Antimicrobial resistance (AMR) is well recognised as one of the most pressing public health threats globally. This chapter assembles the key messages emerging from the publication and discusses the main policy implications from the OECD analysis on the health and economic burden of AMR. The chapter presents the recent trends and projections for 51 OECD countries, European Union (EU) and European Economic Area (EEA) members and Group of 20 (G20) countries. It identifies the main gaps in multi-sectoral policy action to tackle AMR. Results from a special focus on AMR in long-term care settings are also presented. The chapter concludes by summarising an analysis of the effectiveness and cost-effectiveness of 11 modelled policy interventions and 3 policy packages designed in concordance with the One Health approach. Combined, the analyses presented in the chapter make a powerful economic case for continued policy action to tackle AMR.
Embracing a One Health Framework to Fight Antimicrobial Resistance

1. Addressing antimicrobial resistance
Abstract
Key findings
Total antibiotic consumption increased slightly in humans and declined for animals, but worrisome AMR trends persist, particularly for highest-priority antibiotics and certain antibiotic-bacterium pairs
Between 2000 and 2019, on average, the sales of all classes of antibiotics in humans increased slightly by 1.9% across all OECD countries. The OECD forecasts suggest that antibiotic consumption is expected to remain relatively flat across OECD countries between 2019 and 2035. In the OECD, the consumption of highest priority and third-line antibiotics in humans has been increasing relatively faster than total consumption. If left unchecked, resistance to third‑line antimicrobials can more than double by 2035 in the OECD compared to where it was in 2005.
Over the last two decades, on average, the sales of all classes of antimicrobials used in meat production are estimated to have halved across OECD countries, after adjusting for key factors. If historical trends continue, antimicrobial consumption in food animals could decrease an estimated 10% in the OECD and 12% in the EU/EEA by 2035 compared to 2020 while stabilising in the G20 at 2020 levels.
In 2019, resistance proportions across 12 priority antibiotic-bacterium combinations averaged at 20% in OECD countries, 22% in the EU/EEA and 30% in the G20. It is projected that between 2019 and 2035, resistance proportions for these antibiotic-bacterium combinations will remain mostly stable if current trends continue and no new policy actions are taken. A stabilisation of average resistance proportions at 2017 levels is also projected for EU/EEA countries and G20 countries.
Despite the overall stabilisation of resistance proportions, for certain countries, including Greece, India and Türkiye, resistance is expected to remain above 40% by 2035. For certain antibiotic-bacterium pairs, the projected resistance proportions can be as high as nearly 90%.
AMR continues to pose a large burden on population health and the economy. The OECD Strategic Public Health Planning for AMR (SPHeP-AMR) model used data from national surveillance systems and other intergovernmental organisations from 34 OECD and EU/EEA countries, including all 29 EU/EEA countries, as well as Japan, Switzerland, Türkiye, the United Kingdom and the United States, and shows that on average, every year until 2050:
Seventy-nine thousand people (22 000 in the EU/EEA) lose their lives due to resistant infections, corresponding to 2.4 times the number of deaths due to tuberculosis (TB), influenza and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) in 2020. Resistant strains of Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae) and Staphylococcus aureus (S. aureus) are the top killers, causing nearly three in four deaths.
Resistant infections acquired in healthcare settings pose a greater risk of mortality compared to those acquired in the community. Healthcare‑acquired resistant infections account for more than 60% of AMR-related deaths, even though they only represent 31% of total resistant infections.
Deaths due to AMR are concentrated among the elderly populations, with around 2 in 3 AMR‑related deaths occurring among people above 65 years of age. Among the younger population under 20 years of age, nearly 10% of all resistant infections are estimated to occur among newborns and children below 5 years of age.
The annual cost of treating complications caused by AMR is estimated to average more than USD 28.9 billion adjusting for purchasing power parity (PPP) (USD PPP 7.5 billion in the EU/EEA) if all resistant infections were eliminated and USD PPP 5.9 billion (USD PPP 1.6 billion in the EU/EEA) if all resistant infections were replaced by susceptible infections.
AMR is expected to cause a decline in the labour market output of about 734 000 full-time equivalents (FTEs) in the working population every year. Around 84% of the decline in labour market output is due to a reduction in workforce participation. These economic losses cost in total USD PPP 36.9 billion each year (USD PPP 5.8 billion in the EU/EEA).
Despite recent progress, considerable gaps remain in the policy action to tackle AMR
Around 92% (47/51) of OECD, EU/EEA and G20 countries combined already developed their national action plans to tackle AMR by 2020‑21. However, only around 20% (10/51) reached the final stage of implementation, where financial provisions for the implementation of the national action plans to tackle AMR are incorporated in the national action plans and budgets. In 2020, Group of 7 (G7) and OECD countries were the leading sources of development funding allocated to AMR but the current level of development funding is unlikely to fill the existing gaps in domestic funding in low- and middle‑income countries.
Since the launch of the World Health Organization (WHO) Global Action Plan on AMR (AMR-GAP) in 2015, nearly all OECD, EU/EEA and G20 countries rolled out multi-sectoral policies consistent with their national policy priorities and recommendations of the AMR-GAP. However, notable gaps remain in the implementation of interventions, including optimising antibiotic use in human and animal health, monitoring antibiotic use and AMR surveillance, scaling up infection prevention and control programmes, scaling up nationwide activities to raise AMR awareness, incorporating AMR in the training and education of healthcare professionals and implementing good management and hygiene practices in farms and food establishments.
Only 17 out of 32 OECD and EU/EEA countries that reported data in a recent OECD survey indicated having national action plans that reference long-term care facilities (LTCFs). Antimicrobial stewardship and AMR surveillance remain limited, with only nine countries having in place antibiotic guidelines or restrictive lists for antimicrobials in LTCFs and only six countries conducting surveillance of antibiotic consumption and AMR in LTCFs.
Policies to tackle AMR offer an excellent investment
The modelled policies can substantially improve population health. Hospital-based interventions usually offer the highest protective effects, including antimicrobial stewardship programmes (ASPs) and enhancing environmental hygiene and improving hand hygiene. Community-based interventions and One Health interventions, such as delayed antimicrobial prescription, scaling up the use of point-of-care rapid diagnostic tests and enhanced food handling, also offer significant protective effects, albeit of smaller magnitude.
By investing in the modelled interventions, countries can realise substantial savings in healthcare expenditure and productivity gains. Enhancing environmental hygiene and improving hand hygiene are estimated to yield the highest annual savings in health expenditures by eliminating both resistant and susceptible infections, amounting to USD PPP 7.2 billion and USD PPP 6 billion respectively.
Combining single interventions into policy packages addressing many of the policy gaps identified by the OECD analysis can yield considerable health and economic benefits. For instance, investing in a mixed package – improving hand hygiene, scaling up ASPs, delaying antimicrobial prescription, increasing mass media campaigns and enhancing food safety – could generate a gain of 466 000 life years (LYs) per year across all 34 countries included in the analysis and saves about USD PPP 9.4 billion annually in health expenditure.
Benefits of implementing policy packages more than make up for their implementation costs. The annual average cost of implementing the mixed package is around five times lower than the reduction in health expenditure and productivity gains combined. This benefit-to-cost ratio is around 4.7 for a package focusing on hospital-based actions and 2.5 for a package comprising community-based actions.
AMR is a top public health threat that can be prevented by effective policy action at little cost
In 2018, the OECD Stemming the Superbug Tide: Just A Few Dollars More report highlighted the huge benefits of early and comprehensive action to tackle AMR. The report found that under a business-as-usual scenario, in which no policy changes were made, resistance proportions, averaged across 8 priority antibiotic-bacterium combinations, could increase by 1 percentage point between 2015 and 2030 (OECD, 2018[1]). The report also highlighted that the challenge was multifaceted, spanning numerous antibiotic-bacterium combinations, with levels and trends of antimicrobial use and resistance widely disparate across countries and antibiotic-bacterium combinations. At the time of the release of the report, countries were upscaling their action to stem the rise of superbugs fearing the threat of a post-antibiotic world. The report was a loud call to action by producing evidence on the effectiveness and the cost-effectiveness of policies to optimise the use of antimicrobials and prevent the spread of infections in humans.
Building on that seminal work, the OECD has continued working on this top public health threat to extend the scope of its analysis and to provide a more comprehensive assessment of priority actions for the next phase of the fight against AMR. A first major lesson learnt since 2018 is that any credible action should endorse a One Health approach, going beyond human health to include animal and plant health and the environment and by recognising that all of these sectors are closely linked to one another (FAO/WHO, 2021[2]; WHO, 2022[3]). For this reason, actions in one sector alone may not produce any tangible impact if they do not go hand-in-hand with actions in other sectors. Further, the new OECD report draws on newly released data and evidence regarding the effectiveness of policy actions and best practices, which are selected on policy priorities defined by countries in their national action plans and based on an analysis of the still significant gaps in the implementation of policies on the ground. The report also considers the impact of another pandemic, COVID‑19, and how the “new normal” following the most acute phase of COVID‑19 may have affected the AMR pandemic.
An overarching message from this report is that there are some signs that efforts to tackle AMR, rolled out since the release of Stemming the Superbug Tide, went in the right direction and reviewed countries are possibly curbing the growth in AMR, although these efforts do not seem to be yet sufficient to fully reverse trends. Actions to optimise the use of antibiotics may be reaping some initial benefits, particularly in the livestock sector where there has been a significant decline in the sale of antimicrobials. Despite some recent reductions in sales of antibiotics for use in humans – reductions that continued during the initial phase of the COVID‑19 pandemic, at least in many EU/EEA countries – today, antibiotic consumption across the OECD remains higher than 20 years ago, after adjusting for population size and defined daily dose (DDD).1 Projecting these trends, AMR is expected to continue growing but at a slower pace than in the past. The OECD analysis suggests that AMR may stabilise or even slightly decrease by 2035, particularly in the case of some bacteria-drug combinations. However, this should not give any reason for complacency as some worrying trends are forecast for backup antibiotics in some countries such as large non-OECD economies part of the G20 and Mediterranean countries part of the OECD and the EU/EEA. The new input data used to feed the model show a small increase in the health and economic burden caused by AMR, compared to the analyses produced in 2018. At that time, it was calculated that about 2.4 million persons would die as a consequence of AMR in Europe, North America and Australia between 2015 and 2050 and that AMR-related complications could cost up to USD PPP 3.5 billion per year to the health system of the 33 OECD and EU/EEA countries included in the analysist at the time (OECD, 2018[1]).
The AMR national policy landscape has significantly improved since 2018 but gaps in the implementation of the policies on the ground remain. The COVID‑19 pandemic has posed new challenges. For example, many OECD countries reported that programmes aiming to promote the prudent use of antibiotics were severely disrupted in the early phases of the pandemic. At the same time, COVID‑19 has also opened new opportunities placing a spotlight on infection prevention and control (IPC) policies and inducing significant improvements. Based on countries’ responses to the 2021‑22 Tripartite AMR Country Self-assessment Survey (WHO/FAO/OIE, 2021[4]), nearly all OECD countries developed a national action plan for AMR, which was not the case at the time of releasing the previous publication. However, only 20% of OECD countries report the most advanced stage of implementation, which entails integrating the financial provisions for nation plans to tackle AMR in the national budgets and action plans. In addition, the analysis of the level of implementation of policies identifies some key priority areas for action. First, policies promoting prudent use of antibiotics and preventing the spread of infections in humans are too often implemented haphazardly without nationwide coverage and their designs do not reflect best practices. Second, surveillance systems to monitor antibiotic use and AMR are not yet of sufficiently high standards, particularly in the case of monitoring AMR levels, hindering the implementation of other policies, particularly in long-term care settings. Finally, only a minority of countries enforce controls to ensure compliance with regulatory frameworks to promote prudent use of antimicrobials in animals.
To further upscale their action to tackle AMR, countries can count on a comprehensive set of evidence‑based options. The new OECD analysis identifies 29 One Health policy options, ranging from those promoting prudent use of antimicrobials in the human and agriculture sectors (e.g. stewardship programmes, financial incentives, and education and training of healthcare professionals) to those preventing or reducing the incidence of infections, mainly through improved hygiene and to improve vaccination coverage. Environmental interventions also show the potential to reduce the concentration of antibiotics in the environment and AMR levels, particularly in the case of regulatory policies. For a subset of interventions for which evidence is more consolidated and by using the OECD model for Strategic Public Health Planning (SPHeP), the report also calculates the health and economic impact of scaling up interventions and their return on investment.
If the 2018 report argued that just a few dollars more would be sufficient to stem the superbug tide, this report shows that countries, in some cases more than others, have responded to this call and have mobilised investments to tackle AMR, also reaping some initial, albeit small benefits. Extra effort is needed to consolidate the path towards a more positive outlook. While additional investments supporting the development and access to the market of new antimicrobials, vaccines and devices are sorely needed and will require time to produce results, countries should continue investing in public health policies to promote prudent use of antimicrobials and prevent the spread of infections across humans, agriculture and the environment. One Health policies already in place should be fine‑tuned to meet the highest standards and match best practices. Equally important is for countries to make sure that these policies are consistently implemented nationwide. In some cases, innovative policies, with a focus on One Health policies, should be also implemented to ensure a more comprehensive and effective action to cover sectors that, so far, have not been considered of the highest priority such as long-term care and the environment. Countries with higher AMR rates – large G20 non-OECD economies and Mediterranean OECD and EU/EEA countries for example – should make even greater efforts to catch up with countries at the forefront of the fight against AMR.
This chapter summarises the findings and policy recommendations from the report. It starts by discussing the trends and patterns of antimicrobial consumption and AMR across countries. Next, the chapter presents results from the analysis of the health and economic burden of AMR across 34 OECD and EU/EEA countries. It then shifts its focus to an assessment of the AMR policy landscape discussing countries’ priorities based on an analysis of the 2021‑22 Tripartite AMR Country Self-Assessment Survey (WHO/FAO/OIE, 2021[4]) and national action plans using natural language processing techniques. Complementing this analysis, results from a comprehensive review of the latest evidence on the effectiveness of AMR policies in line with the One Health approach are presented. Results from these analyses shed light on the current gaps in policy implementation and put forward a menu of evidence‑based interventions to close such gaps. Next, the chapter places the spotlight on tackling AMR in long-term care settings, an emerging area of interest that saw substantially less policy action compared to other settings (e.g. hospital care) despite being recognised as a significant reservoir of AMR. Finally, the chapter reports results from the cost-effectiveness analysis for 11 One Health interventions making the economic case for investment.
AMR is forecasted to grow at a slower pace than in historical trends, suggesting that recent efforts to optimise antibiotic use may be yielding promising results, particularly in the livestock sector
While many OECD and EU/EEA countries show modest declines in sales of antibiotics for use in humans, possibly due to the promotion of prudent use of antimicrobials, long-term trends still show that sales have increased in the majority of countries. In contrast, sales in the livestock sector have decreased substantially, particularly over the last decade. However, this sector still accounts for a large majority of antibiotic sales and, while data are haphazard and incomplete, some worrying trends regarding antibiotics dispersed in the environment were identified.
