In order to address the growing demand for cancer care, countries need to seek effective and efficient ways of delivering high-quality cancer care. Most EU countries, however, have a shortage of healthcare experts in this field, leading to a shared challenge of ensuring widespread access to highly qualified professionals across their regions. With emerging technologies in cancer medicines and medical equipment, EU countries also face difficulties in securing adequate access and ensuring financial sustainability in the provision of high-quality cancer care. Countries address these challenges through policies that encompass enhancing the healthcare workforce, investing in medical technologies and optimising their utilisation, refining the organisation of cancer care delivery, and ensuring high-quality cancer care.
Beating Cancer Inequalities in the EU

5. Ensuring the sustainability of high-quality cancer care systems
Abstract
Key findings
To ensure the sustainability of healthcare systems, considering growing demand for healthcare services, countries need to put greater policy focus on health promotion, prevention and early diagnosis to reduce the burden of cancer. However, healthcare systems also need to tackle challenges in providing sustainable and high-quality cancer care to increasing numbers of patients, including workforce shortages, unbalanced distribution of resources and concerns about the affordability of new oncology medicines:
Over three‑quarters of EU+2 countries (the 27 European Union Member States, Iceland and Norway) face workforce shortages both in cancer care and in the health sector as a whole. Austria, Estonia, Latvia and Norway reported general shortages of nurses and a resulting negative impact on the delivery of cancer care.
Inadequate geographical distribution of the workforce and of radiotherapy equipment is creating gaps in access to cancer care between urban and remote populations in Cyprus, Greece, Norway, Spain and Sweden.
To tackle workforce shortages, EU+2 countries have increased training capacity (e.g. Slovenia), encouraged task substitutions among healthcare professionals (e.g. Ireland), provided financial incentives (e.g. Malta) and recruited foreign-trained health professionals (e.g. Iceland).
Countries face challenges in making new medicines available and accessible. The time between European Medicines Agency (EMA) approval and a reimbursement decision for a given oncology medicine ranged from less than 100 days in Germany and Sweden to over 3 years in Cyprus, Latvia and Lithuania. The proportion of indications with high clinical benefit in breast and lung cancer that are reimbursed or covered also varies 3-fold across countries.
Addressing barriers that impede patient access to existing reimbursed medicines and new cancer medicines is vital to enhance the quality of care. Alongside the EU regulation on health technology assessment (HTA), tools used by EU+2 countries include joint HTA collaborations and value frameworks developed to support the process of HTA and to assist in rationalising reimbursement decisions.
Encouraging entry and use of generics and biosimilars helps countries reinvest in new cancer medicines and improve the financial sustainability of cancer care delivery (e.g. Germany and Estonia).
Countries have sought ways to organise cancer care delivery to ensure timely access and bolster positive outcomes:
14 EU+2 countries have reorganised cancer care delivery to improve effectiveness and to ensure sustainability in recent years. Among these, a few (e.g. Austria, France, Hungary or Germany) have established comprehensive vertically cancer care systems with national centres of expertise, regional specialty centres and local certified cancer centres.
In 16 countries, cancer care networks are organised horizontally across providers to improve care co‑ordination.
A few countries are developing mobile palliative care for cancer patients at home (e.g. Czechia, Slovenia or Spain).
Policy levers to improve the quality of cancer care include developing multidisciplinary teams (21 countries), clinical guidelines for high standards of care (20 countries), accreditation or certification mechanisms (16 countries) and monitoring performance indicators (16 countries), notably around waiting times (e.g. Denmark, Iceland, Latvia or Sweden) or patient-reported outcomes (e.g. Belgium, the Netherland or Slovenia).
5.1. Challenges in sustaining high-quality cancer care are increasing
The demand for healthcare services for non-communicable diseases is increasing, and cancer is a major public health concern in Europe. The disease burden is expected to increase further as cancer incidence is increasing along with cancer prevalence due to decreased mortality and improved survival probabilities (Chapter 2). To ensure the sustainability of healthcare systems, countries need to place greater policy focus on addressing cancer risk factors (Chapter 3) and improving screening and early diagnosis to reduce the burden of cancer (Chapter 4). Furthermore, to care for an increasing number of people with cancer in a sustainable way, countries need to seek effective and efficient ways of delivering high-quality cancer care. Most European countries, however, face shortages of various types of professionals providing cancer prevention, diagnosis and care services – in particular, general practitioners (GPs) and nurses. Further, securing access to high-quality professionals across regions within countries is a common challenge. With emerging technologies in cancer medicines and medical equipment, EU+2 countries (the 27 European Union Member States, Iceland and Norway) also face financial challenges in securing access to innovative treatments and in providing sustainable, high-quality cancer care.
This concluding chapter describes the challenges faced by countries in securing and utilising human resources for health and medical technologies, and the policy responses and developments adopted to ensure sustainable, high-quality cancer care. These include utilising a high-quality health workforce effectively by increasing training capacities and promoting task reallocation, as well as investing in medical technologies and optimising their use. Finally, the chapter discusses policy levers for efficient and effective delivery of cancer care, such as concentration of cancer care, establishment of cancer care networks, multidisciplinary team practice, and monitoring and feedback mechanisms for cancer care delivery.
5.2. The majority of EU+2 countries have adopted policies to secure professionals providing high-quality cancer care
5.2.1. Health workforce shortages are a common challenge
Various types of healthcare professionals engage in cancer care, reflecting the complexity of the services provided. Nonetheless, most European countries face workforce shortages in the health sector, affecting the delivery of cancer prevention, screening, diagnosis, treatment, follow-up and palliative care, as reported by 21 of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance1 (Table 5.1).
Table 5.1. The majority of EU+2 countries reported shortages of various types of professionals engaging in cancer care
|
|
GPs, family doctors |
Oncologists |
Radiologists |
Radiation therapists |
Inpatient oncology nurses |
Community-based nurses |
Others |
|---|---|---|---|---|---|---|---|
|
Austria |
Yes |
NA |
NA |
Yes |
Yes |
Yes |
|
|
Bulgaria |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Croatia |
Yes |
No |
No |
No |
NA |
NA |
|
|
Cyprus |
No |
No |
No |
No |
No |
NA |
|
|
Czechia |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Denmark |
Yes |
No |
Yes |
Yes |
No |
No |
|
|
Estonia |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
France |
Yes |
No |
No |
No |
Yes |
Yes |
|
|
Germany |
Yes |
No |
No |
No |
NA |
NA |
|
|
Greece |
Yes |
No |
No |
No |
Yes |
Yes |
|
|
Iceland |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Ireland |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Radiographers, clinical psychologists, child and adolescent psychiatrists, neuropsychologists, mental health-trained/psycho‑oncology-trained clinical nurse specialists, medical social workers, music therapists, play therapists, health and social care professionals |
|
Latvia |
Yes |
NA |
Yes |
NA |
NA |
NA |
Specialists in radiology diagnosis, general nurses, chemotherapist-oncologists |
|
Lithuania |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
|
|
Malta |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Medical physicists, oncology/navigator nurses, fast-track and survivorship co‑ordinators |
|
Netherlands |
Yes |
No |
No |
No |
Yes |
Yes |
|
|
Norway |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Poland |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Portugal |
Yes |
No |
NA |
No |
NA |
NA |
Medical physicists |
|
Slovak Republic |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Slovenia |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Clinical psychologists, palliative care team members |
|
Spain |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Sweden |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Dietitians and nutritionists, physiotherapists, psychologists, counsellors and social workers |
|
Total Yes |
22 |
12 |
15 |
14 |
17 |
17 |
Notes: NA means not answered. Information is not available for Belgium, Italy and Luxembourg. Red indicates workforce shortages and blue indicates no workforce shortages.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
While GPs have a critical role to play in cancer care and follow-up care, 22 of the 26 responding countries reported that the number of GPs was not sufficient, and only Cyprus reported a sufficient number. The availability of GPs varies widely across EU+2 countries – from 1.2 per 1 000 population in Belgium to less than 0.5 per 1 000 in Poland, Greece and Hungary (Figure 5.1). However, even the countries with the highest availability – such as Belgium, France and Spain – considered the supply insufficient to meet the demand.
Figure 5.1. The availability of general practitioners in 2021 varied over four‑fold between Belgium and Poland
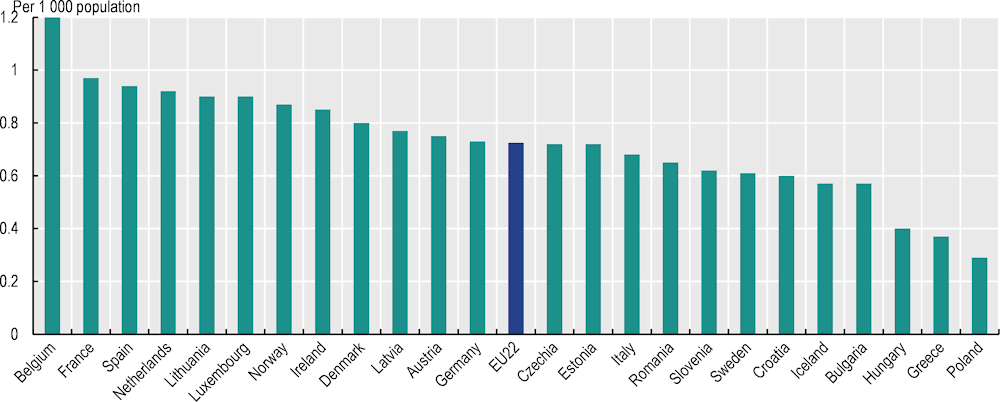
Notes: Data for Luxembourg refer to 2017; data for Denmark, Finland and Sweden refer to 2020. Medical interns and residents who have completed a basic medical university education and are undertaking postgraduate clinical training are included if they are specialising in general practice or if they have not chosen their area of specialisation yet.
Source: OECD Health Statistics 2023, https://doi.org/10.1787/health-data-en.
The 2023 OECD Policy Survey on Cancer Care Performance also revealed that the number of oncologists was not considered sufficient in 12 of the 26 responding countries. In Croatia, where about 9 doctors per 100 000 population work as specialists in radiotherapy, oncology or internal oncology, or have completed sub-speciality training in oncology, availability of oncologists was reported to be sufficient for current needs. On the other hand, among the countries reporting shortages of oncologists, Poland and Sweden reported relatively high availability (of between 7 and 8 oncologists per 100 000 population), while Bulgaria reported relatively low availability (about 2 oncologists per 100 000). Due to cross-country differences in the qualification requirements for doctors providing cancer care, the scope of their responsibilities and availability of data, it is challenging to compare the number of oncologists across countries. Nonetheless, about one‑third of EU+2 countries consider the supply of oncologists inadequate for the demand.
Nurses are also crucial in providing cancer care, but several countries – including Austria, Estonia, Latvia and Norway – reported general shortages of nurses and a resulting negative impact on the delivery of cancer care. Further, 17 of the 26 countries responding to the OECD Policy Survey also reported shortages of oncology nurses, not only in hospitals but also in community and home care settings. In the Netherlands, for example, the supply of paediatric oncology nurses was reported to be insufficient (Box 5.1).
Shortages of other healthcare professionals are also considered challenging in delivering cancer care. Over three in five countries responding to the OECD Policy Survey reported insufficient numbers of radiologists and radiotherapists. Health workforce shortages were reported for specialists in radiology diagnosis in Latvia; medical physicists in Malta and Portugal; dietitians, nutritionists, physiotherapists and psychologists in Sweden; and professionals providing palliative care and clinical psychology in Slovenia. Ireland also reported shortages of clinical psychologists, child and adolescent psychiatrists, neuropsychologists, mental health-trained clinical nurse specialists and medical social workers, among other professionals.
Box 5.1. Health workforce assessment and planning for cancer care takes place in several countries
While assessment of future demand and supply of health professionals is undertaken in many countries to develop health workforce strategies (Ono, Lafortune and Schoenstein, 2013[1]), only a few countries conduct an assessment specifically covering professionals in cancer care. In the Netherlands, the Advisory Committee on Medical Manpower Planning (ACMMP) examines capacity and training programmes for healthcare professionals, produces a national overarching forecast and issues recommendations. The ACMMP also forecasts demand and supply of specific types of professionals who are subject to limited geographic mobility for 12 hospital training programme fund regions; this analysis includes oncology and paediatric oncology nurses. The most recent assessment undertaken in 2018 found that the number of paediatric oncology nurses was lower than the level needed to meet expected healthcare needs (The Capacity Body, 2019[2]).
Other OECD countries also conduct health workforce assessments in cancer care. In the United States, a study found that since 2013 the supply of oncologists had increased by 16% – faster than the number of new cancer cases (American Society of Clinical Oncology, 2020[3]). Australia also conducted a workforce survey in oncology to inform planning of human resource strategies in cancer care, which suggested the need for geriatric oncology professionals, in view of population ageing (Lwin et al., 2018[4]).
