rates were also observed in Croatia, the Slovak Republic, Latvia, Slovenia and Poland (all reporting mortality rates above 277 per 100 000 – the highest quintile). The lowest mortality rates occurred in Luxembourg (203 per 100 000 population, which is 16% lower than the EU27 average), and Cyprus, Finland, Malta, Sweden and Spain all had rates lower than 221 per 100 000 population. Overall, cancer mortality rates varied 1.6‑fold across countries. Between 2010 and 2020, the age‑standardised all-cancer mortality rate decreased by 10% in the EU27. Decreases in cancer deaths were observed in all 29 EU+2 countries except Bulgaria (8% increase) and Cyprus (4% increase).
Figure 2.3. The 10% cancer mortality decrease in 2010‑20 contrasts with cancer incidence increases in most EU+2 countries in 2010‑22
Age‑standardised incidence (estimated) and mortality (observed) rates per 100 000 population, both sexes
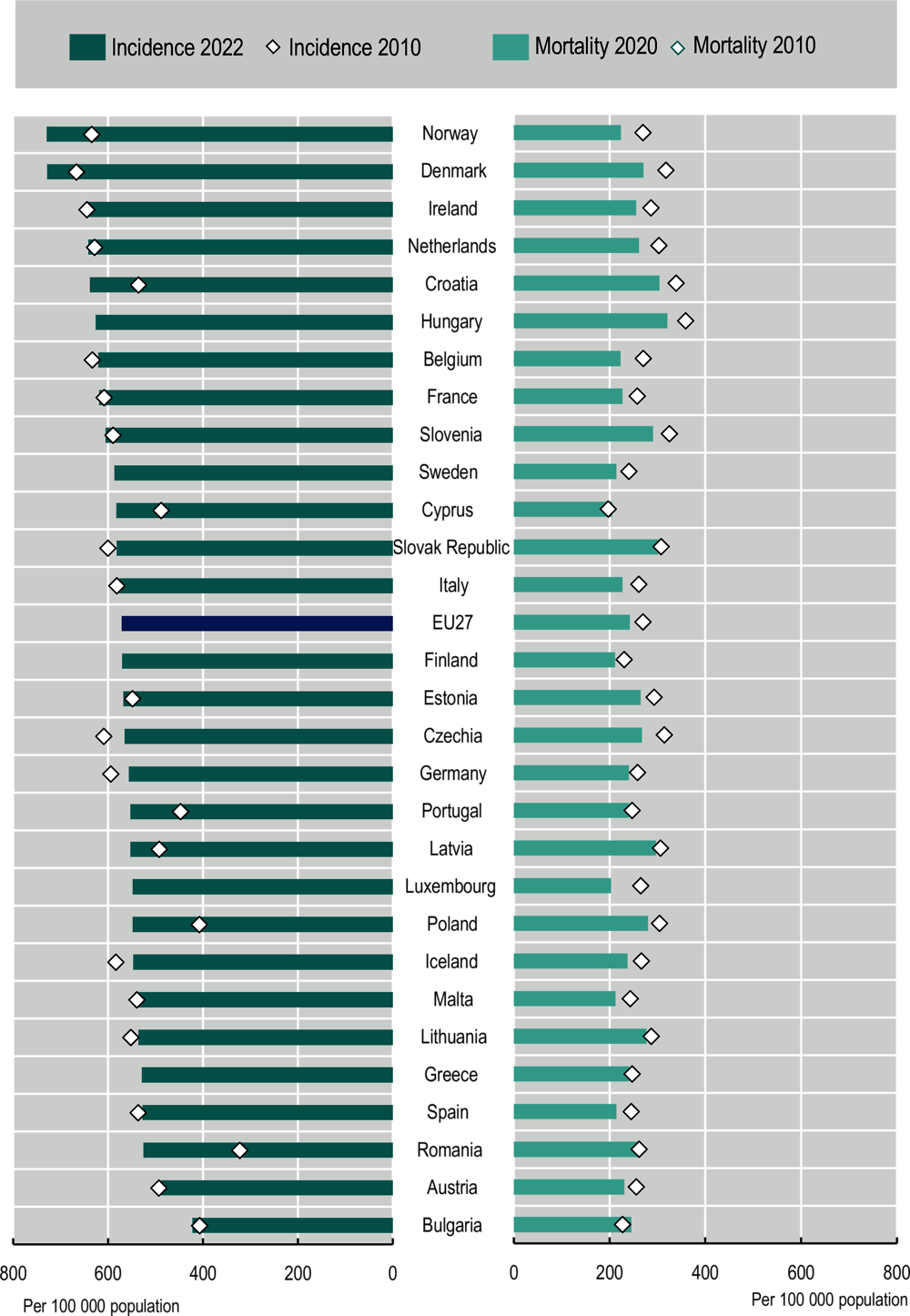
Notes: Estimated national age‑standardised rates (European 2013 edition) per 100 000 population. Incidence estimates were created before the COVID‑19 pandemic, based on incidence trends from previous years, and may differ from observed rates in more recent years. Incidence rates are calculated for all cancer sites except non-melanoma skin, while mortality rates correspond to all malignant neoplasms. The EU average for mortality includes EU Member States and is calculated as a population-weighted average. The 2010 cancer incidence rates are estimated from subnational registries with different population coverage, limiting the international comparability of these estimates: Germany (80% coverage), Spain (27% coverage), France (20% coverage), Italy (57% coverage) and Romania (23% national coverage). Further, these 2010 measures in the graph is weighted to reflect the size of registries present in ECIS 2010 data. In Iceland, the 2020 mortality rate is a five‑year rolling average (2016‑20), and the 2010 mortality rate is a four‑year rolling average (2006‑09) (no data for 2010). Incidence rates in 2010 are missing for Sweden, Hungary, Finland, Luxembourg and Greece.
Source: Incidence data from ECIS (2023[1]), European Cancer Information System, https://ecis.jrc.ec.europa.eu (accessed on 27 April 2023); mortality data from Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
Four cancers are responsible for 50% of all new cancer diagnoses
The most common cancers among those estimated to have been diagnosed in the EU27 in 2022 were breast cancer in women, with 374 836 new cases (148 per 100 000 women), followed by prostate cancer in men (330 492 new cases; 154 per 100 000 men), colorectum cancer (356 154 new cases; 73.5 per 100 000 population) and lung cancer, including trachea and bronchus (319 236 new cases; 66 per 100 000 population). Together, these four cancer sites were responsible for 50% of all new cancer diagnoses in 2022 (Table 2.1).
In terms of mortality, most cancer deaths in 2022 were expected to be caused by breast cancer (17% of cancer deaths among women), lung cancer (23% of cancer deaths among men and 15% among women), colorectum cancer (12% of cancer deaths), prostate cancer (11% of cancer deaths among men) and pancreas cancer (between 7% and 8% of cancer deaths). According to the Eurostat Database, these five cancers were responsible for 52% of all cancer deaths in 2020 in the EU27.
Table 2.1. The ten leading cancer sites affecting men and women in the EU27 in 2022
|
Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Estimated new cases |
Breast |
374 836 |
29% |
|
|
Prostate |
330 492 |
23% |
|
Colorectum |
158 698 |
12% |
Lung |
203 029 |
14% |
|||
|
Lung |
116 207 |
9% |
Colorectum |
197 456 |
13% |
|||
|
Corpus uteri |
69 163 |
5% |
Bladder |
127 640 |
9% |
|||
|
Melanoma skin |
49 509 |
4% |
Kidney |
58 213 |
4% |
|||
|
Pancreas |
50 438 |
4% |
Melanoma skin |
51 998 |
4% |
|||
|
Non-Hodgkin lymphoma |
41 189 |
3% |
Non-Hodgkin lymphoma |
51 518 |
4% |
|||
|
Ovary |
40 714 |
3% |
Pancreas |
49 714 |
3% |
|||
|
Thyroid |
38 503 |
3% |
Stomach |
45 246 |
3% |
|||
|
Brain and other CNS |
19 539 |
2% |
Multiple myeloma |
18 808 |
1% |
|||
|
All cancer sites* |
1 276 601 |
All cancer sites* |
1 465 846 |
|||||
|
Estimated deaths |
Breast |
95 829 |
17% |
|
|
Lung |
164 485 |
23% |
|
Lung |
88 097 |
15% |
Colorectum |
88 585 |
12% |
|||
|
Colorectum |
70 371 |
12% |
Prostate |
76 772 |
11% |
|||
|
Pancreas |
47 744 |
8% |
Pancreas |
47 208 |
7% |
|||
|
Ovary |
27 677 |
5% |
Bladder |
39 318 |
5% |
|||
|
Stomach |
20 262 |
3% |
Liver |
36 406 |
5% |
|||
|
Leukaemia |
20 023 |
3% |
Stomach |
31 519 |
4% |
|||
|
Liver |
17 759 |
3% |
Leukaemia |
25 020 |
3% |
|||
|
Non-Hodgkin lymphoma |
15 865 |
3% |
Kidney |
21 781 |
3% |
|||
|
Brain and other CNS |
15 424 |
3% |
Non-Hodgkin lymphoma |
20 150 |
3% |
|||
|
All cancer sites |
575 326 |
All cancer sites |
717 274 |
|||||
Notes: CNS stands for central nervous system. * Includes all cancer sites except non-melanoma skin cancer. Estimates were calculated based on incidence and mortality trends before the COVID‑19 pandemic and may differ from observed rates in more recent years. Lung also includes bronchus and trachea.
Source: ECIS (2023[1]), European Cancer Information System, https://ecis.jrc.ec.europa.eu (accessed on 27 April 2023).
2.1.2. Cancer mortality rates are decreasing for most cancers, including the five leading causes of cancer death
National efforts to improve cancer prevention and treatment are reflected in an overall downward trend in cancer mortality (Figure 2.4). Stomach cancer mortality declined the most between 2010 and 2020, decreasing by 27%. Lung cancer, which remains the leading cause of cancer death across both sexes, saw a 12% reduction in mortality rates during this period. Significant decreases in mortality rates were also seen for cancers of the cervix uteri (‑16%), colorectum (‑15%) and kidney (‑14%), while breast cancer mortality rates declined by 7%. Among the most lethal cancers, the only increase in mortality rates was seen for pancreatic cancer (6%).
Despite the growing incidence of several cancers, Hashim et al. (2016[13]) suggest that the decrease in cancer mortality is partly explained by reductions in some cancer risk factors, as well as improvements in countries’ early detection and cancer treatment. This is especially the case for breast, cervical and colorectal cancers, for which population-based screening strategies have been introduced in most EU+2 countries. The report explores these topic in detail in Chapters 3 and 4.
Figure 2.4. Age‑standardised mortality rates for most cancers decreased in the last decade
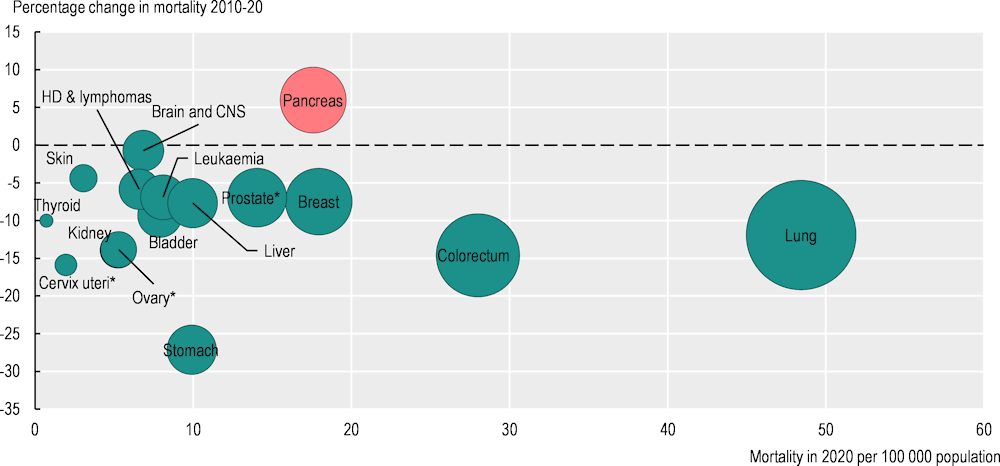
Note: The red bubble signals an increase in the percentage change in the cancer mortality rate during 2010‑20; green bubbles signal a decrease. The size of the bubbles is proportional to the mortality rate in 2020. The mortality rate for some of these cancers is low; hence, the percentage change should be interpreted with caution. * Percentage change for prostate, ovary and cervix uteri cancers refers to 2011‑20. HD stands for Hodgkin disease.
Source: Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
Heterogeneity in cancer mortality highlights the potential for sharing best practices
Table 2.2 presents a dashboard with the age‑standardised mortality rates per 100 000 population for selected cancer sites in EU+2 countries, in a lowest to highest cancer mortality average ranking. The table shows a per-cancer-site colour scale where dark red corresponds to the highest quintile of mortality rates and dark blue corresponds to the lowest quintile. The relative predominance of blue across the top and red across the bottom of the table indicates that countries’ cancer mortality rates are broadly consistent for the 14 cancers examined, suggesting a better performance – with lower cancer mortality rates – in Nordic and Western European countries.
While Sweden, Luxembourg, Spain and Finland have the lowest average mortality rates for the selected cancers, Latvia, Croatia, the Slovak Republic and Slovenia consistently have somewhat higher mortality rates. Outliers from the general country-level trend may indicate cancer sites that countries need to pay special attention to, or those where favourable policies could be replicated to improve cancer outcomes. For example, mortality rates for prostate and pancreas cancers are relatively high in Sweden – a country that otherwise has low mortality rates for other cancers. Diagnosing these cancers at an early stage has a significant impact on their survival (van den Bergh, Loeb and Roobol, 2015[14]; Gheorghe et al., 2020[15]), highlighting the need for greater efforts to improve prevention and early diagnosis to address these challenges.