Currently, the OECD analysis suggests that one in five bacterial infections are resistant to antibiotic treatment in the OECD but the growth rate has been relatively small over the last decade. Assuming that trends continue into the future, calculations from the OECD model suggest that the overall resistance proportions in OECD would remain mostly flat by 2035 but significant differences across countries will persist. For example, 18 countries are projected to experience growth in AMR rates across antibiotic-bacterium combinations. Importantly, in many OECD and EU/EEA countries, resistance to second- and third-line antibiotics, our backup option for difficult-to-treat infections, will grow much more than for first-line antibiotics. While data for 2020, the first year of the COVID‑19 pandemic, are still considered tentative, they suggest that the policy response to contain SARS‑CoV‑2 may have produced a secondary impact on AMR, at least in EU/EEA countries (Box 1.1). It is still too early to say whether this impact will persist in the “new normal” or revert to the pre‑COVID situation.
Box 1.1. Public health policies to contain the COVID‑19 pandemic appears to have had a positive impact on antibiotic consumption in EU/EEA countries
Enhanced hygiene measures and lower use of healthcare services for non-COVID-related hospitalisations reduced the use of antibiotics in hospitals and the community, possibly supporting a future reduction in AMR rates
Preliminary data covering EU/EEA countries in 2020 show that the mean total consumption of antibiotics in humans in the EU/EEA dropped by 17.6% compared to the year before, after adjusting for population size and therapeutic dose (OECD et al., 2022[5]). A majority of countries reported decreases in antibiotic consumption for both the community and the hospital sector and generally larger decreases in the community than in the hospital sector. In the community, the decrease between 2019 and 2020 was proportionally larger in countries with higher antibiotic consumption than in countries with lower antibiotic consumption.
Several reasons were suggested to explain this trend but they generally relate to actions taken by governments, healthcare providers and populations to curb the COVID‑19 pandemic including:
Decreases in antibiotic prescriptions for respiratory infections and to the youngest age groups, following changes in infectious diseases epidemiology.
Non-pharmaceutical interventions intended to limit SARS‑CoV‑2 spread (e.g. restrictions on movement, physical distancing, respiratory etiquette, hand hygiene and international travel restriction).
Lower use of primary care services, due to lockdowns and reprioritisation of healthcare resources, which could have led to changes in antibiotic prescribing patterns.
Higher demand for intensive care beds to treat patients with COVID‑19 significantly reduced the number of admissions for elective surgery or chronic care, a situation for which antimicrobials are used in a significant amount.
The impact of the reduction in the use of antibiotics in humans on AMR rates is still unclear. The AMR surveillance systems of many OECD and EU/EEA countries were severely affected during the initial phases of the pandemic. For EU/EEA countries, statistics for 2020 report a great increase in the number of isolates processed for pathogens commonly responsible for healthcare‑associated infections (HAIs) and a great reduction in the number of isolates for other bacteria not directly linked to the COVID‑19 response and for pathogens in the community (ECDC, 2022[6]). The COVID‑19 pandemic has severely affected the soundness of the statistics and made the observed changes in AMR percentages between 2019 and 2020 difficult to interpret. Robust surveillance systems will continue to be vital to monitor the situation, assess the consequences and inform public health decisions.
Source: OECD et al. (2022[5]), Antimicrobial Resistance in the EU/EEA: A One Health Response, https://www.oecd.org/health/Antimicrobial-Resistance-in-the-EU-EEA-A-One-Health-Response-March-2022.pdf; ECDC (2022[6]), Antimicrobial Resistance Surveillance in Europe, https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf.
This section looks at historical trends and projections of sales for major classes of antibiotics and resistance proportions for 12 priority antibiotic-bacterium combinations, using a new round of data and advanced statistical techniques such as machine learning. Analyses cover the period 2000‑19,2 with projections up to 2035 for 51 countries, including OECD, EU/EEA and G20 countries.
Worrisome trends in antimicrobial consumption in humans and animals remain a serious concern
Misuse of antimicrobials in human and animal health remains one of the key drivers of AMR. While sales of antibiotics for humans in OECD and EU/EEA countries have recently started decreasing, sales are still higher than 20 years ago and sales in non-OECD G20 countries have increased significantly to almost OECD levels. In 2019, just before the COVID‑19 pandemic, sales of antibiotics in OECD countries monitored by ResistanceMap/IQVIA were estimated at 21.8 DDD per 1 000 inhabitants per day, slightly higher (+1.9%) than 19 years before when sales were estimated at 21.4 DDD per 1 000 inhabitants per day. Average trends across the EU/EEA mirror those in the OECD. The average trend across G20 countries shows a convergence towards the OECD levels, driven primarily by a significant increase in antibiotic sales in countries such as Brazil, China, India, Indonesia and Saudi Arabia.
The analysis of the short-term trends suggests a more optimistic picture, possibly because of the impact of increased efforts to promote prudent use of antibiotics. Between 2015 and 2019, antibiotic sales decreased by 6.5% across OECD countries, 8% across EU/EEA and 7.4% in G20 countries. Despite these trends, the analysis of historical data on sales of antibiotics for use in humans across OECD and EU/EEA countries further points to some worrying trends:
Sales of high-priority antibiotics for human health have been increasing faster than total consumption of all types of antibiotics. For example, the consumption of carbapenems and polymixins, two last-resort antibiotics used to treat patients with multi-drug resistant infections, increased by 10% and 67% respectively across EU/EEA countries between 2011 and 2020 (ECDC, 2019[7]). Similarly, an OECD analysis found a seven‑time higher increase in the use of carbapenems than total consumption across OECD countries between 2010 and 2015. The literature also suggests even higher growth rates for low- and middle‑income countries (LMICs), which would account for most of the global growth in antibiotic consumption (Klein et al., 2018[8]).
In 2015, the latest year for which data are available for all the countries included in the analysis, 14 OECD countries did not meet the target set by the WHO of having at least 60% of total national antibiotic consumption made up of antibiotics with lower resistance potential – known as the Access antibiotics in the WHO Access, Watch, Reserve or AWaRe classification of antibiotics3 (Klein et al., 2021[9]). Large economies outside the OECD, such as Brazil, Chine and India were also among the countries that did not meet the WHO target in 2015. Since then, some OECD countries (e.g. Switzerland) have progressed on this indicator and now meet the 60% target.
Across EU/EEA, in 2020, the consumption of broad-spectrum antibiotics in the community was 3.5 times higher than the consumption of narrow-spectrum antibiotics, which should typically be the first-line therapy.4 Between 2011 and 2020, an increasing trend was observed in this ratio for the EU/EEA overall, indicating a shift towards broad-spectrum antibiotics to treat infections in the community (OECD et al., 2022[5]).
Should total antibiotic consumption continue to evolve following the same trends identified by the OECD model between 2015 and 2035, it is estimated that consumption would decrease by 3% in the OECD from 23.3 to 22.6 DDD per 1 000 inhabitants per day respectively (Figure 1.1). EU/EEA member states could see average total consumption decrease by 3.3%, while sales in G20 countries could drop by 6.2%. One of the main reasons underpinning this small but positive trend is that a number of countries experienced reductions in sales of antibiotics in the last few years before the COVID‑19 pandemic. An emergent cause of concern that could put at risk the emerging trends identified by the model is represented by the growing shortages in the availability of antibiotics (Box 1.2).
Figure 1.1. If trends persist, total antibiotic consumption in humans in the OECD could decrease
Total antibiotic consumption in 2015 and 2035*
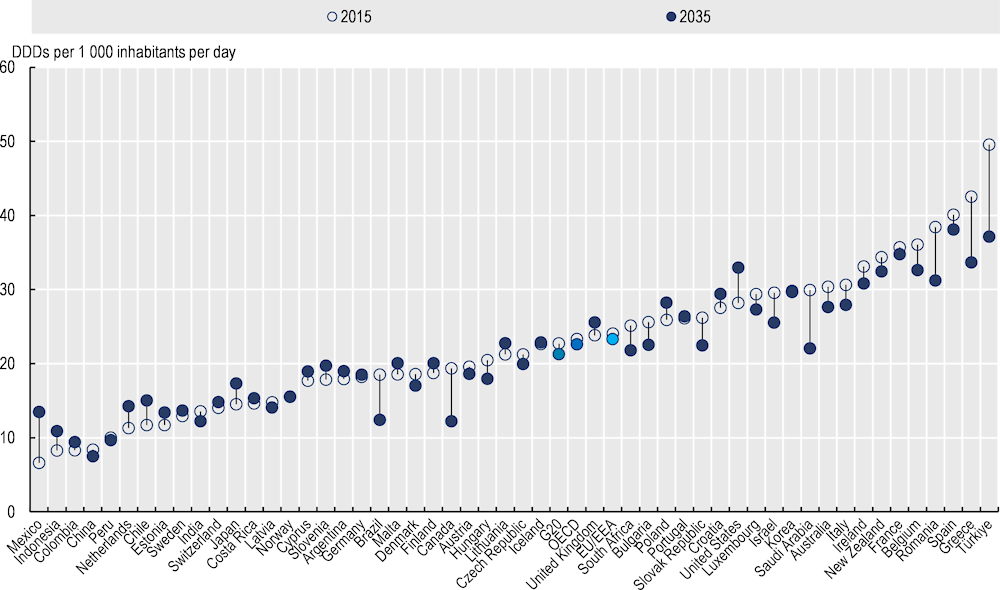
* Original data go as far as 2015; estimates for 2016‑20 were derived through a combination of multiple imputations (data from OECD.Stat on consumption used as priors) and exponential smoothing with a damped trend. Averages for different country groups are unweighted.
Source: Chapter 2, Figure 2.3, https://stat.link/jqv4a0.
Box 1.2. Shortages of antibiotics and unavailability of forgotten antibiotics may negatively affect efforts to promote prudent use of antibiotics
In a 2019 survey of 39 European countries, 95% of participating pharmacists indicated that the shortage of medicines was a major problem in their hospitals (EAHP, 2019[10]). Antimicrobial agents were the leading cause of shortages in medicines from as far back as 2014. In 2019, around 63% of participating pharmacists indicated that they experienced shortages in antimicrobial agents, 5% more pharmacists than in 2014, indicating the situation is not improving.
Availability issues are even more evident for the so-called “forgotten antibiotics”. These are older but still clinically effective antibiotics, which are often categorised as Access antibiotics in the WHO AWaRe classification. These antibiotics are often not available in countries, either because they were never introduced or because they were withdrawn from the market at a certain point. A 2017 study found the availability of these antibiotics was low, with only about 69% (25 out of the 36 considered antibiotics) accessible in about 20 out of the 39 countries, mainly OECD and EU/EEA countries, included in the analysis (Pulcini et al., 2017[11]).
Source: EAHP (2019[10]), 2019 EAHP Medicines Shortages Report: Medicines Shortages in European Hospitals, https://www.eahp.eu/practice-and-policy/medicines-shortages (accessed on 18 June 2022); Pulcini, C. et al. (2017[11]), “Forgotten antibiotics: A follow-up inventory study in Europe, the USA, Canada and Australia”, https://doi.org/10.1016/j.ijantimicag.2016.09.029.
Antimicrobials are used in animals for several purposes (see Chapters 2 and 5). They can be used to treat animals with bacterial infections. Antimicrobials can also be administered to animals who have been in contact with infected animals as a form of disease control (also called metaphylaxis). When no animals exhibit signs of infection, antibiotics can be used prophylactically across groups to prevent disease. Finally, antimicrobials may be used in healthy animals to accelerate weight gain and improve the efficiency of feed utilisation (WHO, 2017[12]). Metaphylaxis, prophylaxis and growth promotion can result in large volumes of antibiotics being used.
Worldwide, the consumption of antibiotics in animals far surpasses consumption in humans, with an estimated 73% of total antimicrobial sales globally being used in animals raised for food (Van Boeckel et al., 2019[13]). It is estimated that in 28 EU/EEA countries that report both animal and human consumption data, approximately 70% of the active substance of antimicrobials was sold for use in food-producing animals (ECDC/EFSA/EMA, 2017[14]). Moreover, last resort antibiotics (e.g. colistin) continue to be used for growth promotion purposes in many countries (Kumar et al., 2020[15]).
Across OECD countries, the average sales of all classes of antimicrobials used in chicken, cattle and pig systems are estimated to have halved over the last two decades, after adjusting for total production and importation of meat products. Most of this observed decline took place around 2014. The trend is similar in the EU/EEA but with the largest part of the reduction starting from 2010. Consumption in animals in the G20 is estimated to have dropped as well over the last 20 years but remains at levels higher than those in the OECD and EU/EEA.
While surveillance systems for antimicrobial consumption in animals are generally less developed than those used to monitor consumption in humans and these figures should be interpreted with caution, this is excellent news for at least two reasons. First, it is a sign that policy efforts by countries and stakeholders produced a significant impact on antimicrobial consumption in the livestock sector. Second, given that worldwide and within the EU/EEA, about 70% of total antimicrobial sales are used in animals raised for food (ECDC/EFSA/EMA, 2017[16]; Van Boeckel et al., 2019[13]), it is conceivable that if these trends continue, they could result in a significant decrease of total sales of antibiotics (i.e. humans and animals). In fact, according to the OECD analyses, if downward trends in the OECD and EU/EEA persist, these regions could see an additional 10% and 12% reduction in antimicrobial sales for food animals per animal biomass by 2035, compared to 2020.
The use of antimicrobials in aquaculture merits attention as one of the next priority areas to continue optimising the use of antimicrobials in livestock production. Aquatic animals represent 17% of global animal protein consumption and nearly 50% of the global supply of fisheries products for human consumption already comes from aquaculture. Given that consumption of aquatic animals is growing faster than the consumption of meat (with the exception of chicken), it is projected that, at current trends, the use of antimicrobials for food-producing aquatic animals will account for almost 6% of total global antimicrobial consumption by 2030, including humans and animals. In the same period, global sales of antimicrobials for use in aquaculture will rise by 33% (by 29.7% in Europe). Even most worryingly, 96% of all antimicrobial use in aquaculture comes from classes classified as highly important and critically important for humans (Schar et al., 2020[17]).
Beyond the use of antimicrobials in humans and the animal sector, other sectors are also contributing to high levels of antibiotics and are underpinning the rise in AMR. Data for these sectors are less accurate than for humans and livestock production but the available evidence suggests that these are all emerging issues deserving further attention and action. Some of the issues identified during the review of the evidence include the following:
At least 20 countries approved the use of antibiotics to treat plant diseases (FAO, 2018[18]). In certain countries with strong regulatory oversight, antibiotic use in plants is minimal but this is not the case everywhere and significant amounts of antimicrobials were found to be used to control plant pests (WHO/FAO/OIE, 2021[4]).
While most high-income countries either ban or restrict the use of antimicrobials in horticulture, this is not the case in many LMICs, where the sale of antimicrobials in plants is either unregulated or insufficiently enforced. Even when regulations are strong and effective, there may be disagreement over the best course of action.