Balanced geographical distribution of the health workforce is important to ensure adequate access to cancer prevention, screening and care across regions. However, a workforce distribution problem is reported in a few EU+2 countries – including Greece, the Netherlands, Norway and Sweden – affecting overall access to healthcare and to cancer care in particular. In Greece, geographically uneven developments of healthcare infrastructure and services have created disparities between urban and rural or remote areas in healthcare, as most doctors – including oncologists – are based in urban areas. There are hospitals with an oncology department that do not have a doctor, and small regional hospitals may also lack other specialists, such as haematologists and lab specialists for biomarker testing (OECD, 2023[5]). Inadequate geographical distribution of oncologists is also reported for Austria, Czechia, Hungary, Italy, Latvia, Norway, Portugal and Romania. In Portugal, medical oncologists are not adequately distributed across regions, leading to variations in workload across oncology departments, and possibly resulting in differences in care experiences and outcomes for people with cancer (OECD, 2023[6]). In some regions of Romania, owing to low supply, one oncologist serves a target population of over 200 000 inhabitants (OECD, 2023[7]).
Compared to specialised oncology care, access to primary care – which plays an important role in cancer prevention, diagnosis, referral and follow-up care – is often more equally distributed across regions within countries (OECD, 2020[8]). Nonetheless, unequal access to primary care is reported to be an issue in some EU countries. In the Netherlands, the density of primary care physicians varied between 6.0 and 7.8 per 10 000 population across regions in 2023. The urban region generally in the western part of the country has a surplus of GPs while some rural areas have a shortage. In France also, there are wide disparities in the density of GPs across regions. Medical deserts - characterised by a very low density of GPs- are located mainly in rural areas and in distant suburbs of small towns and big cities, mostly concentrated in the central and the northwest parts of France.
5.2.2. About half of EU+2 countries are increasing training capacities or reallocating tasks among healthcare professionals
As shown in Figure 5.2, countries are using different policy options to tackle workforce shortages in the health sector, and one of the most common approaches is increasing training capacity. Half of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance took this approach to improve the availability of workforce providing cancer care. In Luxembourg, the University of Luxembourg began offering places for a Bachelor of Medicine degree for the first time in September 2020, with the goal of increasing the overall domestic medical workforce, as well as three postgraduate speciality medical training programmes including oncology. In Slovenia, there has been an increase in training sites for clinical psychologists and palliative care, as the country plans to increase the number of mobile palliative care units and expand the availability of psychological support (OECD, 2023[9]). In Latvia, specialists in oncology and chemotherapy (oncologist-chemotherapists) have been trained not only in diagnosis of cancer and medical treatment including chemotherapy, endocrine therapy, immunotherapy, supportive and symptomatic therapy but also in palliative and rehabilitative care, which are not always widely available and accessible. Such policies have also been adopted in several OECD countries, including a good example from Canada (Box 5.2).
Figure 5.2. EU+2 countries have adopted a range of policies to address health workforce shortages in oncology
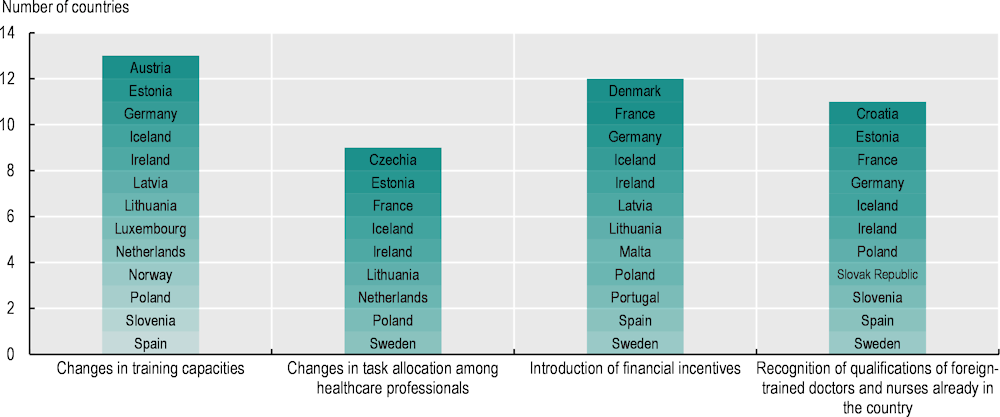
Note: Information is not available for Belgium and Cyprus.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
Box 5.2. Canada has implemented multiple strategies to address shortages of health professionals providing cancer care
In Canada, the COVID‑19 crisis hiked the demand for healthcare, exacerbating shortages of healthcare professionals as their working conditions, health and well-being were compromised, and a large proportion of the workforce entered retirement. To tackle shortages of professionals providing cancer care, Canada has implemented multiple strategies, including hiring back retired nurses, incentivising currently employed technicians and practitioners, bringing more nursing students and international trainees into the system, raising enrolment limits on nursing programmes, and modifying task allocation among healthcare professionals (e.g. having family doctors provide chemotherapy treatment and training paramedics to perform palliative care). The Canadian Partnership Against Cancer is currently rolling out funding to provinces and territories across Canada to implement and evaluate innovative models of care along the cancer continuum, which include an effort to address health workforce shortages. Areas of focus include network models, virtual care and expanded scopes of practice models.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
To address workforce shortages and improve overall efficiency in delivering cancer care while optimising the use of the existing workforce, 9 of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance have reallocated tasks among healthcare professionals (Figure 5.2):
In Czechia, where availability of oncologists is relatively low, a GP training programme was introduced in 2019 to enhance the skills needed to monitor patients with a history of cancer. This initiative aims to improve access to cancer care (OECD, 2023[10]).
In France, to expand the role of nurses in medical practice, a Master’s programme to train advanced practice nurses was created in 2018, offering them the opportunity to become responsible for regular follow-up of cancer patients, in co‑ordination with oncologists.
To support pharmacists in providing high-quality cancer care and identifying their training needs, Ireland has developed the National Competency Framework, which outlines the behaviours, skills and knowledge required for pharmacists working in cancer care. Ireland has also developed a number of educational initiatives – including e‑learning programmes – to equip various types of nurses with adequate knowledge, skills and competencies in areas such as anticancer therapy and psychosocial care to provide cancer care safely and effectively.
Provision of financial incentives is another common approach to resolve health workforce shortages; these had been introduced in 12 of the 26 countries responding to the OECD Policy Survey (Figure 5.2). Malta, for example, has limited capacity for training in certain specialisations, including oncology, so the government funds specialised oncology training abroad, which typically lasts one or two years, in full (OECD, 2023[11]). In 2023, Denmark also allocated funding to pay healthcare professionals for weekend shifts to improve workforce capacities in cancer care.
Of the 26 responding countries, 11 have expanded efforts to recruit foreign-trained health professionals. In Slovenia, recognition of foreign-trained healthcare professionals has been in place for many years, but the level of language requirement was relaxed recently to attract greater numbers. In Iceland, as Icelandic-born doctors receive oncology specialisation training abroad, efforts have been made recently to recruit these doctors to the national health system. Iceland has also tried to increase numbers of foreign-born doctors (OECD, 2023[12]).
Several countries aim to address workforce shortages in cancer care comprehensively through implementation of their ongoing national cancer plans. In Iceland, education and human resources development in cancer care were identified as among the nine priorities of the National Cancer Plan. To improve recruitment and retention of health professionals in cancer care, a comprehensive review of staffing and education is planned to address issues in medical education, nursing graduate education, specialised education, continuous professional education and work environments (OECD, 2023[12]). In the Slovak Republic, the National Oncology Programme Action Plan 2021‑25 aims to develop legislative changes to increase employment and incentives for healthcare professionals in cancer care, alongside specific training of clinical trial co‑ordinators and clinical research nurses. Furthermore, a pilot project funded by the EU Human Resources Operational Programme was approved in 2021 to finance staff for mobile palliative teams, which were newly created to cover all Slovak regions (National Oncology Institute, 2022[13]).
5.3. EU+2 countries aim to balance access to medical technologies and financial sustainability of cancer care delivery
5.3.1. Inequalities in patient access to cancer medicines and ensuring access to new cancer medicines are paramount challenges for healthcare systems
There has been a marked increase in the number of approved cancer medicines and extensions of indications in the past two decades
Between 2004 and 2022, the European Medicines Agency (EMA) granted centralised marketing authorisation to 152 new cancer medicines (EMA, 2023[14]). There has been a marked increase in the number of approved oncology medicines each year. Three distinct periods are noticeable (Figure 5.3). Between 2004 and 2011, the average annual number was close to four. Around ten new medicines per year were approved between 2012 and 2020, while 2021 was an exceptional year, with 17 approvals of new cancer medicines, followed by 15 approvals in 2022. Extensions of the use of existing cancer medicines to new indications (i.e. new patient groups) are also common and subject to approval by the EMA. Between 2020 and 2022, the EMA approved 73 extensions of existing oncology medicines.
Figure 5.3. The annual number of new cancer medicines approved by the EMA increased markedly during 2004‑22
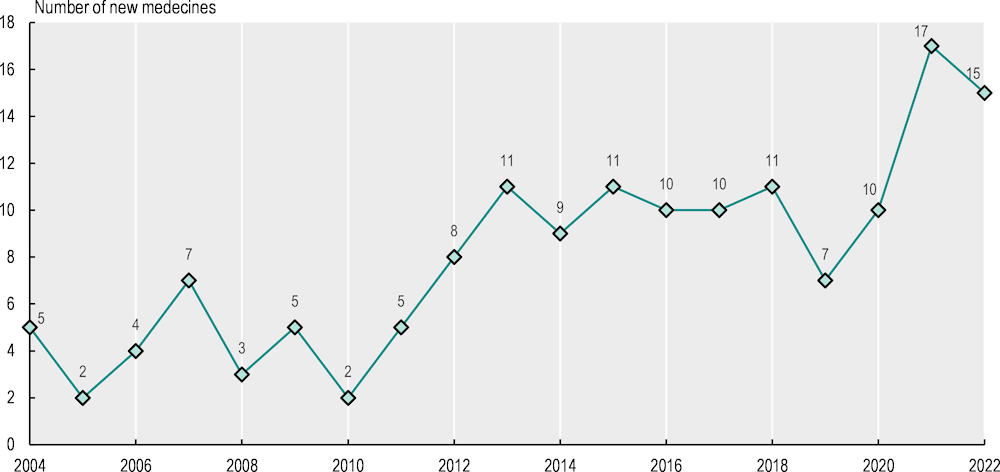
Note: Medicines used for cancer patients in Anatomical Therapeutic Chemical (ATC) classification groups L01, L02 and L04 were included. Radiopharmaceuticals in ATC group V were not included. Medicines with identical active substances were only included for their first instance of marketing authorisation. Six medicines were included that had their authorisation withdrawn after initial approval.
Source: Data from EMA (2023[14]), Download medicine data, www.ema.europa.eu/en/medicines/download-medicine-data, accessed on 3 April 2023).
With rising prices of oncology medicines, the budget impact of new medicines is increasingly influencing reimbursement decisions
Nearly all EU+2 countries, except for Cyprus and Slovenia, have established a health technology assessment (HTA) agency to inform decision making in the pricing and reimbursement of a new medicine/indication (WHO, 2018[15]; OECD/European Observatory on Health Systems and Policies, 2019[16]). The most common criteria for reimbursement decision are the relative therapeutic benefit, medical necessity, (lack of) availability of treatment alternatives and relative cost – effectiveness. However, with rising costs of new medicines, the budget impact is becoming an important criterion in public coverage/reimbursement decisions for oncology medicines.
Recent decades have witnessed rising prices of individual cancer medicines (OECD, 2020[17]) and rising expenditure on cancer medicines as a whole, both in absolute spending and in relative terms as a share of total spending on cancer care (Hofmarcher et al., 2019[18]). This creates an affordability challenge even for more affluent countries – in particular, for publicly funded health systems that operate on constrained budgets (Vogler, 2021[19]; WHO, 2018[20]). Of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance, 17 indicated that the budget impact is increasingly influencing their coverage/reimbursement decisions for various reasons – most importantly, the rising prices of new medicines and the increasing number of new medicines (data not shown). The rising number of cancer patients eligible to receive these new products was also cited as a contributing factor by several countries.
There is a three‑fold difference in public coverage of cancer medicines with a high clinical benefit across EU+2 countries
The availability and coverage of new medicines determine the level of access patients have. The definition of “availability” can vary, ranging from marketing authorisation, approval for reimbursement/coverage, and/or first sales in a particular country. In addition, the time to availability is measured as the difference between the starting point (the date of application for regulatory approval or the date of the actual approval) and the endpoint (the date of reimbursement/coverage or the date of first use in routine clinical practice). For EU countries, the Transparency Directive (Council Directive 89/105/EEC) regulates the issue of time to reimbursement. The Directive mandates maximum time limits for pricing and reimbursement decisions (90 days for pricing, 90 days for reimbursement or 180 days for combined pricing and reimbursement decisions) from the time a pharmaceutical company applies for pricing and reimbursement of a medicine to the country’s competent authorities. However, the pricing and reimbursement process may include “clock stops” (a period of time during which the evaluation of a medicine is officially stopped while the pharmaceutical applicant prepares responses to questions from the competent authority), and therefore usually lasts longer than 180 calendar days after the application was submitted.