Table 2.2. Cancer mortality is consistently higher in Central and Eastern European countries
Age‑standardised mortality rate per 100 000 population, 2020, both sexes
|
Bladder |
Brain and CNS |
Breast |
Cervix uteri |
Colorectum |
HD & lymphomas |
Kidney |
Leukaemia |
Liver |
Ovary |
Pancreas |
Prostate |
Stomach |
Lung |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Sweden |
6.1 |
6.3 |
13.5 |
1.1 |
26.4 |
6.2 |
4.5 |
7.2 |
7.1 |
4.8 |
18.6 ↑ |
21.1 |
5.0 |
33.5 |
|
Luxembourg |
6.3 |
5.2 |
20.3 |
0.3 |
23.9 |
6.3 |
2.9 |
8.8 |
7.4 |
5.1 |
16.3 |
13.3 |
7.2 |
39.2 |
|
Spain |
8.5 |
6.5 |
12.8 |
1.3 |
29.4 |
6.0 |
4.2 |
6.6 |
10.0 |
4.1 |
14.9 ↑ |
11.2 |
9.7 |
44.8 |
|
Finland |
5.0 |
6.3 |
15.8 |
0.8 |
20.8 |
8.9 |
5.7 |
5.4 |
9.1 ↑ |
4.5 |
21.2 ↑ |
14.8 |
6.7 |
36.7 |
|
Belgium |
6.5 |
6.5 ↑ |
17.8 |
1.2 |
21.6 |
6.2 |
4.4 |
8.5 |
8.5 ↑ |
5.1 |
16.0 |
13.2 |
5.8 |
49.4 |
|
Norway |
6.0 |
6.5 |
12.3 |
2.1 ↑ |
32.2 |
6.3 |
5.1 |
6.6 |
6.6 ↑ |
5.0 |
16.0 |
20.2 |
5.8 |
44.6 |
|
France |
7.2 |
5.9 |
18.0 |
1.1 |
23.3 |
6.7 |
4.8 |
8.3 |
12.3 |
4.7 |
17.6 ↑ |
12.0 |
6.0 |
44.4 |
|
Cyprus |
5.9 |
8.8 ↑ |
16.3 |
1.4 ↑ |
18.6 ↑ |
6.8 ↑ |
2.6 |
10.8 ↑ |
8.2 ↑ |
6.4 ↑ |
14.6 ↑ |
12.5 |
8.5 ↑ |
40.9 ↑ |
|
Italy |
7.9 |
6.3 ↑ |
18.0 |
0.7 ↑ |
25.1 |
7.1 |
4.8 |
8.3 |
11.6 |
4.6 |
17.7 ↑ |
10.0 |
11.5 |
44.5 |
|
Portugal |
7.4 |
7.9 ↑ |
15.6 |
1.8 |
32.0 |
7.4 |
4.0 |
7.3 |
11.3 ↑ |
3.4 |
14.2 ↑ |
15.9 |
18.0 |
37.2 ↑ |
|
Malta |
9.3 |
8.6 |
20.1 |
0.8 |
25.6 |
6.0 |
6.6 ↑ |
7.8 |
5.9 |
7.1 |
22.8 ↑ |
8.6 |
5.9 |
35.6 |
|
Greece |
10.1 ↑ |
9.1 |
17.5 |
1.2 |
21.5 |
5.1 ↑ |
4.7 |
9.1 |
11.0 |
4.4 |
16.1 ↑ |
13.0 |
9.3 |
58.0 |
|
Austria |
6.1 |
6.8 |
18.3 |
1.6 ↑ |
23.4 |
7.1 |
4.1 |
9.3 |
9.4 ↑ |
5.4 |
20.4 ↑ |
15.4 ↑ |
8.2 |
44.7 |
|
Netherlands |
8.1 |
5.5 ↑ |
18.1 |
1.4 |
27.1 |
7.8 |
5.3 |
8.1 |
7.3 |
5.9 |
16.8 |
17.8 |
6.6 |
57.2 |
|
Germany |
6.0 |
6.5 |
19.4 |
1.7 |
25.2 |
7.4 |
5.3 |
8.6 |
8.8 |
5.5 |
19.5 ↑ |
15.5 |
8.7 |
47.5 |
|
Iceland |
7.9 |
8.7 |
18.1 |
2.0 ↑ |
25.6 |
6.9 |
6.9 |
6.7 |
7.5 ↑ |
5.4 |
16.7 ↑ |
23.4 ↑ |
6.1 |
50.1 |
|
Denmark |
7.7 |
7.6 |
18.5 |
1.5 |
28.8 |
6.0 |
4.2 |
8.6 |
7.7 |
5.5 |
19.5 ↑ |
23.9 |
7.2 |
57.2 |
|
Romania |
9.1 ↑ |
8.7 ↑ |
18.7 ↑ |
6.9 |
34.3 ↑ |
3.6 |
4.7 ↑ |
6.3 |
14.4 |
5.3 |
15.4 ↑ |
13.5 ↑ |
15.6 |
49.1 |
|
Bulgaria |
8.4 ↑ |
8.9 ↑ |
19.3 ↑ |
4.8 ↑ |
36.0 ↑ |
4.2 ↑ |
5.1 ↑ |
5.8 |
9.1 |
6.0 ↑ |
16.3 ↑ |
16.8 ↑ |
14.4 |
44.8 ↑ |
|
Ireland |
6.9 |
7.8 ↑ |
19.9 |
1.7 |
27.3 |
8.5 |
5.5 |
7.1 |
10.5 ↑ |
7.4 |
16.1 |
17.4 |
7.8 |
52.1 |
|
Czechia |
8.8 |
7.2 |
17.1 |
2.7 |
33.3 |
6.1 ↑ |
8.7 |
9.5 |
8.2 |
6.0 |
21.9 |
15.1 |
9.3 |
48.8 |
|
Poland |
11.8 ↑ |
8.3 |
19.9 ↑ |
4.1 |
35.6 |
5.3 ↑ |
6.7 |
8.0 |
5.9 |
7.4 |
13.8 |
16.9 ↑ |
13.4 |
60.5 |
|
Lithuania |
8.7 |
9.3 ↑ |
19.1 |
6.4 |
30.4 |
5.8 ↑ |
7.8 |
9.3 |
7.9 ↑ |
8.9 |
17.4 ↑ |
18.1 |
20.8 |
41.4 |
|
Estonia |
7.8 |
8.1 |
18.9 |
4.6 |
29.8 |
7.0 |
9.5 ↑ |
8.9 |
9.6 ↑ |
7.2 |
18.7 ↑ |
17.4 |
18.9 |
44.6 |
|
Hungary |
10.7 |
6.6 ↑ |
22.9 |
3.8 |
50.5 |
5.3 |
7.8 |
8.4 |
8.2 |
7.0 |
22.0 ↑ |
14.2 |
13.3 |
81.0 |
|
Slovenia |
11.3 ↑ |
7.1 |
21.9 |
1.8 |
30.9 |
9.8 ↑ |
7.4 |
8.6 |
13.6 ↑ |
4.9 |
18.9 |
20.5 |
14.4 |
53.3 |
|
Slovak Republic |
9.8 ↑ |
8.5 ↑ |
23.8 ↑ |
3.5 |
46.3 |
7.0 ↑ |
8.8 ↑ |
9.8 ↑ |
9.1 |
6.9 ↑ |
20.6 ↑ |
17.7 |
13.7 |
47.5 |
|
Croatia |
10.9 ↑ |
9.7 |
16.8 |
2.9 ↑ |
47.6 |
7.3 |
8.2 |
9.1 |
11.0 |
7.2 ↑ |
17.6 ↑ |
18.4 |
15.3 |
63.0 |
|
Latvia |
11.1 ↑ |
10.0 ↑ |
22.4 ↑ |
5.6 |
33.3 |
6.6 |
9.8 |
8.1 |
9.1 ↑ |
10.5 |
20.6 ↑ |
21.1 ↑ |
20.0 |
46.8 |
|
EU27 average |
7.9 |
6.9 |
18.0 |
2.0 |
28.0 |
6.6 |
5.3 |
8.1 |
10.0 |
5.3 |
17.6 ↑ |
14.0 |
9.9 |
48.4 |
Notes: CNS stands for central nervous system. The colours correspond to quintiles of mortality among the 29 countries, where blue is the quintile with the lowest mortality rate, light blue the second quintile, white the third quintile, light red the fourth quintile and dark red the quintile with the highest mortality rate. The order of countries in the table is determined by the average position of annual mortality rates for each cancer. In Iceland, the 2020 mortality rate is a five‑year rolling average (2016‑20) and the 2010 mortality rate is a four‑year rolling average (2006‑09) (no data for 2010). Arrows indicate an increase greater than 3% in mortality rates between 2010 and 2020; except for Iceland and Denmark, and for cervix uteri, ovary and prostate cancers, which show the difference between 2011 and 2020. EU27 averages include only EU Member States and are calculated as population-weighted averages.
Source: Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
The Slovak Republic and Latvia are in a challenging position, as they have both higher mortality rates for all cancers shown in the dashboard and recent increases in mortality for at least six of these cancers. Croatia has among the highest mortality rates for eight cancers (bladder, brain and central nervous system (CNS), colorectal, kidney, liver, ovary, stomach and lung), but lower mortality rates for breast cancer (in the second lowest quintile). This relatively good performance may be attributable to effective implementation of the national breast cancer early detection plan. The Croatian population-based screening programme was first implemented in 2006, with around 150 000 mammograms performed on women aged 50‑69 every year (Brkljačić and Šupe Parun, 2020[16]).
Sweden, Luxembourg, Spain, Finland and Belgium have the lowest mortality rates, and have seen further decreases in mortality for most cancers over the decade. The decline in cancer mortality since 2010 for most cancers is particularly seen in countries with lower cancer mortality in 2020, with exceptions such as Cyprus, Italy and Portugal. Conversely, countries with higher cancer mortality in 2020 (at the lower end of the dashboard) are more likely to have seen mortality increases between 2010 and 2020. In Slovenia, however, it is important to note that the overall cancer mortality rate had declined substantially in 2019 compared to 2011 (by 10%). Bulgaria and Romania experienced increases in mortality for most cancers between 2010 and 2020, which can be attributed both to a rise in cancer incidence and to improvements in the accuracy of reporting cancer deaths.
Overall, variations in cancer mortality between EU+2 countries are wide. In 2020, breast cancer mortality rates varied almost two‑fold, and the mortality rates for colorectal, liver, prostate, stomach, and lung varied between more than two‑fold and four‑fold. Cervical cancer presents the most extreme variation: Luxembourg has 0.3 deaths per 100 000 population compared to Romania’s 6.9 deaths per 100 000 – a 20‑fold difference.
Patients with rare cancers have worse health outcomes than other cancer patients
Cancers with an annual incidence rate lower than six cases per 100 000 people are considered rare. Together, rare cancers account for around 20‑24% of all cancer diagnoses (Gatta et al., 2011[17]; de Heus et al., 2022[18]). According to the EU-funded Surveillance of Rare Cancers in Europe (RARECAREnet), there are 198 identified types of rare cancers.
The average survival estimates for patients with rare cancers is lower than for those with common cancers. A population-based study in the Netherlands determined that, between 1995‑99 and 2015‑19, five‑year survival estimates increased less for rare cancers (from 46.2% to 52.6% – a 6.4 percentage point increase) than for common cancers (from 56.9% to 70.1% – a 13.2 percentage point increase) (de Heus et al., 2022[18]). Poorer survival estimates for patients with rare cancers may be explained by several challenges, including late or incorrect diagnosis, less access to effective therapies, and a lack of new therapies and research.
More research is needed on country-specific cancer recurrence
Cancer recurrence after its initial remission in individuals is an important factor in the burden of cancer. However, little is known about health system performance in preventing cancer recurrence, as it is widely accepted that recurrence depends more on cancer type than on the effectiveness of treatment. Nevertheless, for several cancer types, effective early detection of recurrence can lead to improvements in outcomes (Israel and Kuten, 2007[19]).
Epithelial ovarian cancer recurrence is observed in almost 25% of cases with early-stage disease, and in more than 80% with more advanced stages (median follow-up of over 4 years) (Salani et al., 2011[20]), which is the highest rate among common cancers that are not diagnosed at a metastatic stage. This is followed by lymphomas, which have a 30% to 75% recurrence rate (median follow-up of over 4 years) (Chihara et al., 2016[21]; Li, Young and Medeiros, 2018[22]; Glimelius and Diepstra, 2016[23]); bladder cancer, with a 50% recurrence rate; and soft tissue sarcomas, with a recurrence rate of 50% (median follow-up of almost 8 years) (Woll et al., 2012[24]) and higher for advanced and rare cases (Casali, 2015[25]).
Cancers for which population-based screening programmes and early detection are widespread often have high recurrence rates. Prostate cancer has a 18‑48% 10‑year recurrence rate, depending on the risk level of the individual (Kurbegovic et al., 2017[26]). Breast cancer has an over 30% recurrence rate (median follow-up of over 8 years), which can be lowered to 5‑9% with surgery or post-surgical medication (Colleoni et al., 2016[27]), and colorectal cancer has a recurrence rate of 17% (median follow-up of 4.4 years) (Pugh et al., 2016[28]). Other cancers with high recurrence rates are glioblastoma (aggressive stage IV brain tumour) (75‑80% after median follow-up of 43.0 months) (Jiang et al., 2020[29]), kidney cancer (13‑49% after median follow-up of 20.2 months (Santini et al., 2016[30]), melanoma (~30% in the 2 years following initial diagnosis) (Tas and Erturk, 2017[31]) and pancreas cancer (36‑46%, after 36 months of follow-up) (Breidert et al., 2012[32]), among others.
2.1.3. Efforts in early detection and treatment have improved cancer survival
Five‑year estimated survival probabilities for most cancers have improved (or changed very little) in most countries for people diagnosed between 2010 and 2014 compared to people diagnosed between 2005 and 2009 (Table 2.3). However, estimated five‑year survival probabilities for acute lymphoblastic leukaemia in children decreased by more than 1 percentage point in six countries (Norway, France, Italy, Slovenia, Croatia and Czechia). It is important to note that the CONCORD‑3 study estimates of five‑year net survival can have large 95% confidence interval for countries with low numbers of cases, such as Slovenia. More recent studies (considering people diagnosed until 2016) of cancer survival probabilities in Slovenia show a five‑year survival estimate for childhood leukaemia of around 88% (Zadnik et al., 2021[33]).
Estimated five‑year survival probabilities for women diagnosed with cervical cancer during 2010‑14 compared to 2005‑09 also decreased in seven countries (Austria, Croatia, Czechia, Iceland, Latvia, Malta and Slovenia). Decreases in cervical cancer survival probabilities can be explained by both challenges in access to cancer treatment and improvements in prevention activities, including human papillomavirus vaccination (see Chapter 3) and cervical cancer screening programmes (see Chapter 4), which increase the likelihood of finding precancerous lesions. Effective treatment for precancerous lesions prevents most non-aggressive cervical cancers that have a negative effect on cancer survival.
