This chapter reports findings from modelling the health and economic impact of scaling up 11 policy interventions to tackle antimicrobial resistance (AMR) consistent with the One Health approach. The selected interventions aim to optimise the use of antibiotics in human health, to promote AMR awareness and understanding and to reduce the incidence of infections in healthcare settings, farms and food establishments. In addition, the chapter reports the impact of three policy packages designed to address the most pressing policy gaps on AMR. The results are presented for 34 countries, including 29 European Union (EU)/European Economic Area (EEA) countries and Japan, Switzerland, Türkiye, the United Kingdom and the United States. The chapter concludes by discussing the implications of the findings.
Embracing a One Health Framework to Fight Antimicrobial Resistance

6. Cost-effectiveness of interventions relevant to tackling antimicrobial resistance
Abstract
Key findings
Policies to tackle AMR reduces deaths and the burden of diseases
The OECD model shows that healthcare‑based interventions, including antimicrobial stewardship programmes (ASPs), improving hand hygiene and enhancing environmental hygiene are expected to yield the greatest health gains, producing, on average, 71 000‑153 000 life years (LYs) gained each year if resistant infections were eliminated across the 34 countries included in the analysis.
ASPs are estimated to result in the greatest gains in terms of the number of averted AMR-related deaths. On average, this intervention is estimated to prevent more than 10 000 deaths per year across the 34 countries included in the analysis if resistant infections were eliminated and more than 3 200 deaths if resistant infections were replaced by susceptible ones. This is equivalent to preventing around 10-30% of deaths due to tuberculosis (TB), influenza and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) in 2020 (or the nearest year for which this information is available).
Policies to tackle AMR can reduce pressure on hospital resources, generate substantial savings in health expenditure and produce gains in workforce productivity
All of the modelled interventions promise to have a significant impact on the use of hospital resources. ASPs promise the greatest reduction in the extra days spent in hospitals, with the estimated reduction ranging from more than 3.7 million fewer days annually if resistant pathogens were to be eliminated and 822 000 fewer hospital days if resistant infections were replaced by susceptible infections. This would be equivalent to freeing up the entire acute bed capacity in Ireland in 2020 for nearly 1 year by eliminating resistant infections and around 2 months if resistant infections were replaced by susceptible infections.
All modelled interventions are expected to result in savings in health expenditure. Across the 34 countries included in the analysis, enhancing environmental hygiene is estimated to yield the greatest amount of savings in health expenditure amounting to more than USD 7.1 billion per year adjusting for purchasing power parity (PPP) by eliminating both resistant and susceptible infections. Following this intervention, improving hand hygiene and scaling up ASPs are associated with expected reductions in health expenditure exceeding USD PPP 6 billion and USD PPP 3.9 billion per year respectively.
Enhancing environmental hygiene practices can generate almost USD PPP 6.4 billion annually in productivity gains, which measures the combined effect of changes in the participation in the workforce and workforce productivity. Improving hand hygiene and scaling up ASPs can potentially yield more than USD PPP 5.2 billion and USD PPP 3.9 billion respectively.
Benefits of implementing policies to tackle AMR as part of a package more than make up for their implementation costs
Countries can achieve greater return on their investments by combining single interventions into a package of interventions. Investing in a hospital-based package can produce an average gain of more than 511 000 LYs and 618 000 disability-adjusted life‑years (DALYs) per year across the 34 countries included in the scope of the analysis. Every year, savings in health expenditure by scaling up this package is estimated to be around USD PPP 11 billion across the countries included in the analysis. These estimated savings in health expenditure is roughly equivalent to half of all health spending in the Czech Republic in 2020. The estimated gains in productivity can exceed USD PPP 14.9 billion if the hospital package was scaled up to desired levels.
A mixed package that combines policy interventions in human health and food sectors also promises health and economic gains. A mixed package is expected to result in a gain of more than 466 000 LYs and 556 000 DALYs every year across all countries included in the analysis. This package will potentially lead to USD PPP 9.4 billion in savings in health expenditure and USD PPP 13.8 billion in productivity gains.
A community-based package is estimated to produce relatively smaller but crucial health and economic benefits. This package is predicted to produce more than 262 000 LYs and 308 000 DALYs annually, save countries more than USD PPP 5.3 billion in health expenditure and result in USD PPP 8.4 billion in productivity gains.
Benefits that can be accrued by upscaling each policy package substantially exceed their implementation costs. The average cost of implementation of the mixed package is around 5 times lower than the estimated benefits accrued through reductions in health expenditure and gains in productivity combined. The cost of the hospital-based package is around 4.7 times lower than its potential benefits, whereas the benefits associated with the scale-up of the community-based package are 2.5 times that of the cost of scaling up this intervention.
It is vitally important to continue to shore up effective policies in line with the One Health approach
Evidence presented in Chapters 3 and 4 underlines the vital importance of continued action to stem AMR. Chapter 3 demonstrated that AMR continues to pose significant health and economic burden across the OECD and EU/EEA countries included in the analysis. This chapter showed that infections caused by resistant organisms could claim around 79 000 lives each year across the 34 countries included in the analysis. Further, it showed that without new policy action, AMR can cost around USD PPP 28.9 billion annually to health systems across the OECD and EU/EEA countries included in the analysis. Subsequently, Chapter 4 found that important progress has been made in recent years in scaling up policies to effectively tackle AMR. Though important gaps remain in the implementation of AMR policies, particularly those aiming to optimise the use of antibiotics, policies to reduce the incidence of infections in various settings including healthcare facilities, farms and food establishments and increase AMR awareness and understanding in the general public and among health professionals. Even in countries where the AMR agenda is more advanced, the design of the AMR policies often does not reflect the best practices and international standards and the implementation is limited to select localities. Exacerbating these challenges, the existing enforcement mechanisms do not always guarantee a high degree of compliance. Combined, findings emerging from earlier chapters suggest that it is paramount to continue to invest in policies to tackle AMR through multi-sectoral action.
Considering these findings, it is important to assess the effectiveness and cost-effectiveness of scaling 11 interventions that can be implemented in the human health and non-human sectors up to the desired levels of health and economic outcomes (Box 6.1). The chapter also quantifies the return on investing in these policies. While the majority of interventions that were selected to be modelled for the purposes of this chapter target the human health sector, the cost-effectiveness of interventions in food safety and agriculture was also examined. The interventions modelled in the scope of this chapter were selected using three criteria:
Consistency with the interventions whose implementation is recommended by the World Health Organization (WHO) Global Action Plan on AMR (2015[1]) and prioritised by OECD and EU/EEA countries in their action plans.
Difference between the current and desired level of implementation of each intervention across OECD and EU/EEA countries.
Availability of high-quality quantitative evidence on the effectiveness of each intervention at the individual level that could be used as inputs to the OECD Strategic Public Health Planning for AMR (SPHeP-AMR) model.
The chapter starts by summarising the design features of the 11 modelled interventions. Next, it presents results that show the estimated health and economic impact of scaling up each intervention when implemented first separately and then as part of a package in which multiple interventions are scaled up at the same time. The chapter concludes by discussing the implications of the key findings.
Box 6.1. Quantifying the return on investment of policy interventions to tackle AMR using the OECD SPHeP-AMR model
The effectiveness and cost-effectiveness estimates of policy interventions modelled in this chapter were generated by deploying the OECD SPHeP-AMR model described in Box 3.1 in Chapter 3. The OECD SPHeP-AMR model recognises whether a policy intervention will work in a given setting and the magnitude of its potential effectiveness is highly sensitive to contextual factors such as the demographic and epidemiological profile of each country, local treatment costs and cost of implementation. When quantifying the potential effectiveness of each policy intervention, the OECD model integrates these factors into its framework by modelling interventions across four key parameters:
Intervention effectiveness at the individual level: Parameter values are extracted based on systematic reviews and meta‑analyses in the existent literature whenever possible. In cases where evidence is taken from single studies, randomised controlled trials were prioritised over observational studies.
Intervention effectiveness over time: A growing body of literature on AMR-relevant policies suggests that the effectiveness of public health interventions may change over time, with some interventions having larger observable effects in the earlier phase of implementation and waning effects over time. In the OECD analysis, it is assumed that the modelled interventions continue to receive investments over time (e.g. repeated training, regular updates to mass media campaigns, etc.) to ensure that their effectiveness remains constant over the simulation period.
Intervention coverage: This parameter involves identifying population groups that are eligible to be covered by the modelled intervention and the level of exposure to the intervention at the outset. For the modelled interventions that have not yet been implemented in the countries included in the analysis, the coverage in the business-as-usual scenario was set to zero. For other interventions that have already been implemented in some capacity, the coverage in the business-as-usual scenario was selected based on information extracted from studies that use data from the 2020‑21 Tripartite AMR Country Self-Assessment Survey (WHO/FAO/OIE, 2021[2]), as well as the 2019 Hand Hygiene Self-Assessment Framework (de Kraker et al., 2022[3]) and the 2019 Infection Prevention and Control (IPC) Assessment Framework survey (Tomczyk et al., 2022[4]) conducted by the WHO. These surveys were selected to calculate intervention coverage in line with the beginning of the projection period.
Implementation costs: Implementation costs combine: i) programme‑level costs associated with administration, training, and other activities; and ii) patient-level costs are associated with individual-level expenditures. Costs were derived using a standardised ingredients-based approach using the WHO Choosing Interventions that are Cost-Effective (WHO-CHOICE) framework (2003[5]) as well as forthcoming OECD publications on infectious diseases that focused on promoting hand hygiene and enhanced environmental hygiene in healthcare services. All costs are expressed in 2020 USD PPP to account for the differences in purchasing power. All costs are calculated from a governmental and healthcare perspective. In effect, this means refers to the assumption that the interventions would be delivered by social health insurance schemes or by hospitals regardless of their ownership status. Costs covered by other agents are excluded from the analysis (e.g. costs for the purchase of personal protective equipment [PPE] bought by farmers in the improved farm hygiene intervention).
Gauging population-level effectiveness and return on investment
The model gauges the population-level effectiveness of the modelled interventions through comparisons against a business-as-usual scenario over time. For the majority of countries, the simulation period is from 2021 to 2050. For a handful of countries, the first year of the simulation ranges between 2015 and 2020 depending on the availability of historical data. Under the business-as-usual scenario, it is assumed that no new AMR-relevant interventions are rolled out throughout the simulation period except for those already in place and the provision of preventive and health services is assumed to remain unchanged. A comparison between the business-as-usual and intervention scenarios yields the impact of an intervention, measured through the differences in health and economic outcomes. The uncertainty around the effectiveness of an intervention is assessed through sensitivity analyses. Combined, results from these analyses are used to quantify the return on investment.
Assessing the effectiveness of modelled interventions using different scenarios
The OECD model recognises that some of the modelled interventions can interrupt the transmission of both susceptible and resistant infections while others are assumed to reduce only the incidence of resistant infections (see below). For interventions that are assumed to target only the resistant infections, results are presented using two scenarios: i) the elimination scenario in which the scale-up of the modelled is assumed to eliminate resistant infections while the burden of susceptible infections remains unchanged; and ii) the replacement scenario whereby resistant infections are assumed to be replaced by susceptible infections.
Note: A detailed description of the OECD SPHeP-AMR model is accessible here: http://oecdpublichealthexplorer.org/sphep-amr-doc/.
Source: WHO/FAO/OIE (2021[2]), Tripartite AMR Country Self-Assessment Survey (TrACSS) 2020‑21, https://www.who.int/publications/m/item/tripartite-amr-country-self-assessment-survey-(tracss)-2020-2021; WHO (2003[5]), Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis, https://apps.who.int/iris/handle/10665/42699; Tomczyk, S. et al. (2022[4]). “The first WHO global survey on infection prevention and control in health-care facilities”, https://www.doi.org/10.1016/s1473-3099(21)00809-4.
A host of multi-sectoral policies offers an important means to tackling AMR in line with the One Health approach
Consistent with the Global Action Plan on AMR (WHO, 2015[1]), the OECD analysis groups the modelled interventions into four domains:
1. Policies to optimise the use of antibiotics in human health.
2. Policies in human health to reduce the incidence of infections.
3. Policies to promote AMR awareness and understanding.
4. Policies outside of human health sector to reduce the incidence of infections.
The next section summarises the design features of the modelled interventions and Table 6.1 denotes the key model parameters associated with each intervention (detailed descriptions of the selected interventions are available in Annex 6.A).
Policies to optimise the use of antibiotics in human health
The modelled interventions in this category can be implemented in hospitals and community settings. All of these interventions are built under the assumption that efforts that aim to optimise the use of antibiotics will reduce AMR in the short term, reflecting the evidence emerging from the literature (Lee et al., 2013[6]). It is also assumed that there exists a perfectly elastic relationship between the consumption of antibiotics in human health and AMR in the short term. In effect, this means a 10% improvement in the use of antibiotics can result in a 10% reduction in AMR in the short term. This assertion is based on a handful of studies that quantify the relationship between antibiotic consumption in human health and AMR (Kaier, Frank and Meyer, 2011[7]; FiRe Network, 2004[8]).