An OECD analysis of a sample of indications in breast and lung cancer with the highest clinical benefit scores and with EMA marketing authorisation after 1 January 2016 shows that the proportion of indications reimbursed/covered varied substantially across countries (Figure 5.4). Germany reported that all indications were covered, followed by the Netherlands (92%) and Bulgaria and Sweden (both 85%). However, it should be noted that the mere inclusion of a medicine/indication in a positive reimbursement list does not mean that all eligible patients may have access in clinical practice. For instance, previous studies found that use of immunotherapies in Bulgaria was among the lowest in the EU in 2018 (Hofmarcher et al., 2019[18]). Budget restrictions might inhibit the widespread use of a reimbursed medicine in practice in some countries. Malta reimbursed none of the indications studied2, and Cyprus and Latvia reported that only small proportions of indications were covered (both 31%).
Figure 5.4. The share of selected indications of newer cancer medicines with public reimbursement/coverage varies widely
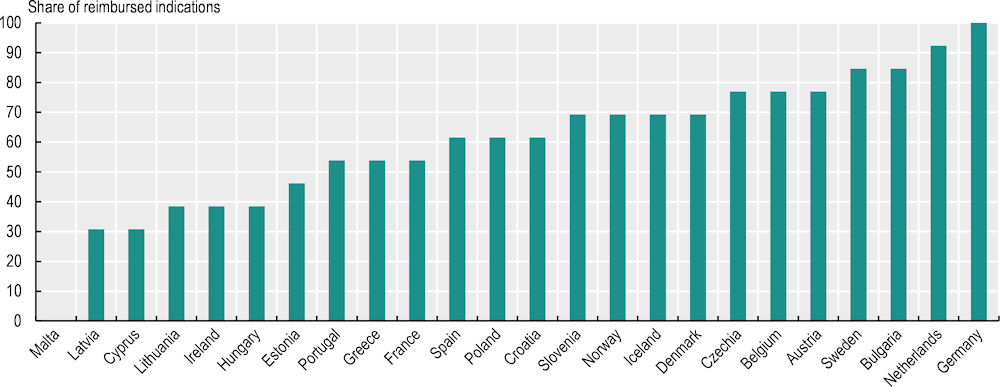
Notes: A total of 24 countries responded to the pilot data collection. Thirteen indications of ten cancer medicines used in the treatment of breast cancer and lung cancer with marketing authorisation by the EMA after 1 January 2016 and active authorisation on 26 March 2023, and with the highest clinical benefit according to the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring system (scores of A and 5), were included in the analysis. The shares show the inclusion status of the indications in the public reimbursement list on 1 April 2023.
Source: 2023 pilot data collection on access to cancer medicines in EU countries.
Time from marketing authorisation to coverage decision ranges from less than 100 days in Germany and Sweden to over 3 years in Cyprus, Latvia and Lithuania
The time between EMA marketing authorisation and application by the pharmaceutical company for coverage is often interpreted as a reflection of companies’ launch strategies, which may in turn be influenced by national pharmaceutical policies. For example, in some countries, the application for coverage can be made prior to receipt of marketing authorisation. Also, the importance of external price referencing as a pricing mechanism in Europe may be a driver of companies’ launch policies,3 alongside the size of the market and the expected profits. Using GDP per capita as a proxy of the size of the market and expected profit, Figure 5.5 shows a negative correlation between GDP per capita and mean time from EMA approval to coverage application. Wide variations in the time to application were found among countries responding to the 2023 OECD Policy Survey on Cancer Care Performance. Denmark and Norway had the shortest mean time between marketing authorisation and application for coverage (with negative values, meaning that application takes place before EMA authorisation), followed by Belgium (15 days after EMA authorisation) and Germany (20 days). Latvia (528 days after EMA authorisation), Greece (530 days) and Cyprus (716 days) had the longest mean times (Figure 5.5).
Figure 5.5. The correlation between time from EMA approval to application for reimbursement and GDP per capita is negative
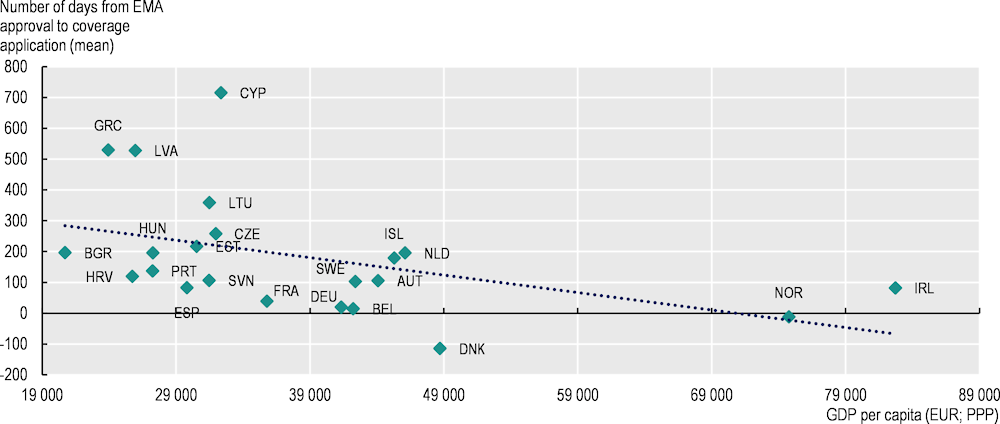
Note: A total of 24 countries responded to the pilot data collection.
Source: 2023 pilot data collection on access to cancer medicines in EU countries.
In addition to the time between EMA authorisation and application by the company for coverage, there is also the timeline between application for coverage and issuing of the reimbursement/coverage decision. This time is influenced by HTA processes and pricing mechanisms. In Germany, there can be no delays between marketing authorisation and reimbursement. Short durations of fewer than 100 days were reported in Greece, Iceland, the Netherlands and Sweden (data not shown). In the Netherlands, the short time difference is partly explained by the fact that certain indications of immunotherapy medicines are automatically covered on EMA authorisation as a result of special agreements (Lawlor et al., 2021[21]). Longer mean periods of more than 3 years were reported in Cyprus, Latvia and Lithuania, and periods of more than 500 days were reported in Czechia, Hungary, Poland and Portugal (see also Chapter 1). Observed delays are in many cases longer than the 180 days maximum defined in the Transparency Directive for EU countries’ reimbursement and pricing procedures. However, in this study, the time measured includes “clock stops” during which pharmaceutical companies are asked to provide additional information. Therefore, these delays cannot be interpreted purely as administrative processing time.
Future timelines for assessment of new cancer medicines and extensions of their indications might see improvements in individual countries. The adoption of Regulation (EU) 2021/2 282 on health technology assessment (HTAR) as part of the EU Pharmaceutical Strategy mandates joint clinical assessments and joint scientific consultations of patients, clinical experts and other relevant experts (European Commission, 2023[22]). This will apply to all new cancer medicines as of 12 January 2025. Joint European HTA and cross-border joint procurement are also good policy options to expedite public reimbursement/coverage decisions in the context of rising cancer medicines costs. Joint evaluations of the (relative) effectiveness of selected cancer medicines by regional collaborations, such as Beneluxa (among Belgium, the Netherlands, Luxembourg, Austria and Ireland) and FINOSE (among the Nordic countries excluding Iceland) have already been conducted. These voluntary collaborations on HTA between European countries might also see changes as a result of the HTAR (OECD, 2020[17]).
At the same time, value frameworks such as the European Society for Medical Oncology (ESMO)-Magnitude of Clinical Benefit Scale (MCBS) have been developed to support the process of HTA and to assist in rationalising reimbursement decisions. The ESMO-MCBS value framework offers a grading system of new indications of cancer medicines and the relative magnitude of clinical benefit that can be anticipated from data derived from pivotal clinical trials or meta‑analyses. ESMO proposed the MCBS to be used as a tool to support the process of prioritisation of access to cancer medicines by national health authorities when resources are constrained (Cherny et al., 2015[23]; Cherny et al., 2017[24]). New medicines with a potentially high clinical benefit could be reviewed on a fast-track basis, whereas new medicines with a potentially low clinical benefit could de‑prioritised.
Some countries restrict reimbursement of oncology medicines to smaller patient populations than those defined in the market authorisation
The reimbursement and coverage decision for a cancer medicine/indication might entail some restrictions to the eligible patient population as defined by the EMA. Restrictions can include criteria related to a patient’s health condition, the stage of treatment, the duration of therapy or a specific threshold for gene expression. This means that the national eligible patient population would be smaller than the patient population defined in the marketing authorisation. The purpose of these restrictions is to limit the uncertainty of clinical effectiveness in the patient group and/or to limit the budget impact (Hofmarcher et al., 2023[25]). Several countries did not report any restrictions to their reimbursed indications, while Estonia, France and Croatia reported that more than half of all reimbursed indications had restrictions, and Czechia reported that all indications had restrictions (Figure 5.6). This information had already been reported in previous studies in Czechia, Poland, Hungary and the Slovak Republic (Hofmarcher et al., 2023[25]).
Figure 5.6. The share of indications of newer cancer medicines with restricted coverage compared to the market authorisation population varies across countries
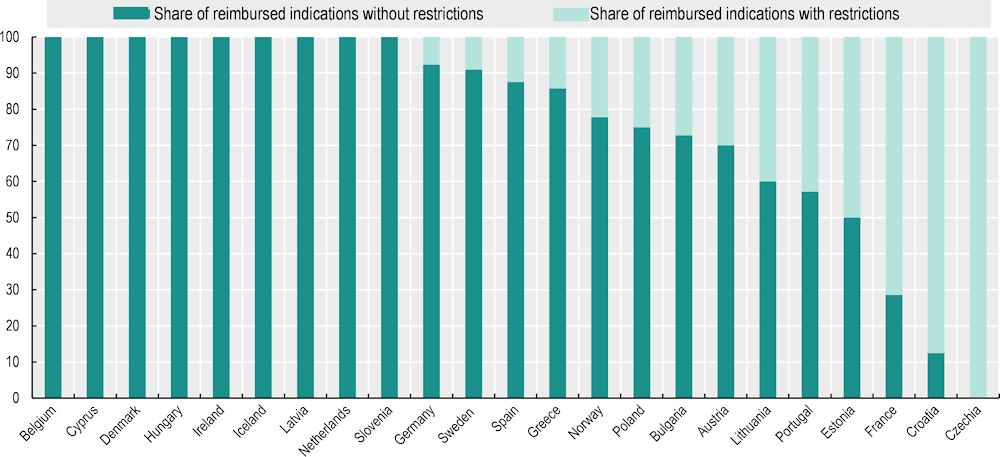
Notes: A total of 24 countries responded to the pilot data collection. Malta is missing because it reimbursed none of the indications studied. Restrictions in reimbursement/coverage were defined as any restriction/limitation in the public reimbursement list on 1 April 2023 compared to the text of the approved indication by the EMA.
Source: 2023 pilot data collection on access to cancer medicines in EU countries.
Central and Eastern European countries tend to have lower use of novel cancer medicines as measured in both cost and volume
Agreed reimbursement/coverage of medicines does not necessarily mean that patients have access to them when seeking care. Countries with high levels of reimbursement/coverage may still be characterised by low use in clinical practice. The use of cancer medicines in terms of costs has been shown to vary widely across countries. Expenditure on all cancer medicines – among countries with complete data – ranged from EUR 13 per capita in Latvia to EUR 108 per capita in Austria in 2018. Countries in Western Europe tended to have the highest expenditure, followed by countries in Northern and Southern Europe, whereas countries in Central and Eastern Europe tended to spend the least (Hofmarcher et al., 2019[18]).
A comparison of the use of medicines in volumes (milligrams) corroborates these findings. An examination of uptake of immunotherapies for lung cancer treatment across Europe suggests much lower utilisation in Central and Eastern European countries (Figure 5.7). Among countries with complete data, Belgium had the highest utilisation, whereas Czechia, Latvia and Lithuania recorded almost no utilisation at all, despite patient needs being roughly equivalent across countries – as approximated by the incidence of lung cancer. Apart from vast country differences in the use of immunotherapies, the analysis (Hofmarcher et al., 2019[18]) also showed that large differences are apparent in cancer types that have seen the recent introduction of many new medicines, such as multiple myeloma and prostate cancer. In contrast, there are much smaller differences in the use of certain older medicines – such as trastuzumab in breast cancer – yet even for these medicines there is a tendency of lower utilisation in Central and Eastern European countries compared to other countries.