In Iceland, estimated five‑year survival probabilities decreased during 2010‑14 compared to 2005‑09 for four cancers (stomach, rectum, liver and cervix), while six other countries (Slovak Republic, Czechia, Croatia, Romania, Slovenia and Cyprus) each had two cancer sites with decreasing estimated five‑year survival probabilities.
Western European and Nordic countries such as Belgium, Norway, Sweden, Iceland, Germany and Portugal consistently have survival estimates in the top quintile (the best performing) for most cancers. Cyprus also has survival estimates in the top quintile for 8 of 11 cancers examined (stomach, colon, rectum, pancreas, breast, cervix, ovary and prostate), suggesting one of the best performances in EU+2 countries.
Bulgaria, the Slovak Republic, Czechia, Croatia, Poland, Romania and Lithuania have some of the lowest estimated five‑year survival across the 11 cancer sites, with estimates in the lowest quintile for at least five cancer sites, suggesting important room for improvement. Countries that have around the average survival are interesting case studies. Estonia has among the lowest estimated five‑year survival probabilities for colon, rectum, liver, prostate and breast cancers, while having some of the highest estimates for cervix, ovary, pancreas and stomach cancers.
Table 2.3. Central and Eastern European countries tend to have the lowest estimated five‑year cancer survival
Age‑standardised five‑year net survival estimates (%) for patients diagnosed during 2010‑14
|
Stomach |
Colon |
Rectum |
Liver |
Pancreas |
Lung |
Breast |
Cervix |
Ovary |
Prostate |
AL. leukaemia in children |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cyprus |
§35.6 |
§72.1 |
§75.9 ↓ |
§10.6 ↓ |
§16.6 |
18.7 |
§92.8 |
§73.3 |
§46.4 |
§99.2 |
§86.6 |
|
Belgium |
37.5 |
67.9 |
66.6 |
20.7 |
12.4 |
18.2 |
86.4 |
65.4 |
43.1 |
93.8 |
90.8 |
|
Norway |
26.5 |
66.7 |
69.2 |
18.7 |
9.5 |
19.0 |
87.7 |
73.3 |
45.5 |
92.9 |
83.0 ↓ |
|
Sweden |
24.8 |
64.9 |
64.7 |
16.6 |
9.7 |
19.5 |
88.8 |
68.3 |
46.5 |
90.7 |
89.0 |
|
Iceland |
28.1 ↓ |
68.2 |
63.0 ↓ |
14.3 ↓ |
20.2 |
89.1 |
80.1 ↓ |
40.3 |
90.8 |
92.4 |
|
|
Germany* (10 registries) |
33.5 |
64.8 |
62.3 |
13.0 |
10.7 |
18.3 |
86.0 |
65.2 |
41.2 |
91.6 |
91.1 |
|
Portugal |
32.2 |
60.9 |
59.6 |
18.7 |
10.7 |
15.7 |
87.6 |
66.2 |
43.6 |
90.9 |
89.8 |
|
Austria |
35.4 |
63.7 |
64.2 |
§14.8 |
§10.5 |
19.7 |
84.8 |
63.9 ↓ |
41.0 |
90.2 |
|
|
France* (21 registries) |
26.7 |
63.7 |
60.9 |
18.3 |
8.6 |
17.3 |
86.7 |
65.0 |
43.5 |
93.1 |
88.6 ↓ |
|
Italy* (43 registries) |
30.5 |
64.2 |
61.3 |
20.3 |
9.2 |
15.9 |
86.0 |
66.8 |
39.4 |
89.5 |
87.8 ↓ |
|
Finland |
25.7 |
64.9 |
64.4 |
§10.4 |
§7.4 |
13.0 |
88.5 |
67.4 |
41.1 ↓ |
93.2 |
95.2 |
|
Netherlands |
25.0 |
63.1 |
65.3 |
15.8 |
7.4 |
17.3 |
86.6 |
67.5 |
37.5 |
88.5 |
90.4 |
|
Latvia |
28.0 |
56.5 |
53.3 |
12.9 |
13.7 |
20.4 |
82.2 |
56.0 ↓ |
45.5 |
90.4 |
84.1 |
|
Spain* (8 registries) |
27.6 |
63.2 |
59.5 |
17.3 |
7.7 |
13.5 |
85.2 |
64.5 |
39.8 |
89.7 |
84.7 |
|
Ireland |
27.6 |
60.5 |
61.7 |
14.2 |
9.6 |
17.5 |
82.0 |
63.6 |
32.8 |
91.1 |
88.3 |
|
Denmark |
19.9 |
61.6 |
64.8 |
7.5 |
8.0 |
16.6 |
86.1 |
69.5 |
39.7 |
85.6 |
94.0 |
|
Estonia |
29.2 |
58.4 |
54.8 |
4.2 ↓ |
10.2 |
16.9 |
76.6 |
66.5 |
42.3 |
86.3 |
87.7 |
|
Slovenia |
28.8 |
61.9 |
60.3 |
7.4 |
6.6 |
14.8 |
83.5 |
65.5 ↓ |
37.0 |
85.0 |
70.1 ↓ |
|
Lithuania |
27.0 |
56.9 |
52.7 |
§8.0 |
§7.0 |
9.9 |
73.5 |
59.2 |
35.0 |
94.3 |
74.7 |
|
Malta |
23.8 |
57.5 |
56.1 |
§0.0 |
§5.5 |
14.9 |
86.9 |
57.4 ↓ |
28.0 |
88.2 |
|
|
Romania* (Cluj) |
§26.0 |
§52.2 ↓ |
58.4 |
§13.2 |
§6.0 |
11.1 |
74.8 |
65.3 |
37.2 |
77.1 ↓ |
53.9 |
|
Poland |
20.9 |
52.9 |
48.4 |
10.8 |
8.0 ↓ |
14.4 |
76.5 |
55.1 |
37.5 |
78.1 |
86.9 |
|
Croatia |
20.0 |
51.1 |
48.2 |
§9.3 |
§8.4 |
10.0 |
78.6 |
63.2 ↓ |
36.0 |
80.9 |
85.2 ↓ |
|
Czechia |
20.6 |
56.1 |
52.3 |
6.7 |
6.1 |
10.6 |
81.4 |
61.0 ↓ |
36.5 |
85.3 |
88.2 ↓ |
|
Slovak Republic |
21.1 |
51.8 |
48.6 |
7.6 |
6.4 |
11.2 |
75.5 ↓ |
60.5 |
33.4 ↓ |
74.7 |
87.0 |
|
Bulgaria |
16.0 |
52.4 |
45.9 |
6.5 |
7.7 |
78.3 |
54.8 |
37.3 |
68.3 |
78.3 |
|
|
EU25 average |
26.8 |
60.2 |
58.8 |
11.9 |
9.0 |
15.1 |
83.2 |
63.8 ↓ |
39.2 |
87.3 |
85.1 ↓ |
Notes: AL leukaemia stands for acute lymphoblastic leukaemia. The colours correspond to quintiles of survival among the 26 countries, where dark red is the quintile with the lowest survival estimate, light red the second quintile, white the third quintile, light blue the fourth quintile and dark blue the quintile with highest survival estimate. Hungary and Luxembourg did not participate in CONCORD‑3; Greece only presents data for AL leukaemia in children, and was excluded from the table. Estimates for adults were age‑standardised using the International Cancer Survival Standard weights. For children (0‑14 years) age‑standardised estimates are derived by assigning equal weights to the three quinary age‑specific estimates (0‑4, 5‑9, 10‑14). § indicates survival estimate considered less reliable. Arrows (↓) indicate a decrease in probability of survival of more than 1 percentage point with respect to people diagnosed in 2005‑09. Five‑year net survival refers estimates to the cumulative probability that the cancer patient would have lived five years after diagnosis after correction for other causes of death. Countries with * present estimates covering only part of the population. EU25 average is a non-weighted average of the 25 EU countries in the dashboard.
Source: Allemani, C. et al. (2018[8]), “Global surveillance of trends in cancer survival 2000‑14 (CONCORD‑3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries”, https://doi.org/10.1016/s0140-6736(17)33326-3.
Denmark and Finland have low estimated five‑year survival probabilities for stomach and liver cancer (in the worst and second worst performing quintiles). At the same time, both countries have among the highest estimates (in the best performing quintiles) for breast, prostate and childhood acute lymphoblastic leukaemia (in Finland), and for cervix, rectum and childhood acute lymphoblastic leukaemia (in Denmark) (see Table 2.3). More recent data on estimated five‑year survival in these two countries are presented in Box 2.2. Updated data indicate a clear upward trend for survival probabilities in all cancers combined, suggesting an overall improvement compared to Table 2.3.
Box 2.2. The NORDCAN survival data combine updated information on five countries
The NORDCAN platform is a valuable compilation of data from the cancer registries of five countries: Denmark, Finland, Iceland, Norway and Sweden. These registries, which include some of the oldest population-based registries in the world, have been providing complete coverage of the now combined population of 27 million for over 60 years. The registries adhere to high quality standards in terms of data completeness and accuracy. The data include or can be linked to a wide range of information such as date of diagnosis, topography, histology, behaviour, method of confirmation, stage at diagnosis and treatment. Despite small variations in registration, screening and coding practices between the countries, information in the Nordic cancer registries is generally similar and more comparable than other international collections of data. Figure 2.5 shows an example of data extracted from NORDCAN.
Figure 2.5. Cancer survival estimates are increasing rapidly in Nordic countries
Age‑standardised five‑year relative survival for ten cohorts of men and women
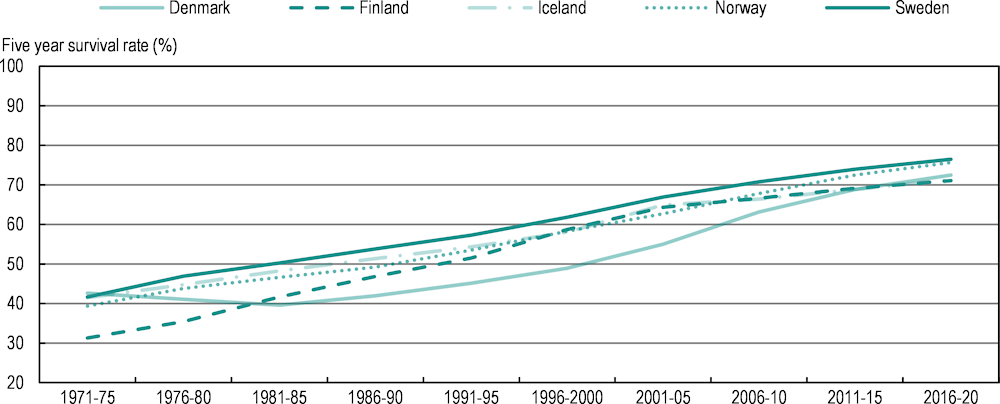
Notes: Relative survival is the ratio of the observed survival proportion to the expected survival proportion for patients diagnosed in several periods of time. The figure presents the simple average between men and women per country.
Source: Pukkala, E. et al. (2017[34]), “Nordic cancer registries – an overview of their procedures and data comparability”, https://doi.org/10.1080/0284186x.2017.1407039; Larønningen, S. et al. (2023[35]), NORDCAN: Association of the Nordic Cancer Registries, https://nordcan.iarc.fr/.
The positive trend in Nordic countries has been attributed to several factors, including new treatments that have improved the probability of curing or improving survival probabilities for several types of cancer (Hemminki, Försti and Hemminki, 2021[36]; Hemminki et al., 2022[37]). At the same time, earlier diagnosis through better imaging, biomarkers and screening strategies has had a direct impact on improving cancer survival, and is central to the cancer strategies of these countries (OECD, 2023[38]; OECD, 2023[39]; OECD, 2023[40]; OECD, 2023[41]; OECD, 2023[42]). Early diagnosis, by definition, increases the length of survival directly as the course of the cancer becomes longer. More importantly, earlier diagnosis can improve survival probabilities by enabling use of treatments that are not available at later stages (such as surgery or adjuvant therapies). Early diagnosis also has implications for tumour size and stage, as smaller tumours in earlier stages may respond better to treatment (Hemminki, Försti and Hemminki, 2021[36]; Burki, 2020[43]).
2.1.4. Disruptions caused by COVID‑19 are expected to increase the cancer burden in the short term
The COVID‑19 pandemic affected cancer care significantly, disrupting prevention efforts, screening, diagnosis, treatment and access to medications. The pandemic led to a decrease in cancer diagnoses, which could indicate a future increase in cases (European Commission, 2022[4]), and the delays in both treatment and diagnosis are expected to reduce survival probabilities and increase cancer costs and mortality.
Since 2020, cancer screening, diagnosis and treatment have faced an unparalleled challenge due to COVID‑19 (WHO, 2023[44]). This was particularly the case during the first wave of infections in early 2020, which led many countries to take containment measures, leading to the slowdown or even cessation of certain healthcare services. Organised cancer screening programmes were significantly reduced (see Chapter 4), which contributed to a major drop in cancer diagnoses over the period (Angelini et al., 2023[45]). For example, comparing April 2019 to April 2020 in Belgium, rates of diagnosis of invasive tumours fell by 44% (Peacock et al., 2021[46]). In Spain, the number of cancers diagnosed at the national level in February 2021 was 13% lower than in March 2019 (Ministry of Health, 2023[47]), and the number in the Catalonia region was 34% lower than expected between March and September 2020 (Sagan et al., 2021[48]). In England (United Kingdom), in April 2020, there were significant reductions in cancer referrals (‑63%) and colonoscopies (‑92%) compared to the 2019 monthly average, leading to a 22% decrease in cases referred for treatment. Although rates returned to 2019 levels by October 2020, around 3 500 fewer colorectal cancer cases were diagnosed and treated in England than would have been expected between April and October 2020 (Morris et al., 2021[49]). Slovenia conducted one of the first studies documenting the effect of the COVID‑19 pandemic on cancer care, exposing reductions of 43% for pathohistological and 29% for clinical cancer notifications between November 2019‑February 2020 and April 2020 (Zadnik et al., 2020[50]). In a follow-up study, it was suggested that new cancer diagnoses in the country dropped by 6% in 2020, 3% in 2021 and 8% in July 2022, with the largest drops seen in the 50‑64 age group (almost 14% in 2020 and 16% in 2021) (Zagar et al., 2022[51]).