Strengthening antimicrobial stewardship programmes (ASPs) in human health: This intervention entails the scale-up of a hospital-based stewardship programme to promote the prudent use of antimicrobials. It involves building multi-disciplinary teams that provide guidance on antimicrobial prescription, coupled with scaling up the monitoring of antimicrobial use and AMR burden in healthcare facilities.
Introducing delayed antimicrobial prescribing: This is a community-based initiative to promote prudent antibiotic prescription. It involves developing or updating clinical guidelines that consider delayed prescribing practices, provider training and education to improve awareness and understanding around best practices in delayed antibiotic prescribing, rollout of a feedback programme to assess prescriber performance over time and the development and distribution of informational materials that serve as best practice reminders.
Scaling up the availability of rapid diagnostic tests (RDTs) (C-reactive protein [CRP] testing): This is a novel community-based programme that aims to increase the use of RDTs by increasing the availability of point-of-care (POC) CRP in ambulatory care settings in line with antibiotic treatment guidelines depending on the CRP levels. In addition, the intervention entails a brief training to disseminate information on the benefits of using POC CRP testing in line with antibiotic prescribing guidelines, dissemination of informational materials for prescribers and monitoring and evaluation of prescribers’ adherence to the antibiotic treatment guidelines.
Using financial incentives to optimise antimicrobial use: This is a nationwide pay-for-performance programme with the aim of promoting the prudent use of antimicrobials in community settings. It involves rewarding lump-sum bonus payments corresponding to around 1% of the base salary of prescribers for meeting pre-set prescribing targets. The intervention also includes setting up a monitoring and evaluation mechanism to assess whether the prescribers are meeting their prescribing targets.
Policies in human health to reduce the incidence of infections
Improving hand hygiene and enhancing environmental hygiene practices are considered by the WHO as the Core Components of IPC practices in healthcare settings (WHO, 2022[9]; 2016[10]). Improvements in vaccination coverage have also been increasingly suggested as another crucial strategy to tackle AMR (WHO, 2015[1]). The OECD model assumes that all of these interventions can reduce the incidence of not only resistant infections but also the incidence of susceptible ones.
Improving hand hygiene: This is a hospital-based intervention with multiple components to improve hand hygiene practices in line with the WHO guidelines. It involves ensuring that physical infrastructure (e.g. alcohol dispensers) is accessible throughout health facilities, designating an IPC focal point that is responsible for organising and co‑ordinating all educational activities around improving hand hygiene practices, providing training and education opportunities for health workers around best practices in hand hygiene, distributing informational materials and monitoring compliance with hand hygiene practices.
Enhancing environmental hygiene practices: This is a hospital-based multimodal programme to enhance routine cleaning practices. It involves substituting disinfectant products already in use with those that have been shown greater effectiveness in line with the WHO guidance, the introduction of no-touch disinfection methods as part of the terminal cleaning of rooms/areas in between occupying patients, a training programme targeting staff members who are responsible for environmental cleaning in healthcare facilities to teach best practices in environmental cleaning and regular audits of environmental cleaning activities.
Increasing coverage of 23‑valent pneumococcal polysaccharide vaccines (PVV23): This is a nationwide community-based campaign of PVV23 targeting 90% coverage across older adults. It involves making sure that there are sufficient stocks of PPV23 in healthcare facilities and disseminating informational materials to ensure high levels of uptake among the target population and setting up a monitoring system to assess the level of vaccine uptake over time.
Policies to promote AMR awareness and understanding
The interventions grouped in this category can be implemented in community settings under the assumption that increasing AMR awareness and understanding can improve behaviours around antibiotic use among prescribers as well as the general public. These interventions also rely on the perfect elasticity assumption between antibiotic consumption in human health and AMR in the short term.
Improving health professional training and education: This is a routine training programme that can be implemented in community settings. Specifically, the modelled intervention aims to improve the communication skills of prescribers, with a particular emphasis on enhancing their proficiency around methods to examine and modify patients’ beliefs and expectations around antibiotic use. The modelled intervention also involves setting up a monitoring mechanism to examine whether the training curriculum yields the intended improvements in the communication skills of prescribers.
Scaling up mass media campaigns: This is a nationwide community-based mass media campaign to enhance AMR awareness and understanding in the general population. The campaign is assumed to be carried out annually during the peak flu season when antibiotic consumption reaches its highest levels. Awareness activities are assumed to include developing communication toolkits and key messages targeting a diverse set of mass media outlets (e.g. television, etc.) and making sure that guidelines and recommendations for prudent antibiotic use developed by public health agencies are accessible on line.
Policies outside the human health sector to reduce the incidence of infections
Adopting a One Health framework is considered to be vital to tackling the complex drivers of AMR that span multiple sectors. In recognition, the OECD model looks at the potential impact of two interventions that can be implemented in farms and food establishments. Similar to the interventions in the human health sector that aim to reduce the incidence of infections, it is assumed that the scale-up of these interventions can reduce the incidence of both resistant and susceptible infections.
Enhancing farm hygiene: This is a novel programme that aims to improve the use of PPE in farm settings in accordance with international guidelines. It involves the introduction of a regulatory framework to facilitate the purchase of PPE (e.g. farm boots, work clothes, etc.) that can be used by farmers and technical visitors (e.g. veterinarians). Informational materials like posters and brochures are assumed to be developed. In addition, a monitoring and evaluation mechanism is assumed to be put in place to increase compliance.
Enhanced hygiene in food handling: This intervention is modelled as a hazard analysis and critical control points (HACCP)-based food safety training programme for food service workers in food establishments. It entails routine training sessions by trainers with expertise in HACCP systems and a focus on personal hygiene, food preparation and storage, and the dissemination of informational materials around food safety to reinforce the key lessons from training sessions and routine inspections of food establishments to assess compliance.
Table 6.1. Inputs used to model the selected policy interventions to tackle AMR
|
Policies to optimise the use of antibiotics in human health |
Policies in human health to reduce the incidence of infections |
Policies to promote AMR awareness and understanding |
Policies outside of human health sector to reduce the incidence of infections |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Strengthen ASPs |
Delayed antimicrobial prescribing |
Scale up RDTs |
Financial incentives |
Enhance hand hygiene |
Enhance environmental hygiene |
Improve vaccination coverage |
Enhance health worker training |
Scale up mass media campaigns |
Improve farm hygiene |
Improve food handling practices |
|
|
Setting |
Hospital |
Community |
Community |
Community |
Hospital |
Hospital |
Community |
Community |
Community |
Farms |
Food establishments |
|
Target population |
Health workers |
Health workers |
Health workers |
Health workers |
Health workers |
Health workers |
General population |
Health workers |
General population |
Farm workers and professional visitors |
Food service workers |
|
Intervention effectiveness at the individual level |
25% decline in antibiotic use |
60% decline in antibiotic prescribing |
32% reduction in immediate antibiotic prescribing in adults and 46% in children <18 years of age |
8% decline in antibiotic prescribing |
33% reduction in risk of infection among people who comply with enhanced hand hygiene practices compared to those who do not |
26% reduction in risk of infection among people who are exposed to enhanced environmental hygiene practices compared to those who do not |
64% decline in the incidence of all serotypes of invasive pneumococcal disease and pneumococcal pneumonia |
39% reduction in antibiotic prescribing in comparison to usual care |
7% decline in antibiotic prescription |
12% reduction in risk of infection among people who use PPE compared to those who do not |
28.6% reduction in microbial count |
|
Intervention effectiveness over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
Observed immediately and sustained over time |
|
Intervention coverage (business-as-usual scenario) (%) |
21‑43 |
0 |
0 |
0 |
18‑54 |
21‑43 |
10‑74 |
46‑56 |
0 |
10‑50 |
10‑50 |
|
Target coverage (%) |
80 |
40 |
70 |
70 |
70 |
70 |
90 |
70 |
100 |
70 |
70 |
|
Implementation cost (per capita USD PPP) |
0.53 ‑6 |
0.06‑1.26 |
0.53‑2.15 |
0.15‑8.01 |
0.02‑1.06 |
0.71‑5.06 |
0.03‑0.57 |
0.05‑0.86 |
0.40‑1.36 |
0.08‑0.78 |
0.02‑0.73 |
Results
Scaling up policy interventions to tackle AMR can prevent thousands of resistant infections every year
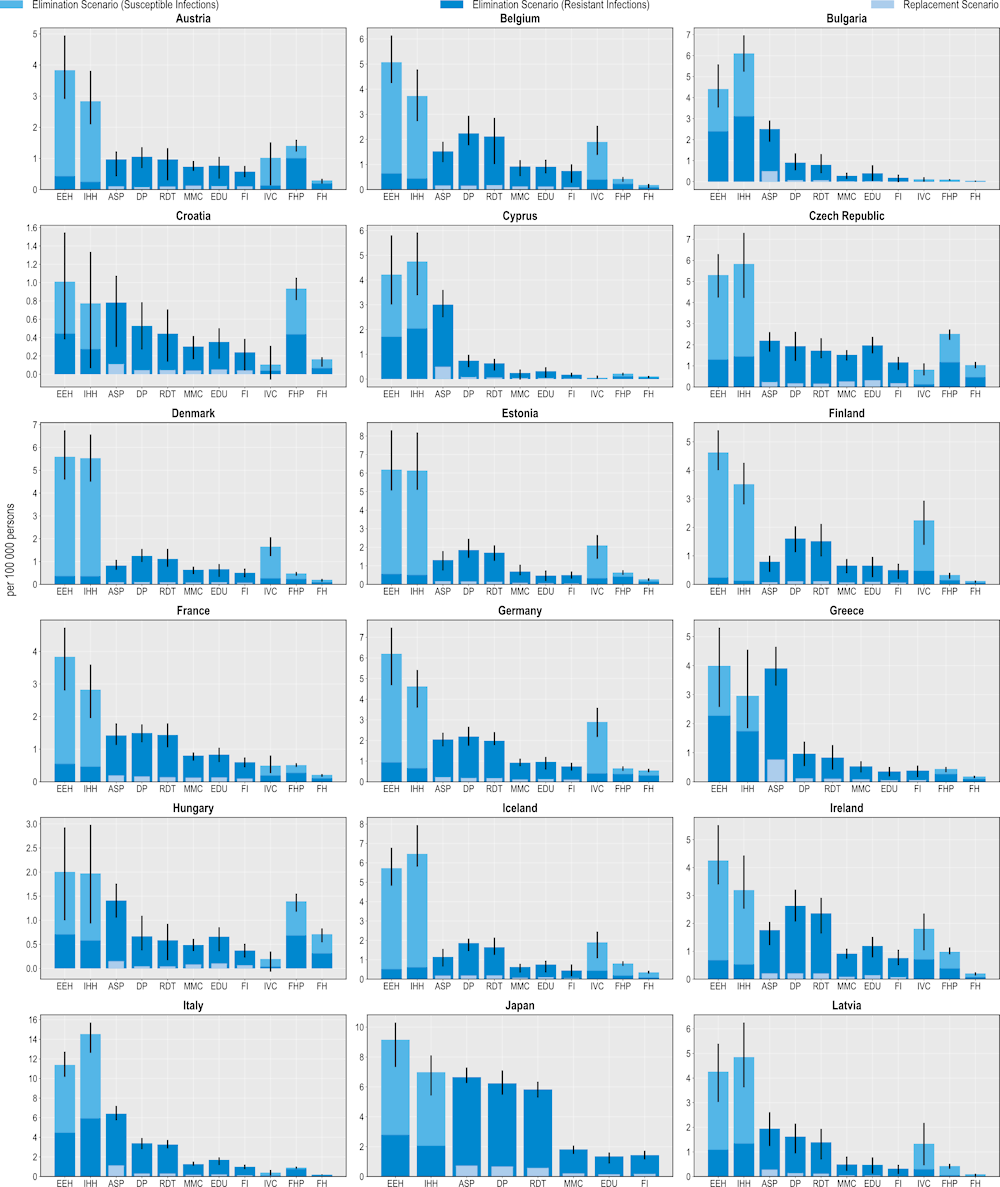
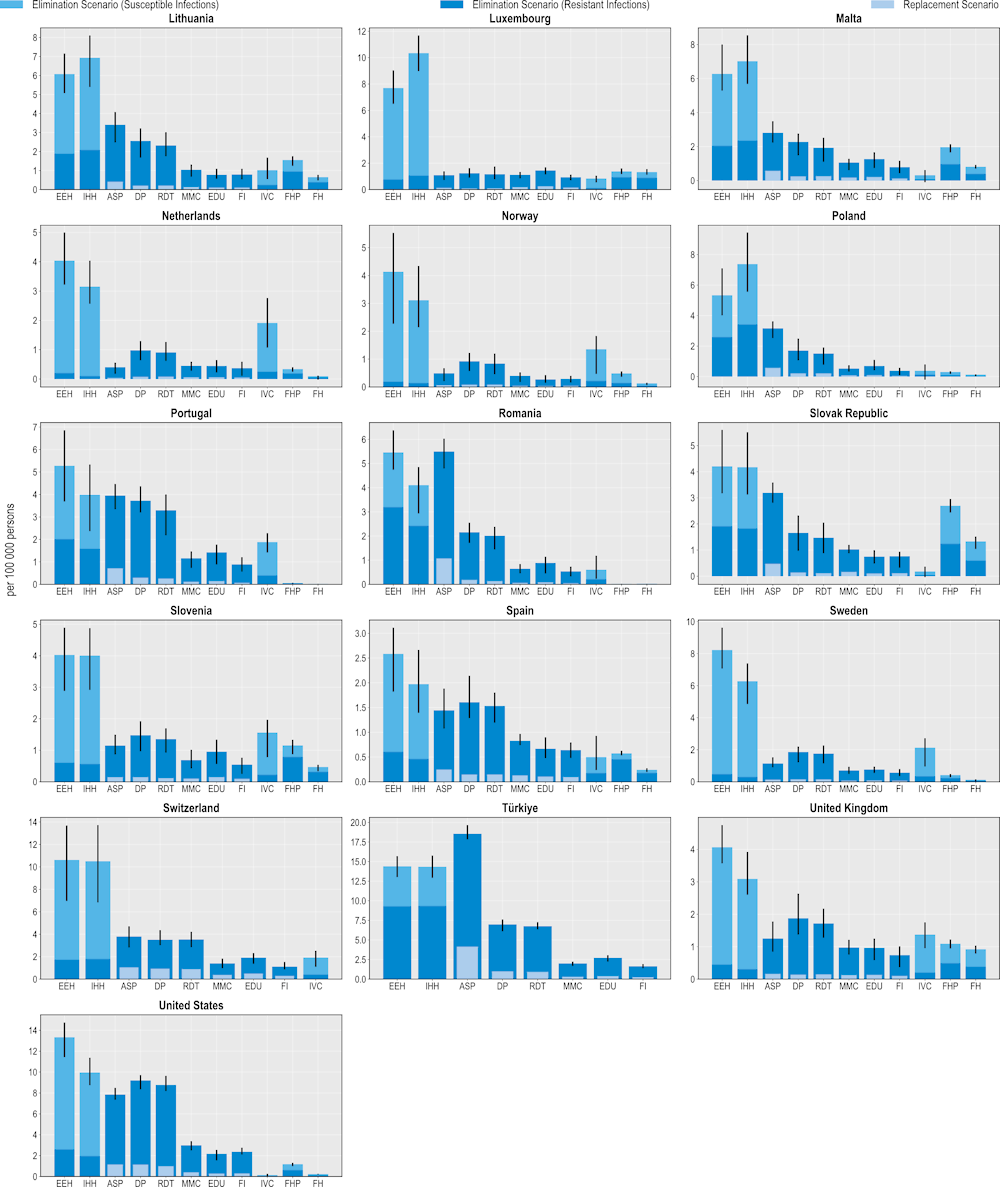
All 11 modelled interventions are estimated to reduce the number of infections each year (Figure 6.1). Scaling up ASPs is expected to yield the greatest reductions in the number of resistant infections whereas increasing the coverage of PVV23 is estimated to produce the smallest reductions. The magnitude of the estimated effectiveness of each intervention varies substantially across countries, reflecting the differences in the incidence of resistant infections, variation in the distribution of the type of resistant infections (i.e. community- vs. healthcare-acquired infections [HAIs]) and healthcare system characteristics.