Figure 5.7. Uptake of immunotherapies by volume in 2018 was much lower in Central and Eastern European countries
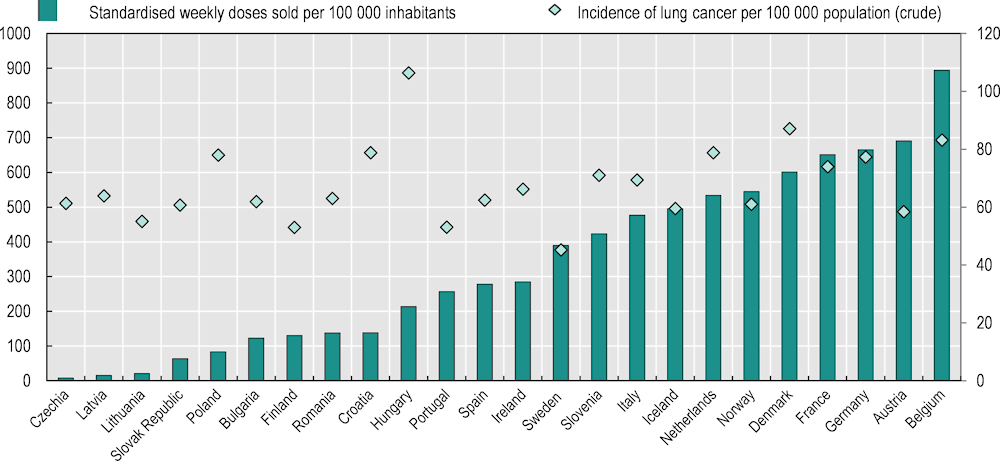
Notes: Data for Cyprus, Estonia, Greece, Luxembourg and Malta are not available. Data for Czechia are incomplete and hence underestimated. Four medicines (atezolizumab, ipilimumab, nivolumab and pembrolizumab) are included. Standardised weekly doses were calculated based on data on milligrams of medicines sold. For each medicine, the total amount of milligrams sold was standardised to the weekly recommended dose in milligrams per patient, which yields the number of weekly doses sold. The weekly doses sold for all medicines were summed up and then divided by the number of inhabitants. Lung cancer incidence is used as an indicator of patient needs for immunotherapy across countries.
Source: Hofmarcher et al. (2019[18]).
It is also important to note that to improve access to novel cancer medicines, countries most often use early access schemes or programmes (and disregard compassionate use programmes regulated by Article 83 of Regulation (EC) No 726/2004 in EU countries). Early access schemes make a medicine available to a patient prior to marketing authorisation and/or the publicly funded coverage/reimbursement decision in a country. These schemes generally apply to promising medicines used in severe conditions with high unmet need and no therapeutic alternatives; see a recent OECD report for a more detailed general description (Chapman, Szklanowska and Lopert, 2023[26]). Early access schemes for cancer medicines existed in 13 of the 26 responding countries to the 2023 OECD Policy Survey on Cancer Care Performance.
While patent expirations in oncology are expected to alleviate part of the financial pressure, there are still important country differences in the proportion of biosimilars being reimbursed
Encouraging the entry and use of generics and biosimilars when the originator product has gone off patent or lost market exclusivity is becoming increasingly important in lowering prices for oncology treatments (Godman et al., 2019[27]), helping to redirect financial resources to pay for newer medicines and to improve financial sustainability. The 2023 pilot data collection on access to cancer medicines in EU countries revealed significant differences in the share of 19 biosimilars for three reference medicines (bevacizumab, rituximab, trastuzumab) with public reimbursement/coverage across the responding countries (Figure 5.8). In Estonia, all biosimilars are used in hospitals, and the particular brand used depends on tender results. In Malta, only three biosimilars (16%) – one for each reference medicine – are available in the government Formulary List,4 following a competitive procurement procedure. All countries with the exception of Cyprus had at least one reimbursed biosimilar for each of the three medicines. Previously, Cyprus had only reimbursed biosimilars for two of the three medicines, but one for bevacizumab was added in May 2023.
The mean time from EMA approval to public reimbursement/coverage of biosimilars also exhibited great variation between countries, ranging from around 200 days in Germany and Spain to between 700 and 835 days in Greece, Iceland, Latvia, Lithuania and Slovenia, and almost 1 400 days in Cyprus. Countries with a higher share of publicly reimbursed/covered biosimilars tended to have shorter time periods between EMA approval and public reimbursement/coverage.
Figure 5.8. The share of biosimilars for cancer medicines with public reimbursement/coverage and the time between EMA approval and reimbursement/coverage vary widely
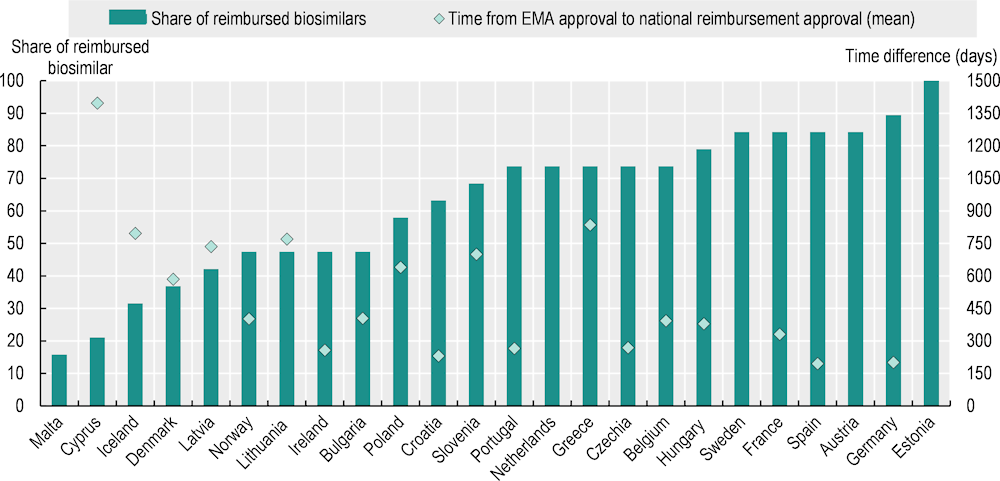
Notes: A total of 24 countries responded to the pilot data collection. Nineteen biosimilars of three cancer medicines (bevacizumab, rituximab, trastuzumab) with active marketing authorisation by the EMA as of 26 March 2023 were included in the analysis. The public reimbursement/coverage status in the countries shows the situation on 1 April 2023. The mean time difference was calculated based on the number of biosimilars with valid reimbursement on 1 April 2023. For Austria, Estonia, Malta, the Netherlands and Sweden, no data on reimbursement dates were provided.
Source: 2023 pilot data collection on access to cancer medicines in EU countries.
5.3.2. Access to medical equipment is uneven due to old equipment and unbalanced distribution in some countries
The availability of medical equipment has improved, but ageing equipment and equitable access are challenges
Reflecting the growing trends of cancer incidence and prevalence (Chapter 2), the availability of medical equipment has improved over the past decade. The volumes of radiotherapy equipment per population have increased by 14% on average in EU countries over the last 10 years. The most notable increase was in Bulgaria, where the volume more than doubled between 2012 and 2021 (OECD, 2023[28]), becoming one of the highest in Europe after Norway, Denmark, France and the Slovak Republic (Figure 5.9). In Hungary, providers have historically received limited public financing for the purchase, maintenance and renewal of medical equipment. Recently, however, several centres have received funds to replace radiation therapy equipment that is over 10 years old (OECD, 2023[29]), increasing the share of new equipment (Box 5.3). The availability of computerised tomography (CT) scanners and magnetic resonance imaging (MRI) units has also increased in almost all EU+2 countries over the last 10 years. The increases were notable in Romania, where the number of CT scanners per population doubled, and in Norway, where the number of MRI units per population increased more than seven‑fold. The increase was also substantial in Latvia, where the numbers per population have increased by about 30% for CT scanners and 80% for MRI units since the implementation of the first National Cancer Control Programme in 2009 (OECD, 2023[30]).
Figure 5.9. Volumes of radiation therapy equipment per population vary three‑fold across EU+2 countries
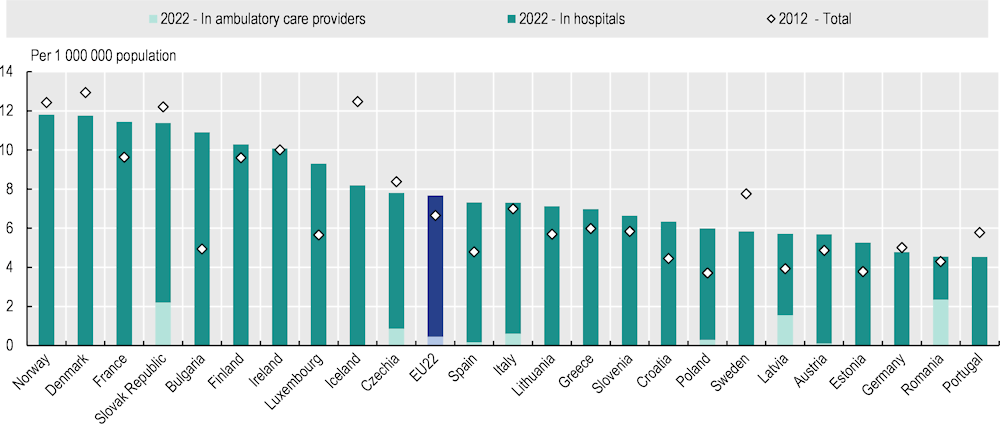
Notes: Data refer to the nearest available year. Radiation therapy equipment includes linear accelerators, Cobalt‑60 units, Caesium‑137 therapy units, low to orthovoltage X-ray units, high-dose and low-dose rate brachytherapy units and conventional brachytherapy units.
Source: OECD Health Statistics 2023, https://doi.org/10.1787/health-data-en.
Box 5.3. The latest medical equipment is not always available in some EU+2 countries
Although the use of outdated equipment is not recommended, old equipment is used widely in some EU+2 countries. The World Health Organization (WHO) reported that the optimal lifespan of radiotherapy equipment is usually 10‑15 years (WHO & IAEA, 2021[31]), and COCIR – the European Trade Association representing the medical imaging, radiotherapy, health information and communication technology and electromedical industries – defined 12 years as the maximum lifespan of equipment, beyond which ideally it should not be in use (COCIR, 2019[32]). In Belgium, Germany, Ireland, Italy, the Netherlands, Portugal and Spain, however, about one‑quarter of radiation therapy equipment is more than 15 years old (Figure 5.10).
Figure 5.10. On average in the EU27, 17% of particle therapy equipment is more than 15 years of age
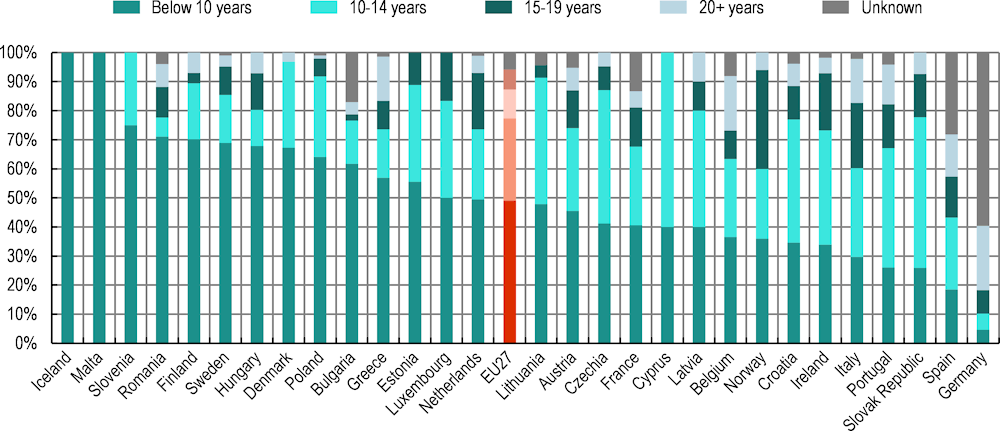
Source: IAEA (2022[33]), DIrectory of RAdiotherapy Centres (DIRAC), https://dirac.iaea.org/ (accessed on 4 October 2023).
The availability of other equipment, such as robot-assisted surgery, has been also expanded over time across countries. Robotic surgery has been shown to lead to faster recovery and shorter hospital stays; less scarring; and lower likelihood of infections at the surgical site, blood loss and postprocedural pain. For colorectal cancer, robotic surgery allows difficult-to‑access sites (such as a narrow pelvis) to be reached, and helps patients retain sexual and urinary function following surgery (Avram et al., 2023[34]). Data from the Medicare population in the United States showed that robotic prostatectomy was associated with four times lower odds of receiving a blood transfusion, and three times lower odds of prolonged hospital stay compared to the traditional open procedure (Gandaglia et al., 2014[35]). In stomach cancer patients, in addition to less invasive endoscopic mucosal resection and endoscopic submucosal dissection (which have become available to detect and treat early gastric cancer), robotic surgery has been used and found to be associated with lower rates of intra‑abdominal infection complications (4.4% versus 9.4%) while keeping survival rates similar to those for laparoscopic surgery (Hikage et al., 2021[36]). Robot-assisted surgery is also used in pancreatic, head and neck, kidney, bladder and gynaecological cancers, among others.