Delays in cancer diagnosis lead to – and the consequences are exacerbated by – delays in medical, surgical or radiotherapeutic treatment, resulting in poorer health outcomes such as higher risk of death (Hanna et al., 2020[52]) and costs. Depending on the type of cancer, a four‑week delay in surgery is associated with a 6‑8% increase in the risk of death, while a four‑week delay in systemic treatment increases the risk of death by between 1% and 28%. For example, in breast cancer, a four‑week delay in surgery increases the risk of death by 8%, which grows to 17% for an eight‑week delay and 26% for a 12‑week delay. Similarly, a four‑week delay in colectomy increases the risk of death by 6%, and a four‑week delay in cervical cancer adjuvant radiotherapy increases the risk of death by 23% (Hanna et al., 2020[52]). A Canadian model predicts that cancer care disruptions during the COVID‑19 pandemic could lead to around 2.0% more cancer deaths in Canada during 2020‑30 (Malagón et al., 2022[53]).
Moreover, many cancer patients worldwide are challenged by a cost-of-living crisis. The costs of treatments that were delayed by the pandemic may force patients to make choices such as cutting back on essentials that can influence health outcomes. This issue spans country income levels. People in high-income and middle‑income countries can spend over 15% of household income on cancer-related out-of-pocket costs, while the figure in lower-income countries can reach 40% (defined by the World Health Organization (WHO) as the level of catastrophic health expenditure). There is a risk that the exacerbated financial burden started by the pandemic and enhanced by the cost-of-living crisis may push cancer patients away from continuing treatment (Lancet Oncology, 2022[54]).
Amid global recovery from COVID‑19, governments and healthcare authorities worldwide must urgently address the challenges in cancer services. Decisive action is imperative, as disrupted referrals and clinical pathways lead to mounting backlogs of undiagnosed patients and overwhelmed healthcare workers. There are high risks of more patients being diagnosed with advanced disease and an increase in avoidable premature deaths. In the United Kingdom, the crisis is described as the most severe in four decades, demanding immediate investments – such as GBP 325 million for diagnostics – to fortify overstretched cancer services (Wilkinson, 2021[55]). In contrast, in some countries, cancer services recovered quickly from the pandemic, resulting in little to no effect of the service delays on cancer outcomes. In the Netherlands, the average two‑year probability of survival among patients diagnosed during the pandemic (2020‑21) was 76%, which is 1% higher than for patients diagnosed before the pandemic (2015‑19). While a long-term negative effect cannot be ruled out, it is unlikely given the strong correlation between short- and long-term cancer survival (IKNL, 2023[56]).
2.2. The burden of cancer differs widely within countries between regions and population subgroups
2.2.1. Regions in the same country often have vastly different cancer burdens
Cancer incidence rates in Bulgaria, Portugal, Latvia and Austria vary markedly between regions
Evaluating patterns of cancer incidence between regions or other geographical areas enables policy makers to examine how the cancer burden varies within a country, helping to improve understanding of the causes and risks associated with cancer. Highlighting differences between geographical areas helps in developing appropriate policy options. In 2023, 18 EU+2 countries provided age‑standardised cancer incidence rates by region. Figure 2.6 illustrates the percentage difference between the region with the highest and lowest incidence rates in each. Geographical variation was most pronounced in Bulgaria, Portugal, Latvia and Austria, with regional variation in incidence rates of more than 50%. Denmark and Norway had the lowest variation in incidence rates. Geographical disparities in cancer incidence reflect variations in the prevalence of cancer risk factors – such as behavioural and environmental factors (see Chapter 3), and social and economic disadvantage (at both individual and ecological levels) – but are also partly determined by access to cancer screening programmes (see Chapter 4).
Figure 2.6. Cancer incidence rates within a country differ between regions
Percentage difference between regions with the highest and lowest cancer incidence rates, latest year
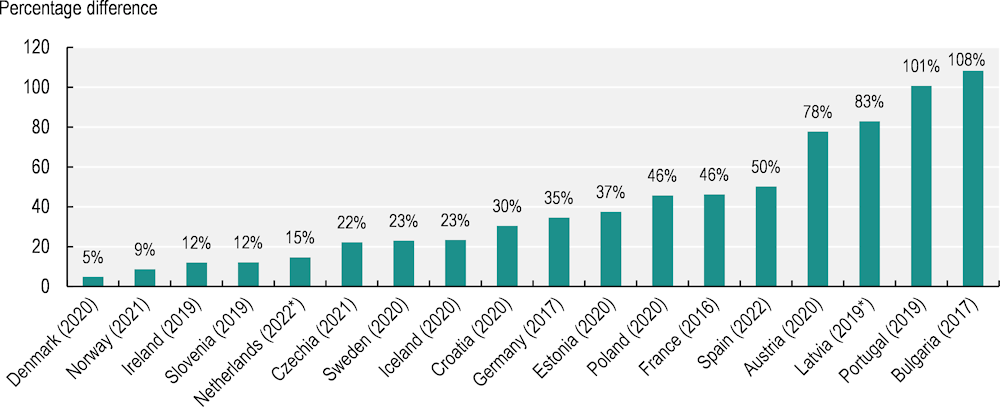
Notes: Data are not directly comparable between EU+2 countries due to different methodologies and years of observation. For Denmark, Norway, Ireland, Sweden, Iceland, Croatia, Germany, Czechia and Bulgaria, an average of the regional differences for men and women was used. Other countries provided a measure for the total population. For Czechia, the regional incidence data only consider breast cancer for women and prostate cancer for men. * Provisional data.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
Regional differences in survival probabilities offer opportunities to target intervention
In the CONCORD‑3 study, regional variations in five‑year net survival probabilities are presented for many cancers in 22 countries (Allemani et al., 2018[8]). For example, for cancers in adults, Portugal has 4 registries (100% population coverage), while Poland has 16 registries (also 100% coverage). Spain provided data from 8 registries (20% coverage), Germany from 10 registries (37% coverage), France from 21 registries (22% coverage) and Italy from 43 registries (58% coverage).
These differences affect the range of variation between registries, and limit the comparability and interpretation of the data. With these limitations in mind, the regional variations in survival probabilities nevertheless offer opportunities for policy makers to use these variations to target interventions.
Regional variations in survival estimates for selected countries are presented in Figure 2.7: each box-plot shows the range of survival estimates among all cancer registries for which suitable estimates could be obtained, for patients diagnosed with liver, breast and cervix cancers in each country (France, Germany, Italy, Poland, Portugal and Spain). The number of registries included is shown in parentheses. The horizontal line inside each box represents the median survival estimate among all contributing registries (50th centile). The rectangular box covers the inter-quartile range (IQR) between the lower and upper quartiles (25th and 75th centiles). The extreme limits of the box-plot are 1.5*IQR below the lower quartile and 1.5*IQR above the upper quartile. Dots indicate “outlier” values outside this range. Overall, larger within-country differences in survival are found for liver and cervical cancer.
Figure 2.7. The range of age‑standardised five‑year net survival estimates in six countries for patients diagnosed during 2010‑14 is wide
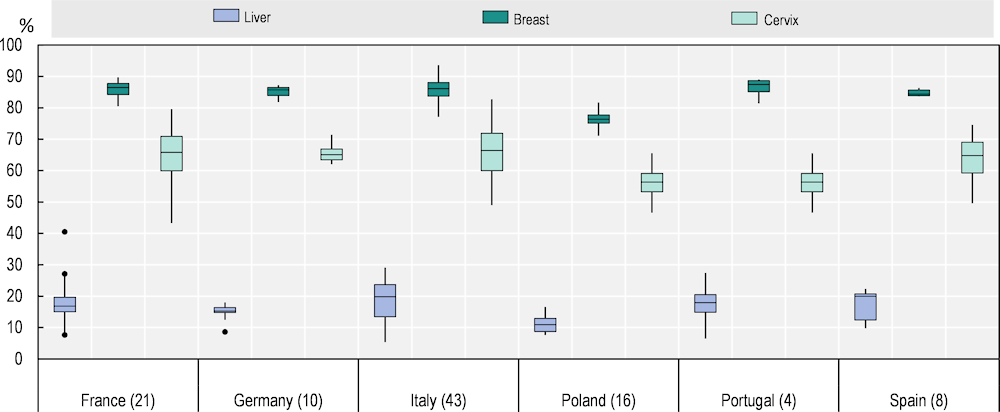
Notes: Outliers identified using Tukey’s rule (below or above ). Survival estimates considered less reliable by CONCORD‑3 were excluded.
Source: Allemani, C. et al. (2018[8]), “Global surveillance of trends in cancer survival 2000‑14 (CONCORD‑3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries”, https://doi.org/10.1016/s0140-6736(17)33326-3.
Cancer mortality rates vary by up to 37% between regions within a country
The large geographical disparities in cancer incidence and survival are consistent when considering cancer mortality rates by European NUTS2 regions (Figure 2.8). The largest within-country differences in overall cancer mortality (excluding outermost regions as defined by the EU4) can be found in Romania, where Bucuresti-Ilfov had 37% higher cancer mortality rates than Sud-Vest Oltenia in 2020. There were also large regional disparities in overall cancer mortality in Poland, France, Spain and Germany, with at least a 30% variation in mortality rates. By contrast, Slovenia, Ireland, the Slovak Republic and Lithuania had smaller geographical disparities in cancer mortality in 2020. As shown in Table 2.2, the map shows a clear disadvantage in Central and Eastern European countries, which have the highest cancer mortality rates, while rates are lower in the Nordic countries.
Figure 2.8. Cancer death rates vary significantly by region in Romania, Poland, France, Spain and Germany
Age‑standardised cancer mortality rate per 100 000 population by NUTS2 region, 2020
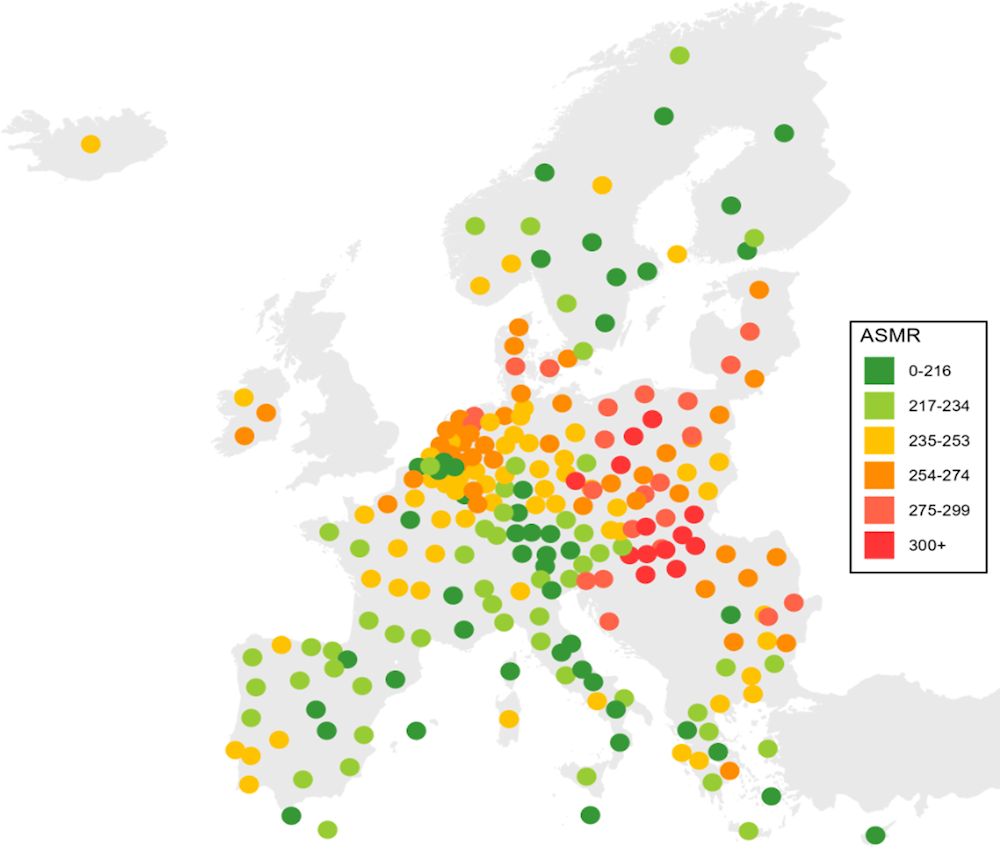
Note: The map is based on cancer mortality rates in 2020. In Iceland, the 2020 mortality rate is a five‑year rolling average (2016‑20).
Source: Eurostat (2023[57]), Causes of Death – Standardised Death Rate by NUTS 2 Region of Residence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ASDR2__custom_6414996/default/table.
2.2.2. The proportion of deaths attributable to cancer is highest among the population aged 50‑69
It is estimated that nine out of ten new cancers in the 29 EU+2 countries in 2022 occurred among people aged over 50. This trend is consistent around the globe, with cancer cases and cancer deaths in this age group accounting for more than 85% of the cancer burden (Lin et al., 2021[58]).
However, the proportion of all deaths attributed to cancer is higher in the group aged 50‑69: 37% of all deaths among this age group were attributable to cancer in the 29 EU+2 countries in 2020, compared to 19% among those aged 70‑85 and over (Figure 2.9). Among children (aged less than 15 years), cancer represented 7% of all deaths. This highlights the need to prevent cancer for middle‑aged population groups and to identify the disease at an earlier stage.
Figure 2.9. The share of cancer deaths among all deaths is highest among the 50‑69 age group
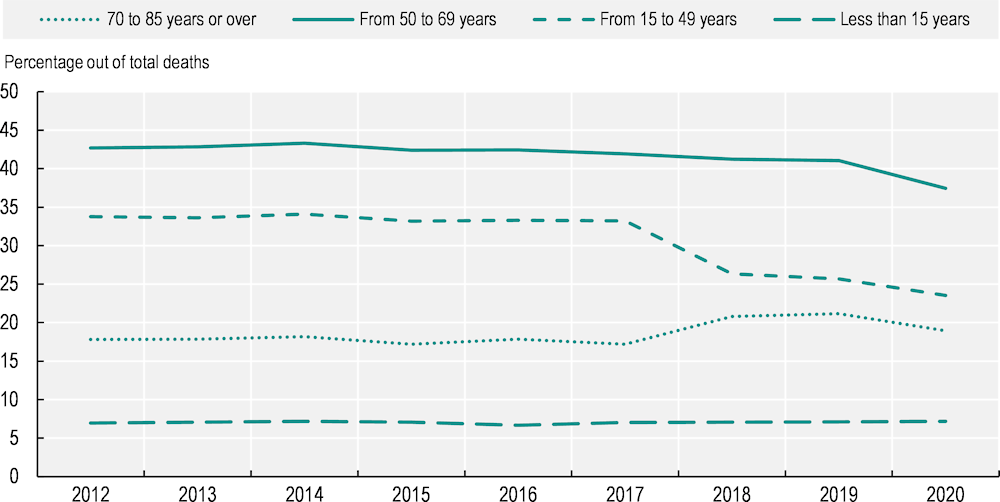
Note: The graph shows the total number of cancer deaths divided by the total number of deaths per age group in EU+2 countries.