Antimicrobials may be dispersed in the environment by manufacturing plant run-off. This is particularly problematic in China and India where most antimicrobials are produced. Studies have found concentrations of antimicrobials in water downstream of manufacturing sites that were higher than blood concentrations in humans taking antimicrobials (WHO/FAO/OIE, 2020[19]). While there are no international guidelines on this matter, out of the 17 companies assessed in the Access to Medicine Foundation’s report (2020[20]), 13 had an environmental risk-management strategy to address AMR and 12 set antimicrobial discharge limits at their facilities. However, only six companies asked their suppliers to set discharge limits and no company made any data from monitoring limits publicly available. The report also found that none of the 17 companies monitored the discharge levels of private waste‑treatment plants that are contracted to dispose of their manufacturing waste (Access to Medicine Foundation, 2020[20]).
Antimicrobials may also be present in the environment at large, from soil to waterways, for different reasons. A large part of the antibiotic volume ingested by both humans and animals (estimates vary, but around 80% in animals) is excreted in its active form, depending on the class of antimicrobial and how it is used. Antibiotics that have expired or are no longer necessary are also often discarded in general waste or wastewater.
Overall, AMR will grow at a slower pace than expected but worrying trends are forecasted for backup antibiotics and in certain countries
Across OECD countries, one in five infections in humans is resistant to antimicrobials, with a tenfold difference between countries with the highest and lowest resistance proportion. According to the OECD analyses of data from surveillance networks collated in ResistanceMap:
In 2019, the estimated resistance proportions across 12 priority antibiotic-bacterium combinations were 20% in OECD countries. Denmark and Norway had the lowest estimated average resistance proportions, at nearly 6%, whereas in Greece and Türkiye, around 44% of infections were estimated to be resistant.
In 2019, for some antibiotic-bacterium combinations such as fluoroquinolone‑resistant and carbapenem-resistant Acinetobacter baumannii (A. baumannii), over 90% of infections were due to resistant bacteria in the countries with the highest resistance proportions.
In 2019, resistance proportions in EU/EEA countries were evaluated to be similar to the OECD, with average resistance rates evaluated at 22% and higher across G20 countries at 30%.
Data on resistance proportions in humans for infections with a large animal reservoir, such as Salmonella and Campylobacter, remain very limited but the available evidence suggests a worrying situation. In the United States, the resistance of Salmonella typhi was estimated to average 18% in 2018 (CDC, 2022[21]).
Resistance to ciprofloxacin, a Watch antibiotic in the WHO AWaRe classification, was 13% in Salmonella spp. in 12 EU member states and 16 out of 19 EU/EEA countries reported very high or extremely high resistance to ciprofloxacin in Campylobacter (EFSA/ECDC, 2020[22]).
A small average increase in resistance proportions between 2009 and 2019 masks wide cross-country variation. Resistance proportions for 12 priority antibiotic-bacterium combinations5 slightly increased across the OECD between 2009 and 2019, from 18% to 20%. The growth rate across EU/EEA and G20 was similar, at around 3%. Across all countries, the average largest increases in resistance proportions were for A. baumannii resistant to fluoroquinolone (+12.6%) while the largest projected reductions were in methicillin-resistant S. aureus (MRSA; ‑3.2%). While in 8 countries average resistance proportions for all the 12 antibiotic-bacterium combinations went down (‑1.4 percentage points), the majority of OECD countries experienced an increase by as much as 8 percentage points between 2009 and 2019 (e.g. the Czech Republic and Italy). It is also estimated that no country has reduced resistance proportions for all 12 antibiotic-bacterium combinations between 2009 and 2019. Salmonella resistant to ciprofloxacin, a zoonosis, had a threefold increase in 2 years in EU member states for which data are available (from 1.7% in 2016 to 4.6% in 2018). In the United States, resistance of Salmonella typhi increased from close to 0 in 1999 to 18% in 2018 (CDC, 2022[21]).
By using machine‑learning techniques on updated historical data on resistance proportions and correlates of AMR (e.g. antimicrobial consumption in humans and animals), the OECD projected that between 2019 and 2035, resistance proportions averaged across 12 priority antibiotic-bacterium combinations will remain relatively flat, if trends continue into the future (Figure 1.2). Similar reductions of around 1 percentage point for average resistance proportions are also projected for EU/EEA and G20 countries. The OECD analyses also suggest that:
Resistance proportions averaged across 12 antibiotic-bacterium combinations, are projected to increase in 18 countries, remain at their 2019 average levels in 1 country and decrease in 32 countries.
Countries with historically low average resistance proportions are likely to maintain these into 2035. Conversely, countries with historically high average resistance proportions are estimated to have experienced most of the growth between 2009 and 2019, with average resistance proportions either flattening or dropping slightly by 2035.
Resistance proportions for some antibiotic-bacterium combinations is expected to be dangerously high. For example, the average resistance proportions for fluoroquinolone‑resistant A. baumannii and carbapenem-resistant A. baumannii can be as high as 45% and 30% across OECD by 2035 (51% and 37% in EU/EEA respectively). The projected resistance proportions for these antibiotic-bacterium combinations by 2035 are expected to exceed 70% in countries where the average resistance proportions were already high in 2019 such as India, Türkiye and Greece.
In absolute terms, China, Luxembourg and Poland could see the largest percentage point increases, on average across 12 antibiotic-bacterium combinations, between nearly 3 and 6 percentage points higher in 2035 than in 2019. Conversely, the Czech Republic, Germany and Sweden could see the largest percentage point drops in average resistance proportions, projected to decrease around 4 to 5 percentage points.
Compared to the previous round of estimates, including data up to 2015, the new round of projections suggests a lower growth rate for future resistance levels. Keeping in mind the challenges around data availability and uncertainty related to the extrapolation process, the revised projections seem also to suggest some initial impact of the global efforts to tackle AMR. The revised estimates suggest that, compared to the previous set of analyses, there are more countries exhibiting a downward trend across the 12 antibiotic-bacterium combinations. In addition, antibiotic consumption in humans in the EU/EEA decreased between 2010 and 2019. Antimicrobial consumption in animals, which was included in the estimation procedure for the first time under a One Health approach, has also shown a downward trend in the OECD and the EU/EEA in the last few years. Finally, recent trends in AMR in the EU/EEA between 2016 and 2020 show some reductions.
Figure 1.2. Projected average proportion of infections caused by bacteria resistant to antimicrobial treatment for 12 antibiotic-bacterium combinations in 2009, 2019 and 2035
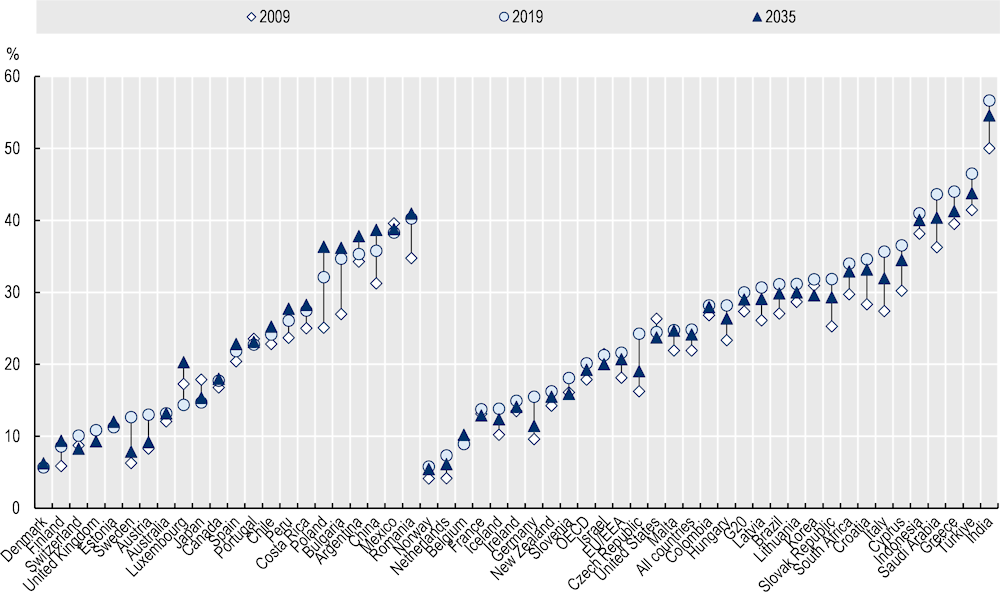
Note: For countries on the left of this graph, resistance proportions are higher in 2035, compared to 2019. For countries on the right, rates are lower in 2035. Otherwise, countries are sorted left to right based on ascending resistance proportions in 2019. Averages for different country groups are unweighted.
Source: Chapter 2, Figure 2.6, https://stat.link/8l5h7e.
Despite a projected overall stabilisation of resistance proportions, AMR is projected to remain dangerously high for certain countries and certain antibiotic-bacterium combinations. The top causes of concerns and reasons for an even tighter implementation of AMR policies include the following:
By 2035, around half of the infections due to A. baumannii in G20 countries could be resistant to either fluoroquinolones or carbapenems. In the OECD, Greece and Türkiye are likely to continue to exhibit very significant average resistant proportions, with around 85% of infections in these countries due to A. baumannii projected to be resistant to either fluoroquinolones or carbapenems by 2035.
Relative growth rates for resistance to second- and third-line antibiotics are forecasted to grow much more quickly than for resistance to first-line treatments. By 2035, resistance to third-line antimicrobials are projected to be 2.1 times higher in OECD countries (3.3 times in EU/EEA, 1.6 times in the G20) compared to what it was in 2005, albeit from still mostly low levels. Similarly, resistance to second-line antimicrobials is forecast to be 23‑45% higher in 2035 across the same groups of countries, compared to 2005 levels.
Antimicrobial resistance damages population health and the economy
Much has already been written on a so-called “post-antibiotic” world, in which virtually no antibiotic would be effective, but AMR is already causing significant health and economic burden to the population and the economy of OECD countries and EU/EEA member states. Patients with resistant infections are more likely to develop complications and face a lower probability of recovery and a higher risk of death. Typically, resistant infections are costlier to treat compared to susceptible infections as they are more likely to require more intensive medical procedures and more aggressive antimicrobial therapies. As a result, patients with resistant infections spend a longer time in hospital, if they are hospitalised. Combined, these features of resistant infections lead to lower workforce participation and productivity.
In the longer-term, the burden caused by AMR in a post-antibiotic scenario could be significantly worse because even small infections could lead to death. In such a scenario, the burden of AMR would be greater than its direct impact because many non-essential treatments requiring the use of antibiotics (e.g. elective surgery) could be delayed or even avoided as the risk of death would be greater than the disability caused by the absence of treatment. The OECD had previously calculated that in a scenario where antibiotics would become almost completely ineffective, the ten most common procedures carried out in hospitals in the European region in 2014 would have produced an additional 435 000 infections leading to an additional 30 000 deaths (OECD, 2018[1]). A similar analysis for the United States concluded that the same scenario would produce an additional 400 000 infections and 21 000 deaths in 2010 (Teillant et al., 2015[23]). Such estimates roughly correspond to the yearly number of deaths due to motor vehicle accidents in the same regions and in the same period. While the “post-antibiotic” scenario remains a longer-term threat, it is crucial to assess the health and economic burden of AMR as new data come and evidence emerges.
The new iteration of the OECD analysis extends its previous assessment in a number of directions. For example, the new analysis increased the number of antibiotic-bacterium combinations that now include infections with a significant animal reservoir. This analysis also extended the geographical coverage to a total of 34 countries including all 29 EU/EEA countries, as well as Japan, Switzerland, Türkiye, the United Kingdom and the United States. The number of policy options modelled increased to include 11 One Health interventions. Finally, it quantified the impact of AMR on workforce productivity and the broader economy.
The analyses were carried out within the OECD SPHeP Framework using data from national surveillance systems obtained from relevant governmental agencies or other intergovernmental organisations (Box 1.3). For each country, the OECD model evaluates the impact of AMR under two different scenarios:
A first – the elimination scenario – uses the classical burden of disease approach and assumes that antibiotic-resistant bacteria are eliminated. In practical terms, the scenario evaluates how the assessed outcomes change as a result of a fictitious elimination of the risk factor and, consequently, of all its consequences.
A second – the replacement scenario – assumes that bacteria do not develop resistance. In this scenario, people that were infected by resistant bacteria would continue to be infected by bacteria that are susceptible to antibiotics. Outputs from this scenario are more conservative because susceptible bacteria increase the risk of complications and deaths but less than resistant bacteria.
Box 1.3. The OECD SPHeP framework – A tool to assess the medium- and long-term effects of top public health threats, including antimicrobial resistance
The OECD Strategic Public Health Planning for AMR (SPHeP-AMR) framework is an advanced systems modelling tool for public health policy and strategic planning. It is used to predict the health and economic outcomes of the population of a country, or a region, up to 2050. The model for AMR simulates synthetic populations of 34 countries, including all 29 EU/EEA countries, as well as Japan, Switzerland, Türkiye, the United Kingdom and the United States and many OECD countries.
The AMR model covers 28 antibiotic-bacterium combinations, including 6 HAIs and 7 community-acquired infections (CAIs), out of which 2 are infections with a significant zoonotic reservoir. Some infections can be both hospital- and community-acquired and some infections can be resistant to multiple antibiotics.
The incidence and prevalence of diseases in a specific country’s population are calibrated to match estimates from the European Centre for Disease Prevention and Control (ECDC) estimates and official statistics obtained by national authorities and the WHO. Data provided to the OECD are collected by national surveillance systems and generally reflect the national official statistics. This approach has many advantages. Data gathered from the ECDC and official statistics are aligned with the information presented by countries in their national reports and evaluations, as well as assessments generated by the ECDC. Data from the ECDC are collated from laboratories and hospitals in countries based on procedures and methodologies that aim to harmonise data collection and management efforts across countries. On the other hand, the results presented in this chapter should be considered conservative. While there has been notable progress in recent years to strengthen AMR surveillance, detection and reporting capacity across many OECD and EU/EEA countries, important cross-country differences persist. These differences can mean that countries with more accurate reporting systems may show a greater AMR burden because they face a lower risk of under-reporting. The links between infections and complications, including deaths, are modelled through probability rates retrieved from the literature. The impact of infections on workforce productivity is also simulated through relative risk retrieved from the literature.
The model was used to simulate various scenarios, including the burden related to resistant infections (two scenarios described earlier) and policy scenarios (described in Chapter 6). Policy scenarios were modelled on evidence of the highest quality across four key dimensions, including: i) effectiveness of interventions at the individual level; ii) effectiveness over time; iii) eligible population and exposure; and iv) cost of running the intervention.
To assess the population-level impact of a scenario, model outputs were evaluated against a business-as-usual scenario, in which age‑ and sex-specific exposure to AMR is assumed to remain unchanged over the simulation period and the provision of preventive and health services is assumed to be implemented at the current levels in each country. A comparison of the business-as-usual scenario and the analysis scenario yields the impact on health outcomes, health expenditure and workforce productivity. The impact on workforce productivity is evaluated using the human capital approach, which is based on several assumptions including, for example, those on reserve labour force, friction costs and the impact on reserve wages.