Hospital-based interventions offer the greatest benefits compared to interventions that can be implemented in other settings. For example, scaling up ASPs is estimated to help avoid, on average, more than 298 000 resistant infections per year across the 34 countries included in the analysis. IPC measures such as enhancing environmental hygiene and improving hand hygiene are also highly effective. On average, enhancing environmental hygiene can prevent more than 123 000 resistant infections each year whereas improving hand hygiene is estimated to prevent more than 113 000 infections. It is important to note that the beneficial impact of these two IPC measures goes beyond preventing only resistant infections. The OECD analysis suggests that, in addition to the impact on resistant infections, an average of more than 461 000 susceptible infections can be eliminated per year by improving environmental hygiene. Similarly, enhancing hand hygiene is expected to avoid more than an additional 392 000 susceptible infections every year.
Boosting the implementation of community-based interventions also leads to reductions in the number of resistant infections. Delayed antibiotic prescription is estimated to prevent, on average, more than 279 000 resistant infections each year. Scaling up mass media campaigns, improving prescriber training and education and financial incentives to optimise antimicrobial use are also effective in reducing resistant infections, with the estimated impact ranging from almost 157 000 to 196 000 resistant infections across these interventions. Countries such as the Czech Republic and Luxembourg that have a relatively higher burden of community-acquired infections (see Chapter 3) are poised to make greater gains from investing in these interventions.
Scaling up the coverage of PVV23 can produce health gains by reducing the number of resistant infections caused by Streptococcus pneumoniae (S. pneumoniae) among older populations. On average, this intervention is estimated to avert almost 3 000 infections each year across the countries included in the analysis. Importantly, there are notable differences in the effectiveness of PVV23. For instance, Germany stands to make the greatest gains across all of the countries included in the analysis, averting around 7.5 resistant infections per 100 000 persons every year. This corresponds to more than 6 000 resistant infections prevented among the target population reflecting the relatively higher incidence of S. pneumoniae compared to the other countries and the low levels of PVV23 coverage among the target population (Bahrs et al., 2021[11]).
The lower impact of PVV23 coverage compared to the other assessed interventions is not surprising given the relatively low incidence of S. pneumoniae across the 34 countries included in the analysis (see Chapter 3). While little global evidence reporting the burden of S. pneumoniae among the elderly population is sparse, findings from available studies suggest that morbidity and mortality due to S. pneumoniae are particularly pressing in non-OECD countries such as those in the WHO African and Eastern Mediterranean Regions (Wahl et al., 2018[12]) and in countries where vaccination coverage against S. pneumoniae remains low. Combined, evidence suggests that increasing the coverage of vaccines that target S. pneumoniae promises greater benefits in settings with a relatively higher burden of S. pneumoniae and lower vaccination rates.
Outside of the human health sector, enhancing food safety and improving farm biosecurity are both associated with reductions in the number of infections, highlighting the importance of the One Health approach. Each year, improving food safety is expected to prevent, on average, more than 424 000 resistant and susceptible infections in humans. Approximately 48% of this attributable reduction would be driven by preventing resistant infections. The Czech Republic and the Slovak Republic stand to achieve the greatest reductions in the number of resistant infections by investing in this intervention, preventing around 139 and 128 resistant infections per 100 000 persons every year. Improving biosecurity in farm settings can also safeguard population health: on average, more than 150 000 infections in humans can be averted through scaling up this intervention, with nearly half of the observed reductions occurring through preventing resistant infections.
Figure 6.1. Antimicrobial stewardship programmes are the most effective modelled policy intervention to avert resistant infections
Number of infections averted per 100 000 persons annually up to 2050
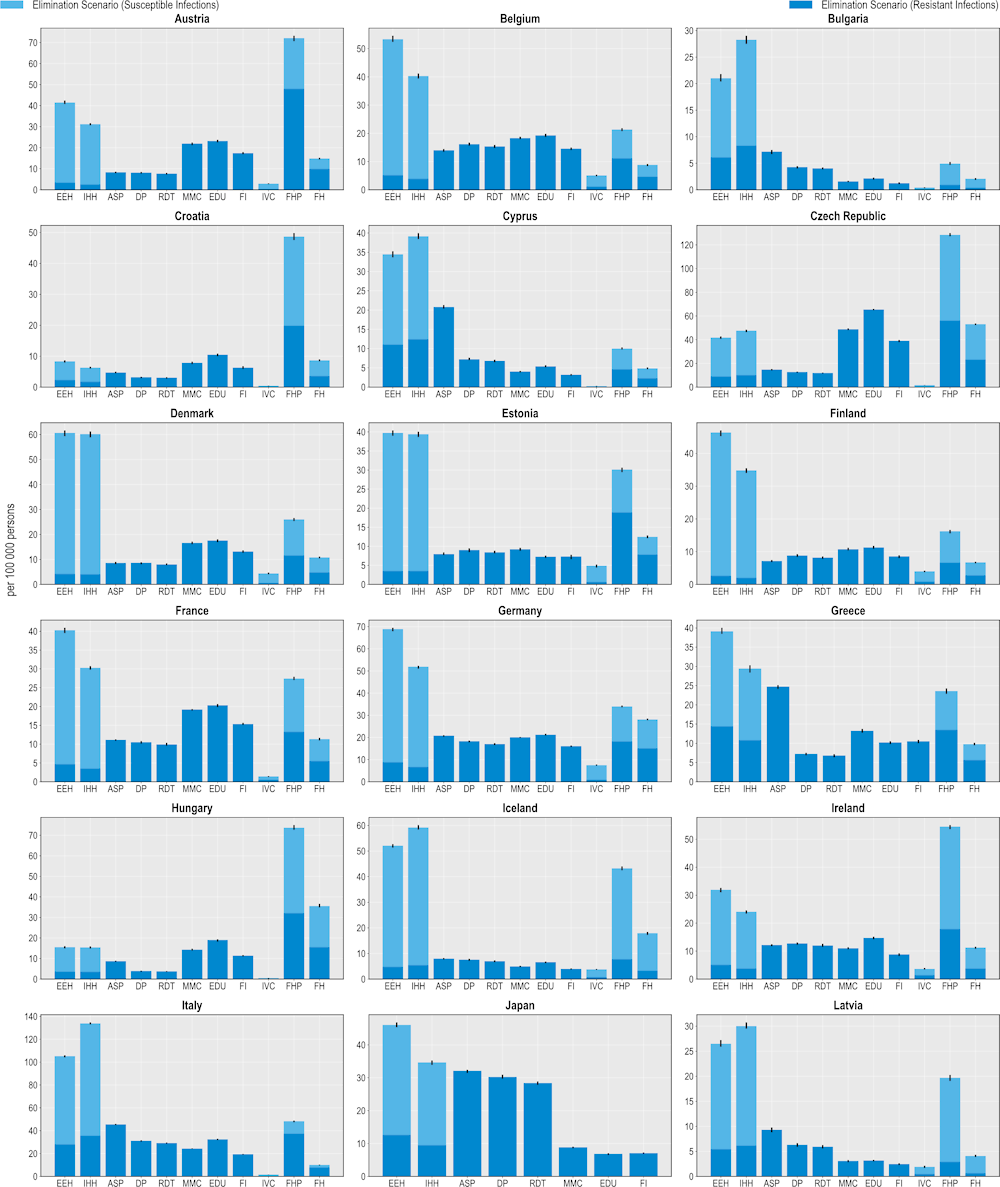
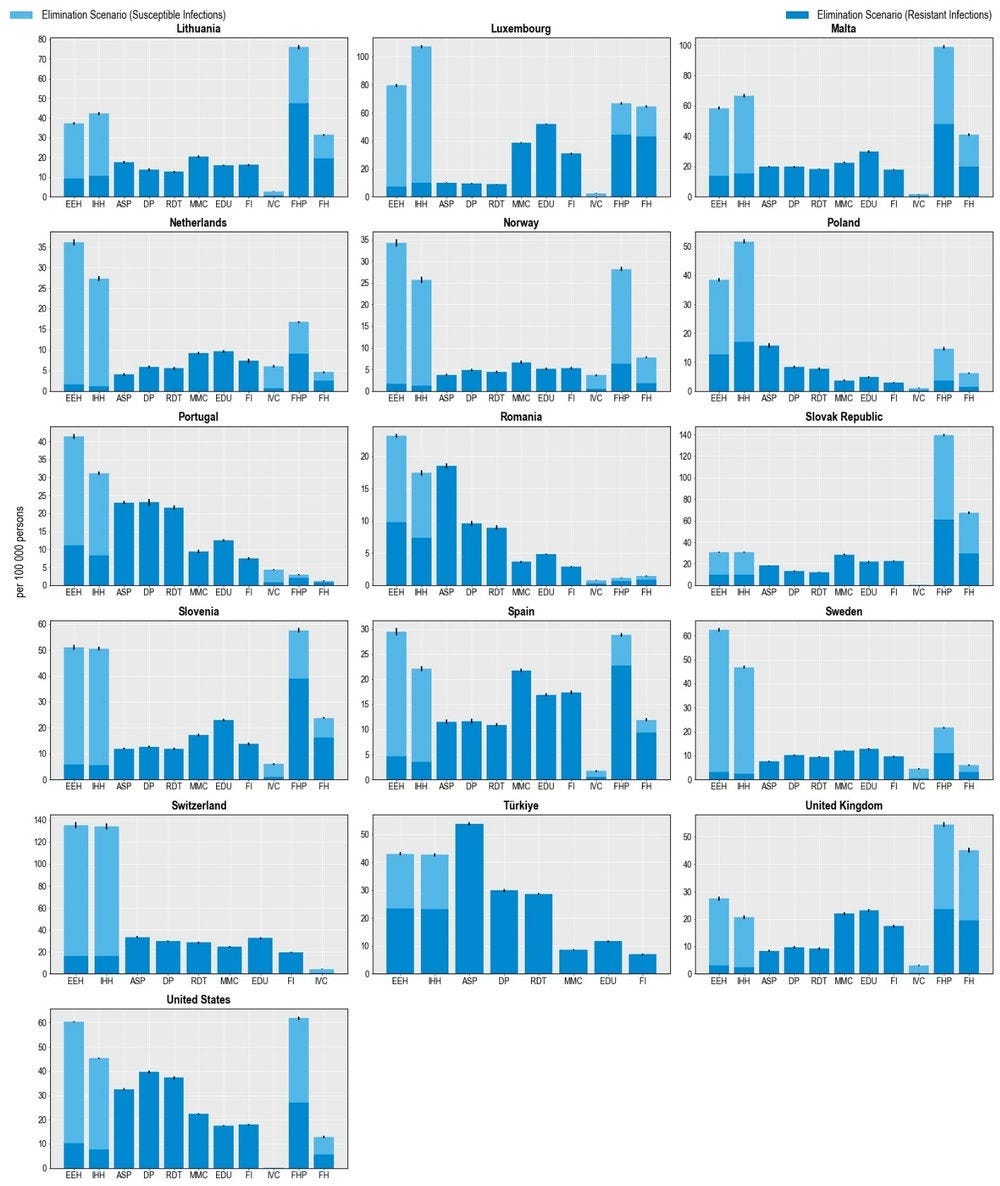
Note: ASP: Antimicrobial stewardship programme; DP: Delayed prescribing; EDU: Education and training of healthcare professionals; EEH: Enhancing environmental hygiene; FH: Farm hygiene; FHP: Food handling practices; FI: Financial incentives; FMS: Improving farm hygiene practice; IHH: Improving hand hygiene; IVC: Increasing vaccine coverage; MMC: Mass media campaigns; RDT: Rapid diagnostic testing capacity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Investing in policy interventions to tackle AMR can safeguard population health by preventing thousands of deaths
All 11 modelled interventions are associated with reductions in the number of deaths caused by resistant infections (Figure 6.2). ASPs are estimated to prevent the highest number of deaths. On average, ASPs are estimated to avoid more than 10 000 deaths each year using the elimination scenario and more than 3 200 deaths using the replacement scenario. In other words, scaling up ASPs to the desirable levels across the 34 countries included in the analysis can be equivalent to preventing around 10-30% of deaths due to TB, influenza and HIV/AIDS in 2020 (or the nearest year for which this information is available).