Source: Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
The most common cancers vary by age group in the EU27, highlighting the need for age‑appropriate awareness, prevention and early detection programmes (see Chapters 3 and 4). Leukaemia is the most common malignancy in children (aged less than 15 years), accounting for around 33% of new malignancies among boys and 30% among girls (Figure 2.10). Among young adults (15‑49 years), testicular cancer and skin melanoma are the most common new cancers in men, while breast and thyroid cancers are the most common in women. According to ECIS data (2023[1]), up to 83% of testicular cancers arise in young adult men, and 18% of breast cancers in young adult women. Cervical cancers are also common among women in the same age group (36% of cervical cancers are detected in women aged 15‑49), necessitating amplified awareness and effective screening strategies (ECIS, 2023[1]). For adults aged 50‑69, cancers of the breast, prostate and lung are the most common. From the age of 70, prostate and breast cancers continue to dominate, followed closely by colorectal and lung cancers.
Figure 2.10. The most common cancers vary by age group
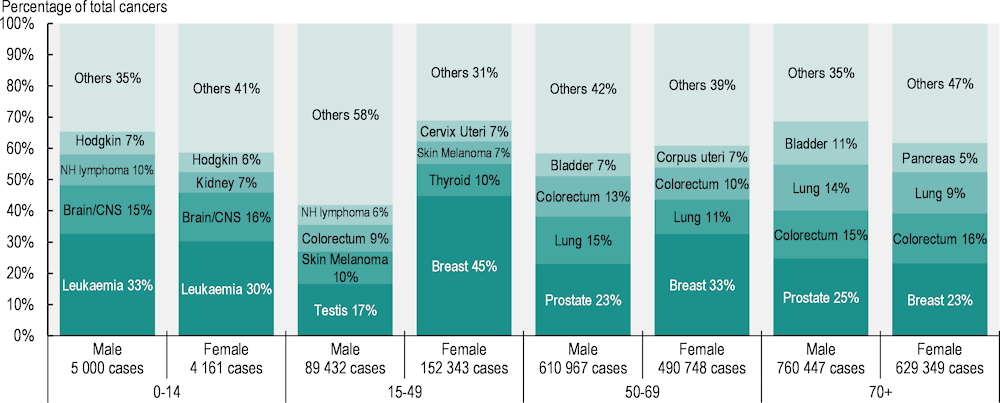
Notes: Data relate to all cancer sites except non-melanoma skin cancer. Panels show the proportion of all new cancer cases estimated in 2022 in the EU27 by age at diagnosis. Lung cancer includes trachea and bronchus. CNS stands for central nervous system; NH lymphoma stands for Non-Hodgkin lymphoma; Hodgkin refers to Hodgkin disease (Hodgkin lymphoma).
Source: ECIS (2023[1]), European Cancer Information System, https://ecis.jrc.ec.europa.eu (accessed on 27 April 2023).
2.2.3. Men have a higher cancer burden than women in all EU+2 countries
Men were more likely to be diagnosed with cancer in 2022 (see also Table 2.1). Across the EU27, cancer incidence rates were 40% higher among men than women, with incidence for men estimated at 684 per 100 000 compared to 488 per 100 000 for women. Incidence was higher in 2022 for men than for women in all age groups, apart from those aged 15-49. Among young adults, estimated cancer diagnoses were substantially higher for women, with incidence estimated at 156.5 per 100 000 women aged 15-49 compared to 90.8 per 100 000 men of this age. Furthermore, the difference in expected age‑adjusted incidence rates between the sexes in 2022 was slightly less than it was in 2020 (42%). This is because cancer incidence was expected to remain unchanged among men, while it was expected to increase by 1% among women between 2020 and 2022.
Similarly, in 2020, cancer mortality in the EU27 was 69% higher among men than women (Figure 2.11). While the gender gap in cancer mortality was still prominent, it had steadily decreased since 2010, when it stood at 84% (Eurostat, 2023[2]). Countries with the highest gender gaps in cancer mortality were the Baltic countries (Lithuania, Latvia, Estonia), Portugal and Spain. Some Nordic countries (Iceland, Denmark and Sweden) and Ireland had the smallest gender gaps among EU+2 countries.
Figure 2.11. Men are more likely to be diagnosed with and die from cancer
Relative difference between men and women (%), estimated cancer incidence in 2022 and observed cancer mortality in 2020
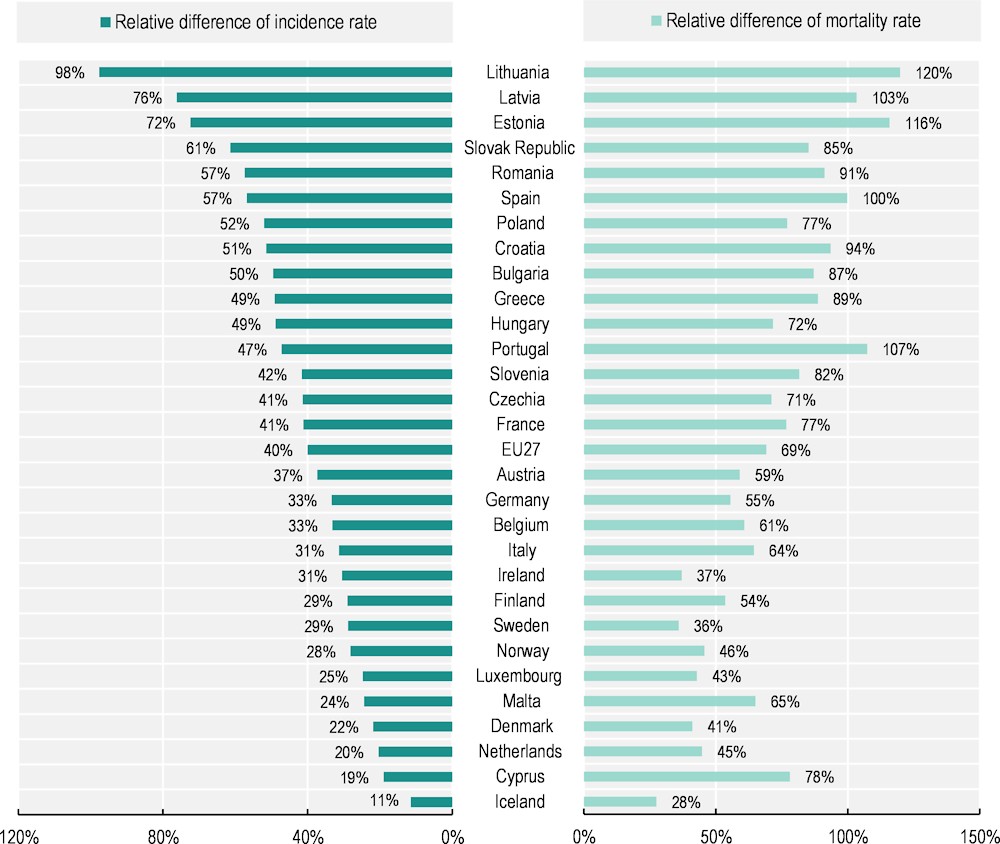
Notes: Estimated national age‑standardised rates (European new) per 100 000 population. Incidence estimates were created before the COVID‑19 pandemic, based on incidence trends from previous years, and may differ from observed rates in more recent years. Incidence rates are calculated for all cancers except non-melanoma skin cancer, while mortality rates correspond to all malignant neoplasms. The EU27 average for mortality rate is calculated as a population-weighted average.
Source: Incidence data from ECIS (2023[1]), European Cancer Information System, https://ecis.jrc.ec.europa.eu (accessed on 27 April 2023); mortality data from Eurostat (2023[2]), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table.
2.2.4. The burden of cancer falls disproportionately on socio‑economically disadvantaged groups
Education-related inequalities in cancer mortality are higher in Baltic, Central and Eastern European countries
Socio‑economic inequalities in the cancer burden have been identified in several EU+2 countries (Vaccarella et al., 2023[59]; Launoy, Zadnik and Coleman, 2021[60]). Systematic differences in cancer incidence, survival and mortality are observed between social groups, most often assessed on the basis of education levels, income levels, ethnicity or migration background.
A recent study of 18 European countries confirmed that people with lower education levels diagnosed during 1990‑2015 had higher mortality rates for nearly all cancer types than their more educated counterparts (Vaccarella et al., 2023[59]). For total cancer, the age‑standardised mortality rates in men were more than twice as high among those in lower than higher education groups in Czechia, Estonia, Hungary and Poland. Overall, the analyses show that education-related inequalities were generally higher in Baltic, Central and Eastern European countries and smaller in Southern Europe. Among women, the largest inequalities in cancer mortality were found in Nordic countries.
Inequalities are especially notable for tobacco-related and infection-related cancers. Preliminary findings from the EUCanIneq study, which aims to develop relevant indicators of socio‑economic inequality in cancer mortality in the EU as part of the European Cancer Inequalities Registry, shows that lung cancer mortality rates were 2.6 times as high among men with lower than higher levels of education, and 1.7 times as high among women with lower than higher levels of education. However, the magnitude of inequalities varied significantly between countries. For men, the net difference in all-cancer mortality rates per 100 000 population between those with lower and higher education levels varied widely, ranging from 50 in Sweden to 203 in Estonia (Figure 2.12). Among women, the difference between education groups was highest in Denmark (102) and Norway (108).
Figure 2.12. Lung cancer mortality rates among men vary with education level in all countries
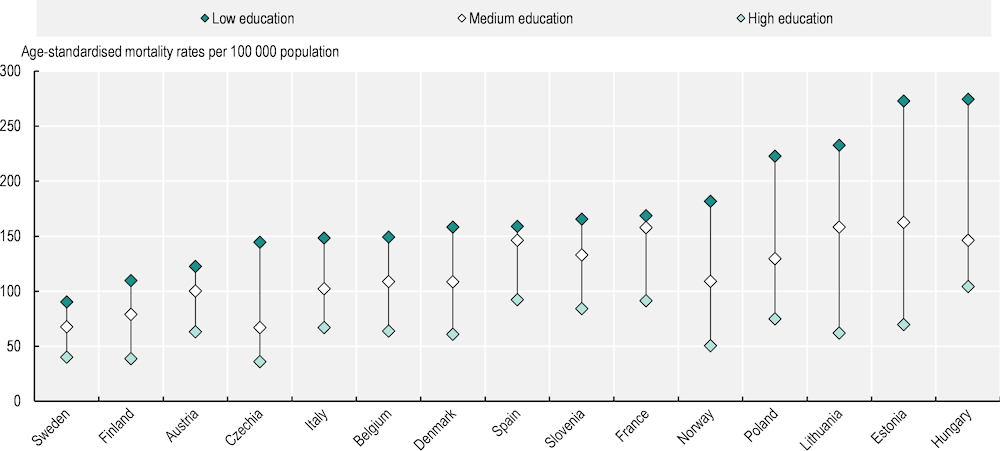
Note: Caution is recommended when interpreting results, as the data are based on predictions for 2015‑19, with different methodology across countries and varying level of population coverage.
Source: Preliminary findings from the EUCanIneq study.
A national study in Italy also showed that, in virtually all regions, cancer mortality was higher among those with lower education levels than among those with higher education levels in 2012‑14, for both men and women (Petrelli et al., 2019[61]).
A clear social gradient in cancer survival has also been observed in several countries. In Slovenia, Ireland and Germany, people living in more deprived areas have consistently lower five‑year survival probabilities than those living in less deprived areas (Box 2.3).
Box 2.3. National studies provide evidence on the association between deprivation level and cancer survival – examples from Slovenia, Ireland and Germany
In Slovenia, the European Deprivation Index (a composite measure of socio‑economic environments based on education, employment status and household composition, among other variables) has been associated with survival trend (Figure 2.13). Survival for all cancer sites was considerably higher for the more affluent population diagnosed in 2014‑18 than for patients from more deprived groups. The five‑year survival estimate for all cancer combined was 9 percentage points higher in the most affluent (63%) than the most deprived (54%) population group.
Figure 2.13. The five‑year net survival estimate is significantly higher in the least socio‑economically deprived group in Slovenia
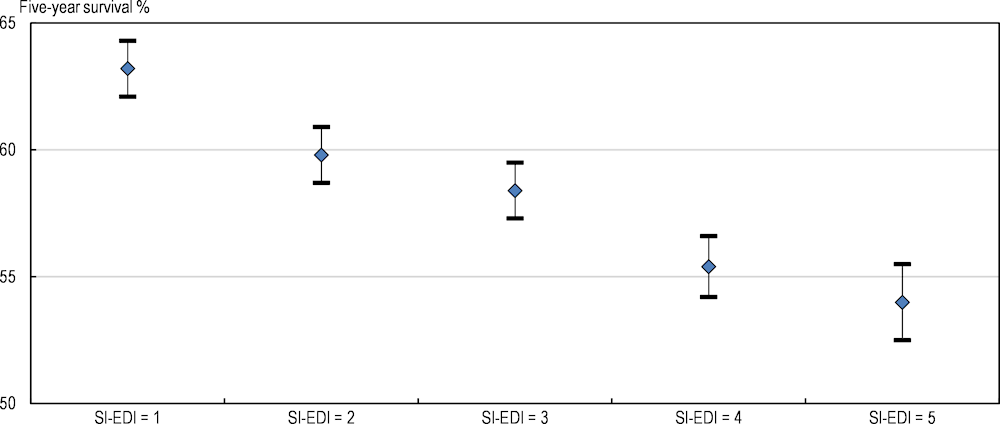
Notes: Shows 95% confidence interval. All adult cancer patients diagnosed between 2014 and 2018. SI-EDI is the Slovenian version of the European Deprivation Index (EDI) which is an index of relative deprivation. SI-EDI = 1 is the most affluent group and SI-EDI = 5 is the most deprived group.