For more information on the OECD SPHeP-AMR framework, see Box 3.1 in Chapter 3 and Box 6.1 in Chapter 6 and the SPHeP-AMR Technical Documentation (http://oecdpublichealthexplorer.org/amr-doc/).
Antimicrobial resistance worsens population health and decreases life expectancy
Findings from the OECD SPHeP-AMR model suggest that across 34 EU/EEA and OECD countries starting from 2021 (or earlier depending on the availability of historical data) up to 2050, AMR is expected to cause the following detrimental impacts on health:
On average, every 7.3 seconds someone is infected by a resistant bacterium, most often in the community. Nearly 4.3 million infections are estimated to occur each year in the 34 countries included in the analysis (Almost 1.7 million across the EU/EEA) due to resistant infections. Around 2 in 3 resistant infections (around 69% in the EU/EEA) are acquired in the community with the remaining cases developing in healthcare settings.
Every year, on average, 79 000 people (nearly 22 000 in the EU/EEA) die due to resistant infections. This corresponds to about 2.4 times the number of deaths due to TB, influenza and HIV/AIDS in 2020 combined. Countries in southern Europe and Mediterranean countries face a greater burden, with most of the cross-country variability explained by a higher incidence of infections as well as other factors like clinical management practices.
Resistant strains of E. coli, K. pneumoniae and S. aureus are the top killers causing around three in four deaths. Resistant E. coli alone represents about one‑third of all deaths caused by AMR, while resistant K. pneumoniae accounts for about 21% of all deaths due to AMR. In contrast, resistant strains of Salmonella spp., Campylobacter jejuni (C. jejuni), Campylobacter coli (C. coli) and Mycobacterium tuberculosis represent a small share of the AMR burden. However, these bacteria remain a top public health threat elsewhere: diarrheal diseases caused more than 1.5 million deaths worldwide in 2019, with a high burden in children under 5 years of age (Vos et al., 2020[24]). Similarly, TB was estimated to kill 1.6 million people worldwide in 2021 (WHO, 2022[25]).
Resistant HAIs present a greater risk of death compared to those acquired in the community. HAIs account for more than 60% of AMR-related deaths even though they only represent about 31% of resistant infections. For instance, hospital-acquired K. pneumoniae represents only around 4% of all resistant infections but causes around 13% of all AMR-related deaths. In contrast, community-acquired C. jejuni and C. coli cause about 36% of all resistant infections but account for less than 1% of all AMR-related deaths. These findings underline the importance of hospital-based measures to reduce the burden of HAIs.
Deaths due to AMR are concentrated among the elderly populations, with around 2 in 3 AMR‑related deaths occurring among people above 65 years of age. About 4% of deaths due to AMR occur among people under 20 years of age, particularly in newborns or young children.
AMR is linked with reductions in life expectancy at birth in the order of magnitude of about 2.6 months (1.6 months across the EU/EEA). This is roughly equivalent to one‑third of the impact caused by COVID‑19 between 2019 and 2020, which was estimated to be around 7.5 months across the 34 countries included in the analysis based on OECD data (OECD, 2022[26]).
AMR accounts for a significant share of total health expenditure
As for the analyses on the health burden, the OECD model was run on the same group of 34 countries for the period from 2021 (or earlier depending on the availability of historical data) up to 2050 to calculate the use of healthcare resources and the related costs caused by the growing rates of AMR. Under the elimination scenario, the model calculates that:
Resistant infections are estimated to result in more than 32.5 million extra days spent in hospital every year across the 34 included countries (more than 9.5 million extra hospital days across the EU/EEA countries). The total amount of extra hospital days due to AMR is roughly equivalent to using the entire acute bed capacity in Spain for the whole of 2020.
The annual cost of treating complications caused by AMR is estimated to average more than USD 28.9 billion adjusting for PPP across all of the countries included in the analysis, corresponding to almost USD PPP 26 per capita. In the EU/EEA, the healthcare cost of AMR is estimated to reach around USD PPP 7.5 billion every year corresponding to around USD PPP 15.3 per capita.
The cost of inaction to tackle AMR up to 2050 is expected to exceed treatment costs due to COVID‑19 in 2020. In 17 OECD countries and EU/EEA countries for which data are available, the total health expenditure incurred each year due to AMR is about 19% of the total health expenditure due to treating COVID‑19 patients in 2020.
AMR negatively affects workforce productivity and the economy
The OECD SPHeP-AMR model was also used to quantify the impact of AMR on workforce productivity, which is measured as a combination of: i) participation (assessed through employment rate); and ii) productivity (measured through absenteeism and presenteeism). Changes in labour supply and workforce productivity are translated to monetary losses using the human capital approach, whereby the duration of foregone work is multiplied by the estimated national average wage in the simulation period, to provide a high-level impact of AMR on the broader economy. As for the other analyses, the OECD SPHeP‑AMR model was run on the same group of 34 countries for the period from 2021 (or earlier depending on the availability of historical data) up to 2050.
Under the elimination scenario, the model evaluates the following:
AMR is expected to cause a decline in the labour market output of about 734 000 FTEs in the working population every year, which corresponds to about a 0.12% decline in the labour market output. In the EU/EEA, the average yearly loss in labour market output stands at around 161 000 FTEs, which is equivalent to about a 0.06% decline in productivity. The magnitude of this decline may seem smaller compared to other public health threats. However, as discussed in the section on the impact on population health, resistant infections primarily develop among people aged 65 and over who – very often – have already left the labour market.
Around 84% of the decline in labour market output is due to a reduction in workforce participation – mainly caused by the death of people in active employment – with most of the remaining share attributable to increased absenteeism.
The estimated declines in workforce productivity translate into considerable financial losses. The model estimates a total economic loss of USD PPP 36.9 billion each year across the 34 countries included in the analysis, corresponding to around USD PPP 32.7 per capita. In the EU/EEA, AMR is estimated to depress workforce productivity by around USD PPP 5.8 billion per year by 2050, corresponding to approximately USD PPP 11.8 per capita every year.
Italy, Ireland and Malta are estimated to incur the greatest losses in per capita labour market output across the EU/EEA countries, with losses ranging from around USD PPP 16.5 in Malta to USD PPP 23.8 in Italy. Across the non-EU/EE member OECD countries, the greatest losses occur in the United States (USD PPP 61.8 per capita per year) and Türkiye (USD PPP 56.9 per capita per year).
Figure 1.3 summarises the health and economic burden of AMR across the 34 countries included in the analysis and key findings for the replacement scenario are presented in Box 1.4, together with the results for all the other dimensions.
Box 1.4. The health and economic impact in the replacement scenario
As discussed earlier, the replacement scenario assumes that resistant infections would be replaced by infections caused by susceptible bacteria. This is the more conservative assumption as susceptible infections are generally less dangerous but still pose significant a burden of disease. Results from the OECD SPHeP-AMR model suggest that across the 34 countries included in the analysis:
The estimated deaths due to resistant infections exceed 24 000 every year up to 2050, with around 6 000 of these deaths occurring across the EU/EEA countries.
Resistant infections acquired in healthcare settings are estimated to represent about 3 in 4 (73%) of all deaths due to resistant infections. This figure is 11% higher than in the elimination scenario, underlying the significantly higher mortality caused by HAIs compared to CAIs.
Resistant infections are estimated to result in 6.9 million extra days spent in hospitals every year up to 2050, corresponding to around USD PPP 5.9 billion in healthcare expenditure (USD PPP 5.2 per capita).
Annual losses in the labour market productivity are estimated to average around 119 000 FTEs (18.7 FTEs per 100 000 working population) and nearly 27 000 (9.6 FTEs per 100 000 working population) across the EU/EEA countries.
After converting reductions in workforce productivity in labour market outputs, resistant infections are estimated to produce losses of more than USD PPP 6.6 billion (USD PPP 5.9 per capita) up to the year 2050. Across the EU/EEA countries, annual losses amount to nearly USD PPP 960 million (USD PPP 1.9 per capita).
Figure 1.3. Summary of health and economic impact of AMR across the 34 countries included in the analysis

Notes: The infographic above summarises the health and economic impact of AMR under the elimination scenario.
OECD countries have national action plans for AMR (AMR-NAP) that are aligned with the Global Action Plan on AMR (AMR-GAP) but only nine of the counties put in place financial provisions for implementation in national plans and budgets
In recent years, the global community has made important strides to tackle AMR. In May 2015, all members of the WHO made a commitment to tackling AMR by adopting the AMR-GAP (WHO, 2015[27]). Since then and up to 2021‑22, 149 countries released their own action plan (WHO/FAO/OIE, 2021[4]) although only 10% (17/166) of action plans proceeded to the most advanced stage of implementation, including financial provisions for the implementation of AMR-NAPs in national action plans and budgets. OECD, EU/EEA and G20 countries report a more advanced stage of implementation (Figure 1.4). Nonetheless, only around 20% (10/51) of OECD countries and key partners and EU/EEA countries had proceeded to the final stage of implementation by 2020‑21, where financial provisions for the implementation of the AMR-NAP are incorporated in national action plans and budgets (WHO/FAO/OIE, 2021[4]).
Figure 1.4. National action plans for AMR are usually well-developed but there are significant gaps in policy implementation
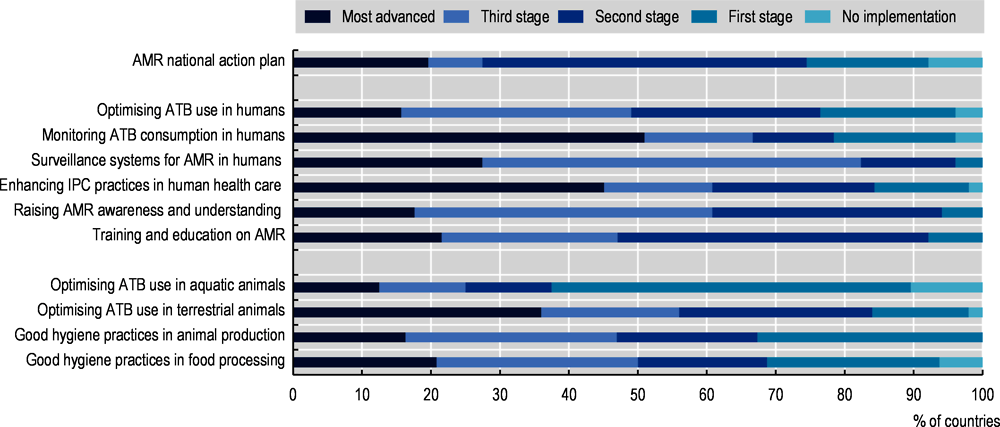
Note: The data presented in the graph are based on 51 countries included in the OECD analysis. ATB: Antimicrobial.
Source: OECD analysis based on WHO/FAO/OIE (2021[4]), Tripartite AMR Country Self-Assessment Survey (TrACSS) 2020‑21, https://www.who.int/publications/m/item/tripartite-amr-country-self-assessment-survey-(tracss)-2020-2021.
A multi-sectoral approach has been endorsed by the majority of countries while developing their AMR‑NAPs. In nearly all OECD countries, EU/EEA and G20 members, at least two sectors actively participated in the development and implementation of these action plans by 2021‑22, with animal health and food safety being the two sectors most often involved. Conversely, plant health was the sector less often involved in the development process with, respectively, only 63% and 55% of OECD and EU/EEA countries involving these sectors. Involvement of the various sectors was most often sought by establishing multi-sectoral co‑ordination mechanisms, such as steering committees or joint working groups, which are considered best practices.
By using natural language processing techniques, the OECD has assessed the level of alignment between the GAP-AMR and 21 national action plans from OECD, EU/EEA and G20 countries as well as the level of emphasis that each plan places on key policy dimensions to contain AMR (Özçelik et al., 2022[28]). The considered policy dimensions were selected based on their recognised role in driving success in tackling AMR (Ogyu et al., 2020[29]; Chua et al., 2021[30]; Anderson et al., 2019[31]) and include: i) funding and budgetary considerations; ii) optimising use of antimicrobials; iii) strengthening surveillance mechanisms; iii) strengthening AMR surveillance; iv) IPC policies; v) promoting research and development (R&D); and vi) enhancing AMR awareness and understanding. The key findings of this analysis include the following:
There is a high degree of convergence between AMR-NAPs and the AMR-GAP in terms of their strategic objectives. Optimising the use of antimicrobials in human and animal health is the most frequently featured strategic objective, followed by strengthening AMR surveillance, reducing the incidence of infections and making an economic case for sustainable investments. In comparison, improving awareness and understanding of AMR is the least frequently discussed objective.
Only 12 out of 21 AMR‑NAPs from OECD, EU/EEA and G20 countries discuss budgetary considerations and less than half refer to the cost-effectiveness of AMR-relevant interventions.
With respect to strategies to optimise antimicrobial use, strengthening antimicrobial stewardship, improving the availability of antibiotic prescribing guidelines, encouraging the use of older antimicrobials and scaling up electronic prescribing programmes are the most emphasised interventions.
Strengthening AMR surveillance is widely recognised as a top priority across the AMR-NAPs but countries would benefit from deepening their engagement with global and regional AMR surveillance networks, enhancing laboratory network capacity and integrating information from new data sources into AMR surveillance.
In terms of reducing the incidence of infections, the highest emphasis is placed on improving water, sanitation, hygiene and waste management practices and vaccination coverage in human health. There is a need to put more emphasis on veterinary vaccines and enhancing biosecurity.
In terms of strategies to spur AMR-related R&D, AMR-NAPs primarily focus on incentivising the early stages of drug development, whereas emerging evidence points to the need to supplement these incentives with incentives that can help improve the expectations around future revenues.
With respect to strategies to enhance AMR awareness and understanding, frequently highlighted interventions include those targeting medical professionals and the general public while less emphasis is given to interventions targeting young children.
Important gaps exist in the implementation of AMR-relevant policies
By using data from the latest wave of the 2021‑22 Tripartite AMR Country Self-Assessment Survey (WHO/FAO/OIE, 2021[4]), the OECD has assessed the level of actual implementation of the policy actions across OECD, EU/EEA and G20 countries (Figure 1.4). Findings from this analysis point to significant gaps in implementation:
In 76% of countries, guidelines for the appropriate use of antimicrobials are available at the national level. But, in only eight countries, policies promoting the optimal use of antibiotics are implemented for all major syndromes and data are used systematically to provide feedback to prescribers.
Around half of the countries, most often part of the OECD or EU/EEA, have developed monitoring and surveillance systems that are able to collect data and report on antibiotic sales or consumption at the national level for human use, as well as antibiotic prescribing and appropriate use in a representative sample of public and private health facilities.
Only 61% of countries report having IPC programmes developed in accordance with the WHO IPC core components and functioning at the national and health facility levels. These countries also confirm that actions are evaluated and updated on a regular basis. An additional 16% of countries report having IPC programmes meeting the highest standards but implementation cannot be assured nationwide.