According to the 2023 OECD Policy Survey on Cancer Care Performance, 21 of the 26 responding countries used robotic or robot-assisted surgery that utilises artificial intelligence. For example, in France, surgical robots have been used for breast, ear, nose, throat, thyroid, gynaecological and digestive cancers, and Germany, Ireland and Slovenia have introduced robot-assisted surgery for prostatectomy. In other OECD countries, Canada also offers robot-assisted surgery for certain types of cancer surgery, including rectal, gynaecological, prostate and kidney cancers, and is studying the cost-benefit ratio for other types of cancer. While robotic and robot-assisted surgery are not currently used in Croatia, Estonia and Lithuania, these countries are exploring their use in the near future.
National cancer plans have been a catalyst for investment in technology in several countries. In Hungary, under the National Cancer Plan, investments were made in new molecular pathology tests, radiotherapy procedures and robot-assisted surgery, and these procedures have become publicly available in recent years (OECD, 2023[29]).
Although within-country differences in the supply of medical equipment have decreased over time, unequal distribution of medical equipment remains, leading to unequal access to medical technologies including the latest clinical procedures across population groups in a few countries. In Cyprus, for example, the majority of medical equipment is in private sector institutions, leading to long waiting times for public healthcare services and financial barriers to access for lower income groups (OECD, 2023[37]). In Spain, six provinces and the two autonomous cities (Ávila, Huesca, Palencia, Segovia, Soria, Teruel, Ceuta and Melilla) do not have radiotherapy units in their territories, creating substantial barriers to access to cancer care among vulnerable groups due to long journeys or accommodation costs (OECD, 2023[38]).
Countries need to take comprehensive approaches to optimise the use of medical technologies
Shortages and unequal distribution of medical equipment can lead to delayed diagnosis and treatment of cancer, but investment in purchasing equipment is not always sufficient to ensure timely access. In Sweden, a recent review of radiation therapy points to restrictions on utilisation of the equipment because of a lack of radiation oncologists and specialist nurses (Bergfeldt et al., 2022[39]). Similarly, in Poland, increases in equipment have not been matched with sufficient supplies of specialised medical personnel capable of performing radiotherapy (OECD, 2023[40]).
To ensure timely access to and optimal use of medical equipment, comprehensive approaches are needed. Countries need to invest not only in purchasing and renewing equipment but also in maintaining it, and adequate workforce capacity and organisation of cancer care delivery also need to be sought. To improve access to high-quality radiation therapy, Belgium, for example, established the Iridium Network, a highly specialised radiotherapy network based on a multidisciplinary approach. The Network, which involves eight partner hospitals, makes structured collaboration possible between doctors from different hospitals in the Greater Antwerp and Waasland area (OECD, 2023[41]), facilitating access to radiation therapy among cancer patients.
5.4. Organisation of cancer care delivery is improving through care concentration, structured networks, multidisciplinary teams and better availability of home care
5.4.1. Half of EU+2 countries have concentrated cancer care delivery
The clinical benefits of concentration of care are well known: a higher volume of cases that hospitals or doctors treat is known to be associated with a lower risk of perioperative morbidity and mortality (Weitz et al., 2004[42]), better long-term outcomes (Hillner, Smith and Desch, 2000[43]) and higher quality of end-of-life care (Morishima et al., 2013[44]). For example, a recent study found that patients in Ireland with rectal cancer treated at a cancer centre had significantly higher five‑year cancer-specific survival probabilities (81.1%) versus those not treated at cancer centres (76.3%) (O’Connell et al., 2022[45]). Furthermore, higher surgeon volume is found to be associated with lower costs for cancer surgical procedures such as pneumonectomy, esophagectomy and pancreaticoduodenectomy (Ho and Aloia, 2008[46]). However, a systematic review found that the overall economic impact of centralisation is unclear, as it also results in greater costs of accessing cancer care by patients and their carers (Ke, Hollingworth and Ness, 2012[47]).
About half of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance had concentrated cancer care delivery (Figure 5.11). This had often involved established comprehensive cancer centres, which serve as a vital hub that bridges the gap between research and clinical care, and aim to provide state‑of-art cancer care (Oberst, 2019[48]).
Figure 5.11. Most countries have sought efficient ways of providing high-quality care in recent years
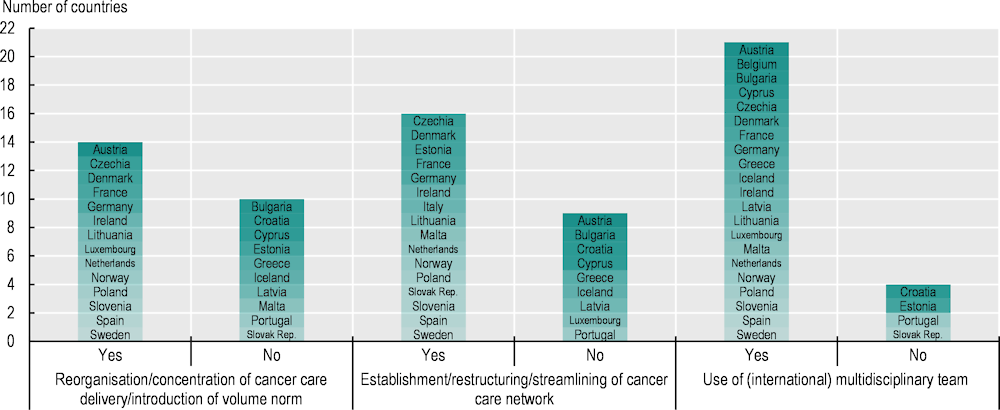
Note: Information is not available for Belgium (apart from “Use of (international) multidisciplinary team”). According to (OECD, 2023[29]), Hungary has also concentrated cancer care.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
Among the countries that concentrate cancer care, several had established vertically structured cancer care delivery systems:
In Austria, the most specialised treatment is provided by oncology reference centres, which should have a catchment area of at least 500 000 people and be reachable by any patient in the area within 120 minutes; and specialist centres, which cover at least 300 000 people within 60 minutes. Associated oncology centres mainly support specialist centres with diagnosis, referral and continuity of care, as do office‑based specialists. Research, teaching and treatment of rare cancers and high-risk patients are centralised at reference centres (OECD, 2023[49]).
In Hungary, cancer care delivery is structured and provided at national, regional and county centres. The National Institute of Oncology provides care for rare cancers, and four regional centres provide comprehensive care for medium-incidence cancers. Complex and rare procedures – including recently introduced molecular pathology tests and robot-assisted surgery – are provided in these national and regional centres. County centres, located in each of 19 counties, are responsible for provision of care for high-incidence cancers, and 14 of these also operate as radiotherapy centres (OECD, 2023[29]).
In Germany, there are 15 certified comprehensive cancer centres, which constitute the third tier and provide care for a broad spectrum of cancer types across all clinical aspects. They also lead research and teaching. Certified oncology centres (currently 141) constitute the second tier and provide care across several cancer types and specialties. At the first tier, 1 130 certified organ cancer centres specialise in one cancer type or specialty (OECD, 2023[50]).
Centralisation of cancer care in sites has been credited with great improvements in paediatric cancer outcomes over the last decades (American Academy of Pediatrics, 2014[51]). A study including Belgium, Bulgaria, Finland, Ireland, the Netherlands and Slovenia found that survival outcomes were significantly better if treatment was mainly given at hospitals with high volumes of patients, particularly for central nervous system tumours (Gatta et al., 2019[52]). A recent study also found that centralisation of cancer care led to improvement in outcomes of paediatric cancers in the Netherlands (van der Steeg et al., 2023[53]). Reflecting these findings, among the countries that reported having concentrated cancer care, almost all reported that care for rare cancers including paediatric cancers is centralised. In Lithuania for example, paediatric cancer care is concentrated in two centres (OECD, 2023[54]). In Ireland, all children are referred to the national Paediatric Haematology and Oncology Centre at Children’s Health Ireland on diagnosis, to establish treatment and follow-up plans in a centralised manner (OECD, 2023[55]).
In countries with a small population size, however, concentration of care for rare cancers does not lead to accumulation of sufficient knowledge and expertise due to the low number of cases. Some of these benefit from expertise accumulated abroad for rare cancers including paediatric cancer (Box 5.4).
Box 5.4. Countries seek international collaboration to care for patients with rare cancers
Countries with low population numbers – including Austria, Denmark, Estonia, Iceland and Norway – can benefit from international collaboration to improve access to care, particularly for rare cancers. In Austria, treatment of rare cancers is subject to the National Action Plan for Rare Diseases, which ensures access to treatment at hospitals in the European Reference Networks (OECD, 2023[49]). In Estonia, doctors can rely on international collaboration when skills or equipment are lacking – as in the case of proton therapy, which plays an important role in paediatric cancer and is not currently available in the country (OECD, 2023[56]). For rare cancers, including childhood cancers for which specific treatment is not available, Iceland actively collaborates with university hospitals in Scandinavian countries – particularly in Sweden. To ensure access to care for rare cancers abroad, funding is made available to cover travel and healthcare costs (OECD, 2023[12]). In Norway, patients are entitled to treatment abroad for rare cancers or others with expertise gaps, and the government funds treatment in hospitals abroad if the patient meets the requirements (OECD, 2023[57]). Denmark also allocated additional funding for referrals abroad in 2023.
To improve treatment outcomes, some countries focus on centralising the delivery of specific cancer care at certain centres. Belgium, for example, delivers pancreatic surgery, complex oesophageal surgery and breast cancer care in a concentrated and centralised manner (OECD, 2023[41]). In Portugal, resources for radiation therapy are concentrated in ten high-volume centres (Directorate-General for Health, 2019[58]), and complex stereotactic treatments are concentrated in five centres (OECD, 2023[6]).
In a few countries, including Czechia, France, Germany, the Netherlands and Spain, a volume norm is set to pay for cancer care or for a facility to be authorised to deliver certain treatment, contributing to cancer care concentration:
In Germany, hospitals are allowed to provide certain plannable services if the minimum volume is expected to be achieved in the next calendar year based on justified volume expectations. The annual minimum quantity per hospital site is, for example, 20 for complex interventions on the pancreas organ system for adults, 40 for allogeneic stem cell transplantation, 75 for thoracic surgical treatment of lung cancer in adults and 100 for surgical treatment of breast cancer (Gemeinsamen Bundesausschusses, 2023[59]). Furthermore, centre regulation by the Joint Federal Committee requires minimum case numbers, and OnkoZert – a voluntary certification programme of the German Cancer Society – stipulates minimum case numbers for their certification requirement (OECD, 2023[50]).
In the Netherlands, following the advice of the Quality of Cancer Care Taskforce of the Dutch Cancer Society about the importance of concentrating complex services in specialised settings with adequate resources, expertise and volumes to enhance quality of care, formal agreements were established on minimum patient/procedure volumes in 2007. Currently, minimum volumes are determined by Oncology-SONCOS (Foundation of Co‑operation in Oncology), which is part of the Federation of Medical Specialists. Monitoring of volume norms occurs for some cancer types as part of quality assessment by the Dutch Institute of Clinical Auditing (OECD, 2023[60]).
Organisation and delivery of cancer care has been an important feature in a number of countries’ national cancer plans. In Spain’s 2021 update to its National Cancer Strategy, a key priority area is centralisation of care for rare and paediatric tumours and for highly complex procedures (OECD, 2023[38]). In order to centralise care expertise, Estonia’s Cancer Control Plan 2021‑30 set a target that 95% of patients with a haematological cancer should be diagnosed in a cancer centre (OECD, 2023[56]).
5.4.2. Over half of EU+2 countries have established cancer care networks to provide high-quality care
Cancer care networks provide a structure for healthcare providers, including individual professionals, to work closely across care settings and professional types. They facilitate better co‑ordination and flow of knowledge about high-quality care between organisations and individuals, and are associated with improved access to and quality of cancer care (Brown et al., 2016[61]). For example, according to a study in France (Ray-Coquard et al., 2005[62]), the rate of compliance with clinical guidelines increased significantly for breast cancer (from 12% to 36%) and colon cancer (from 14% to 46%) at regional cancer network hospitals after dissemination of clinical practice guidelines, while such improvement was not observed in hospitals that were not part of the cancer network. Another study in Scotland (United Kingdom) found that managed clinical networks led to significant improvements in waiting times between referral and initial assessment, and in the proportion of patients undergoing appropriate diagnostic procedures (McCullough et al., 2014[63]).