The OECD analysis points to substantial cross-country variation. For example, across the EU/EEA member OECD countries, Italy and Greece can avert, on average, 1.6 and 0.9 deaths per 100 000 persons each year respectively by investing in this intervention, whereas Türkiye can prevent 3.9 deaths per 100 000 persons each year, representing the highest potential gains across non-EU/EEA OECD countries.
IPC measures are also effective in preventing AMR-related deaths. On average, improving environmental hygiene is estimated to reduce more than 4 800 deaths every year across the 34 countries included in the analysis. Similarly, improving hand hygiene could help avoid more than 4 500 deaths per year. Importantly, improving environmental hygiene and hand hygiene practices can also prevent thousands of deaths each year by eliminating susceptible infections (more than 8 500 and 7 400 deaths respectively). Italy and Luxembourg are the two EU/EEA countries that can avoid the greatest number of deaths due to AMR by investing in these IPC measures. Across the non-EU/EEA member OECD countries, Switzerland and Türkiye are poised to prevent the highest number of AMR-related deaths per 100 000 persons every year by investing in improvements in environmental and hand hygiene practices.
Interventions that can be implemented in community settings also offer a valuable means for reducing AMR-related deaths. Delaying antibiotic prescribing could prevent the greatest number of deaths across all of the community-based interventions. On average, this intervention is estimated to prevent around 7 800 deaths per year under the elimination scenario and nearly 2 000 deaths using the replacement scenario. This is followed by scaling up RDTs, increasing mass media campaigns and improving prescriber education. Increasing vaccination coverage can also help reduce mortality due to AMR. Similar to its relative effectiveness in terms of preventing resistant infections, the impact of this intervention in terms of preventing mortality is estimated to be more modest compared to others in the human health sector due to the relatively low estimated incidence of deaths attributable to S. pneumoniae across the countries included in the analysis.
Figure 6.2. All modelled policy interventions can avert deaths due to AMR
Number of deaths due to AMR averted per 100 000 persons annually up to 2050
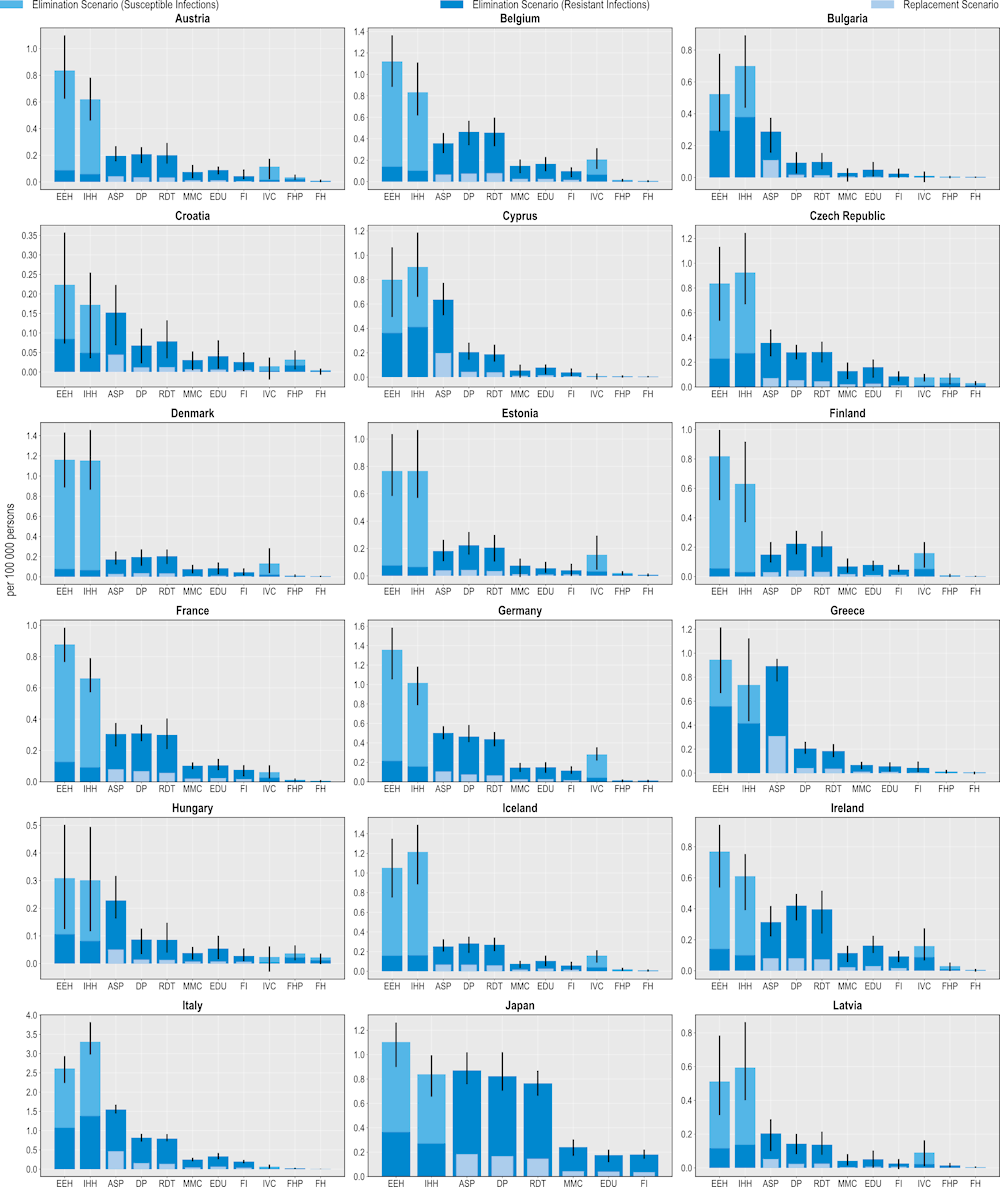
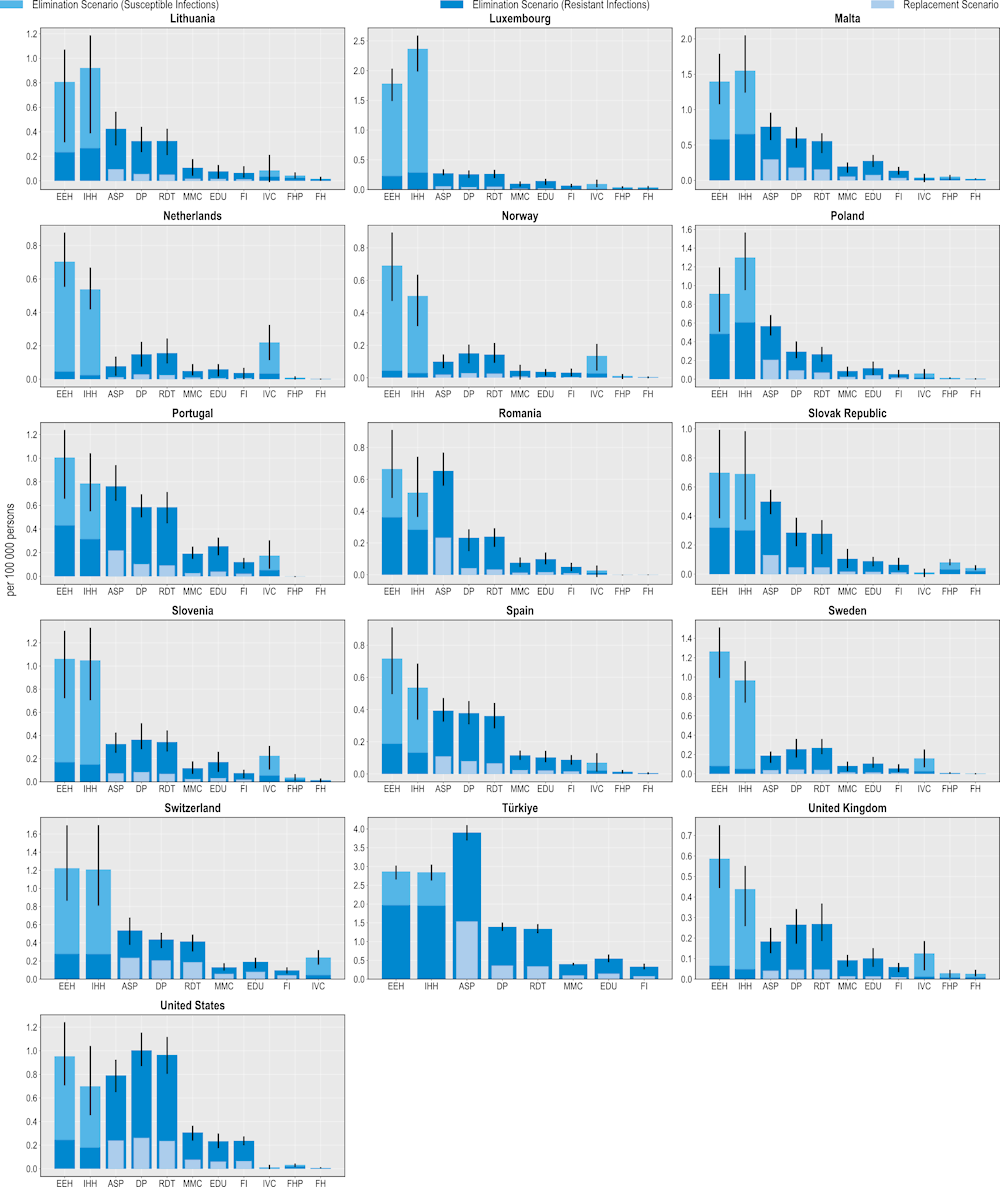
Note: ASP: Antimicrobial stewardship programme; DP: Delayed prescribing; EDU: Education and training of healthcare professionals; EEH: Enhancing environmental hygiene; FH: Farm hygiene; FHP: Food handling practices; FI: Financial incentives; FMS: Improving farm hygiene practice; IHH: Improving hand hygiene; IVC: Increasing vaccine coverage; MMC: Mass media campaigns; RDT: Rapid diagnostic testing capacity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Investing in policies to tackle AMR leads to gain in years of life
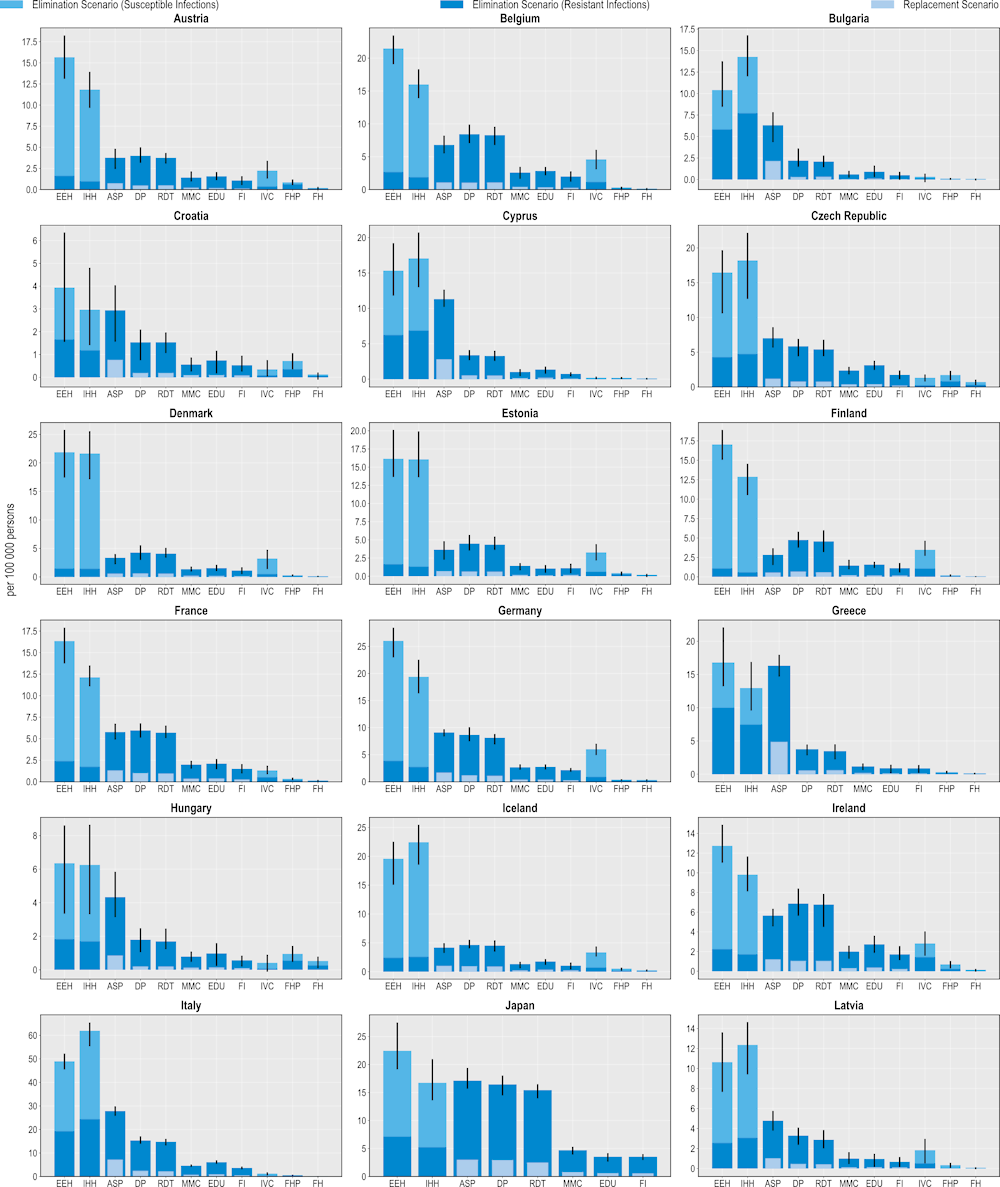
All of the modelled interventions are linked to improvements in the number of LYs lived (Figure 6.3). Similar to their beneficial impact on mortality, interventions to scale up ASPs promise the greatest savings in LYs gained whereas interventions to improve biosecurity practices in farm settings offer the lowest gains. Importantly, the effectiveness of all interventions on morbidity as measured in DALYs surpasses their effectiveness on mortality as measured in LYs gained.