Information campaigns for the public and training for healthcare personnel are implemented nationwide and at a high standard in 61% and 47% of countries respectively. Figures do not substantially differ for OECD and EU/EEA countries.
More than 90% of countries confirm that they have in place high-quality regulatory frameworks to promote prudent use of antimicrobials in animals though around 1 in 3 countries confirm that controls are in place to ensure compliance with legislation.
Around half of the countries – similar but slightly better results are also found for OECD and EU/EEA countries – report that their plans for good management and hygiene practices in animal production and food processing are not implemented nationwide.
While OECD countries and the European Commission remain the main source of funding for AMR innovations, additional funding is crucial to promote the development, particularly the later stages, and to bring to the market new antimicrobials, vaccines and devices (Box 1.5).
Box 1.5. OECD countries are the leading source of financing for R&D relevant to AMR
Between 2017 and 2020, the total spending on R&D for AMR declined slightly from USD 1.67 billion in 2017 to USD 1.92 billion in 2020 (Global AMR R&D Hub, 2023[32]). In 2020, OECD countries, including Germany, the United Kingdom and the United States, as well as the EU/EEA countries, were the lead source of financing for R&D allocated to AMR. Most of the R&D funding for AMR is allocated to funding basic research, development of therapeutics, operational and implementation research that can help support decision-making and management strategies, and diagnostics and capacity-building activities. This finding is coherent with studies that examined earlier periods, which concluded that the majority of R&D funding for AMR is allocated to supporting basic research and preclinical trials (Simpkin et al., 2017[33]). While this emphasis on the early stage of antimicrobial development is essential, increasing financial resources available for the later stages of clinical development can offer an important incentive that facilitates timely access to pharmaceutical markets in newly developed antibiotics. Moreover, increasing late‑stage incentives can help attract greater private investments.
Source: Simpkin, V. et al. (2017[33]), “Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps”, https://doi.org/10.1038/ja.2017.124.
Countries can count on a comprehensive set of policy options to tackle antimicrobial resistance in human health, agriculture and the food supply chain
To inform the next phase of the fight to tackle AMR and help countries implement evidence‑based interventions to close the gaps identified in the previous section, the OECD carried out a review of best practices and innovative policy options. Available evidence and datasets identify a comprehensive set of policy actions that countries can implement to tackle AMR (Figure 1.5). Policies are categorised on the target area span across four domains: i) human health; ii) agriculture; iii) food supply chain; and iv) the environment; and based on whether the policy aimed to promote prudent use of antibiotics or prevent the spread of infections. In total 29 policies were identified, with the highest number of policies targeting human health and the promotion of prudent use of antibiotics.
Figure 1.5. Multi-sectoral AMR-relevant strategies included in the OECD review
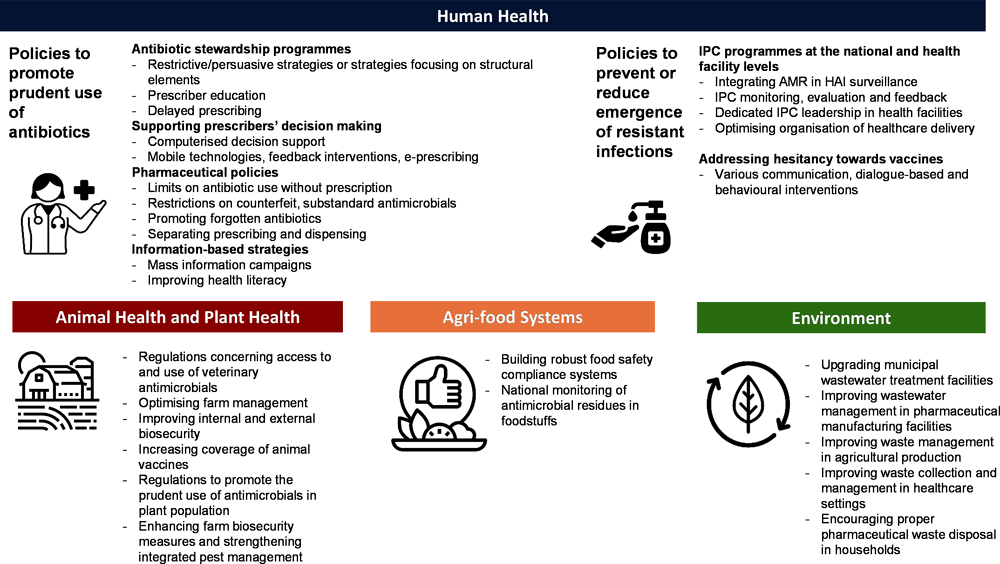
Policies focusing on human health
Most policies that tackle AMR in human health recognise that behaviours and choices made by individuals play an important part in promoting prudent use of antimicrobials and preventing the spread of infections. Health professionals’ behaviours – whether related to antibiotic prescription or the correct implementation of IPC practices – are influenced by a range of factors including their training, whether the system supports the clinical decision-making process, provider compensation methods, professional and social preferences and norms. Similarly, patient knowledge, preferences and attitudes play an important role in antibiotic use and the decision to be vaccinated. Complex interactions between healthcare providers and patients have also been shown to influence behaviours around antibiotics.
The review identified three categories and six policy interventions (Table 1.1). Overall, the available evidence supports the implementation of all policies identified. The use of new technologies, for example, to support prescribers’ decision making or for surveillance purposes, and removal of barriers to action emerge as successful enablers of policy implementation. The use of behavioural approaches also shows a promising impact in supporting policy actions. Finally, the evidence suggests that the design of the interventions is another very important determinant of success, particularly in the case of awareness-raising or education-based interventions that should deliver clear and consistent messages.
Table 1.1. Key findings on the impact of policy actions to tackle AMR in human health
|
Category of intervention |
Policy interventions |
Key findings |
|---|---|---|
|
Policies to promote prudent use of antibiotics |
Antibiotic stewardship programmes (ASPs) |
|
|
Supporting prescribers’ decision making |
|
|
|
Pharmaceutical policies |
|
|
|
Information-based strategies |
|
|
|
Policies to reduce the incidence of infections |
IPC programmes at the national and health facility levels |
|
|
Policies to improve vaccination coverage |
Addressing hesitancy towards vaccines |
|
Policies focusing on animal health, plant health and agri-food systems
Much like policies to tackle AMR in humans, policies in the agriculture domain aim to both promote prudent use of antimicrobials and prevent the spread of infections in livestock and crop production. Most of the attention in terms of policy implementation is focused on livestock production with much less evidence on plant production, which is another potentially important driver of antimicrobial use in agriculture. Previous OECD work in the field identified five key recommendations in this domain (Ryan, 2019[34]), which emphasised the need to adopt a flexible and step-by-step approach based on a mix of management and biosecurity measures. A strong call for more evidence on the economic benefits of each intervention as well as a need to ensure rapid availability of the evidence was also made.
The current analysis is designed by taking into consideration these recommendations, specifically those related to the type of policy and approaches, and identified four main areas of action in the agriculture and food supply chain and seven policy interventions (Table 1.2). Overall, the available body of evidence seems to be smaller and less consolidated than for human health, particularly in the case of plant health. Nonetheless, interventions such as regulation and optimising farm management emerge as effective in decreasing the use of antimicrobials in agriculture settings and decreasing AMR emergence and transmission. Supporting the sector in the transition towards tighter implementation of best practices, for example, by increasing the accessibility of alternatives or by supporting market mechanisms pushing in the desired direction, would help enhance the overall coverage and effectiveness of the interventions.
Table 1.2. Key findings on the impact of policy actions to tackle AMR in animal health, plant health and agri-food systems
|
Category of intervention |
Policy interventions |
Key findings |
|---|---|---|
|
Policies to promote prudent use of antimicrobials in animals |
Regulations concerning access to and use of veterinary antimicrobials |
|
|
Policies to prevent the emergence and spread of infectious diseases in animals |
Optimising farm management |
|
|
Improving internal and external biosecurity |
||
|
Increasing the coverage of animal vaccines |
||
|
Plant health |
Policies to promote prudent use of antimicrobials in plants |
|
|
Policies to prevent the emergence and spread of diseases in plants |
||
|
Agri-food systems |
Scaling up food safety compliance systems |
|
Policies to tackle AMR in the agri-food systems are also gaining momentum, given the non-negligible burden of foodborne diseases, including in high-income countries, and the risk that resistant bacteria can spread through the farm-to-fork chain. The review identified the hazard analysis critical control points (HACCP) system as the key code of practices that can help minimise such burden and disrupt the AMR transmission in the food supply chain. For this reason, some OECD countries have started incorporating the implementation of the HACCP system in their AMR-NAPs. Evidence also highlights how strong surveillance systems are a key factor in supporting the implementation of an effective HACCP approach as they can identify antimicrobial residues throughout the chain in a timely manner.
Policies focusing on the environmental reservoir
Policies to tackle AMR in the environment generally focus on improving the management of waste produced by sectors at high risk for contamination with antimicrobials or high prevalence of AMR such as the agriculture and health sectors and pharmaceutical production. Identified interventions generally focus on improving industry standards or coverage of policies already in place. For example, only about 20% of wastewater that is directly discharged into the environment is treated at the global level (FAO, 2018[35]). Production of guidelines and support of self-regulatory approaches are also identified among the most common policy practices.
The review identified five policy actions falling in this category (Table 1.3). Overall, available evidence appears to be in development, with a smaller number of studies and study designs that, often due to the own nature of the interventions, tend to be of lower quality than for interventions targeting humans or the agriculture sector. Even so, the evidence does suggest that interventions in this domain are associated with reductions in the transmission of AMR. However, at least for now, these interventions should be seen as complementary to others as, in isolation, they are unlikely to halt AMR transmission in the environment due to limitations in existing technologies. For this reason, the use of new technologies is consistently identified as a very promising approach to improving the effectiveness of action. Additional investments in waste management programmes, whether these are for sewage systems or more effective disposal systems for antimicrobials, are also identified as a top priority for upscaling action in this domain.
Table 1.3. Key findings on the impact of policy actions to tackle AMR in the environment
|
Category of intervention |
Policy interventions |
Key findings |
|---|---|---|
|
Measures to dispose and remove antibiotics from the environment |
Upgrading municipal wastewater treatment facilities |
|
|
Improving waste management in agricultural production |
|
|
|
Improving wastewater management in pharmaceutical manufacturing facilities |
|
|
|
Improving waste collection and management in healthcare settings |
|
|
|
Encouraging proper pharmaceutical waste disposal in households |
|
Long-term care is an emerging priority area for tackling AMR with a great potential for improvement
Tackling AMR and inappropriate antibiotic use in LTCFs is a key part of addressing the threat of AMR in settings other than acute care facilities more broadly. It is recognised that inappropriate antibiotic use and AMR in LTCFs are not just a problem for their residents but they can have negative consequences for the broader community, putting wider populations at risk. When staff, visitors and residents move in and out of LTCFs, so do organisms, including resistant pathogens. The movement of residents between LTCFs and acute care facilities is particularly important, as LTCFs can act as an incubator and reservoir for resistant infections. Some of the key underpinning reasons include:
The majority of residents in LTCFs are old and frail, very often with multiple morbidities requiring the use of invasive devices. All this significantly increases the likelihood of developing hospital-acquired infections, including resistant infections (Bonomo, 2000[36]; Moyo et al., 2020[37]; Tandan et al., 2018[38]; Nicolle, 2001[39]).
IPC practices are more difficult to implement in LTCFs than in hospital settings due to a number of issues such as longer stays, increased number of interactions between staff and patients and increased risk of cross-contamination, more limited budget for IPC policies and lower staff-to-resident ratios (Marra et al., 2018[40]; Stone et al., 2018[41]).
In LTCFs, antibiotics are frequently prescribed for prevention rather than to treat infections. In Europe, between 54% and 96% of antibiotic prescriptions in LTCFs are given without laboratory or diagnostic testing and up to one in four antibiotic prescriptions is unnecessary or inappropriate in terms of choice and duration (Patterson et al., 2019[42]; Furuno and Mody, 2020[43]; Latour et al., 2012[44]).
Patients in LTCFs are more likely to be infected by resistant pathogens, including multi-drug resistant organisms, than community-dwelling older adults due to the high probability of concurrence of all of the factors mentioned above (Cassone and Mody, 2015[45]).
Surveillance and monitoring of antibiotic use and AMR in LTCFs are still limited in many countries (Haenen et al., 2019[46]) – much more than in the hospital sector – severely hindering the implementation of benchmarking and auditing practices as well as goal setting.
Residents of LTCFs show high consumption of antibiotics driving high rates of AMR
Around 5% of residents of LTCFs are under treatment with systemic antibiotics – antibiotics that impact the whole body – at any moment across OECD countries for which data are available. Data from point prevalence surveys for 25 OECD and EU countries carried out in 2016‑17 show that around 1 in 20 patients were under treatment with systemic antibiotics at the time of the survey, with the share of patients, ranging from 0.7% in Lithuania to 10.5% in Denmark and Spain. This figure was similar but slightly lower in 2013‑14. Analyses on a longer time perspective conclude that, over a year, about 62% of residents of LTCFs and up to 4 in 5 residents in certain OECD countries are expected to be prescribed antibiotics at least once (Raban et al., 2021[47]).
Prescription of antibiotics in LTCFs may have decreased during the COVID‑19 pandemic. While there is still a paucity of data on changes in prescriptions of antimicrobials during the COVID‑19 pandemic in LTCFs, data from specific studies suggest that antibiotic consumption in this setting may have decreased in 2020, compared to previous years, due to a reduction in procedures and treatments as well as potential changes in the resident population. For example, a the United States study on almost 2000 LTCFs finds a 16% reduction in overall antibiotic use between January and June 2020 – compared to the 9% seasonal decrease observed in 2019 (Gouin et al., 2022[48]).
The OECD analysis suggests that the majority of antibiotics prescribed in LTCFs are unnecessary or inappropriate. Despite it being crucial to ensure that antibiotics are used wisely, up to three in four antibiotic prescriptions in LTCFs are unnecessary or inappropriate. One of the main drivers of inappropriate prescription relates to the decision on whether a patient needs to be treated with antibiotics or not, followed by the length of the therapy. One of the key reasons behind these worrying statistics is that, in Europe, between 54% and 96% of antibiotic prescriptions in LTCFs are given without laboratory or diagnostic testing and medical decisions are not always in alignment with evidence‑based guidelines (Latour et al., 2012[44]; Szabó and Böröcz, 2014[49]).