Given these benefits, networks of clinical experts have regularly been established in Europe, and over half of EU+2 countries have created cancer care networks in recent years to promote evidence‑based practice and drive improvement in standards of patient care (see Figure 5.11). However, networks are organised differently across countries. In some countries – including Czechia, France and Italy – cancer care networks are organised horizontally across providers at regional levels to improve quality of cancer care, including care co‑ordination:
In Czechia, accredited comprehensive cancer centres form and lead networks called regional oncology groups; within these, oncology care providers co‑operate with each other, consult on treatment decisions and co‑ordinate follow-up care. Contractual co‑operation of cancer centre providers with a regional oncology group promotes compliance with common clinical protocols and guidelines and a standardised oncology care management system. Since 2019, GPs are also included in these collaborative oncology networks, as they have started to gain new competencies for monitoring patients with a history of cancer (OECD, 2023[10]).
In Italy, regional oncological networks of care were established in 2019 with the aim of ensuring a multidisciplinary approach to cancer care. Networks consist of care providers with different specialisations that co‑ordinate care to ensure adequate access to the most appropriate services – from prevention and diagnosis to treatment and palliative care (OECD, 2023[64]).
The National Cancer Institute in France published new organisational guidelines for regional cancer networks and created one network per region, with the aim of co‑ordinating the organisation of healthcare services at a regional level.
At the national level, networks sometimes focus on specialised cancer care – such as for rare cancers – to optimise the use of expertise, which is limited within countries. Rare cancers represent approximately one‑quarter of all cancers, and their treatment often encounters common issues such as lack of expertise and quality of care, discrepancies in outcomes and limitations in research (Frezza et al., 2019[65]). To address these issues, the European Reference Networks (ERNs), established in 2017, facilitate cross-border collaborations between specialists for diagnosis and treatment of low-prevalence complex diseases, including rare cancers (Héon-Klin, 2017[66]). ERNs for rare cancers5 aim to increase equity in access to care in Europe.
The majority of EU+2 countries take part in these ERNs. In Germany, for example, comprehensive cancer centres and certified oncology centres treat patients with rare cancers; they are typically part of ERNs and participate in clinical studies (OECD, 2023[50]). Portugal has also established reference centres for the management of seven oncological specialisations, including paediatric cancer. Other countries have established networks specific to rare cancers at the national level, such as France (OECD, 2023[67]) and Italy (OECD, 2023[64]). Lastly, several countries have also developed a network for specific types of cancer care, including Poland (for breast, lung, ovarian, colon and prostate cancers, and palliative care), Portugal (for palliative care) and Slovenia (for skin melanoma).
At the European level, additional networks are being established to promote high-quality cancer care across countries. The Joint Action on network of Comprehensive Cancer Centres (CraNE) currently undertakes preparatory activities with the aim of creating an EU network linking recognised national comprehensive cancer centres by 2025, as defined in Flagship 5 of Europe’s Beating Cancer Plan. The network aims to facilitate uptake of high-quality diagnosis and treatment, including training, research and clinical trials across European countries (European Commission, 2022[68]). To bring together the best resources available in Europe, the EU Joint Action on Networks of Expertise (JANE) also plans to establish seven new EU networks of expertise in areas such as personalised primary prevention, survivorship, palliative care, omic technologies,6 high-tech medical resources, complex and poor prognosis cancers, and adolescents and young adults with cancer.
5.4.3. Over two‑thirds of EU+2 countries provide cancer care in multidisciplinary teams
Multidisciplinary teams (MDTs) have been recommended for cancer treatment and care, as they improve the quality of care and outcomes (Selby et al., 2019[69]). According to a systematic review, MDTs resulted in improved patient outcomes in terms of diagnosis, treatment planning and patient satisfaction, and in improvements in survival probabilities for various cancers including colorectal, head and neck, breast, oesophageal and lung cancers (Prades et al., 2015[70]). MDT practice is also helpful in alleviating shortages in the cancer health workforce and in facilitating provision of integrated cancer care. However, providing MDTs entails considerable costs, and studies on their cost effectiveness are still limited and not yet conclusive (Ke et al., 2013[71]; Edney, Gray and Karnon, 2020[72]).
According to the 2023 OECD Policy Survey on Cancer Care Performance, 21 of the 26 responding countries use MDTs – typically including oncologists, surgeons, radiologists and pathologists – to provide high-quality cancer care in an efficient and effective manner (Figure 5.11). In Luxembourg, through the country’s first National Cancer Plan, multidisciplinary oncology consultation boards were developed in 2016 to facilitate evaluation and improvement of practice. There are formal MDT boards for common cancer types at hospitals and specialised MDT boards for rare cancers and complex cases at the National Cancer Institute (OECD, 2023[73]). In the Netherlands, all new diagnosed cancer cases are discussed in MDT meetings organised according to the type of cancer. The meetings aim to establish comprehensive and inclusive decision-making processes for people with cancer, to strengthen communication between specialists on managing evidence‑based treatment and to ensure timely initiation of treatment. MDTs also work in palliative care (OECD, 2023[60]). In Slovenia, weekly online multidisciplinary tumour boards and consultations are organised in bigger secondary and tertiary hospitals for all cancer types, where the majority of cancer cases are presented by treating physicians to determine their optimal care (OECD, 2023[9]). Additionally, as illustrated in Section 5.2.2, some countries – including Czechia, Ireland and Latvia – have enhanced inter-speciality training among different healthcare professionals to improve the availability of high-quality multidisciplinary cancer care.
To facilitate multidisciplinary learning and practices in cancer care, some countries have expanded the use of teleconsultation, as in Croatia, Estonia, and the Slovak Republic, for example. In the Slovak Republic, plans were made to establish an online platform for multidisciplinary tumour boards based at the National Cancer Institute. This platform can be consulted by oncologists throughout the country, and can facilitate care co‑ordination among cancer care providers, improving the quality of cancer care across regions (OECD, 2023[28]). In other OECD countries, Canada has implemented connected models of care that support improved care co‑ordination between cancer specialists and primary care providers, multidisciplinary clinics and cancer care networks. These bring together multiple care specialties and expanded use of virtual technology to support virtual consultations, patient navigation and other areas of care.
5.4.4. Countries are expanding availability of home care
To respond to the needs of patients whose preference is to receive care in the community where they live, countries are expanding availability of cancer care in home settings. Using video consultations, healthcare professionals provide follow-up care to their patients at home after surgery, examine the healing process of a surgical wound or have a psychotherapeutic conversation. Uptake of telemedicine has accelerated recently, particularly since the COVID‑19 pandemic. In Hungary, the National Cancer Plan describes specific objectives, actions and measures for general psycho‑oncological support, and stresses the need to leverage telemedicine and other digital solutions in cancer care (OECD, 2023[29]). France also has several ongoing projects to support home care, such as the use of telemonitoring for patients on oral therapies based on wearable devices, which allow MDTs to monitor side effects and pain management. In Germany, legislation and regulations were further developed for the use of video consultations, teleconsultations and tele‑expertise, which are also used in cancer treatment.
Home palliative care is also becoming available in an increasing number of EU+2 countries, including Belgium, Cyprus, Czechia, Estonia, France, Hungary, Iceland, Italy, Latvia, Lithuania, Luxembourg, the Netherlands, Poland, Slovenia, Spain and Sweden. In the Netherlands, palliative care is mainly organised at the community level. It is led by GPs and nurses, who are mainly responsible for providing home palliative care, while palliative care specialists are available to support and share their expertise if needed (OECD, 2023[60]). In Spain, palliative care is implemented at the regional level and is covered throughout the country by the National Health Service. In Czechia, 15 accredited comprehensive cancer centres have a contract for palliative home care, and since 2017 coverage of mobile palliative care teams providing home care has been covered by the country’s health insurance funds (OECD, 2023[10]). France made a large investment to expand home palliative care in recent years: the 2021‑24 National Plan for the Development of Palliative Care and Support at the End of Life aims to guarantee access for all citizens as close as possible to where they live. It allocated EUR 5 million to healthcare facilities to strengthen mobile palliative care teams, and an additional EUR 3 million to regional health agencies to provide palliative care support systems accessible by health professionals practising at home (OECD, 2023[67]).
Despite these recent expansions, however, availability of and access to home care are still suboptimal in most EU+2 countries. In Cyprus, for example, only one voluntary non-governmental organisation provides palliative care at home to patients and families via the nurses it employs (OECD, 2023[37]), and in Italy, access to palliative care at home is limited in some regions, resulting in regional disparities (OECD, 2023[64]).
5.5. Countries need to intensify efforts to deliver high-quality people‑centred cancer care
5.5.1. Two-thirds of EU+2 countries have developed and use clinical guidelines for standardised delivery of high-quality cancer care
Clinical guidelines are key to providing standardised high-quality cancer care across providers throughout a country, and 20 of the 26 countries responding to the 2023 OECD Policy Survey on Cancer Care Performance reported having developed clinical guidelines for cancer care (Figure 5.12). Among these, several countries have taken a systematic approach to developing clinical guidelines for cancer care. In Germany, for example, the National Guideline Programme of Oncology, launched in 2008 by the Association of Scientific Medical Societies, brings together various specialist societies, the German Cancer Society and German Cancer Aid Foundation to formulate and maintain guidelines. Currently, 32 clinical guidelines in oncology are used at specialised cancer centres, in agreement with social health insurance funds, providing quality standards for all major cancer types across early detection, diagnosis, therapy, follow-up and palliative care (OECD, 2023[50]). In the Netherlands, national guidelines on care quality have been introduced over recent years for cancer care (including rare cancers), underpinned by an auditing system facilitated by the non-profit organisation the Dutch Institute of Clinical Auditing (OECD, 2023[60]).
Several countries benefit from clinical guidelines developed in other countries or at the international level. In Iceland, due to limited national capacity, professionals use international clinical guidelines, including those developed by the National Institute for Health and Care Excellence (United Kingdom), the National Comprehensive Cancer Network (United States) and specialist associations in Nordic and other European countries (OECD, 2023[12]). In Romania, European and international clinical guidelines are transposed into national recommendations (OECD, 2023[7]).
Figure 5.12. Most countries focus on clinical guideline development, accreditation and certification to improve cancer care quality
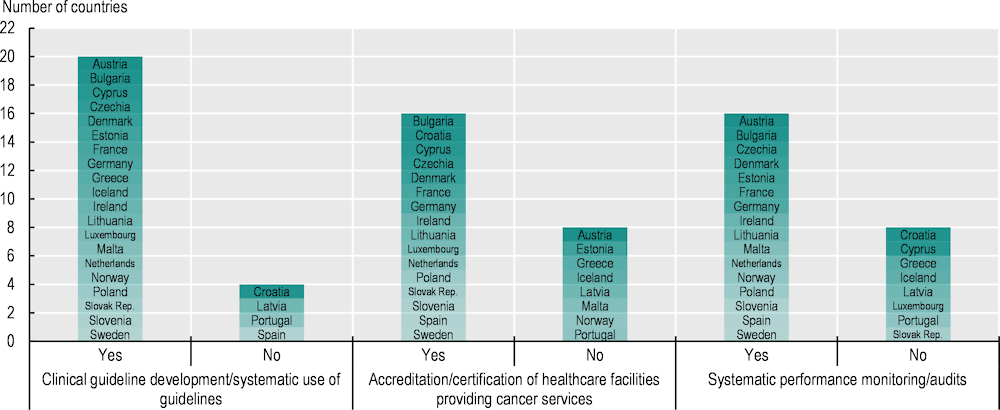
Note: Information is not available for Belgium and Italy.
Source: 2023 OECD Policy Survey on Cancer Care Performance.
5.5.2. Provider accreditation is commonly used to ensure high-quality cancer care
Provider accreditation has a positive impact on healthcare outcomes, such as process-related performance measures, safety culture, hospital efficiency (Hussein et al., 2021[74]) and hospital performance – including breast radiation and chemotherapy, and colon chemotherapy and nodal yield (Schroeder et al., 2022[75]). To improve quality of cancer care, 16 EU+2 countries use accreditation or certification mechanisms for healthcare facilities in cancer care (see Figure 5.12). In Spain, centres are designated as reference centres for rare cancers for adult and children during the accreditation procedure via onsite audits if criteria in relation to the volume of activity, initial and continuous training of professionals, specific resources (staff and equipment) and research are met (OECD, 2023[38]). Bulgaria accredits healthcare facilities that fulfil the requirements, related to equipment and presence of specialist staff, of the national medical oncology standard for provision of complex specialised services for cancer patients, including outpatient and hospital care, rehabilitation and registration (OECD, 2023[76]).