On average, scaling up ASPs is estimated to save more than 153 000 LYs annually using the elimination scenario and more than 47 000 LYs using the replacement scenario. Better environmental hygiene and hand hygiene practices also result in LYs gained by eliminating resistant infections. On average, enhanced environmental hygiene can help save more than 71 000 LYs whereas improved hand hygiene can generate around 67 000 LYs per year. Much like previous health outcomes examined, these interventions can also yield gains in LYs by eliminating susceptible infections.
Delaying antimicrobial prescription is the leading community-based intervention that yields the greatest number of LYs gained. On average, the annual number of LYs gained through this intervention is estimated to exceed more than 121 000 LYs using the elimination scenario and more than 27 000 LYs under the replacement scenario. Scaling up the use of RDTs is the second most impactful intervention in terms of LYs gained. This intervention is associated with more than 114 000 LYs and more than 24 000 LYs gained using the elimination and replacement scenarios respectively. Scaling up mass media campaigns, improving prescriber education and financial incentives also lead to gains in LYs. In comparison, scaling up human vaccination programmes, improving food safety and enhancing biosecurity in farm settings yield the lowest number of LYs gained.
Figure 6.3. All modelled interventions can generate gains in years of life
Number of LYs saved per 100 000 persons annually up to 2050
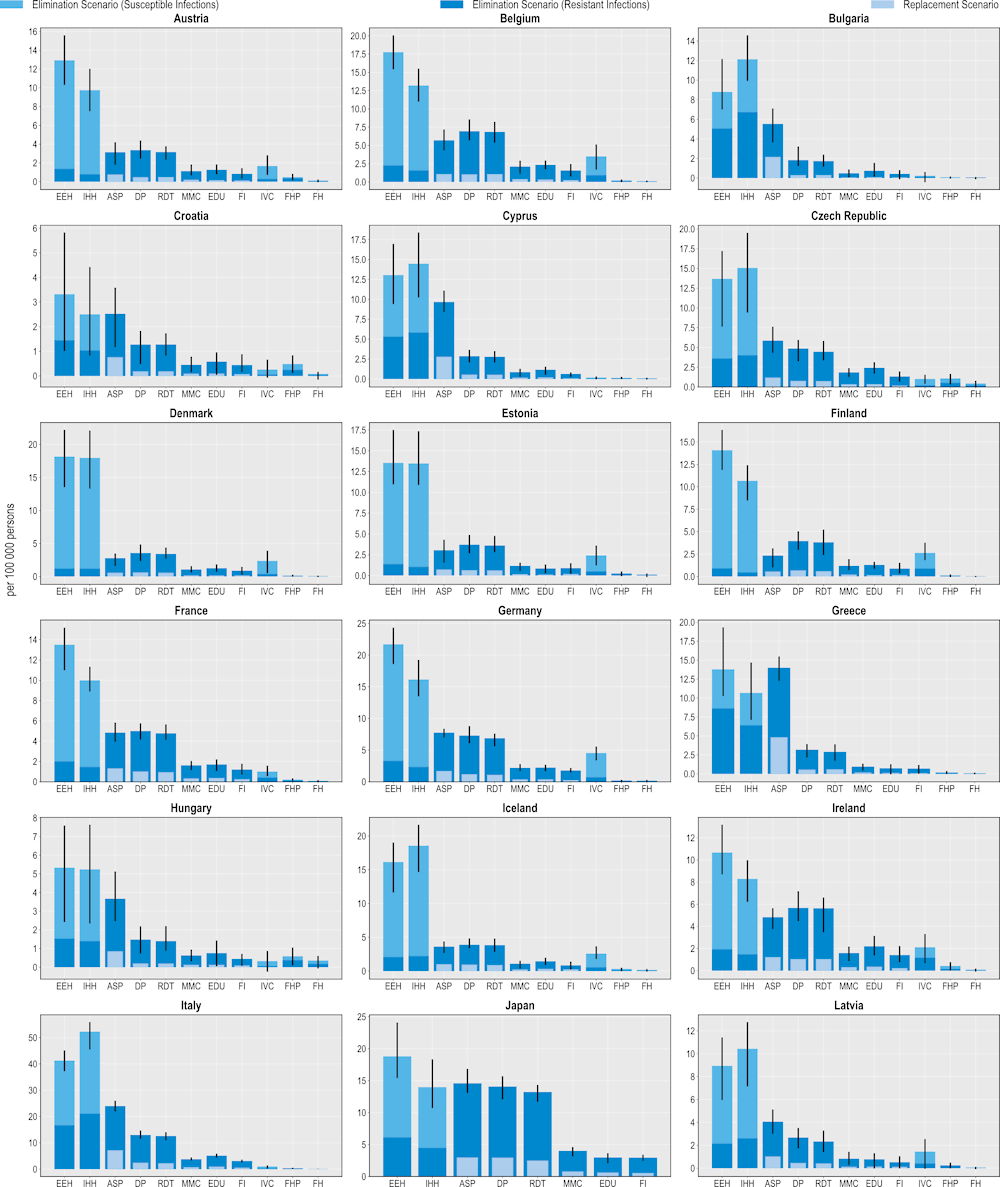
Note: Countries are sorted in alphabetical order.
ASP: Antimicrobial stewardship programme; DP: Delayed prescribing; EDU: Education and training of healthcare professionals; EEH: Enhancing environmental hygiene; FH: Farm hygiene; FHP: Food handling practices; FI: Financial incentives; FMS: Improving farm hygiene practice; IHH: Improving hand hygiene; IVC: Increasing vaccine coverage; MMC: Mass media campaigns; RDT: Rapid diagnostic testing capacity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
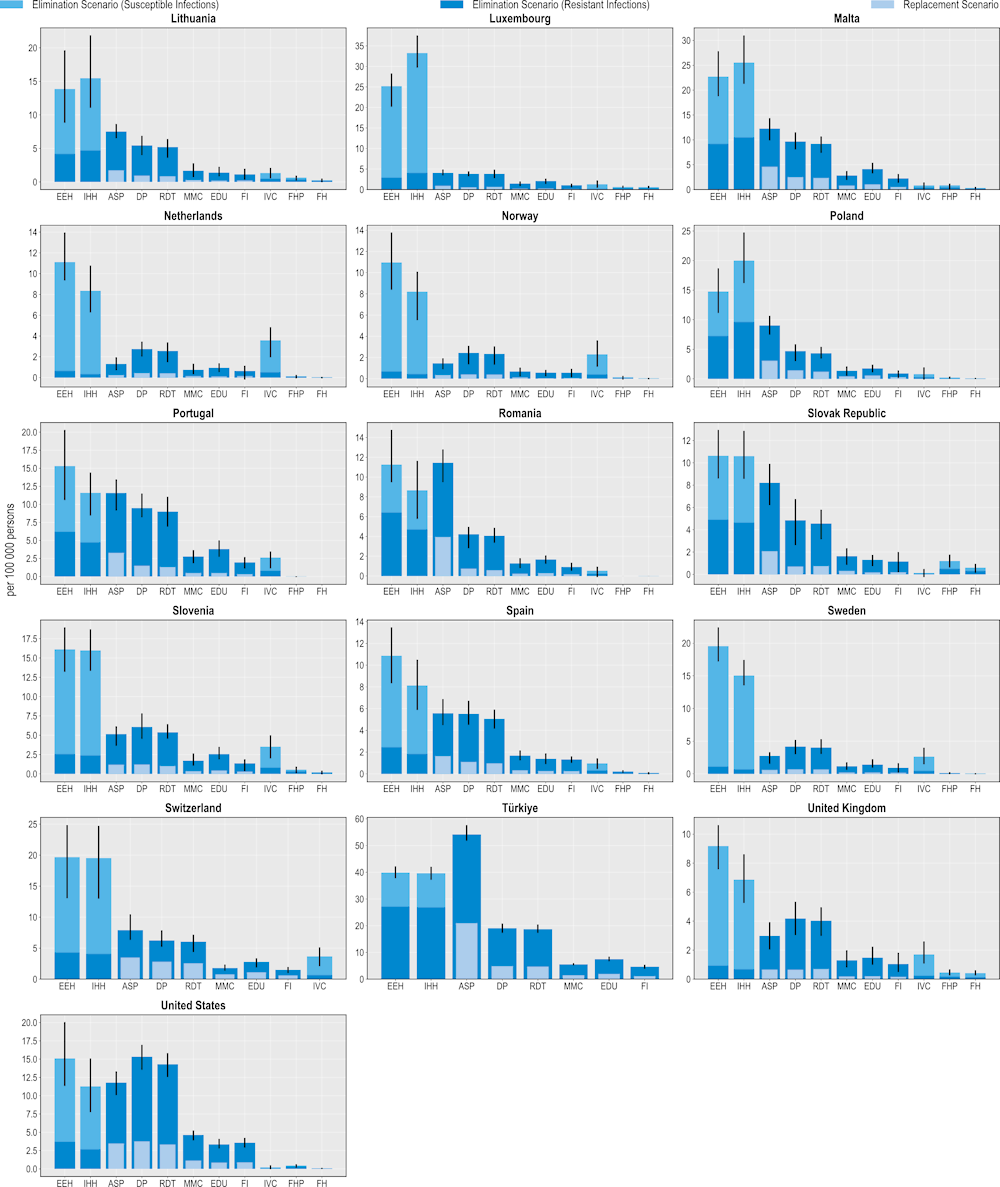
The modelled interventions also yield health benefits as measured in DALYs (Figure 6.4). In a scenario where resistant infections are eliminated, more than 178 000 DALYs would be gained, on average, every year by scaling up ASPs across the countries included in the scope of the analysis. This figure stands at more than 47 000 DALYs if resistant infections were to be replaced by susceptible ones. Improving environmental hygiene and enhancing hand hygiene are also highly effective. For example, improving environmental hygiene promises gains in more than 83 000 DALYs per year by eliminating resistant infections whereas enhancing hand hygiene is estimated to generate more than 78 000 DALYs. Community-based interventions are also associated with estimated health gains, though the magnitude of these gains is smaller than for hospital-based interventions.