Residents of LTCFs are at high risk of developing infections that are often resistant to first-line antibiotics. In 2016‑17, 3.8% of residents of LTCFs sampled for a point prevalence survey of 25 OECD and EU countries reported being affected by a hospital-acquired infection. An analysis carried out by the ECDC for a sub-group of ten European countries, all OECD members, concluded that almost one in three isolates from hospital-acquired infections among LTCF residents were resistant to first-line antibiotic treatments. The percentages of isolates resistant to first-level AMR markers in hospital-acquired infections from LTCF residents ranged from 6.8% in Finland to 42.9% in Poland (Suetens et al., 2018[50]). High levels of resistance to first-line antibiotics increase the chances of prescription of second- and third-line antibiotics, eventually driving up resistance rates for these backup therapeutic options. While no cross-country consistent analysis exists, a study in the United States suggests that, over 11 years, the percentage of K. pneumoniae isolates resistant to carbapenems and third-generation cephalosporins increased from 5.3% to 11.5% (Braykov et al., 2013[51]). In Italy, urine cultures from LTCF residents found a prevalence of carbapenem-resistant Enterobacteriaceae of 20% (Marinosci et al., 2013[52]).
Country response to tackling AMR in LTCFs is still limited
Many countries have legislation and policies to tackle AMR in LTCFs but there are important gaps in the effective use of ASPs and IPC practices. To assess the policy response to AMR in LTCFs, the OECD has rolled out a survey among member countries investigating policies in place and plans for the next steps. The survey also assessed how the COVID‑19 pandemic affected the implementation of AMR policies in these settings, which was shown as one of the most critical during the pandemic (Rocard, Sillitti and Llena-Nozal, 2021[53]).
A total of 33 countries, including both OECD and EU/EEA countries, participated in the survey. Findings from the survey show a growing interest in policy making in this field (Figure 1.6). The main findings include the following:
Just over half of reporting countries (17 out of 33 countries) have an AMR-NAP that specifically references LTCFs, while an additional 6 countries report addressing AMR in LTCFs through other legislation or programmes. Among countries reporting no policy in place, five countries confirm they plan to include references to LTCFs in their next AMR-NAP. Only 12 out of 25 reporting countries confirmed they monitor and evaluate such plans.
Antimicrobial stewardship action is very limited with only nine countries that report having either guidelines on the use of antimicrobials in LTCFs or restrictive lists for antimicrobials. Among this group of countries, only three report a specific budget dedicated to stewardship in LTCFs. Usually, such policies are implemented at the national level but some countries instead enforce policies at the subnational or, even, institutional level. Other related interventions include training on antimicrobial prescription, antimicrobial committees and reminders with each policy generally enforced by four to five countries, most often at the national level.
IPC measures are more likely to be implemented, with 21 countries reporting to have a programme in place, in 14 cases at the national level. However, only around 50% of the countries reporting such programmes (i.e. 11 countries) confirm that there is a dedicated budget for IPC in LTCFs. Appointment of focal points in LTCFs, specific surveillance programmes for residents with multi-drug resistant infection and training for personnel are implemented by 13‑17 countries, depending on the programme.
Surveillance is a weak spot in preventing AMR in LTCFs. Just nine countries conduct surveillance of antibiotic consumption and eight countries track AMR (either AMR or AMR for multi-drug resistant pathogens) in LTCFs. Additionally, four countries monitor AMR or multi-drug resistant pathogens but not antibiotic consumption. Implementation of policies is even less monitored, with only four countries having surveillance programmes for both IPC and stewardship interventions.
Figure 1.6. Summary findings from the OECD LTCF survey
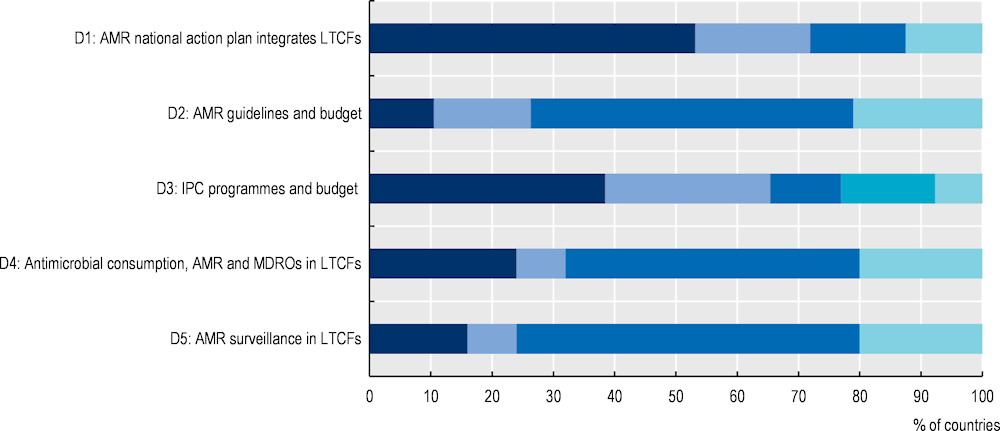
Note: D: Dimension; MDRO: Multidrug-resistant organism.
Across all questions, the last colour indicates “no answer”, “do not know” and other responses.
For D1, dark blue indicates that AMR-NAP mentions LTCFs; light blue indicates there is no mention of LTCFs in the AMR-NAP but there are other relevant legislation and programmes; medium blue indicates that the next AMR-NAP will mention LTCFs but that the current action plan does not and there are no other relevant legislation and programmes.
For D2, dark blue indicates that there are either antimicrobial guidelines or restrictive lists for antimicrobials in LTCFs and a specific budget dedicated to LTCFs; light blue indicates that there are either antimicrobial guidelines or restrictive lists for antimicrobials in LTCFs but there is no budget dedicated to LTCFs; medium blue indicates that there are neither antimicrobial guidelines nor restrictive lists for antimicrobials in LTCFs.
For D3, dark blue indicates that there is an IPC programme and a dedicated IPC budget; light blue indicates that there is an IPC programme but no dedicated IPC budget; medium blue indicates that there is an IPC programme but whether there is a dedicated IPC budget is unknown; and the last colour indicates that there is no IPC programme.
For D4, dark blue indicates that antimicrobial consumption is being monitored as well as AMR and multi-drug resistant organisms; light blue indicates that AMR and multi-drug resistant organisms are being monitored but antimicrobial consumption is not; medium blue indicates that antimicrobial consumption is not being monitored, neither are AMR nor multi-drug resistant organisms.
For D5, dark blue indicates that there are surveillance programmes both for antimicrobial stewardship and IPC; light blue indicates that there are surveillance programmes for IPC but not for antimicrobial stewardship; medium blue indicates that there is no surveillance programme for IPC or antimicrobial stewardship.
The COVID‑19 pandemic had a significant impact on policy actions related to antibiotic use and AMR in LTCFs. Predictably, the major level of disruption relates to the developing, approving or operationalising of the AMR-NAPs as infectious diseases experts in charge of this task were diverted to work on the pandemic. Among countries reporting this information, delays ranged between six months and one year but the survey was carried out before the end of the most acute phase of the pandemic so further delays may be possible. Countries also reported varying impacts of COVID‑19 on ASPs (11 countries) and vaccination campaigns (10 countries). Many countries also reported a positive impact of the pandemic on the adoption of IPC components, such as hand hygiene.
Investing in better surveillance and promoting ASPs and IPC measures should be top priorities to tackle AMR in LTCFs
With all of the countries responding to the questionnaire reporting that they plan to include reference to LTCF in their next national action plan on AMR, it is clear that OECD and EU/EEA countries recognise that tackling AMR and inappropriate antibiotic use in LTCFs requires targeted policy actions. However, as illustrated in the previous section, there are a number of important gaps in the design, adoption and effective use of ASPs, IPC and surveillance in LTCFs. Policy options for countries seeking to reduce the threat of inappropriate antibiotic use and AMR in LTCFs include:
Setting up routine surveillance systems that can collect and report data on antibiotic use and AMR in LTCFs. Routine surveillance is needed to establish a baseline situation, design policies that are fit for LTCFs and monitor and evaluate the impact of those policies.
Promoting the design, implementation and effective use of ASP programmes that are fit for LTCFs, including more integration with prescribers (e.g. general practitioners), better feedback on antibiotic use and AMR profiles, regular training and a budget specifically dedicated to ASPs.
Incentivising adoption and compliance with IPC practices that are tailored to LTCFs, emphasising the need for budgets specifically earmarked for IPC, the creation of IPC committees and adoption of procedures for surveillance and auditing of IPC processes in LTCFs.
Guidelines and centralised policy advice are helpful but may be insufficient to ensure change at scale. Many LTCFs face enormous challenges, from staff shortages to limited financial resources, to significant and complex demands from their residents. A survey of over 1 000 LTCFs in the United States concluded that LTCFs may not follow voluntary IPC guidelines if doing so requires significant financial investment, such as recruiting staff or investing in infrastructure (Ye et al., 2015[54]). Without appropriate financial and technical support, it is unlikely that all LTCFs will be able to implement the surveillance, ASPs and IPC protocols that can make a difference in the fight against AMR.
A combination of well-funded mandates and financial incentives may be a way forward. Financial strategies targeting healthcare providers to promote the prudent use of antibiotics have been shown to improve the appropriateness of antibiotic prescribing in various healthcare settings (Yoshikawa et al., 2021[55]). Both financial penalties and rewards can be effective and the choice of whether to use financial rewards or penalties should be informed by the context (Yoshikawa et al., 2021[55]). More research is needed on whether such strategies could work in LTCFs so pilot projects and experimentation could be useful.
Upscaling public health actions to tackle AMR offers an excellent investment with positive impacts on population health and economies
To tackle AMR, countries should upscale their efforts both by implementing new policy options and by strengthening policies currently in place. Drawing on available evidence, the OECD used its microsimulation model to assess the impact of a comprehensive set of highly effective policy actions on population health, health expenditure and the broader economy (Box 1.6). The analysis assumes that interventions are implemented at the beginning of 2021 (or the first year of the simulation period) and the impact of interventions is assessed to 2050. The analysis covers 34 OECD and EU/EEA countries for which data were available.
Box 1.6. Policy actions to tackle AMR included in the analysis
The OECD analysis covers 11 policy actions selected on a number of criteria including: i) availability of quantitative evidence to feed the OECD model; ii) consistency with actions highlighted in the AMR-GAP and featured among countries’ policy priorities, as identified in the analysis of AMR-NAPs discussed in the previous section; and iii) help to bridge the current gaps in policy implementation by covering a multitude of targets and by providing a comprehensive menu of alternatives.
The modelled interventions can be implemented in hospitals, community settings and agrifood systems. As much as possible, the modelled policies are designed following international standards when available, as in the case of the WHO Core Components for IPC policies such as improving hygiene practices in healthcare settings, best practices from countries or, for more innovative policies, on available evidence discussed with experts. In line with the AMR-GAP, policies can be classified in four categories (Table 1.4), including actions to optimise the use of antibiotics in human health, to reduce the incidence of infections, to promote AMR awareness and understanding, and One Health policies to reduce the incidence of infections in agrifood systems.
Table 1.4. One Health policy actions to tackle AMR included in the analysis, by sector of implementation
|
Policies to optimise the use of antibiotics in human health |
Policies in human health to reduce the incidence of infections |
Policies to promote AMR awareness and understanding |
Policies outside of human health sector to reduce the incidence of infections |
|---|---|---|---|
|
Strengthen antimicrobial stewardship |
Enhance hand hygiene practices |
Enhance health professional training on communication skills |
Improve biosecurity practices in farms |
|
Financial incentives |
Enhance environmental hygiene practices |
Scale up mass media campaigns |
Improve food handling practices |
|
Delayed antimicrobial prescription |
Improve vaccination coverage |
||
|
Scale up use of rapid diagnostic tests |
A brief explanation of the policies modelled can be found below, with a more comprehensive description of the evidence and the interventions’ characteristics presented in Chapter 6 in Annex 6.A.
Strengthening antimicrobial stewardship entails the scaling up of a hospital-based programme with multidisciplinary teams providing antibiotic stewardship and the monitoring of antibiotic consumption.
Delayed antimicrobial prescription looks at the potential impact of the roll-out of antimicrobial prescribing guidelines promoting the use of delayed prescription – patients unlikely to have a bacterial infection can collect the antibiotic only a few days after the prescription – in primary healthcare settings.
Scaling up the use of rapid diagnostic tests (RDTs) entails increasing the availability of point-of-care RDTs in ambulatory care settings in combination with antibiotic treatment guidelines.
Using financial incentives to optimise antimicrobial use entails the implementation of a nationwide pay-for-performance programme in primary care settings by rewarding bonuses to prescribers that meet preset antibiotic prescribing targets.
Improving hand hygiene practices involves the nationwide scale‑up of a facility-based intervention in all healthcare settings that enhances the standards of hand hygiene practices among health workers.
Enhancing environmental hygiene practices in healthcare facilities models the potential impact of the nationwide scale‑up of a facility-based intervention that supplements standard cleaning strategies.
Improving 23‑valent polysaccharide vaccine (PVV23) coverage shows the potential impact of a nationwide campaign for an existing vaccine with low levels of coverage against a bacterium susceptible to developing resistance.
Enhancing health professional training on enhanced communication skills entails the rollout of a nationwide training programme to improve communication on prudent use of antibiotics during consultations with patients in outpatient care settings.
Scaling up mass media campaigns involves the rollout of a nationwide mass media campaign involving traditional and social media to raise AMR understanding and awareness across key stakeholders and the general population.
Enhancing biosecurity practices in farms entails the rollout of a procurement programme facilitating farmers to buy personal protective equipment in farm settings by farmers and professional visitors like veterinarians.
Enhancing food handling practices entails the scale‑up of a food safety control training programme targeting food service workers in food establishments, coupled with visual reminders and regular audits based on checklists.
Substantial health gains may be achieved by scaling up the assessed policies
All 11 modelled interventions are estimated to reduce the number of yearly infections, with hospital-based infections offering the greatest reductions ranging from 113 000 to 298 000 resistant infections per year across the 34 countries included in the analysis.
Among hospital-based interventions, ASPs have the highest level of effectiveness. IPC measures such as enhancing environmental hygiene and improving hand hygiene are also highly effective. Enhancing environmental hygiene and improving hand hygiene are estimated to prevent more than 123 000 and 113 000 resistant infections respectively. In addition, these two interventions can also prevent susceptible infections. On average, improving hand hygiene can help avoid an additional 392 000 susceptible infections every year whereas enhancing environmental hygiene can prevent more than 461 000 susceptible infections each year.
Community-based interventions also lead to reductions in the number of resistant infections but at a lower level, particularly in the case of interventions that already have some grade of implementation across countries or because they target specific population groups. For example, this is the case of campaigns to increase PVV23 coverage for Streptococcus pneumoniae, which is often implemented, although at a sub-optimal level, across OECD countries and is targeted at the elderly population.
Outside of the human health sector, enhancing food safety and improving farm biosecurity are both associated with reductions in the number of infections, highlighting the importance of the One Health approach. Each year, improving food safety is expected to prevent, on average, more than 424 000 resistant and susceptible infections in humans. Improving farm biosecurity would instead avert more than 150 000 infections per year in humans.