Source: Zadnik, V. et al. (2022[62]), “Cancer patients’ survival according to socioeconomic environment in a high-income country with universal health coverage”, https://doi.org/10.3390/cancers14071620.
A recent study in Germany examined five‑year survival probabilities for patients diagnosed during 2012‑14 using the German Index of Multiple Deprivation. It found that survival probabilities were significantly higher in the most affluent quintile than in the most deprived quintile for 17 of 25 cancers, and for all cancer combined (with an average deprivation gap of 2.6 percentage points) (Finke et al., 2021[63]).
Similar gaps are seen in Ireland (Figure 2.14), where the five‑year survival estimate for invasive cancers was 68% among those in the most affluent quintile compared with 59% among those in the most deprived quintile for cancers diagnosed during 2014‑18. While survival estimates have improved over time for all deprivation levels, a deprivation gap in five‑year survival of 8‑10 percentage points persists between the most and least deprived groups (Bambury et al., 2023[64]).
Figure 2.14. Cause‑specific five‑year survival estimates for invasive cancer vary by deprivation quintile and diagnosis period in Ireland
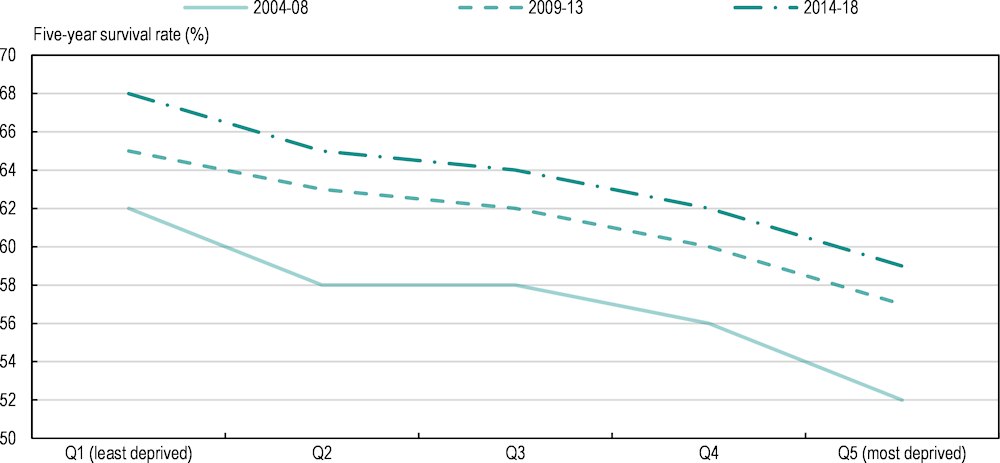
Note: Excludes non-melanoma skin cancer; both sexes combined.
Source: Bambury, N. et al. (2023[64]), Cancer Inequalities in Ireland by Deprivation, 2004-2018: A National Cancer Registry report, NCRI, Cork..
Among socially disadvantaged populations (those on lower income or with fewer years of education) or those living in more economically deprived areas, a handful of studies have shown higher incidence of various cancers in European countries. These include lung cancer (e.g. in Denmark, France, Slovenia and the United Kingdom (England and Scotland)), cervical cancer (e.g. in Denmark, England (United Kingdom) and France), oesophagus (e.g. in Denmark, France and Slovenia) and lip, oral cavity and pharynx (e.g. in Denmark and France) (Dalton et al., 2008[65]; Bryere et al., 2014[66]; Bryere et al., 2018[67]; Derette et al., 2022[68]; Lokar, Zagar and Zadnik, 2019[69]; Shack et al., 2008[70]; Tweed et al., 2018[71]). In France, socially disadvantaged men have higher incidence rates of larynx, pancreas and bladder cancers, and the relative risk between the lowest and highest socio‑economic groups can reach 1.9 for cancers of the lip, oral cavity and pharynx (Bryere et al., 2014[66]; Bryere et al., 2018[67]). A higher incidence of head and neck cancer among disadvantaged groups has been reported in Slovenia (Lokar, Zagar and Zadnik, 2019[69]).
Conversely, for some cancers, there is a reverse social gradient. These include prostate cancer (e.g. in Denmark, France, Scotland (United Kingdom) and Slovenia), breast cancer (e.g. in Denmark, France, Ireland and the United Kingdom (England and Scotland)) and malignant melanoma (e.g. in England (United Kingdom), France, Denmark, Ireland and Slovenia) (Dalton et al., 2008[65]; Bryere et al., 2014[66]; Bryere et al., 2018[67]; Lokar, Zagar and Zadnik, 2019[69]; Tweed et al., 2018[71]; Shack et al., 2008[70]; Bambury et al., 2023[64]). A reverse social gradient in breast cancer incidence can be explained by several factors, including differences in hormonal patterns, childbearing practice or other biological factors.
Beyond differences in access to healthcare and cancer treatment, possible explanations for the relationship between cancer mortality rates and deprivation level are health behaviour differences and higher environmental exposure to risk factors. Smoking behaviour, diet and physical activity vary between socio‑economic groups (see Chapter 3). Participation in screening programmes also plays a role in the differences in mortality rates. Individuals from lower socio‑economic groups are less likely to participate in screening programmes (Chapter 4), leading to later diagnosis and higher mortality (Poiseuil et al., 2023[72]).
A healthy migrant effect can be observed in many EU+2 countries
Because of a lack of information on ethnicity, nationality or country of birth in many cancer registries, there is a crucial gap in research on inequalities in cancer outcomes by ethnicity or migration status. In several EU+2 countries, studies suggest a lower risk of cancer or cancer mortality for foreign-born people compared to native‑born people. In Denmark, Finland, Iceland and Norway, non-Western immigrant women have a lower risk than the native‑born population to develop breast (‑29%), colorectal (‑28%) and lung cancer (‑45%) initially after migration; however, the likelihood increases with the length of stay in the host country (Lamminmäki et al., 2023[73]). They also have a lower risk of dying from breast (‑36%), colorectal (‑34%) and lung cancer (‑49%) than native women. Similar findings have been shown in Spain, where the risk of premature cancer mortality (after controlling for individual characteristics) is lower among migrants than natives, but the advantage decreases with increasing length of residence in the host country (Grande, Garcia-Gonzalez and Stanek, 2023[74]). These results corroborate the so-called “healthy migrant effect”, which suggests that migrants are often in better health than the native‑born population on arrival in the host country, but that their health deteriorates with length of residence. This worsening health status over time may occur as a result of lifestyle changes, wherein migrants change from more traditional to Westernised lifestyles (such as by increasing smoking rates, gaining excess body weight and changing to less healthy diets) and become more sedentary (Labree et al., 2011[75]). In addition, challenges in access to healthcare for migrants – including cost, language and cultural barriers, poor health literacy and discrimination – may all contribute to the decline in health status (Bradby, Hamed and Lebano, 2019[76]). Low socio‑economic status and weaker social networks may also contribute to the worsening of migrants’ health status (Berchet and Jusot, 2012[77]).
Data from the Survey of Health, Ageing and Retirement in Europe (waves 4 to 8) – which inquires whether people currently have a cancer diagnosis – also suggest a “healthy migrant effect” in countries with available data. Controlling for all core individual characteristics and country-specific effects, the analysis confirms the negative association between citizenship and self-reported cancer diagnosis (Table 2.4). Pooled estimations suggest that non-citizen populations are less likely to report a cancer diagnosis than citizens of the country of residence. This may be because non-citizen populations have less access to cancer diagnosis services than citizen populations (as shown in Chapter 4). The analysis also points to the importance of income: in many countries, people with higher income are less likely to report a cancer diagnosis than people on lower income. On the other hand, people with higher education are more likely to report cancer diagnoses, which may be because they are more likely to participate in screening programmes (as shown in Chapter 4).
Table 2.4. Non-citizen populations are less likely to report having a cancer diagnosis in EU+2 countries
|
Individual characteristics |
Likelihood of reporting a cancer diagnosis |
|
|---|---|---|
|
Controls |
Age, sex, household |
All demographic, household, socio‑economic and lifestyle characteristics |
|
Older ages compared to younger ages |
↑ (***) |
↑ (***) |
|
Women compared to men |
↓ (***) |
↓ (***) |
|
Non-citizens compared to citizens |
↓ (**) |
↓ (***) |
|
Rural areas compared to urban areas |
↓ (*) |
↓ (NS) |
|
Highest income quartile compared to lowest quartile |
↓(**) |
|
|
Highest education level compared to lowest level |
↑ (***) |
|
Notes: Probit estimation with N = 139 551 longitudinal observations of 50+ individuals living in a private household in 20 countries. * p < 0.10, ** p < 0.05, *** p < 0.01, NS stands for non-significant result. An up arrow indicates positive marginal effects, and a down arrow indicates negative marginal effects (for example, non-citizen populations have a lower likelihood of reporting a cancer diagnosis than citizen populations).
Source: The Survey of Health, Ageing and Retirement in Europe, waves 4, 5, 6, 7 and 8.
Outside the EU27, in the United Kingdom, a recent analysis showed that Asian and Black people have lower cancer incidence rates for most cancers than White people (Cancer Research UK, 2022[78]), with significantly lower risk for melanoma of the skin and smoking-related cancers. These differences may be explained by genetic and biological factors (such as skin susceptibility) and ultraviolet exposure behaviours for melanoma risk, but also by preventable risk factors such as smoking, overweight and obesity rates, which are often higher among White people than other ethnic groups.
Nevertheless, given the higher prevalence of infection-driven cancer risks among migrants and ethnic minority groups, as well as reduced access to prevention and other healthcare services, and exposure to unhealthy environments in host countries (including air pollution, nutrition and physical activity challenges, among others), the health risks faced by migrant populations in Europe warrant monitoring (Chapter 3). More research is needed to monitor inequalities in cancer risk and survival probabilities by migration status and ethnicity. Data on race and place of birth would need to be captured routinely by cancer registries, but recording of ethnicity is not permitted in some countries (e.g. France and Germany). Analysis of such data would help to improve understanding of the differences between population groups, monitor trends in inequalities and inform targeted policy to improve access to prevention, early diagnosis and treatment.
2.3. Cancer care policies are converging in EU+2 countries
2.3.1. Europe’s Beating Cancer Plan defines an overarching strategic vision to help the EU27 tackle cancer
Officially launched in February 2021 by the European Commission, Europe’s Beating Cancer Plan is the EU’s response to the cancer burden. The main objectives of this comprehensive initiative are to reduce the burden of cancer by focusing on prevention, early detection, diagnosis and treatment, and to improve the quality of life of cancer patients and survivors. The plan comprises eight key components that address various aspects of cancer care. First, it emphasises primary prevention by promoting healthier lifestyles, including tobacco control, improved nutrition and increased physical activity. Second, it aims to enhance cancer screening and early detection programmes to ensure timely diagnosis and treatment. The plan also focuses on improving access to affordable, high-quality cancer care for all patients, with a particular emphasis on reducing inequalities in treatment and improving cancer patients’ quality of life. Additionally, it promotes research and innovation in cancer prevention and treatment, fostering collaboration among EU Member States and encouraging the development of innovative therapies and technologies. Finally, the plan puts a special focus on childhood cancer.
The plan is supported by ten flagship initiatives and various actions planned between 2021 and 2030 (European Commission, 2022[4]). These include establishment of a European Cancer Imaging Initiative to improve the quality and accessibility of imaging technologies for cancer diagnosis, adoption of the Regulation on Health Technology Assessment, publication of the EU Country Cancer Profiles (OECD, 2023[10]) of the European Cancer Inequalities Registry, and adoption of the 2022 recommendation of the European Council on cancer screening. Europe’s Beating Cancer Plan has facilitated exchange of best practices among Member States and encouraged collaboration between stakeholders, including patients, healthcare professionals and research institutions. In addition, the EU’s Cancer Mission is another key effort to provide better understanding of cancer, to facilitate earlier diagnosis and optimisation of treatment, and to improve cancer patients’ quality of life. The Mission is supporting Europe’s Beating Cancer Plan by enabling and accelerating research and dialogue with both Member States and stakeholders.
2.3.2. National cancer plans have been implemented in 25 EU+2 countries
The European Partnership for Action Against Cancer (EPAAC, 2011[79]) played a significant role in promoting the development and implementation of national cancer plans within the EU. It created a collaborative platform for Member States to exchange knowledge, share best practices and align their efforts in addressing cancer. This work resulted in the European Guide for Quality National Cancer Control Programmes (Albreht et al., 2014[80]).
In 2009, the EU agreed that Member States would each implement an integrated national cancer plan by 2013 (European Commission, 2009[81]). However, in 2023, only 25 of the 29 EU+2 countries have effectively put one in place. The Netherlands, Finland, Bulgaria and Greece have not yet implemented a national cancer plan, but the situation in each of these four countries is substantially different. In the Netherlands, the government invests in initiatives that are aligned with Europe’s Beating Cancer Plan, and a Dutch cancer agenda is co‑ordinated by the Netherlands Cancer Collective (organised by the Dutch Cancer Society, the Netherlands Comprehensive Cancer Organisation and the Netherlands Patient Association). This is supported by a comprehensive national cancer registry and a robust clinical auditing system (OECD, 2023[82]). In Finland, the cancer agenda is managed by government initiatives and several other stakeholders, including the Cancer Society of Finland and the National Cancer Centre, which serves as a centre of cancer expertise (OECD, 2023[38]). Despite an organised cancer strategy, organisations in these countries – such as the Cancer Survivorship Care Taskforce in the Netherlands – are lobbying for a national cancer plan to improve the organisation of cancer care. Bulgaria is in the process of approving and implementing a national cancer strategy that will follow the guidelines established by Europe’s Beating Cancer Plan. The national cancer plan was approved by the Council of Ministers in January 2023, but implementation remains a challenge. Coverage of national screening programmes is low, and the data infrastructure to monitor the burden of cancer and outcomes of care is not fully operational, resulting in structural challenges to using cancer and screening registries (OECD, 2023[83]). Greece halted its efforts to create a national cancer care plan in 2012 because of budgetary cuts due to austerity measures, and comprehensive cancer screening programmes have not been developed. The lack of an organised cancer strategy has affected the country’s capacity to prioritise, organise and fund programmes (OECD, 2023[84]).