These findings suggest that the modelled interventions promise a greater beneficial impact on quality of life than premature mortality. This result is partly due to the finding presented in Chapter 3 that resistant infections pose a greater risk of mortality for the elderly population above 65 years of age than other population groups due to a range of factors such as physiological changes (e.g. weakened immune response) and comorbidities. Even after a successful recovery from a resistant infection, elderly individuals continue being more prone to the competing risks of mortality than younger population groups (e.g. greater risk of HAIs and infections acquired in long-term care settings due to longer time spent in these facilities, etc.) (Nelson et al., 2021[13]).
Figure 6.4. All modelled interventions can yield savings DALYs
Number of DALYs gained per 100 000 persons annually up to 2050
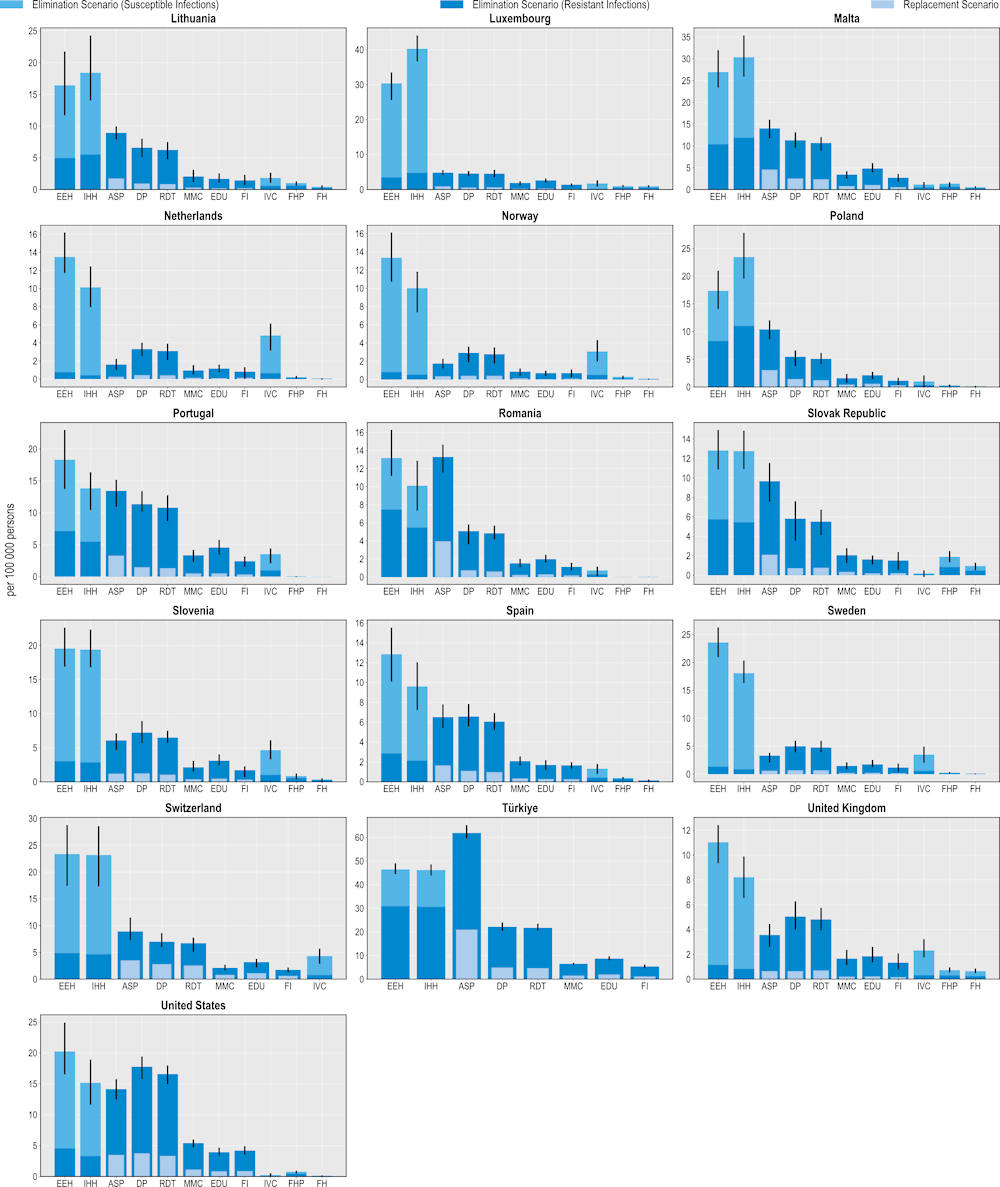
Note: ASP: Antimicrobial stewardship programme; DP: Delayed prescribing; EDU: Education and training of healthcare professionals; EEH: Enhancing environmental hygiene; FH: Farm hygiene; FHP: Food handling practices; FI: Financial incentives; FMS: Improving farm hygiene practice; IHH: Improving hand hygiene; IVC: Increasing vaccine coverage; MMC: Mass media campaigns; RDT: Rapid diagnostic testing capacity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Policy interventions to tackle AMR can reduce the use of hospital resources
Investing in AMR policies can also help reduce the pressure on hospital resources (Figure 6.5). ASPs promise the greatest number of reductions in the use of hospital resources using both modelling scenarios. On average, this intervention is predicted to result in more than 3.7 million fewer extra days spent in hospital using the elimination scenario and more than 822 000 additional days using the replacement scenario. This would be equivalent to freeing up the entire acute bed capacity in Ireland in 2020 for nearly 1 year by eliminating resistant infections and around 2 months if resistant infections were replaced by susceptible infections.
Across EU/EEA member OECD countries, Italy and Portugal are expected to have the largest annual reduction in the number of additional days spent in hospital (576 and 294 additional hospital days avoided per 100 000 persons respectively) whereas Türkiye and the United States can achieve the greatest gains among non-EU/EEA member OECD countries (840 and 388 additional hospital days avoided per 100 000 persons). Investing in better environmental hygiene could prevent nearly 1.7 million additional hospital days by eliminating resistant infections, whereas investing in better hand hygiene would prevent more than 1.5 million extra days spent in hospital due to infections.
Community-based interventions could also contribute to less frequent use of hospital resources. On average, delayed antibiotic prescribing could avert more than 3.1 million additional days spent in hospital if resistant infections are eliminated and avert more than 476 000 additional hospital days if resistant infections were to be replaced by susceptible infections. Following delayed prescribing, greater reliance to RDTs, mass media campaigns, prescriber education and financial incentives also promise non-negligible reductions in the number of extra days spent in hospital due to AMR, with these interventions preventing between nearly 726 000 to more than 2.9 million additional hospital days using the elimination scenario and between more than 111 000 to nearly 450 000 additional hospital days using the replacement scenario.
Figure 6.5. Investing in policies to tackle AMR can reduce additional days spent in hospitals due to treating resistant infections
Number of additional days spent in hospital avoided per 100 000 persons annually up to 2050
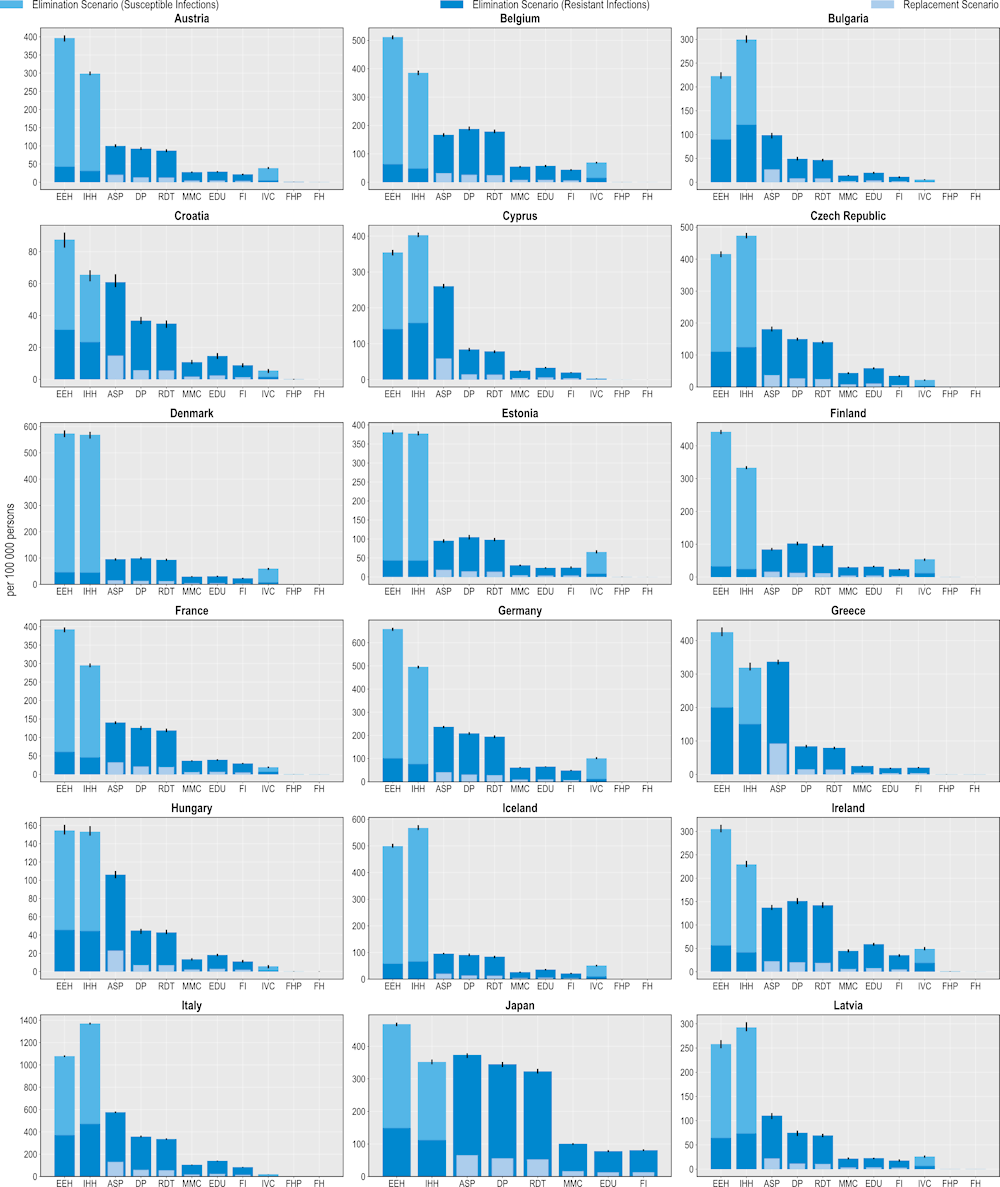
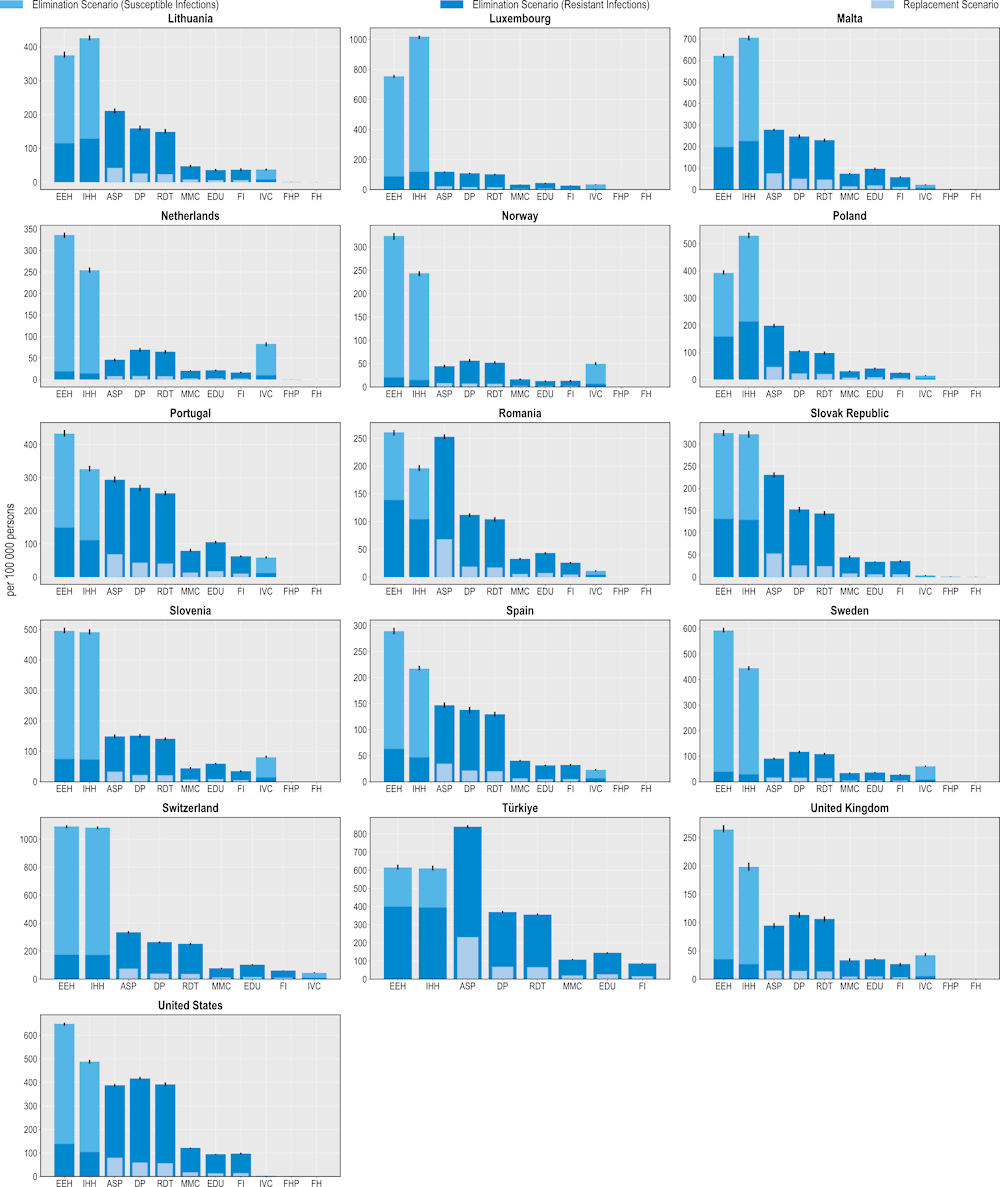
Note: ASP: Antimicrobial stewardship programme; DP: Delayed prescribing; EDU: Education and training of healthcare professionals; EEH: Enhancing environmental hygiene; FH: Farm hygiene; FHP: Food handling practices; FI: Financial incentives; FMS: Improving farm hygiene practice; IHH: Improving hand hygiene; IVC: Increasing vaccine coverage; MMC: Mass media campaigns; RDT: Rapid diagnostic testing capacity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Costs associated with implementing each policy intervention are expected to be offset by reductions in health expenditure and gains in workforce productivity
The OECD analysis demonstrates that implementation costs associated with all of the modelled policy interventions are estimated to be offset by reducing health expenditure while increasing participation in the workforce and improving productivity at work (Figure 6.6). Across the 34 countries included in the analysis, the average implementation annual costs associated with improving hand hygiene are expected to be around 24.6 times lower than the savings generated by estimated reductions in health expenditures and productivity gains made through increased participation in the workforce and productivity at work. Scaling up delayed prescription practices in primary healthcare settings is another highly attractive intervention, with a benefit to cost ratio of around 17. Enhancing food handling practices and improving environmental hygiene practices in healthcare settings are also promising investments. The average annual cost of scaling up each of these interventions across all countries included in the analysis is around five times lower than the expected savings from reducing health expenditure and productivity gains.