In line with the reductions in infections, all 11 modelled interventions are associated with reductions in AMR-related mortality. Once again, hospital-based interventions are the most effective, with the number of deaths prevented ranging more than 4 500 and 10 000 deaths per year across the 34 countries included in the analysis by preventing resistant infections. ASPs are estimated as the most effective intervention in avoiding more than 10 000 deaths per year in the elimination scenario, which is roughly equivalent to preventing around 30% of deaths due to TB, influenza and HIV/AIDS in 2020 (or the latest year for which data are available). In comparison, community-based interventions can be expected to avoid up to a maximum of 8 000 deaths per year. The EU/EEA member states in the southern part of Europe, as well as Japan, Switzerland and Türkiye, are among the countries most often showing the highest reductions in AMR-related deaths following a coverage increase of the interventions.
All of the modelled interventions increase the number of life years (LYs) lived and the number of disability-adjusted life years (DALYs), which is a measure accounting both for an increase in life expectancy and quality of life. Specifically, under the elimination scenario, the OECD SPHeP-AMR model finds that:
When the impact of health is measured in LYs and DALYs, the ranking of the interventions from the most effective to the least effective broadly mirrors the ranking identified when considering mortality. ASPs promise the greatest gains in LYs (153 000) and DALYs (178 000), whereas IPC interventions show the highest gains in absolute terms but a significant share of such gains are derived from averting susceptible infections. For example, improving environmental hygiene, the most effective IPC intervention produces a gain of more than 206 000 LYs (including 71 000 LYs from preventing resistant infections) and more than 253 000 DALYs (including more than 83 000 DALYs from preventing resistant infections).
Interventions implemented in community settings and One Health interventions offer lower but still significant gains. Interventions such as delaying antimicrobial prescription and scaling up the use of RDTs show significant gains both in terms of LYs (121 000 and 114 000 respectively) and DALYs (141 000 and 133 000 respectively), which places them among the most effective actions. The produced gains are lower for the remaining community-based interventions with interventions to improve biosecurity practices in farm settings offering the lowest gains.
The effectiveness of all interventions on morbidity as measured in DALYs surpasses their effectiveness on mortality as measured in LYs saved. This means that interventions are more effective in improving the quality of life of individuals after they have developed a resistant infection than their probability of dying. One of the reasons for this finding is that many infections develop in patients aged 65 and above and such patients continue being more prone to the competing risks of mortality for other causes, even after a successful recovery from an infection.
Many interventions have a significant impact on health expenditure and are cost‑saving
Investing in AMR policies can also help reduce the pressure on hospital resources and improve the resilience of healthcare services as described below:
Interventions such as ASPs could avoid more than 3.7 million extra days spent in hospital per year across the 34 countries included in the analysis, which is equivalent to freeing up the entire acute bed capacity in Ireland in 2020 for nearly 1 year. Community-based interventions can also contribute to shorter hospital stays, with predicted impacts ranging between more than 3.1 million (delayed antibiotic prescribing) and 40 000 (increasing vaccination coverage) days of hospitalisation avoided.
Reductions in the number of days that patients spend in hospital due to a lower incidence of resistant infections translate into savings in health expenditure. IPC interventions such as improving environmental hygiene and hand hygiene practices promise the greatest impacts by eliminating both resistant and susceptible infections, with yearly savings across the 34 countries included in the analysis estimated at nearly USD PPP 7.2 billion (corresponding to USD PPP 6.3 per capita) and more than USD PPP 6 billion (corresponding to USD PPP 5.3 per capita) respectively. Scaling up ASPs is also expected to reduce health expenditure by more than USD PPP 2.7 billion annually, corresponding to USD PPP 1.2 per capita. This is roughly equivalent to 10% of healthcare spending in Greece in 2020.
Broadly, countries with higher incidences of resistant infections stand to achieve the greatest reductions in health expenditure by investing in the modelled interventions. For instance, Italy can reduce health expenditure by USD PPP 9.9 per capita per year by investing in improved hand hygiene practices.
All of the interventions show the potential to increase workforce participation and productivity
All of the modelled policy interventions yield productivity gains that can be achieved primarily through increasing workforce participation, followed by reducing absence from work due to ill health and presenteeism at work. Scaling up ASPs is associated with the highest estimated gains in productivity. On average, this intervention is estimated to generate close to 67 000 FTEs per year combined across the 34 countries included in the analysis. Of these potential gains, more than 56 000 FTEs are expected to be produced through increased participation in the workforce while more than 9 300 FTEs can be gained by reducing absenteeism. Combined, these productivity gains would amount to around USD PPP 3.9 billion (corresponding to USD PPP 3.5 per capita) each year across all of the countries included in the analysis. In many countries, the estimated productivity gains exceed savings in health expenditure.
All of the interventions are affordable and, in the majority of cases, the return on investment is significantly greater than the implementation costs
The average annual cost of implementing the assessed interventions varies between USD PPP 0.2 to USD PPP 2.6 per capita. These are all affordable investments, given the level of income of the assessed countries, corresponding only to a fraction of the healthcare budget of these countries. Using financial incentives to optimise antimicrobial use has the highest estimated annual implementation cost per capita, given that the intervention includes a rewarding bonus corresponding to 1% of the base salary of medical practitioners in primary care that achieves a preset antibiotic prescribing target. For ASPs, expenses associated with building multidisciplinary stewardship teams, which include both salaries and training expenses, are the main cost drivers. Enhancing environmental hygiene and increasing the use of RDTs are, respectively, the third and fourth most expensive interventions, mainly due to the cost of purchasing all of the disposables needed to upscale the implementation of the interventions. The costs associated with implementing other interventions each average below USD PPP 1 per capita. Improving vaccination coverage has the lowest estimated annual cost of implementation per capita.
Gains produced by upscaling the implementation of policies to tackle AMR are substantially higher than their implementation costs when both savings in healthcare expenditure and gains in workforce productivity are considered (Figure 1.7). Across the 34 countries included in the analysis, the average implementation annual costs associated with improving hand hygiene are expected to be around 24.6 times lower than the savings generated by estimated reductions in health expenditures and productivity gains made through increased participation in the workforce and productivity at work. Scaling up delayed prescription practices in primary healthcare settings is another highly attractive intervention, with a benefit to cost ratio of around 17. The average annual cost of scaling up each of these interventions across all countries included in the analysis is around five times lower than the expected savings from reducing health expenditure and productivity gains.
Figure 1.7. Health and economic impacts of interventions to tackle antimicrobial resistance
Average per year for the period 2020‑50,* 34 countries included in the analysis
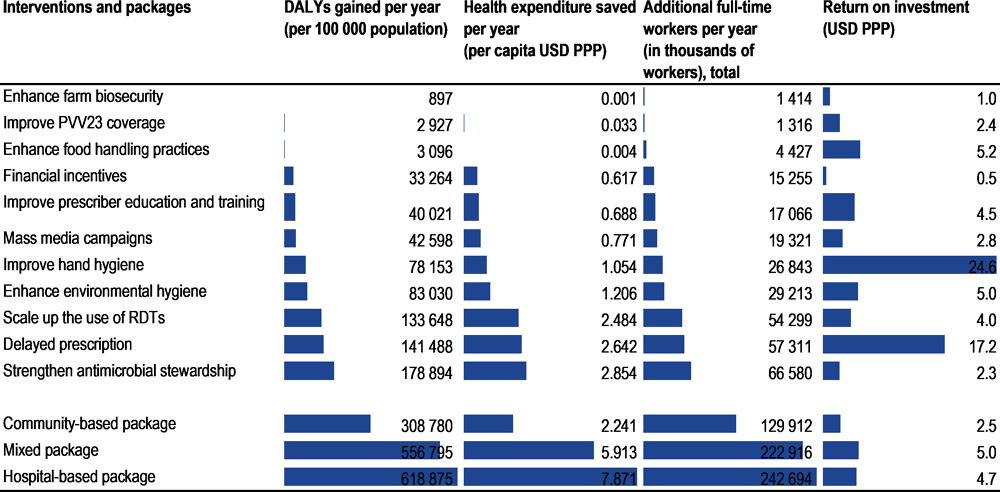
Note: * For some countries, the first year of analysis is earlier than 2020 depending on the availability of historical data. The figure above presents health and economic outcomes attributable to each policy under the replacement scenario. Estimates for the return on investment are the result of the total savings in healthcare expenditure and productivity gains in the 34 countries produced by the policy divided by the total cost of implementing the policy.
Source: OECD analyses based on the OECD SPHeP-AMR model.
Combining policies into a coherent prevention strategy helps countries reach a critical mass with a greater impact
Policy packages offer important advantages over implementing single policies. By scaling up policies as packages, multiple drivers of AMR can be addressed at the same time. Policy packages can also target different population groups and sectors simultaneously while facilitating and reinforcing desirable changes in behaviour. Combined, the potential protective effects of policy packages can go beyond simply adding up the effectiveness of each intervention (i.e. super-additivity of policy packages). Across all modelled policy packages, the elimination scenario was used for interventions that impact antibiotic prescription patterns. The OECD analysis evaluated the effectiveness and cost-effectiveness of three policy packages:
The hospital-based package includes improving hand hygiene, enhancing environmental hygiene and scaling up ASPs, and has an estimated per capita cost ranging between USD PPP 1.4 and USD PPP 9.4.
The community-based package includes delayed antimicrobial prescriptions, introducing financial incentives to optimise antimicrobial use, scaling up the use of RDTs, scaling up mass media campaigns and scaling up prescriber training, and has an estimated cost ranging between USD PPP 0.8 and USD PPP 11.9 per capita.
The mixed package includes improving hand hygiene, scaling up ASPs, delaying antimicrobial prescription, increasing mass media campaigns and enhancing food handling practices and has an estimated cost ranging between USD PPP 0.7 and USD 3.7 per capita.
Results from the OECD SPHeP-AMR model suggest that the choice of the policy packages has important implications:
The results suggest that the different packages respond to different policy objectives and priorities. For example, depending on their own resistant infection burden, countries may choose to prioritise tackling AMR in hospitals or the community. The hospital-based package shows the highest impact on population health and the economy but the mixed package avoids the highest number of infections, many of which are in the community.
Another important implication of the OECD analysis is that hospital infections tend to be more costly to treat and more likely to lead to fatal outcomes. Consistently, the hospital-based package shows the highest impact across multiple outcomes, including life expectancy, quality of life and healthcare costs. At the same time, the average implementation cost for this package tends to be higher than for the mixed package.
Third, a high number of infections develops in the community but health outcomes from these infections are generally milder compared to those from HAIs, particularly in the case of foodborne diseases. This explains the significantly higher impact of the mixed package on the number of infections but its lower – nevertheless significant – impact on morbidity-related dimensions.
Finally, the lower but still considerable impact of community-based interventions should not discourage investments given that many of the interventions included in this package help reinforce the implementation and effectiveness of the other two packages while preventing hospitalisations, which can expose people to the risk of HAIs.
More in detail, the OECD model quantifies that the three packages produce the following yearly impact across the 34 countries included in the analysis:
The mixed package had the highest impact in terms of reducing the number of resistant infections (more than 1.6 million per year), followed by the hospital- (around 1.3 million) and community-based packages (more than 900 000 infections).
The hospital-based package prevents the highest number of deaths (more than 33 000 per year) compared to the mixed package (around 30 000) and the community package (more than 17 000). In effect, the hospital package would prevent a number of deaths equivalent to preventing all deaths due to TB, influenza and HIV/AIDS in 2020 (or the nearest year for which data is available).
The hospital-based package also produces the highest gains in terms of LYs (more than 511 000) and DALYs (more than 618 000). The mixed package also offers important health gains amounting to more than 466 000 LYs and 557 000 DALYs. The community-based package is expected to generate gains equivalent to nearly 263 000 LYs and 308 000 DALYs per year.
The hospital-based package is estimated to have the greatest impact on health expenditures, saving more than USD PPP 11 billion each year (or USD PPP 9.8 per capita), roughly corresponding to half of all health spending in the Czech Republic in 2020. The mixed package would save USD PPP 9.4 billion (corresponding to USD PPP 8.3 per capita) while USD PPP 5.3 billion (corresponding to USD PPP 4.7 per capita) would be saved by the community-based package.
The hospital-based package is predicted to yield the greatest productivity gains amounting to USD PPP 14.9 billion (corresponding to USD PPP 13.2 per capita). In comparison, the mixed package is expected to produce productivity gains amounting to around USD PPP 13.8 billion (corresponding to USD PPP 12 per capita).
The average cost of implementing the mixed package is around five times lower than the estimated benefits accrued through the reduction in health expenditure and productivity gains. This is followed by the hospital-based and community-based packages where the potential benefits are around 4.7 and 2.5 times that of the cost of implementing these packages respectively.
Conclusion: Tackling AMR remains a top public health priority with important health and economic consequences
Tackling antimicrobial resistance is widely acknowledged as a top public health priority with important implications for population health and the economies of the OECD countries and EU/EEA countries. There has been a significant global effort to scale up a wide array of policy interventions in line with the AMR‑GAP since the last OECD analysis in 2018. The new OECD analysis suggests that these efforts may have borne fruit in terms of limiting the health and economic burden of AMR. Yet, important gaps persist in policy action against AMR. The current and projected health impact of AMR remains high, with important cross-country variation. Mirroring the health burden, the cost of AMR to health systems and economies remains worrisome. The real burden of AMR to society is likely to be substantially greater considering its impact on non-human health sectors such as the environment and animal health, for which assessments are ongoing (OIE, 2023[56]).
The OECD analysis demonstrates that more can be achieved by investing in policies in line with the One Health approach. To tackle AMR, policy makers can choose from a wide range of options across sectors. In the human health sector, hospital-based interventions such as scaling up ASPs and improving environmental hygiene and hand hygiene yield important health and economic gains. Community-based interventions are also effective interventions. Beyond the human health sector, the OECD analysis highlighted that enhancing farm biosecurity and improving food handling practices can yield reductions in the number of reduction infections and prevent deaths while resulting in savings in healthcare expenditures and improving workforce participation and productivity.
Investments in policy packages that combine individual interventions can potentially save thousands of lives and yield sizeable savings that far exceed implementation costs. The mixed package promises the highest impact in terms of reducing the number of resistant infections whereas the hospital-based package prevents the highest number of deaths. The implementation of all three policy packages assessed by the OECD can more than make up for their costs.
The OECD analysis underlines the importance of adopting a One Health approach. For example, the simulations show that every USD PPP 1 invested in a mixed policy package that brings together policies which could be implemented in healthcare and community settings as well as in the agriculture and food sectors can return USD PPP 5 in economic benefits. Combined, results from the OECD analysis demonstrate that policy action that is grounded in the One Health approach offers excellent investments to tackle AMR.
References
[20] Access to Medicine Foundation (2020), Antimicrobial Resistance Benchmark 2020, Access to Medicine Foundation, Amsterdam, https://accesstomedicinefoundation.org/resource/2020-antimicrobial-resistance-benchmark (accessed on 18 June 2022).
[31] Anderson, M. et al. (2019), “A governance framework for development and assessment of national action plans on antimicrobial resistance”, The Lancet Infectious Diseases, Vol. 19/11, pp. e371-e384, https://doi.org/10.1016/s1473-3099(19)30415-3.