Over 65 cancer care centres in EU+2 countries are also accredited at the international level. The Organisation of European Cancer Institutes (OECI), the largest network of cancer centres and institutes in the world, developed the Accreditation and Designation Programme to provide equal access to high-quality cancer care for patients. The criteria used for this Programme include availability of a MDT, conducting research, and education and training functions (Boomsma et al., 2023[77]).
In a few countries, cancer care providers need to be accredited for reimbursement. Since 2019, Belgium’s National Institute of Health and Disability Insurance reimburses complex pancreatic surgery provided at centres meeting certain criteria, and complex oesophageal surgery operations performed in an accredited centre (there are ten in total) or in a hospital meeting agreed criteria. In France, since the end of 2009, healthcare providers require authorisation by their regional health agency to treat cancer patients. The authorisation system is designed to guarantee the same level of safety, quality and accessibility of cancer care throughout the country (OECD, 2023[67]). In Bulgaria, a hospital with a contract with the National Health Insurance Fund is reimbursed for cancer care provided only if it fulfils the requirements of clinical standards and the clinical pathway set as part of accreditation (OECD, 2023[76]).
5.5.3. To reduce waiting times, at least one‑third of EU+2 countries have set targets
Beyond developing fast-track pathways to improve timely cancer diagnosis (see Chapter 4 for more details), at least one‑third of EU+2 countries have set waiting time targets to improve access to cancer care (Table 5.2). In most cases these are general guidelines across cancer sites; however, in some countries – such as Ireland and Luxembourg – the guidelines depend on the type of cancer. There are variations in maximum waiting times for the same service across countries. For instance, while Hungary has set waiting time targets of 2 weeks for CT and MRI scans in suspected cases of cancer, the maximum waiting time set for such diagnostic tests in Lithuania is twice as long (4 weeks). The use of waiting time targets is different. In some countries, such as Finland, providers are penalised if targets are not met. In Portugal, patients are able to choose alternative health providers – including in the private sector – if their waiting time approaches or exceeds the maximum (OECD, 2020[78]). In Denmark, if a region cannot provide treatment within the maximum waiting time, it is obliged to refer patients to another hospital within the country or abroad that can do so.
Table 5.2. Countries set various maximum waiting time targets for cancer care
|
Country |
Maximum waiting times |
|---|---|
|
Denmark |
Waiting time for examination: 14 days from the day the hospital received the patient referral Waiting time for surgery after final examination: 14 days from the patient’s consent Waiting time for medical treatment or radiation therapy: 14 days from the patient’s consent |
|
Estonia |
Waiting time for an outpatient consultation/visit: 6 weeks Waiting time for planned home nursing care for cancer patients: 2 weeks |
|
Finland |
Interval between the arrival of a referral concerning a suspected case of cancer and the start of primary treatment: 6 weeks Interval between surgical treatment and adjuvant therapies: 4 weeks (although this depends on the patient’s state of health) |
|
Iceland |
Time between a decision to treat and first cancer treatment: 31 days Time between a referral with a highly suspected case of cancer and first cancer treatment: 62 days |
|
Ireland |
Time between receipt of a referral and an appointment for patients with breast cancer symptoms meeting clinical criteria for urgent referral to a symptomatic breast disease clinic: 2 weeks (target of 95% of patients) Time between receipt of a referral and an appointment for patients with breast cancer symptoms meeting clinical criteria for non-urgent referral to a symptomatic breast disease clinic: 12 weeks (target of 95% of patients) Time between receipt of a referral and an appointment at a rapid access clinic for patients with suspected lung cancer: 10 working days (target of 95% of patients) Time between receipt of a referral and an appointment at a rapid access clinic for men with suspected prostate cancer: 20 working days (target of 90% of patients) |
|
Latvia |
Waiting time for an examination after a screening programme: 30 days Waiting time for a primary diagnostic test of malignant tumour from the date of referral by a family doctor or gynaecologist: 10 working days Waiting time for a specialist visit for secondary diagnosis of malignant tumour after an oncological consultation following primary diagnostics: 10 working days Waiting time for treatment strategies for a patient (surgery, chemotherapy, radiotherapy) after secondary diagnosis of malignant tumour: 1 month |
|
Lithuania |
Waiting time between the first visit to a specialist and the date of cancer diagnosis: 28 calendar days Waiting time from diagnosis to initiation of therapy: 14 calendar days Waiting time from registration to receive an expensive diagnostic test (CT and/or MRI and/or positron emission tomography scan) to the date the diagnostic test is performed: 30 calendar days Waiting time from registration to receive chemotherapy, radiotherapy or haematology services to the date services are received: 30 calendar days Waiting time from registration to receive surgery to the date of the operation: 60 calendar days |
|
Luxembourg |
Waiting time between chemotherapy and radiotherapy for gynaecological cancers: 4 weeks, or 2 weeks after the anatomical pathology analytical report has been received (Conseil Scientifique Domain de la Santé, 2018[79]) Specific targets also available for patients affected by prostate cancer, breast cancer and colorectal cancer |
|
Portugal |
Waiting time for a referral from primary healthcare to specialist care: 24 hours in cases of suspected or confirmed oncological disease Waiting time for the first specialist consultation: within 7, 15 or 30 days, based on priority tiers |
Note: This table includes only waiting time targets specific to cancer care pathways; it excludes general targets for diagnostic or elective procedures that may also apply to cancer care.
Source: OECD Waiting Times Policy Questionnaire 2019 and 2023 OECD Policy Survey on Cancer Care Performance.
5.5.4. Over half of EU+2 countries monitor quality of cancer care for continuous quality improvement
Over half of EU+2 countries systematically monitor the performance of cancer care (see Figure 5.12). In Poland, systematic monitoring of cancer care at the central level has been in place since 2019, using indicators developed to measure the quality of oncological care and patient safety. Monitoring is conducted in parallel by a number of stakeholders, including the Association of Polish Oncologists, the Cancer Society, the Ministry of Health and – importantly – the Patients’ Association. Shared ownership and patient involvement have enhanced rigorous monitoring (OECD, 2023[40]). The National Cancer Framework Programme in Austria has established quality control processes, as well as evaluation of criteria based on structural, process and outcome measures (OECD, 2020[78]). In Germany, a directive on data-supported quality assurance across facilities of the Joint Federal Committee monitors some service areas (e.g. breast surgery and gynaecological surgery) including cancer care, and the German Cancer Society also publishes annual anonymised reports about the results of audits, which includes adherence to national clinical guidelines and case volume targets (OECD, 2023[50]). In Slovenia, clinical cancer registries and their multidisciplinary expert groups were established for the five most common cancers (breast, colorectal, lung, prostate, and malignant melanoma) through the National Cancer Control Plan, and a set of performance quality indicators were developed for regular monitoring (OECD, 2023[9]).
With the aim of improving delivery of people‑centred cancer care, a growing number of countries – including Belgium, Estonia, Germany, Iceland, Ireland, Italy, Latvia, the Netherlands, Slovenia and Sweden – also measure and monitor patient-reported measures. In Norway, annual meetings with patient representatives take place to discuss annual reports and quality indicators (OECD, 2023[57]). In the Netherlands, patient organisations are involved in discussing what data are collected and for what purpose. (OECD, 2023[60])
In countries where waiting time targets for cancer care have been developed (see Table 5.2), waiting times are regularly monitored and assessed. For instance, Denmark has established integrated patient pathways for cancer patients, and monitors these to see whether patients are examined and/or treated within recommended time periods. These data are monitored quarterly and disaggregated by cancer and region. In Iceland, the number of people on waiting lists and the percentage of people waiting for more than three months are reported every three months by the hospital or clinic for specific procedures related to cancer care. Latvia also monitors and prepares annual reports on waiting times for colonoscopy, mammography, chemotherapy and radiation therapy in day care, as well as on oncologists by medical institution (OECD, 2020[78]).
Among the countries with systematic monitoring of cancer care, a few also provide feedback to providers for further quality improvement. These include Estonia, France, the Netherlands, Norway, Slovenia and Sweden. In Estonia, the Advisory Board for the Development of Quality Indicators, established under the Estonian Health Insurance Fund’s supervision in 2014, publishes indicators on access to and quality of diagnosis and care for breast, colorectal, prostate and cervical cancer by hospitals (OECD, 2023[56]). In the Netherlands, cancer registries are implemented nationwide, giving participating healthcare providers access to their performance metrics via reports and a dedicated dashboard, and providing other stakeholders – such as patients and carers – with transparency regarding quality of care (OECD, 2023[60]). In Slovenia, feedback based on quality indicators covering different parts of the patient pathway is given to healthcare institutions and professionals involved in cancer care for several cancers (OECD, 2023[9]).
5.6. Conclusion
The landscape of cancer care in EU+2 countries is marked by increasing challenges, given the rising incidence of cancer. Supporting sustainability and high-quality cancer care entails having an adequate level of cancer resources – including workforce, oncology medicine and medical equipment – to facilitate efficient and effective delivery of cancer care throughout patient pathways. Countries need to invest not only in purchasing or renewing equipment but also in adequate workforce capacity and organisation of cancer care delivery to ensure timely access to and optimal use of medical equipment. To guarantee patient access to oncology medicines, countries need to capitalise on HTAR and ongoing and future joint European HTA collaborations, and to ensure efficient use of generics and biosimilars. Prioritising systematic delivery of cancer care through care concentration, implementation of standardised care based on guidelines and MDTs, and monitoring of cancer care performance (most notably around waiting times and patient-reported experience and outcome measures) are important policy options to ensure continuous quality improvement.
References
[51] American Academy of Pediatrics (2014), “Standards for pediatric cancer centers”, Pediatrics, Vol. 134/2, pp. 410-414, https://doi.org/10.1542/peds.2014-1526.
[3] American Society of Clinical Oncology (2020), Key Trends in Tracking Supply of Demand for Oncologists, American Society of Clinical Oncology, Alexandria, VA, https://old-prod.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2020-workforce-information-system.pdf (accessed on 1 October 2023).
[34] Avram, M. et al. (2023), “Artificial intelligence in improving the outcome of surgical treatment in colorectal cancer”, Frontiers in Oncology, Vol. 13, p. 1116761, https://doi.org/10.3389/fonc.2023.1116761.
[39] Bergfeldt, K. et al. (2022), Nordisk Strålbehandling: En benchmarkingstudie, Regional Cancer Centres in Sweden, Stockholm, https://cancercentrum.se/globalassets/vara-uppdrag/kunskapsstyrning/stralbehandling/rapport_benchmark-stralbehandling-rcc---220926.pdf (accessed on 4 October 2023).
[77] Boomsma, F. et al. (2023), Accreditation and Designation User Manual, OECI, Brussels, https://www.oeci.eu/documents/oeci_accreditation.pdf (accessed on 4 October 2023).
[61] Brown, B. et al. (2016), “The effectiveness of clinical networks in improving quality of care and patient outcomes: A systematic review of quantitative and qualitative studies”, BMC Health Services Research, Vol. 16/1, https://doi.org/10.1186/s12913-016-1615-z.
[26] Chapman, S., A. Szklanowska and R. Lopert (2023), “Exploring the feasibility of monitoring access to novel medicines: A pilot study in EU Member States”, OECD Health Working Papers, No. 151, OECD Publishing, Paris, https://doi.org/10.1787/8c1d16c4-en.
[24] Cherny, N. et al. (2017), “ESMO-Magnitude of Clinical Benefit Scale version 1.1”, Annals of Oncology, Vol. 28/10, pp. 2340-2366, https://doi.org/10.1093/annonc/mdx310.
[23] Cherny, N. et al. (2015), “A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS)”, Annals of Oncology, Vol. 26/8, pp. 1547-1573, https://doi.org/10.1093/annonc/mdv249.
[32] COCIR (2019), Radiotherapy Age Profile & Density, COCIR, Brussels, https://www.cocir.org/fileadmin/Publications_2019/19107_COC_Radiotherapy_Age_Profile_web4.pdf (accessed on 4 October 2023).
[79] Conseil Scientifique Domain de la Santé (2018), Référentiel pour les Cancers Gynecologiques, Conseil Scientifique Domain de la Santé, Luxembourg, https://institutnationalducancer.lu/wp-content/uploads/2018/04/r%C3%A9f%C3%A9rentiel-cancers-gyn%C3%A9cologiques-2.pdf (accessed on 14 November 2023).
[58] Directorate-General for Health (2019), Programa Nacional para as Doenças Oncológicas: Recursos do SNS em oncologia – Relatório de inquérito 2019, Directorate-General for Health, Lisbon.
[72] Edney, L., J. Gray and J. Karnon (2020), “A scoping review of the economics of multidisciplinary teams in oncology care”, Journal of Cancer Policy, Vol. 26, p. 100257, https://doi.org/10.1016/j.jcpo.2020.100257.
[14] EMA (2023), Download medicine data, European Medicines Agency, Amsterdam, https://www.ema.europa.eu/en/medicines/download-medicine-data (accessed on 3 April 2023).