As shown in Figure 6.6, using financial incentives to optimise antimicrobial use and ASPs have the highest estimated annual implementation cost per capita (USD PPP 2.6 and 2.3 respectively), followed by enhancing environmental hygiene (USD PPP 2.2) and increasing the use of RDTs (USD PPP 1.3). The costs associated with implementing other interventions each average below USD PPP 1 per capita. There are notable cross-country differences in the cost of policy implementation. For example, across 29 EU/EEA countries, the estimated cost of implementing financial incentives is the highest in Iceland and Luxembourg. Across the non-EU/EEA member OECD countries, the estimated per capita costs associated with financial incentives are the highest in the United States.
All modelled policy interventions are expected to yield reductions in health expenditure (Figure 6.6). IPC interventions such as improving environmental hygiene and hand hygiene practices promise the greatest impacts by reducing both resistant and susceptible infections. Across the 34 countries included in the analysis, enhancing environmental hygiene and improving hand hygiene in healthcare facilities are estimated to reduce health expenditure by nearly USD PPP 7.2 billion (corresponding to USD PPP 6.3 per capita) and more than USD PPP 6 billion (corresponding to USD PPP 5.3 per capita) respectively. Specifically, the reduction in health expenditure attributable to reducing resistant infections is expected to reach nearly USD PPP 1.4 billion (corresponding to USD PPP 1.2 per capita) and USD PPP 1.2 billion (corresponding to USD PPP 1.1 per capita) by enhancing environmental hygiene and improving hand hygiene respectively.
Scaling up ASPs and delayed prescription practices in primary healthcare settings also lead to notable reductions in health expenditure. Ramping up ASPs is expected to reduce health expenditure by more than USD PPP 2.7 billion annually, corresponding to USD PPP 1.2 per capita. This is roughly equivalent to 10% of the health expenditure in Greece in 2020. Whereas implementing delayed prescription practices can result in a decline in health expenditure by USD PPP 2.5 billion every year, corresponding to a USD PPP 1.05 reduction in per capita health expenditure.
Broadly, countries with higher incidences of resistant infections stand to achieve the greatest reductions in health expenditure by investing in the modelled interventions. For instance, as shown in Chapter 3, Italy and Luxembourg are two EU/EEA countries with the highest number of resistant infections every year. By investing in improved hand hygiene practices, Luxembourg can reduce health expenditure by USD PPP 22.6 per capita each year, the highest annual reduction across the EU/EEA countries. This is followed by Italy where the estimated annual reduction in health expenditure is estimated to average at around USD PPP 9.9 per capita.
Figure 6.6. Benefits accrued by scaling up policy interventions to tackle AMR outweigh costs
Cost of interventions and their impact on savings in health expenditure and productivity gains
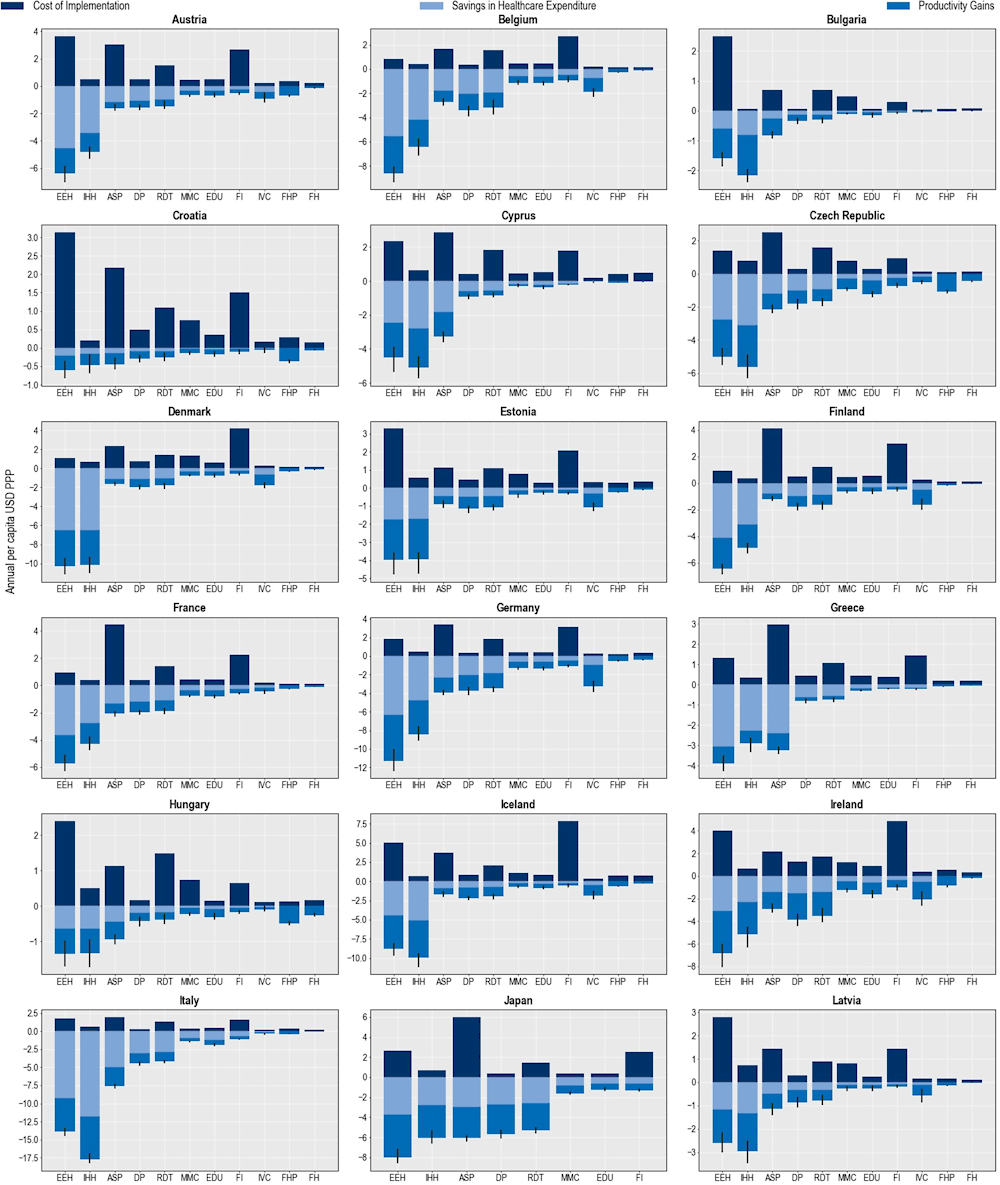
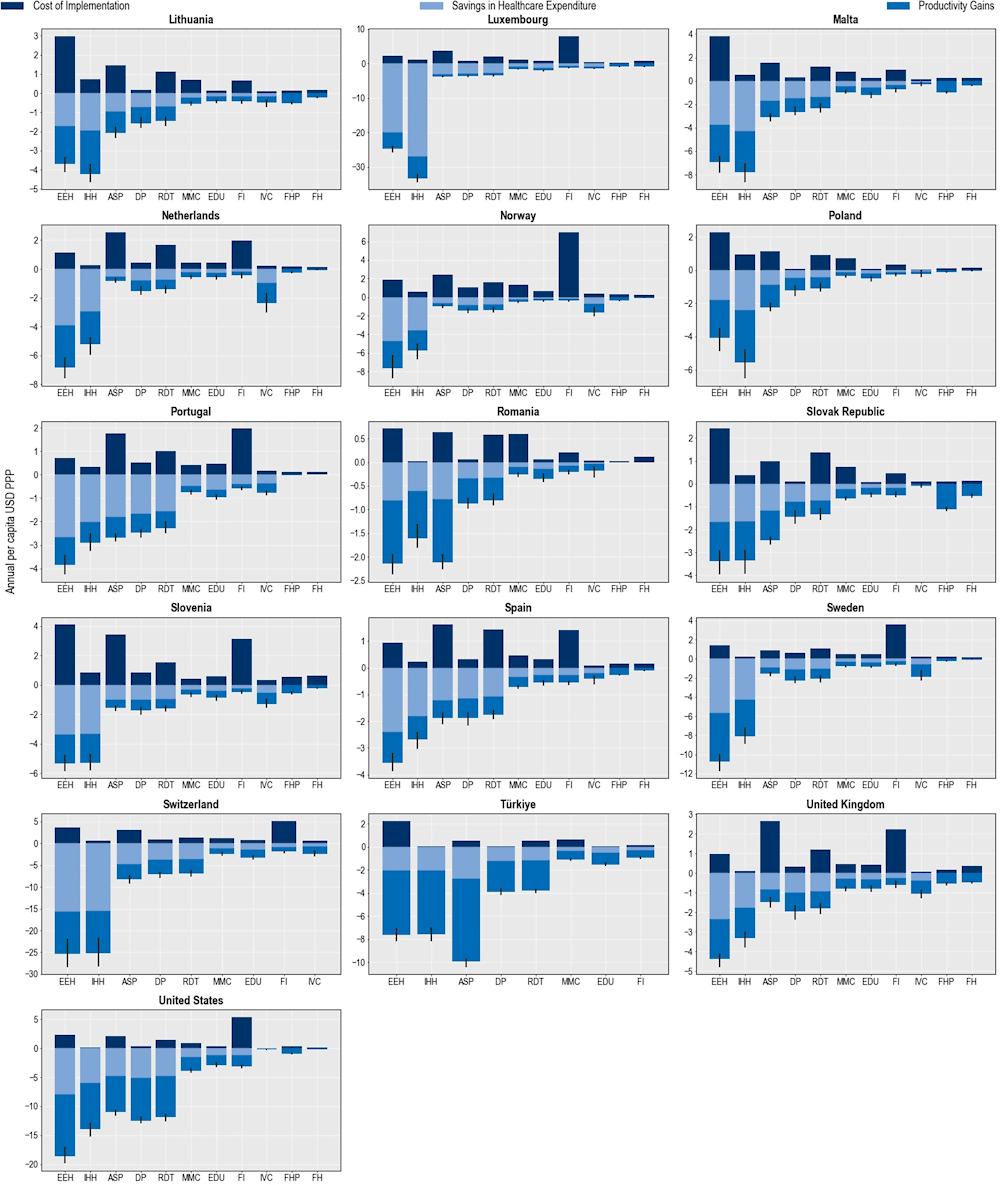
Note: ASP: Antimicrobial stewardship programme; DP: Delayed prescribing; EDU: Education and training of healthcare professionals; EEH: Enhancing environmental hygiene; FH: Farm hygiene; FHP: Food handling practices; FI: Financial incentives; FMS: Improving farm hygiene practice; IHH: Improving hand hygiene; IVC: Increasing vaccine coverage; MMC: Mass media campaigns; RDT: Rapid diagnostic testing capacity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
All of the modelled policy interventions yield productivity gains (Figure 6.7). These gains can be achieved primarily through increasing workforce participation, followed by reducing absence from work due to ill health and presenteeism at work. Scaling up ASPs is associated with the highest estimated gains in productivity. On average, this intervention is estimated to generate close to 67 000 full-time equivalents (FTEs) per year combined across the 34 countries included in the analysis. Of these potential gains, more than 56 000 FTEs are expected to be produced through increased participation in the workforce while more than 9 300 FTEs can be gained by reducing absenteeism. Combined, these productivity gains would amount to around USD PPP 3.9 billion (corresponding to USD PPP 3.5 per capita) each year across all of the countries included in the analysis. In many countries, the estimated productivity gains exceed savings in health expenditure.