National cancer care plans in EU+2 countries follow the general guidelines of the European Guide for Quality National Cancer Control Programmes (Albreht et al., 2014[80]), and are aligned with Europe’s Beating Cancer Plan. However, differences remain. Figure 2.15 presents the ten most commonly prioritised areas in the current national cancer control programme, national health policies or strategies on cancer care, as noted by experts in 21 EU+2 countries. Most countries prioritised screening (19), treatment (19), prevention (17) and the quality of cancer care (16). Rare cancers were only prioritised in nine countries, and the needs of vulnerable populations in only seven countries, despite the proven relationship between deprivation and the cancer burden (see Section 2.2.4). Cancer in children, adolescents and young adults was prioritised less frequently, as were cancer networks, digitalisation and health information.
The ten areas prioritised most frequently were all mentioned in Austria, Czechia, Malta, Slovenia and Spain. Portugal and Sweden prioritised nine of these: rare cancers were the exception in Sweden and the needs of vulnerable people were the exception in Portugal. Other countries had a narrower set of top priorities, such as Italy and Greece (screening and treatment) and Croatia (treatment and prevention). This does not mean that the other areas are not addressed in the national cancer plan; rather, these two areas have a more important role in the national strategy.
Figure 2.15. Screening, treatment and prevention are the most commonly prioritised areas in national cancer strategies
Note: Areas prioritised by only one or two countries are not displayed in the graph, including cancer in children, adolescents and young adults; cancer networks; digitalisation and health information; and early diagnosis.
Source: 2023 OECD Policy Survey on Cancer Care Performance and EU Country Cancer Profiles.
2.3.3. National cancer registries have been established in 24 EU+2 countries
Cancer registries in Europe have evolved into indispensable instruments for assessing the cancer burden and facilitating evidence‑based decision making in cancer control (Albreht, Kiasuwa and Van den Bulcke, 2017[85]). They have among the best population coverage in the world, with most countries covering 100% of their population (Allemani et al., 2018[8]). In 2014, 19 of the 29 EU+2 countries provided data covering their entire population to the CONCORD‑3 study. Five other countries confirmed 100% coverage, via either the 2023 OECD Policy Survey on Cancer Care Performance (Table 2.5) or the IARC (Forman et al., 2014[86]). This leaves only five countries (France, Greece, Italy, Romania, Spain) without a population-based cancer registry covering the entire population. However, the French Senate approved a law supporting the creation of a national cancer registry in June 2023, to be implemented in the short term (Sénat, 2023[87]).
Although most population-based cancer registries in Europe are well equipped for robust cancer surveillance, the scope of information and the extent of data quality and utilisation vary widely between countries. Besides Greece, which lacks a population-based cancer registry, diagnosis data is collected or linked in the cancer registries in at least 26 of the 29 EU+2 countries. The same number of registries has access to population mortality rates. Similarly, cancer stage and survival data are contained or linked in 25 and 26 EU+2 countries respectively, while treatment data are captured by population-based registries in 24 EU+2 countries.
Most registries can link their individual cancer registrations to national data on deaths, but linkage with data from national screening programmes is less common. Screening-detected cancers are flagged in the registries of only 18 EU+2 countries. The timeliness of the data contained in EU+2 countries’ cancer registries can also vary significantly between countries. For example, the latest data available for the Slovak Republic are from 2014, while countries like Spain and the Netherlands (provisional) already have 2022 data available.
Expanding the scope of cancer registries holds the potential to yield stronger epidemiological insights and identify factors contributing to disparities in cancer survival and the quality of life for people living with cancer. However, genetic information and patient-reported experiences or outcomes measures (PREMS/PROMS) are only included in 5 cancer registries of the 26 countries that responded to the 2023 OECD Policy Survey on Cancer Care Performance.
Table 2.5. Most EU+2 countries’ cancer registries include or can be linked to incidence, stage at diagnosis, treatment and survival data
Population coverage and type of data directly contained or linked in European cancer registries
|
Country |
National coverage |
Incidence (new cases) |
Screening (Screen detected) |
Cancer stage data |
Genetic information |
Treatment data |
Survival data |
Patient-reported indicators |
Population mortality rate |
|---|---|---|---|---|---|---|---|---|---|
|
Austria |
Total |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Belgium |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
|
Bulgaria |
Total |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Croatia |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Cyprus |
Total |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Czechia |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Denmark |
Total |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Estonia |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Finland (*) |
Total |
Yes |
Yes |
Yes |
NA/NC |
Yes |
Yes |
Yes |
Yes |
|
France |
Partial (23%) |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Germany |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Greece |
No registry |
No |
No |
No |
No |
No |
No |
No |
No |
|
Hungary |
Total |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
|
Iceland |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Ireland |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Italy |
Partial (70%) |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Latvia |
Total |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Lithuania |
Total |
Yes |
No |
Yes |
No |
No |
Yes |
No |
Yes |
|
Luxembourg |
Total |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
|
Malta |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Netherlands |
Total |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Norway |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
|
Poland |
Total |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Portugal |
Total |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
|
Romania |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
NA/NC |
|
Slovak Republic |
Total |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Slovenia |
Total |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
|
Spain |
Partial (28%) |
Yes |
Yes |
No |
No |
No |
Yes |
No |
Yes |
|
Sweden |
Total |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
Note: (*) Information for Finland was obtained from Forman et al. (2014[86]) and online from the Cancer Society of Finland (2023[88]). NA/NC stands for Not answered/Not confirmed.
Source: 2023 OECD Policy Survey on Cancer Care Performance; Allemani, C. et al. (2018[8]), “Global surveillance of trends in cancer survival 2000‑14 (CONCORD‑3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries”, https://doi.org/10.1016/s0140-6736(17)33326-3.
Information contained in EU+2 countries’ cancer registries follows the same patterns as those in other OECD countries. In the United States and Canada, for example, information on all the categories except PREMS/PROMS is included. The collection of screening data is a recurrent challenge, as it is missing in Japanese, Costa Rican and Mexican registries as well. New Zealand has the only national cancer registry among OECD countries that presents information in all the categories. Efforts to enhance the quality and standardisation of data collected by cancer registries are ongoing. The European Network of Cancer Registries and the Joint Research Centre (JRC) have collaborated to establish a comprehensive and standardised list of data quality checks for European cancer registries. The publication of the JRC Technical Report in 2014, followed by an updated version in 2018 and its latest publication in 2023 (Martos et al., 2023[89]), represents a significant milestone in this endeavour. In addition, efforts to unlock the benefits of health data for research and improved patient care for rare cancers on a pan-European basis are underway with the IDEA4RC project (begun in 2022), which spans cancer registries, national registries, and biobank data across European healthcare systems.
However, continued efforts are needed to strengthen the integration of screening data; enhance data quality checks; and expand the scope of cancer registries to include survivorship, genetic information and PREMS/PROMS. In addition, expanding the scope of registries to collect or allow linkage to socio‑economic data would facilitate better monitoring of cancer inequalities. For example, although a number of countries report national incidence information by region, few do so by socio‑economic status or deprivation level (only France, Ireland, Italy, and Sweden).
While Europe’s General Data Protection Regulation (GDPR) assisted in ensuring data rights, privacy and patient trust in health data sharing, it has created challenges in data sharing and conducting important individual-level data analyses that could inform decision making (Vukovic et al., 2022[90]). Partly because of this shortcoming, on 3 May 2022, the European Commission introduced a proposal for a regulation known as the European Health Data Space (European Commission, 2022[91]). The main objectives of the draft proposal include empowering individuals with greater digital access and control over their personal health data, establishing standards for electronic medical record systems to enable interoperability, and constructing a coherent framework that governs the secondary use of health data.
2.3.4. Conclusion: High-quality cancer registries are key to supporting policy to improve cancer prevention, early detection and care
The current policy frameworks in place in EU+2 countries under the umbrella of Europe’s Beating Cancer Plan and national cancer strategies signify a critical step in confronting the cancer burden. These collaborative efforts are necessary to address growing cancer burden and the significant disparities in cancer survival and mortality discussed in this chapter. The next two chapters will delve into the significant disparities in cancer prevention and screening programmes across different countries and population groups. They will also explore comprehensive and targeted policies aimed at reducing these disparities.
Despite the strides made in cancer policy, critical gaps remain unaddressed. The absence of fully operational registries in Greece and the lack of timeliness and completeness of several cancer registries underscores a dire need for sustained funding and support. One important policy option in the years to come to support research and healthcare improvement will be allocating support for maintaining and developing cancer registries, harmonising standards, and improving interoperability across databases to ensure that essential data on cancer burden and care are both current and actionable.
Comprehensive data from national cancer registries, linked to data from other relevant sources, will be vital in shaping more effective and inclusive strategies to reduce cancer risks, to improve screening and early detection, and to improve survival. Advances in such data linkages will facilitate evaluation of the effectiveness of the healthcare system for cancer patients (Chapter 5) and continuous monitoring of international, regional and socio‑economic disparities in cancer care quality indicators. These developments will be essential in reducing the unequal cancer burden in Europe.
References
[80] Albreht, T. et al. (2014), European Guide for Quality National Cancer Control Programmes, European Partnership for Action Against Cancer, Ljubljana, http://www.epaac.eu/images/WP_10/European_Guide_for_Quality_National_Cancer_Control_Programmes_EPAAC.pdf.
[85] Albreht, T., R. Kiasuwa and M. Van den Bulcke (2017), European Guide on Quality Improvement in Comprehensive Cancer Control, National Institute of Public Health, Ljubljana, https://cancercontrol.eu/archived/uploads/images/Guide/pdf/CanCon_Guide_FINAL_Web.pdf.
[8] Allemani, C. et al. (2018), “Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries”, The Lancet, Vol. 391/10125, pp. 1023-1075, https://doi.org/10.1016/s0140-6736(17)33326-3.
[45] Angelini, M. et al. (2023), “Decrease of cancer diagnosis during COVID-19 pandemic: A systematic review and meta-analysis”, European Journal of Epidemiology, Vol. 38/1, pp. 31-38, https://doi.org/10.1007/s10654-022-00946-6.
[64] Bambury, N. et al. (2023), Cancer Inequalities in Ireland by Deprivation, 2004-2018: A National Cancer Registry report, NCRI, Cork.
[77] Berchet, C. and F. Jusot (2012), “Inégalités de santé liées à l’immigration et capital social: une analyse en décomposition”, Économie publique/Public Economics, Vol. 24-25, https://doi.org/10.4000/economiepublique.8484.
[76] Bradby, H., S. Hamed and A. Lebano (2019), Migrants’ access to health care in Europe: A literature review, European Commission, Brussels, https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5c40201b2&appId=PPGMS.
[32] Breidert, M. et al. (2012), “Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy”, Saudi Journal of Gastroenterology, Vol. 18/2, p. 118, https://doi.org/10.4103/1319-3767.93815.
[16] Brkljačić, B. and A. Šupe Parun (2020), “Croatian success in early breast cancer detection: Favorable news in Breast Cancer Awareness Month”, Croatian Medical Journal, Vol. 61/5, pp. 389-390, https://doi.org/10.3325/cmj.2020.61.389.
[66] Bryere, J. et al. (2014), “Socioeconomic environment and cancer incidence: A French population-based study in Normandy”, BMC Cancer, Vol. 14, pp. 1-10, https://doi.org/10.1186/1471-2407-14-87.
[67] Bryere, J. et al. (2018), “Socioeconomic status and site-specific cancer incidence: A Bayesian approach in a French Cancer Registries Network study”, European Journal of Cancer Prevention, Vol. 27, pp. 391-398, https://doi.org/10.1097/CEJ.0000000000000326.
[43] Burki, T. (2020), “Less Survivable Cancers Taskforce calls for faster diagnosis”, The Lancet Oncology, Vol. 21/10, pp. 1265-1266, https://doi.org/10.1016/s1470-2045(20)30545-3.
[78] Cancer Research UK (2022), First Data in a Decade Highlights Ethnic Disparities in Cancer, https://news.cancerresearchuk.org/2022/03/02/first-data-in-a-decade-highlights-ethnic-disparities-in-cancer/ (accessed on 21 September 2023).
[88] Cancer Society of Finland (2023), The Cancer Registry Monitors, Evaluates and Serves, https://www.cancersociety.fi/publications/reports/cancer-in-finland-2016/the-cancer-registry-monitors-evaluates-and-serves/ (accessed on 10 August 2023).
[25] Casali, P. (2015), “Adjuvant chemotherapy for soft tissue sarcoma”, American Society of Clinical Oncology Educational Book 35, pp. e629-e633, https://doi.org/10.14694/edbook_am.2015.35.e629.
[21] Chihara, D. et al. (2016), “The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma”, British Journal of Haematology, Vol. 176/5, pp. 750-758, https://doi.org/10.1111/bjh.14477.
[6] Cho, H. et al. (2014), “When do changes in cancer survival mean progress? The insight from population incidence and mortality”, Journal of the National Cancer Institute Monographs, Vol. 49, pp. 187-97, https://doi.org/10.1093/jncimonographs/lgu014.
[27] Colleoni, M. et al. (2016), “Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the International Breast Cancer Study Group Trials I to V”, Journal of Clinical Oncology, Vol. 34/9, pp. 927-935, https://doi.org/10.1200/jco.2015.62.3504.
[65] Dalton, S. et al. (2008), “Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: Summary of findings”, European Journal of Cancer, Vol. 44, pp. 2074-2085, https://doi.org/10.1016/j.ejca.2008.06.018.
[18] de Heus, E. et al. (2022), “The gap between rare and common cancers still exists: Results from a population-based study in the Netherlands”, European Journal of Cancer, Vol. 167, pp. 103-111, https://doi.org/10.1016/j.ejca.2022.03.001.
[68] Derette, K. et al. (2022), “Evolution of socioeconomic inequalities in cancer incidence between 2006 and 2016 in France: A population-based study”, European Journal of Cancer Prevention, Vol. 31, pp. 473-481, https://doi.org/10.1097/CEJ.0000000000000732.
[1] ECIS (2023), European Cancer Information System, https://ecis.jrc.ec.europa.eu (accessed on 27 April 2023).
[7] Ellis, L. et al. (2014), “Cancer incidence, survival and mortality: Explaining the concepts”, International Journal of Cancer, Vol. 135, pp. 1774–1782, https://doi.org/10.1002/ijc.28990.
[79] EPAAC (2011), European Partnership for Action Against Cancer, http://www.epaac.eu/.
[4] European Commission (2022), Europe’s Beating Cancer Plan: Communication from the Commission to the European Parliament and the Council, European Commission, Brussels, https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf.