[36] Bonomo, R. (2000), “Multiple antibiotic-resistant bacteria in long-term-care facilities: An emerging problem in the practice of infectious diseases”, Clinical Infectious Diseases, Vol. 31/6, pp. 1414-1422, https://doi.org/10.1086/317489/2/31-6-1414-FIG002.GIF.
[51] Braykov, N. et al. (2013), “Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999-2010”, Infection Control and Hospital Epidemiology, Vol. 34/3, pp. 259-268, https://doi.org/10.1086/669523.
[45] Cassone, M. and L. Mody (2015), “Colonization with multidrug-resistant organisms in nursing homes: Scope, importance, and management”, Current Geriatrics Reports, Vol. 4/1, pp. 87-95, https://doi.org/10.1007/S13670-015-0120-2.
[21] CDC (2022), NARMS Now: Human Data, Centers for Disease Control and Prevention, https://wwwn.cdc.gov/NARMSNow/ (accessed on 18 June 2022).
[30] Chua, A. et al. (2021), “An analysis of national action plans on antimicrobial resistance in Southeast Asia using a governance framework approach”, The Lancet Regional Health - Western Pacific, Vol. 7, p. 100084, https://doi.org/10.1016/j.lanwpc.2020.100084.
[10] EAHP (2019), 2019 EAHP Medicines Shortages Report: Medicines Shortages in European Hospitals, European Association of Hospital Pharmacists, Brussels, https://www.eahp.eu/practice-and-policy/medicines-shortages (accessed on 18 June 2022).
[6] ECDC (2022), Antimicrobial Resistance Surveillance in Europe, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf.
[7] ECDC (2019), Antimicrobial Consumption in the EU/EEA - Annual Epidemiological Report 2019, European Centre for Disease Prevention and Control, Stockholm, https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2019.
[16] ECDC/EFSA/EMA (2017), “ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals”, EFSA Journal, Vol. 15/7, https://doi.org/10.2903/j.efsa.2017.4872.
[14] ECDC/EFSA/EMA (2017), Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-producing Animals, Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report, European Centre for Disease Prevention and Control, European Food Safety Authority, European Medicines Agency, https://doi.org/10.2903/j.efsa.2017.4872.
[22] EFSA/ECDC (2020), “The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018”, EFSA Journal, Vol. 18/3, https://doi.org/10.2903/J.EFSA.2020.6007.
[18] FAO (2018), Antimicrobial Resistance and Foods of Plant Origin, Summary report of an FAO meeting of experts, Antimicrobial Resistance Working Group, Food and Agriculture Organization of the United Nations, Rome, https://www.fao.org/3/BU657en/bu657en.pdf.
[35] FAO (2018), More People, More Food, Worse Water? A Global Review of Water Pollution from Agriculture, Water, Water, Land and Ecosystems (WLE) Program of the CGIAR, International Water Management Institute (IWMI), Food and Agriculture Organization, http://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/1144303/.
[2] FAO/WHO (2021), Code of Practice to Minimize and Contain Foodborne Antimicrobial Resistance, Food and Agriculture Organization and World Health Organization, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B61-2005%252FCXC_061e.pdf.
[43] Furuno, J. and L. Mody (2020), “Several roads lead to Rome: Operationalizing antibiotic stewardship programs in nursing homes”, Journal of the American Geriatrics Society, Vol. 68/1, pp. 11-14, https://doi.org/10.1111/JGS.16279.
[32] Global AMR R&D Hub (2023), Global AMR R&D Hub Dashboard, https://dashboard.globalamrhub.org/reports/investments/overview (accessed on 2 January 2023).
[48] Gouin, K. et al. (2022), “Trends in prescribing of antibiotics and drugs investigated for Coronavirus Disease 2019 (COVID-19) treatment in US nursing home residents during the COVID-19 pandemic”, Clinical Infectious Diseases, Vol. 74/1, pp. 74-82, https://doi.org/10.1093/CID/CIAB225.
[46] Haenen, A. et al. (2019), “Surveillance of infections in long-term care facilities (LTCFs): The impact of participation during multiple years on health care-associated infection incidence”, Epidemiology and Infection, Vol. 147, p. e266, https://doi.org/10.1017/S0950268819001328.
[9] Klein, E. et al. (2021), “Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000-15: An analysis of pharmaceutical sales data”, The Lancet Infectious Diseases, Vol. 21/1, pp. 107-115, https://doi.org/10.1016/s1473-3099(20)30332-7.
[8] Klein, E. et al. (2018), “Global increase and geographic convergence in antibiotic consumption between 2000 and 2015”, Proceedings of the National Academy of Sciences, Vol. 115/15, pp. E3463-E3470, https://doi.org/10.1073/pnas.1717295115.
[15] Kumar, H. et al. (2020), “Understanding of Colistin Usage in Food Animals and Available Detection Techniques: A Review”, Animals, Vol. 10/10, p. 1892, https://doi.org/10.3390/ani10101892.
[44] Latour, K. et al. (2012), “Indications for antimicrobial prescribing in European nursing homes: Results from a point prevalence survey”, Pharmacoepidemiology and Drug Safety, Vol. 21/9, pp. 937-944, https://doi.org/10.1002/PDS.3196.
[52] Marinosci, F. et al. (2013), “Carbapenem resistance and mortality in institutionalized elderly with urinary infection”, Journal of the American Medical Directors Association, Vol. 14/7, pp. 513-517, https://doi.org/10.1016/J.JAMDA.2013.02.016.
[40] Marra, F. et al. (2018), “A decrease in antibiotic utilization for urinary tract infections in women in long-term care facilities”, Canadian Geriatrics Journal, Vol. 21/3, pp. 262-263, https://doi.org/10.5770/CGJ.21.303.
[37] Moyo, P. et al. (2020), “Risk factors for pneumonia and influenza hospitalizations in long-term care facility residents: A retrospective cohort study”, BMC Geriatrics, Vol. 20/1, pp. 1-13, https://doi.org/10.1186/S12877-020-1457-8/TABLES/3.
[39] Nicolle, L. (2001), “Preventing infections in non-hospital settings: Long-term care”, Emerging Infectious Diseases, Vol. 7/2, p. 205, https://doi.org/10.3201/EID0702.010210.
[26] OECD (2022), OECD Health Statistics, OECD, Paris, https://data.oecd.org/ (accessed on 11 July 2022).
[1] OECD (2018), Stemming the Superbug Tide: Just A Few Dollars More, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/9789264307599-en.
[5] OECD et al. (2022), Antimicrobial Resistance in the EU/EEA: A One Health Response, OECD, Paris, https://www.oecd.org/health/Antimicrobial-Resistance-in-the-EU-EEA-A-One-Health-Response-March-2022.pdf.
[29] Ogyu, A. et al. (2020), “National action to combat AMR: A One-Health approach to assess policy priorities in action plans”, BMJ Global Health, Vol. 5/7, p. e002427, https://doi.org/10.1136/bmjgh-2020-002427.
[56] OIE (2023), Antimicrobial Resistance, World Organisation for Animal Health, https://www.woah.org/en/what-we-do/global-initiatives/antimicrobial-resistance/#ui-id-2.
[28] Özçelik, E. et al. (2022), “A comparative assessment of action plans on antimicrobial resistance from OECD and G20 countries using natural language processing”, Health Policy, https://doi.org/10.1016/j.healthpol.2022.03.011.
[42] Patterson, L. et al. (2019), “Evidence of a care home effect on antibiotic prescribing for those that transition into a care home: A national data linkage study”, Epidemiology and Infection, Vol. 147, https://doi.org/10.1017/S0950268818003382.
[11] Pulcini, C. et al. (2017), “Forgotten antibiotics: A follow-up inventory study in Europe, the USA, Canada and Australia”, International Journal of Antimicrobial Agents, Vol. 49/1, pp. 98-101, https://doi.org/10.1016/j.ijantimicag.2016.09.029.
[47] Raban, M. et al. (2021), “Temporal and regional trends of antibiotic use in long-term aged care facilities across 39 countries, 1985-2019: Systematic review and meta-analysis”, PLOS ONE, Vol. 16/8, p. e0256501, https://doi.org/10.1371/JOURNAL.PONE.0256501.
[53] Rocard, E., P. Sillitti and A. Llena-Nozal (2021), “COVID-19 in long-term care: Impact, policy responses and challenges”, OECD Health Working Papers, No. 131, OECD Publishing, Paris, https://doi.org/10.1787/b966f837-en.
[34] Ryan, M. (2019), “Evaluating the economic benefits and costs of antimicrobial use in food-producing animals”, OECD Food, Agriculture and Fisheries Papers, No. 132, OECD Publishing, Paris, https://doi.org/10.1787/f859f644-en.
[17] Schar, D. et al. (2020), “Global trends in antimicrobial use in aquaculture”, Scientific Reports, Vol. 10/1, https://doi.org/10.1038/S41598-020-78849-3.
[33] Simpkin, V. et al. (2017), “Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps”, The Journal of Antibiotics, Vol. 70/12, pp. 1087-1096, https://doi.org/10.1038/ja.2017.124.
[41] Stone, P. et al. (2018), “Nursing home infection control program characteristics, CMS citations, and implementation of antibiotic stewardship policies: A national study”, Inquiry (United States), Vol. 55, pp. 1-7, https://doi.org/10.1177/0046958018778636.
[50] Suetens, C. et al. (2018), “Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017”, Eurosurveillance, Vol. 23/46, p. 1800516, https://doi.org/10.2807/1560-7917.ES.2018.23.46.1800516.
[49] Szabó, R. and K. Böröcz (2014), “Antimicrobial use in Hungarian long-term care facilities: High proportion of quinolone antibacterials”, Archives of Gerontology and Geriatrics, Vol. 59/1, pp. 190-193, https://doi.org/10.1016/J.ARCHGER.2014.02.011.
[38] Tandan, M. et al. (2018), “Antimicrobial prescribing and infections in long-term care facilities (LTCF): A multilevel analysis of the HALT 2016 study, Ireland, 2017”, Eurosurveillance, Vol. 23/46, p. 1800278, https://doi.org/10.2807/1560-7917.ES.2018.23.46.1800278.
[23] Teillant, A. et al. (2015), “Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study”, The Lancet Infectious diseases, Vol. 15/12, pp. 1429-37, https://doi.org/10.1016/S1473-3099(15)00270-4.
[13] Van Boeckel, T. et al. (2019), “Global trends in antimicrobial resistance in animals in low- and middle-income countries”, Science, Vol. 365/6459, https://doi.org/10.1126/science.aaw1944.
[24] Vos, T. et al. (2020), “Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019”, The Lancet, Vol. 396/10258, pp. 1204-1222, https://doi.org/10.1016/s0140-6736(20)30925-9.
[3] WHO (2022), A Health Perspective on the Role of the Environment in One Health, World Health Organization Regional Office for Europe, https://apps.who.int/iris/handle/10665/354574.
[58] WHO (2022), Adopt AWaRe: Handle Antibiotics with Care, World Health Organization, https://adoptaware.org/ (accessed on 18 June 2022).
[25] WHO (2022), Global Tuberculosis Report 2022, World Health Organization, https://apps.who.int/iris/handle/10665/363752.
[57] WHO (2019), Critically Important Antimicrobials for Human Medicine, 6th Rev, World Health Organization, https://apps.who.int/iris/handle/10665/312266.
[12] WHO (2017), WHO Guidelines on Use of Medically Important Antimicrobials in Food-producing Animals, World Health Organization, https://apps.who.int/iris/handle/10665/258970.
[27] WHO (2015), Global Action Plan on Antimicrobial Resistance, World Health Organization, https://apps.who.int/iris/handle/10665/193736.
[59] WHO (2003), Introduction to Drug Utilization Research, World Health Organization, https://apps.who.int/iris/handle/10665/42627.
[4] WHO/FAO/OIE (2021), Tripartite AMR Country Self-Assessment Survey (TrACSS) 2020-2021, World Health Organization, https://www.who.int/publications/m/item/tripartite-amr-country-self-assessment-survey-(tracss)-2020-2021.
[19] WHO/FAO/OIE (2020), Technical Brief on Water, Sanitation, Hygiene and Wastewater Management to Prevent Infections and Reduce the Spread of Antimicrobial Resistance, World Health Organization, https://apps.who.int/iris/handle/10665/332243.
[54] Ye, Z. et al. (2015), “Healthcare-associated pathogens and nursing home policies and practices: Results from a national survey”, Infection Control & Hospital Epidemiology, Vol. 36/7, pp. 759-766, https://doi.org/10.1017/ICE.2015.59.
[55] Yoshikawa, Y. et al. (2021), “Financial strategies targeting healthcare providers to promote the prudent use of antibiotics: A systematic review of the evidence”, International Journal of Antimicrobial Agents, Vol. 58/6, p. 106446, https://doi.org/10.1016/J.IJANTIMICAG.2021.106446.
Notes
← 1. DDD is a standard measure for drugs, calculated as the assumed average maintenance dose per day for a drug used for its main indication in adults (WHO, 2003[59]). The unit used throughout this chapter is DDD per 1 000 inhabitants per day.
← 2. 2020 data were not used to feed the analysis due to their preliminary status and limited geographical coverage.
← 3. As part of the Model Lists of Essential Medicines, the WHO Access, Watch, Reserve or AWaRe classification of antibiotics is a tool to improve the use of antibiotics and has the ultimate goal of reducing antimicrobial resistance. The tool classifies 180 antibiotics (WHO, 2022[58]). Access antibiotics are mostly first-line and second-line therapies with lower resistance potential than other antibiotics. Watch antibiotics have higher AMR potential and should be prioritised in stewardship and monitoring efforts. Watch antibiotics include most of the highest-priority agents in the WHO Critically Important Antimicrobials for Human Medicine (WHO, 2019[57]). Reserve antibiotics include antibiotics of last resort and should be saved for treatment of confirmed or suspected infections due to multi-drug-resistant organisms.
← 4. Broad-spectrum antibiotics include: broad-spectrum penicillins (ATC groups J01CR, J01CD), broad‑spectrum cephalosporins (J01DC, J01DD), macrolides (J01 FA) except erythromycin (J01FA01), and fluoroquinolones (J01MA); and narrow-spectrum antibiotics such as narrow-spectrum penicillins (J01CA, J01CE, J01CF), narrow-spectrum cephalosporins (J01DB) and erythromycin (J01FA). Consumption expressed in DDD per 1 000 inhabitants per day.
← 5. Twelve priority antibiotic-bacterium combinations included in the analysis are vancomycin-resistant Enterococcus faecalis (E. faecalis), vancomycin-resistant E. faecium, third-generation cephalosporin-resistant E. coli, carbapenem-resistant K. pneumoniae, third-generation cephalosporin-resistant K. pneumoniae, carbapenem-resistant Pseudomonas aeruginosa (P. aeruginosa), meticillin-resistant S. aureus, penicillin-resistant Streptococcus pneumoniae (S. pneumoniae), fluoroquinolone‑resistant A. baumannii, carbapenem-resistant A. baumannii, fluoroquinolone‑resistant E. coli and carbapenem-resistant E. coli.