[22] European Commission (2023), Regulation on Health Technology Assessment, European Commission, Brussels, https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment_en (accessed on 12 July 2023).
[68] European Commission (2022), Europe’s Beating Cancer Plan, European Commission, Brussels, https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf.
[65] Frezza, A. et al. (2019), “Networking in rare cancers: What was done, what’s next”, European Journal of Surgical Oncology, Vol. 45/1, pp. 16-18, https://doi.org/10.1016/j.ejso.2018.03.030.
[35] Gandaglia, G. et al. (2014), “Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era”, Journal of Clinical Oncology, Vol. 32/14, pp. 1419-1426, https://doi.org/10.1200/jco.2013.53.5096.
[52] Gatta, G. et al. (2019), “The European study on centralisation of childhood cancer treatment”, European Journal of Cancer, Vol. 115, pp. 120-127, https://doi.org/10.1016/j.ejca.2019.04.024.
[59] Gemeinsamen Bundesausschusses (2023), Regelungen des Gemeinsamen Bundesausschusses gemäß § 136b Absatz 1 Satz 1 Nummer 2 SGB V für nach 108 SGB V zugelassene Krankenhäuser, Federal Joint Committee, Berlin, https://www.g-ba.de/downloads/62-492-3099/Mm-R_2023-02-16_iK-2023-01-01.pdf (accessed on 4 October 2023).
[27] Godman, B. et al. (2019), “Pricing of oral generic cancer medicines in 25 European countries: Findings and implications”, Generics and Biosimilars Initiative Journal, Vol. 8/2, pp. 49-70, https://doi.org/10.5639/gabij.2019.0802.007.
[66] Héon-Klin, V. (2017), “European Reference Networks for rare diseases: What is the conceptual framework?”, Orphanet Journal of Rare Diseases, Vol. 12/1, https://doi.org/10.1186/s13023-017-0676-3.
[36] Hikage, M. et al. (2021), “Robotic gastrectomy compared with laparoscopic gastrectomy for clinical stage I/II gastric cancer patients: A propensity score-matched analysis”, World Journal of Surgery, Vol. 45/5, pp. 1483-1494, https://doi.org/10.1007/s00268-020-05939-8.
[43] Hillner, B., T. Smith and C. Desch (2000), “Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care”, Journal of Clinical Oncology, Vol. 18/11, pp. 2327-2340, https://doi.org/10.1200/jco.2000.18.11.2327.
[18] Hofmarcher, T. et al. (2019), Comparator Report on Cancer in Europe 2019: Disease burden, costs and access to medicines, IHE, Lund, https://ihe.se/en/publicering/comparator-report-on-cancer-in-europe-2019/ (accessed on 15 June 2023).
[25] Hofmarcher, T. et al. (2023), “Access to novel cancer medicines in four countries in Central and Eastern Europe in relation to clinical benefit”, ESMO Open, Vol. 8/4, p. 101593, https://doi.org/10.1016/j.esmoop.2023.101593.
[46] Ho, V. and T. Aloia (2008), “Hospital volume, surgeon volume, and patient costs for cancer surgery”, Medical Care, Vol. 46/7, pp. 718-725, https://doi.org/10.1097/mlr.0b013e3181653d6b.
[74] Hussein, M. et al. (2021), “The impact of hospital accreditation on the quality of healthcare: A systematic literature review”, BMC Health Services Research, Vol. 21/1, https://doi.org/10.1186/s12913-021-07097-6.
[33] IAEA (2022), DIrectory of RAdiotherapy Centres (DIRAC), https://dirac.iaea.org/ (accessed on 4 October 2023).
[71] Ke, K. et al. (2013), “Are multidisciplinary teams in secondary care cost-effective? A systematic review of the literature”, Cost Effectiveness and Resource Allocation, Vol. 11/1, p. 7, https://doi.org/10.1186/1478-7547-11-7.
[47] Ke, K., W. Hollingworth and A. Ness (2012), “The costs of centralisation: A systematic review of the economic impact of the centralisation of cancer services”, European Journal of Cancer Care, Vol. 21/2, pp. 158-168, https://doi.org/10.1111/j.1365-2354.2011.01323.x.
[21] Lawlor, R. et al. (2021), “Accelerating patient access to oncology medicines with multiple indications in Europe”, Journal of Market Access & Health Policy, Vol. 9/1, p. 1964791, https://doi.org/10.1080/20016689.2021.1964791.
[4] Lwin, Z. et al. (2018), “The Australian Medical Oncologist Workforce Survey: The profile and challenges of medical oncology”, Seminars in Oncology, Vol. 45/5-6, pp. 284-290, https://doi.org/10.1053/j.seminoncol.2018.06.004.
[63] McCullough, A. et al. (2014), “The impact of a managed clinical network on referral patterns of sarcoma patients in Grampian”, Scottish Medical Journal, Vol. 59/2, pp. 108-113, https://doi.org/10.1177/0036933014529241.
[44] Morishima, T. et al. (2013), “Impact of hospital case volume on quality of end-of-life care in terminal cancer patients”, Journal of Palliative Medicine, Vol. 16/2, pp. 173-178, https://doi.org/10.1089/jpm.2012.0361.
[13] National Oncology Institute (2022), State of Oncology in Slovakia: Annual report for 2021, National Oncology Institute, Bratislava, https://www.noisk.sk/files/2022/2022-06-09-vyrocna-sprava-2021-stav-onkologie-v-sr-en-v02.pdf (accessed on 4 October 2023).
[48] Oberst, S. (2019), “Bridging research and clinical care – the comprehensive cancer centre”, Molecular Oncology, Vol. 13/3, pp. 614-618, https://doi.org/10.1002/1878-0261.12442.
[45] O’Connell, E. et al. (2022), “Centralisation of rectal cancer care has improved patient survival in the Republic of Ireland”, European Journal of Surgical Oncology, Vol. 48/4, pp. 890-895, https://doi.org/10.1016/j.ejso.2021.10.031.
[49] OECD (2023), EU Country Cancer Profile: Austria 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/798b21c9-en.
[41] OECD (2023), EU Country Cancer Profile: Belgium 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/9a976db3-en.
[76] OECD (2023), EU Country Cancer Profile: Bulgaria 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/f6915046-en.
[37] OECD (2023), EU Country Cancer Profile: Cyprus 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/86732eb6-en.
[10] OECD (2023), EU Country Cancer Profile: Czech Republic 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/c37fd099-en.
[56] OECD (2023), EU Country Cancer Profile: Estonia 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/8434f41c-en.
[67] OECD (2023), EU Country Cancer Profile: France 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/7abfbfad-en.
[50] OECD (2023), EU Country Cancer Profile: Germany 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/fcc8586e-en.
[5] OECD (2023), EU Country Cancer Profile: Greece 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/30b7e1f9-en.
[29] OECD (2023), EU Country Cancer Profile: Hungary 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/ccaf0398-en.
[12] OECD (2023), EU Country Cancer Profile: Iceland 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/263d7eb3-en.
[55] OECD (2023), EU Country Cancer Profile: Ireland 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/f31164f8-en.
[64] OECD (2023), EU Country Cancer Profile: Italy 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/a0a66c1d-en.
[30] OECD (2023), EU Country Cancer Profile: Latvia 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/3b2c7642-en.
[54] OECD (2023), EU Country Cancer Profile: Lithuania 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/31316f70-en.
[73] OECD (2023), EU Country Cancer Profile: Luxembourg 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/6c6cdb7d-en.
[11] OECD (2023), EU Country Cancer Profile: Malta 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/cff97a9a-en.
[60] OECD (2023), EU Country Cancer Profile: Netherlands 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/89b32870-en.
[57] OECD (2023), EU Country Cancer Profile: Norway 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/0fcf5d28-en.
[40] OECD (2023), EU Country Cancer Profile: Poland 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/04cfc3ee-en.
[6] OECD (2023), EU Country Cancer Profile: Portugal 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/2f84bf79-en.
[7] OECD (2023), EU Country Cancer Profile: Romania 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/267467c6-en.
[28] OECD (2023), EU Country Cancer Profile: Slovak Republic 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/01a8d361-en.
[9] OECD (2023), EU Country Cancer Profile: Slovenia 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/cd631ba0-en.
[38] OECD (2023), EU Country Cancer Profile: Spain 2023, EU Country Cancer Profiles, OECD Publishing, Paris, https://doi.org/10.1787/260cd4e0-en.
[17] OECD (2020), Addressing Challenges in Access to Oncology Medicines: Analytical report, OECD Publishing, Paris, https://www.oecd.org/health/health-systems/addressing-challenges-in-access-to-oncology-medicines.htm (accessed on 15 June 2023).
[8] OECD (2020), Realising the Potential of Primary Health Care, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/a92adee4-en.
[78] OECD (2020), Waiting Times for Health Services: Next in Line, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/242e3c8c-en.
[16] OECD/European Observatory on Health Systems and Policies (2019), Greece: Country Health Profile 2019, State of Health in the EU, OECD Publishing, Paris, https://doi.org/10.1787/4ab8ea73-en.
[1] Ono, T., G. Lafortune and M. Schoenstein (2013), “Health workforce planning in OECD countries: A review of 26 projection models from 18 countries”, OECD Health Working Papers, No. 62, OECD Publishing, Paris, https://doi.org/10.1787/5k44t787zcwb-en.
[70] Prades, J. et al. (2015), “Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes”, Health Policy, Vol. 119/4, pp. 464-474, https://doi.org/10.1016/j.healthpol.2014.09.006.
[62] Ray-Coquard, I. et al. (2005), “Persistence of medical change at implementation of clinical guidelines on medical practice: A controlled study in a cancer network”, Journal of Clinical Oncology, Vol. 23/19, pp. 4414-4423, https://doi.org/10.1200/jco.2005.01.040.
[75] Schroeder, M. et al. (2022), “The impact of Commission on Cancer accreditation status, hospital rurality and hospital size on quality measure performance rates”, Annals of Surgical Oncology, Vol. 29/4, pp. 2527-2536, https://doi.org/10.1245/s10434-021-11304-3.
[69] Selby, P. et al. (2019), “The value and future developments of multidisciplinary team cancer care”, American Society of Clinical Oncology Educational Book 39, pp. 332-340, https://doi.org/10.1200/edbk_236857.
[2] The Capacity Body (2019), Recommendations 2021-2024 Advisory Committee on Medical Manpower Planning: Main report, The Capacity Body, Utrecht, https://capaciteitsorgaan.nl/app/uploads/2020/04/2020_02_12-Capaciteitsplan-2021-2024-Hoofdrapport-DEFINITIEF-EN.pdf (accessed on 4 October 2023).
[53] van der Steeg, A. et al. (2023), “The results of concentration of care: Surgical outcomes of neuroblastoma in the Netherlands”, European Journal of Surgical Oncology, Vol. 49/2, pp. 505-511, https://doi.org/10.1016/j.ejso.2022.10.005.
[19] Vogler, S. (2021), “Can we achieve affordable cancer medicine prices? Developing a pathway for change”, Expert Review of Pharmacoeconomics & Outcomes Research, Vol. 21/3, pp. 321-325, https://doi.org/10.1080/14737167.2021.1898951.
[42] Weitz, J. et al. (2004), “Impact of volume and specialization for cancer surgery”, Digestive Surgery, Vol. 21/4, pp. 253-261, https://doi.org/10.1159/000080198.
[15] WHO (2018), Medicines Reimbursement Policies in Europe, WHO Regional Office for Europe, Copenhagen, https://apps.who.int/iris/handle/10665/342220.
[20] WHO (2018), Pricing of cancer medicines and its impacts, World Health Organization, Geneva, https://apps.who.int/iris/handle/10665/277190.
[31] WHO & IAEA (2021), Technical Specifications of Radiotherapy Equipment for Cancer Treatment, World Health Organization, Geneva, https://iris.who.int/handle/10665/339912.
Notes
← 1. Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, France, Germany, Greece, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, the Slovak Republic, Slovenia, Spain and Sweden responded to the 2023 OECD Policy Survey on Cancer Care Performance.
← 2. In Malta, however, many indications included in this analysis are available through an early access scheme on a named-patient basis, but are not formally endorsed as part of the positive list.
← 3. External price referencing involves the use of prices of a medicine in a predefined set of countries to calculate a national reference price for the medicine. This is typically used as a starting point to initiate price negotiations with the pharmaceutical manufacturers during the pricing and reimbursement process. Launching new medicines first in countries where prices are the highest appears a logical strategy in this context.
← 4. The Government Formulary List contains all medicinal products, vitamins, food supplements and borderline substances.
← 5. The networks are ERN EURACAN for rare adult solid cancers, ERN PaedCan for paediatric oncology, and ERN-EuroBloodNet and ERN GENTURIS for rare genetic tumour risk syndromes.
← 6. Omic technologies are used to comprehensively measure the profile of genes (transcriptomics), proteins (proteomics) or small molecule metabolite (metabolomics) within cells or tissues.