[91] European Commission (2022), Proposal for a regulation – The European Health Data Space, https://health.ec.europa.eu/publications/proposal-regulation-european-health-data-space_en (accessed on 27 November 2023).
[81] European Commission (2009), Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions of 24 June 2009 on Action against Cancer: European Partnership, European Commission, Brussels, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52009DC0291.
[2] Eurostat (2023), Causes of Death – Deaths by Country of Residence and Occurrence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ARO__custom_6537139/default/table (accessed on 16 June 2023).
[57] Eurostat (2023), Causes of Death – Standardised Death Rate by NUTS 2 Region of Residence, https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ASDR2__custom_6414996/default/table (accessed on 16 June 2023).
[9] Eurostat (2013), Revision of the European Standard Population: Report of Eurostat’s task force, Publications Office of the European Union, Luxembourg, https://doi.org/10.2785/11470.
[63] Finke, I. et al. (2021), “Small‐area analysis on socioeconomic inequalities in cancer survival for 25 cancer sites in Germany”, International Journal of Cancer, Vol. 149/3, pp. 561-572, https://doi.org/10.1002/ijc.33553.
[86] Forman, D. et al. (2014), Cancer Incidence in Five Continents, Vol. X., International Agency for Research on Cancer, Lyon.
[17] Gatta, G. et al. (2011), “Rare cancers are not so rare: The rare cancer burden in Europe”, European Journal of Cancer, Vol. 47/17, pp. 2493-2511, https://doi.org/10.1016/j.ejca.2011.08.008.
[15] Gheorghe, G. et al. (2020), “Early diagnosis of pancreatic cancer: The key for survival”, Diagnostics, Vol. 10/11, p. 869, https://doi.org/10.3390/diagnostics10110869.
[23] Glimelius, I. and A. Diepstra (2016), “Novel treatment concepts in Hodgkin lymphoma”, Journal of Internal Medicine, Vol. 281/3, pp. 247-260, https://doi.org/10.1111/joim.12582.
[74] Grande, R., J. Garcia-Gonzalez and M. Stanek (2023), “Differences in the risk of premature cancer mortality between natives and immigrants in Spain”, European Journal of Public Health, Vol. 33/5, pp. 803-808, https://doi.org/10.1093/eurpub/ckad102.
[52] Hanna, T. et al. (2020), “Mortality due to cancer treatment delay: Systematic review and meta-analysis”, BMJ, Vol. 371, p. m4087, https://doi.org/10.1136/bmj.m4087.
[13] Hashim, D. et al. (2016), “The global decrease in cancer mortality: Trends and disparities”, Annals of Oncology, Vol. 27/5, pp. 926-933, https://doi.org/10.1093/annonc/mdw027.
[37] Hemminki, J. et al. (2022), “Survival trends in solid cancers in the Nordic countries through 50 years”, European Journal of Cancer, Vol. 175, pp. 77-85, https://doi.org/10.1016/j.ejca.2022.08.015.
[36] Hemminki, K., A. Försti and A. Hemminki (2021), “Survival in colon and rectal cancers in Finland and Sweden through 50 years”, BMJ Open Gastroenterology, Vol. 8/1, p. e000644, https://doi.org/10.1136/bmjgast-2021-000644.
[3] Henley, S. et al. (2022), “COVID-19 and other underlying causes of cancer deaths – United States, January 2018–July 2022”, MMWR. Morbidity and Mortality Weekly Report, Vol. 71/50, pp. 1583-1588, https://doi.org/10.15585/mmwr.mm7150a3.
[5] IARC (2023), GLOBOCAN 2020, https://gco.iarc.fr/ (accessed on 23 November 2023).
[56] IKNL (2023), Little Impact of COVID-19 on the Survival of Cancer Patients, https://iknl.nl/nieuws/2023/overleving-na-covid (accessed on 24 November 2023).
[19] Israel, O. and A. Kuten (2007), “Early detection of cancer recurrence: 18F-FDG PET/CT can make a difference in diagnosis and patient care”, Journal of Nuclear Medicine, Vol. 48/1 suppl, p. 28S, http://jnm.snmjournals.org/content/48/1_suppl/28S.abstract.
[29] Jiang, H. et al. (2020), “Classification of progression patterns in glioblastoma: Analysis of predictive factors and clinical implications”, Frontiers in Oncology, Vol. 10, p. 590648, https://doi.org/10.3389/fonc.2020.590648.
[26] Kurbegovic, S. et al. (2017), “The risk of biochemical recurrence for intermediate-risk prostate cancer after radical prostatectomy”, Scandinavian Journal of Urology, Vol. 51/6, pp. 450-456, https://doi.org/10.1080/21681805.2017.1356369.
[75] Labree, L. et al. (2011), “Differences in overweight and obesity among children from migrant and native origin: A systematic review of the European literature”, Obesity Reviews, Vol. 12/5, pp. e535-e547, https://doi.org/10.1111/j.1467-789x.2010.00839.x.
[73] Lamminmäki, M. et al. (2023), “A population-based cohort study on changes in breast, lung and colorectal cancer incidence and mortality among non-Western immigrant women”, BMC Cancer, Vol. 23/1, p. 665, https://doi.org/10.1186/s12885-023-11140-6.
[54] Lancet Oncology (2022), “Cancer, health-care backlogs, and the cost-of-living crisis”, The Lancet Oncology, Vol. 23/6, p. 691, https://doi.org/10.1016/s1470-2045(22)00302-3.
[35] Larønningen, S. et al. (2023), NORDCAN: Association of the Nordic Cancer Registries, https://nordcan.iarc.fr/.
[60] Launoy, G., V. Zadnik and M. Coleman (2021), Social Environment and Cancer in Europe: Towards an evidence-based public health policy, Springer, Cham, https://doi.org/10.1007/978-3-030-69329-9.
[58] Lin, L. et al. (2021), “Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990–2019”, Journal of Hematology & Oncology, Vol. 14/1, https://doi.org/10.1186/s13045-021-01213-z.
[22] Li, S., K. Young and L. Medeiros (2018), “Diffuse large B-cell lymphoma”, Pathology, Vol. 50/1, pp. 74-87, https://doi.org/10.1016/j.pathol.2017.09.006.
[69] Lokar, K., T. Zagar and V. Zadnik (2019), “Estimation of the Ecological Fallacy in the Geographical Analysis of the Association of Socio-Economic Deprivation and Cancer Incidence”, International Journal of Environmental Research and Public Health, Vol. 16, p. 296, https://doi.org/10.3390/ijerph16030296.
[53] Malagón, T. et al. (2022), “Predicted long‐term impact of COVID‐19 pandemic‐related care delays on cancer mortality in Canada”, International Journal of Cancer, Vol. 150/8, pp. 1244-1254.
[89] Martos, C. et al. (2023), A Common Data Quality Check Procedure for European Cancer Registries, European Commission, Ispra, https://www.encr.eu/sites/default/files/Recommendations/JRC132486_cancer_data_quality_checks_procedure_report_2.0.pdf.
[47] Ministry of Health (2023), Estudio de Impacto de la Pandemia por COVID-19 sobre la Prevención y el Control del Cáncer en el Sistema Nacional de Salud, Ministry of Health, Madrid, https://www.sanidad.gob.es/areas/calidadAsistencial/estrategias/cancer/docs/Estudio_impacto_publicado.pdf.
[49] Morris, E. et al. (2021), “Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: A population-based study”, The Lancet Gastroenterology & Hepatology, Vol. 6/3, pp. 199-208, https://doi.org/10.1016/s2468-1253(21)00005-4.
[83] OECD (2023), EU Country Cancer Profile: Bulgaria 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/f6915046-en.
[39] OECD (2023), EU Country Cancer Profile: Denmark 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/ee590fe8-en.
[38] OECD (2023), EU Country Cancer Profile: Finland 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/427186d4-en.
[84] OECD (2023), EU Country Cancer Profile: Greece 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/30b7e1f9-en.
[40] OECD (2023), EU Country Cancer Profile: Iceland 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/263d7eb3-en.
[82] OECD (2023), EU Country Cancer Profile: Netherlands 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/89b32870-en.
[41] OECD (2023), EU Country Cancer Profile: Norway 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/0fcf5d28-en.
[12] OECD (2023), EU Country Cancer Profile: Poland 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/55f07000-en.
[11] OECD (2023), EU Country Cancer Profile: Romania 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/267467c6-en.
[42] OECD (2023), EU Country Cancer Profile: Sweden 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/7b5ff594-en.
[10] OECD (2023), EU Country Cancer Profiles 2023, https://www.oecd.org/health/eu-cancer-profiles.htm.
[46] Peacock, H. et al. (2021), “Decline and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium: A year-long, population-level analysis”, ESMO Open, Vol. 6/4, p. 100197, https://doi.org/10.1016/j.esmoop.2021.100197.
[61] Petrelli, A. et al. (2019), “Italian atlas of mortality inequalities by education level”, Epidemiologia & Prevenzione, Vol. 43/1 (Suppl 1), pp. 1-120, https://acrobat.adobe.com/link/review?uri=urn:aaid:scds:US:e32d9c3b-c607-3b15-a2ea-54ff8f8c782a.
[72] Poiseuil, M. et al. (2023), “Survival after breast cancer according to participation in organised or opportunistic screening and deprivation”, Cancer Epidemiology, Vol. 82, p. 102312, https://doi.org/10.1016/j.canep.2022.102312.
[28] Pugh, S. et al. (2016), “Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival”, Annals of Surgery, Vol. 263/6, pp. 1143-1147, https://doi.org/10.1097/sla.0000000000001351.
[34] Pukkala, E. et al. (2017), “Nordic cancer registries – an overview of their procedures and data comparability”, Acta Oncologica, Vol. 57/4, pp. 440-455, https://doi.org/10.1080/0284186x.2017.1407039.
[48] Sagan, A. et al. (eds.) (2021), Health Systems Resilience During COVID-19: Lessons for building back better, European Observatory on Health Systems and Policies, Copenhagen, https://eurohealthobservatory.who.int/publications/i/health-systems-resilience-during-covid-19-lessons-for-building-back-better.
[20] Salani, R. et al. (2011), “Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations”, American Journal of Obstetrics and Gynecology, Vol. 204/6, pp. 466-478, https://doi.org/10.1016/j.ajog.2011.03.008.
[30] Santini, D. et al. (2016), “Risk of recurrence and conditional survival in complete responders treated with TKIs plus or less locoregional therapies for metastatic renal cell carcinoma”, Oncotarget, Vol. 7/22, pp. 33381-33390, https://doi.org/10.18632/oncotarget.8302.
[87] Sénat (2023), Proposition de Loi No. 704. Visant à mettre en place un registre national des cancers, https://www.senat.fr/leg/ppl22-704.html (accessed on 27 November 2023).
[70] Shack, L. et al. (2008), “Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England”, BMC Cancer, Vol. 8, pp. 1-10, https://doi.org/10.1186/1471-2407-8-271.
[31] Tas, F. and K. Erturk (2017), “Recurrence behavior in early-stage cutaneous melanoma: pattern, timing, survival, and influencing factors”, Melanoma Research, Vol. 27/2, pp. 134-139, https://doi.org/10.1097/CMR.0000000000000332.
[71] Tweed, E. et al. (2018), “Socio-economic inequalities in the incidence of four common cancers: A population-based registry study”, Public Health, Vol. 154, pp. 1-10, https://doi.org/10.1016/j.puhe.2017.10.005.
[59] Vaccarella, S. et al. (2023), “Socioeconomic inequalities in cancer mortality between and within countries in Europe: A population-based study”, The Lancet Regional Health - Europe, Vol. 25, https://doi.org/10.1016/j.lanepe.2022.100551.
[14] van den Bergh, R., S. Loeb and M. Roobol (2015), “Impact of early diagnosis of prostate cancer on survival outcomes”, European Urology Focus, Vol. 1/2, pp. 137-146, https://doi.org/10.1016/j.euf.2015.01.002.
[90] Vukovic, J. et al. (2022), “Enablers and barriers to the secondary use of health data in Europe: General Data Protection Regulation perspective”, Archives of Public Health, Vol. 80/1, https://doi.org/10.1186/s13690-022-00866-7.
[44] WHO (2023), Cancer Services Disrupted by up to 50% in All Countries Reporting: A deadly impact of COVID-19, https://www.who.int/europe/news/item/03-02-2022-cancer-services-disrupted-by-up-to-50-in-all-countries-reporting-a-deadly-impact-of-covid-19.
[55] Wilkinson, E. (2021), “UK Government urged to recognise post-COVID-19 cancer backlog”, The Lancet Oncology, Vol. 22/7, p. 910, https://doi.org/10.1016/s1470-2045(21)00330-2.
[24] Woll, P. et al. (2012), “Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial”, The Lancet Oncology, Vol. 13/10, pp. 1045-1054, https://doi.org/10.1016/s1470-2045(12)70346-7.
[50] Zadnik, V. et al. (2020), “Impact of COVID-19 on cancer diagnosis and management in Slovenia – preliminary results”, Radiology and Oncology, Vol. 54/3, pp. 329-334, https://doi.org/10.2478/raon-2020-0048.
[33] Zadnik, V. et al. (2021), “Trends in population-based cancer survival in Slovenia”, Radiology and Oncology, Vol. 55/1, pp. 42-49, https://doi.org/10.2478/raon-2021-0003.
[62] Zadnik, V. et al. (2022), “Cancer patients’ survival according to socioeconomic environment in a high-income country with universal health coverage”, Cancers, Vol. 14, p. 1620, https://doi.org/10.3390/cancers14071620.
[51] Zagar, T. et al. (2022), “Impact of the COVID-19 epidemic on cancer burden and cancer care in Slovenia: A follow-up study”, Radiology and Oncology, Vol. 56/4, pp. 488-500, https://doi.org/10.2478/raon-2022-0050.
Notes
← 1. Potential years of life lost is a summary measure of premature mortality that provides an explicit weighting of deaths occurring at younger ages, which are, a priori, preventable. The calculation sums up deaths occurring at each age and multiplies by the number of remaining life‑years up to a selected age limit (75 years for OECD calculations).
← 2. Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, France, Germany, Greece, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, the Slovak Republic, Slovenia, Spain and Sweden responded to the 2023 OECD Policy Survey on Cancer Care Performance.
← 3. In Belgium, the decrease in cancer incidence is not confirmed using official data published by the Belgian Cancer Registry.