Figure 6.7. Investing in AMR policies can improve workforce productivity equivalent to adding thousands of full-time workers every year
Number of FTEs gained per 100 000 persons annually up to 2050
Note: ASP: Antimicrobial stewardship programme; DP: Delayed prescribing; EDU: Education and training of healthcare professionals; EEH: Enhancing environmental hygiene; FH: Farm hygiene; FHP: Food handling practices; FI: Financial incentives; FMS: Improving farm hygiene practice; IHH: Improving hand hygiene; IVC: Increasing vaccine coverage; MMC: Mass media campaigns; RDT: Rapid diagnostic testing capacity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The impact of AMR policies can be enhanced by implementing them as a package of interventions
In recognition of the complexities surrounding AMR, the OECD analysis looks at the effectiveness of bundling multiple policies into a policy package. Policy packages have several important advantages over implementing single policies. By implementing multiple policies as a package, countries can attempt to address various drivers of AMR at the same time. Further, different population groups can be targeted simultaneously if policies are scaled up simultaneously as part of the same package. In addition, various policies within the same package can facilitate and reinforce desirable changes in behaviour. In turn, these changes could generate protective effects that go beyond simply adding up the effectiveness of each intervention (i.e. super-additivity of policy packages). The results presented in the remainder of the chapter consider the first two advantages of policy packages, though the OECD analysis adopts a conservative approach by refraining from using the super-additivity assumption (i.e. no additional effect is considered).
Three packages were assessed:
Hospital-based package: This package focuses on hospital-based interventions that have the greatest estimated impact on health outcomes, including improving hand hygiene, enhancing environmental hygiene and scaling up ASPs. The design and implementation of these interventions vary substantially across countries. The estimated per capita cost of this package ranges between USD PPP 1.4 and USD PPP 9.4.
Community-based package: This package examines the combined impact of community-based interventions with the greatest impact on population health and includes delayed antimicrobial prescriptions, introducing financial incentives to optimise antimicrobial use, scaling up the use of RDTs, mass media campaigns and prescriber training. This package is estimated to cost between USD PPP 0.8 and USD PPP 11.9 per capita.
Mixed package: This package entails a One Health approach by incorporating action across human and non-human health sectors. It includes improving hand hygiene, scaling up ASPs, delaying antimicrobial prescription, increasing mass media campaigns and enhancing food handling practices. The per capita cost of this package is estimated to vary between USD PPP 0.7 and USD PPP 3.7.
Across all three policy packages, the elimination scenario was used for interventions that influence antibiotic prescription.
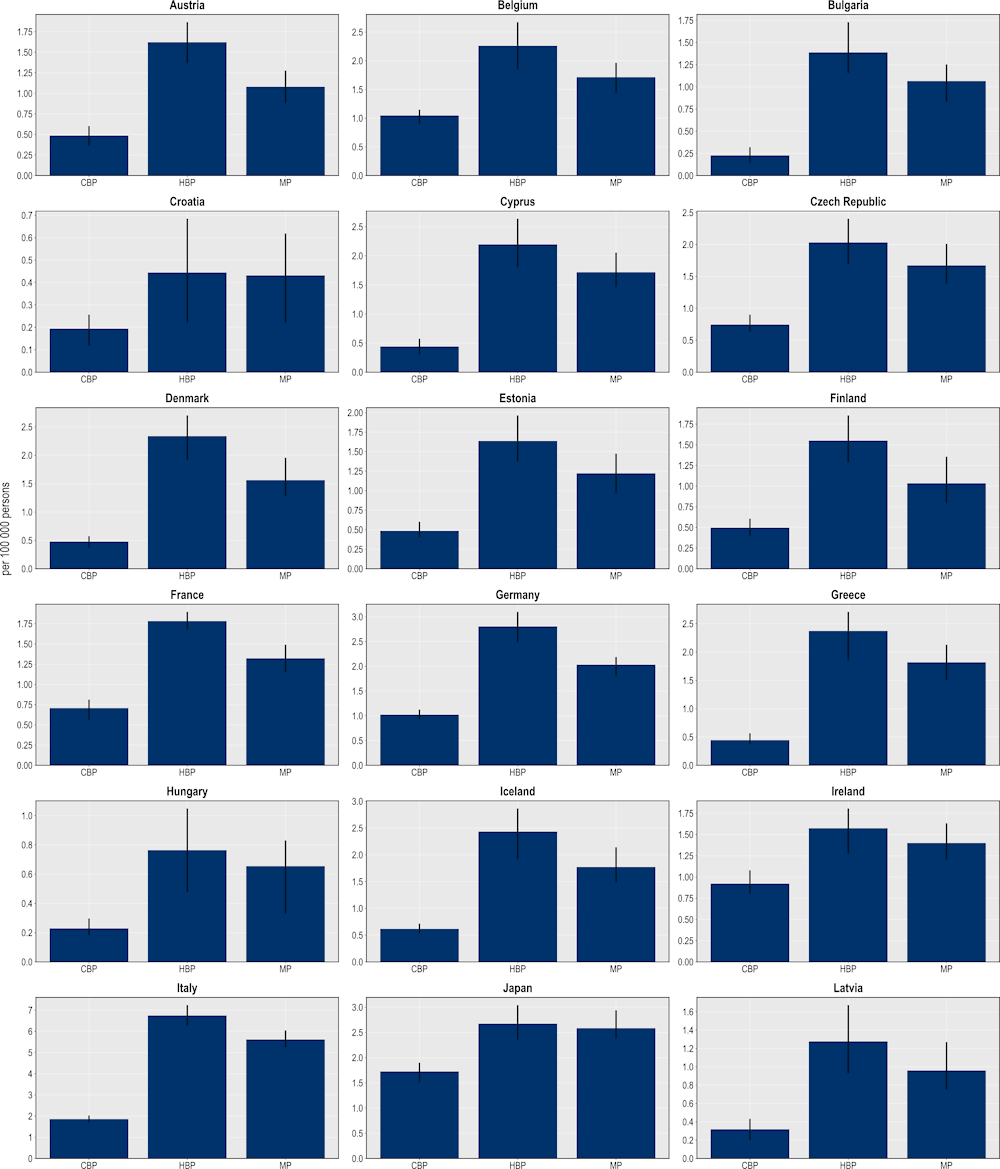
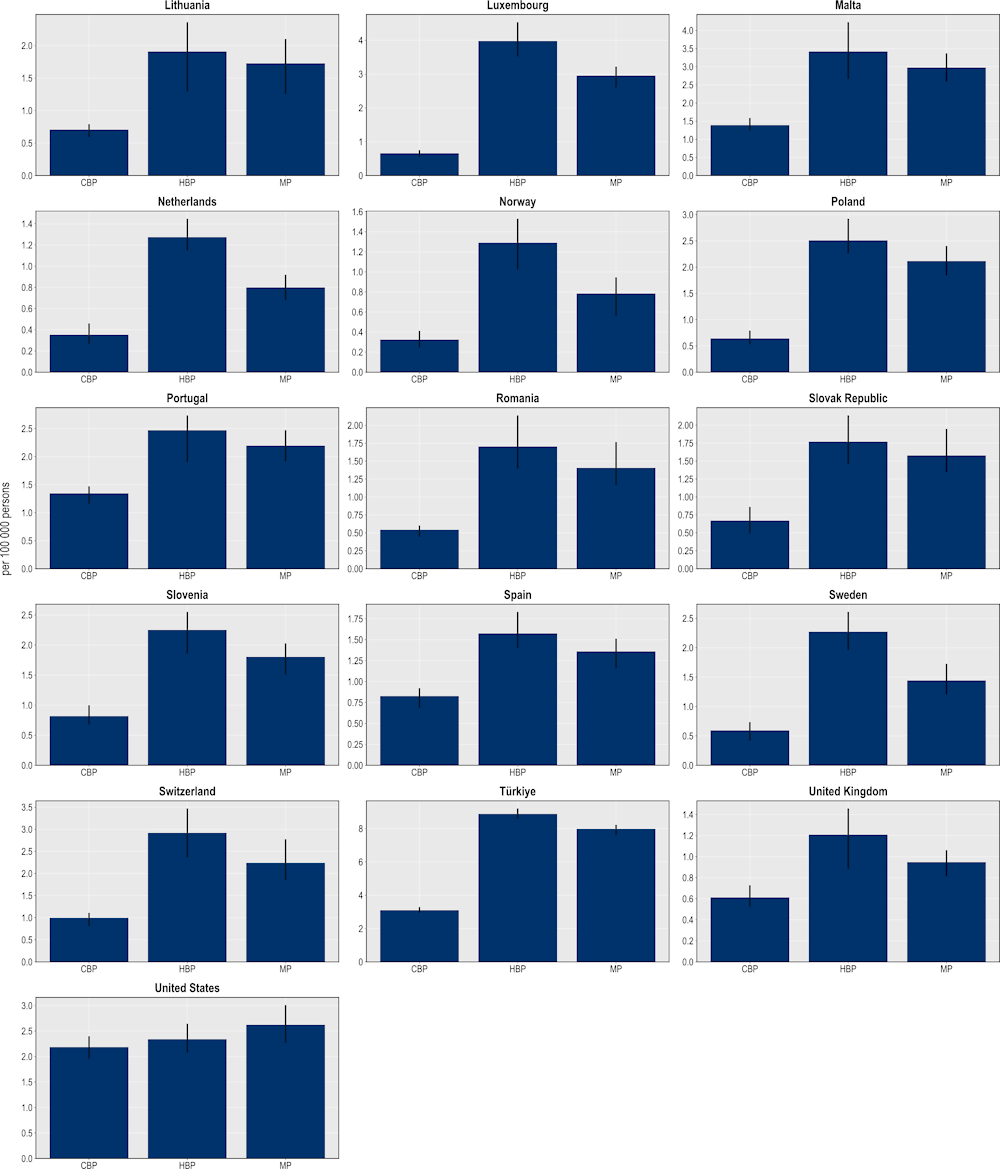
The mixed package provides the largest reductions in the number of resistant infections (Figure 6.8), followed by the hospital- and community-based packages. On average, the mixed package is estimated to reduce more than 1.6 million resistant infections annually across all 34 countries included in the analysis. The hospital-based package is also highly effective, with the number of resistant infections eliminated through this intervention averaging around 1.3 million every year. Finally, the estimated number of resistant infections that can be eliminated through the community-based package is more than 900 000 infections. Much like single interventions, there is substantial cross-country variation in the effectiveness of each policy package.
Figure 6.8. The mixed package yields the largest reductions in the number of resistant infections
Number of resistant infections averted per 100 000 persons annually up to 2050


Note: CBP: Community-based package; HBP: Hospital-based package; MP: Mixed package.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Upscaling the hospital-based package promises to prevent the largest number of deaths compared to the other two packages (Figure 6.9). On average, the hospital-based package is estimated to prevent more than 33 000 deaths each year compared to around 30 000 deaths by the community-based package and more than 17 000 deaths by the mixed package. In effect, scaling up the hospital package to the desirable levels is almost equivalent to preventing all deaths due to TB, influenza and HIV/AIDS in 2020 (or the nearest year for which data are available) across the 34 countries included in the analysis. Across the EU/EEA countries, Italy and Luxembourg are expected to avert the highest number of deaths by investing in a hospital-based package, preventing each year around 6.7 and 4 deaths per 100 000 persons respectively. Whereas Türkiye can avoid 8.9 deaths per 100 000 persons, the highest number of AMR‑related deaths averted across the non-EU/EEA member OECD countries.
Figure 6.9. The hospital-based package prevents the highest number of deaths due to resistant infections
Number of deaths due to AMR averted per 100 000 persons annually up to 2050
Note: CBP: Community-based package; HBP: Hospital-based package; MP: Mixed package.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The hospital-based package also produces the highest gains in terms of LYs and DALYs gained (Figure 6.10). On average, a hospital-based package can produce a gain of more than 511 000 LYs and 618 000 DALYs per year across the 34 countries included in the scope of the analysis. A mixed package also offers important health gains. On average, a mixed package is expected to save more than 466 000 LYs and 556 000 DALYs every year across all countries included in the analysis. A community-based package is expected to generate relatively smaller gains. On average, this package is predicted to produce nearly 263 000 LYs and more than 308 000 DALYs per year.
Figure 6.10. The hospital-based package promises the highest savings in LYs and DALYs
Number of LY and DALYs gained per 100 000 persons annually up to 2050
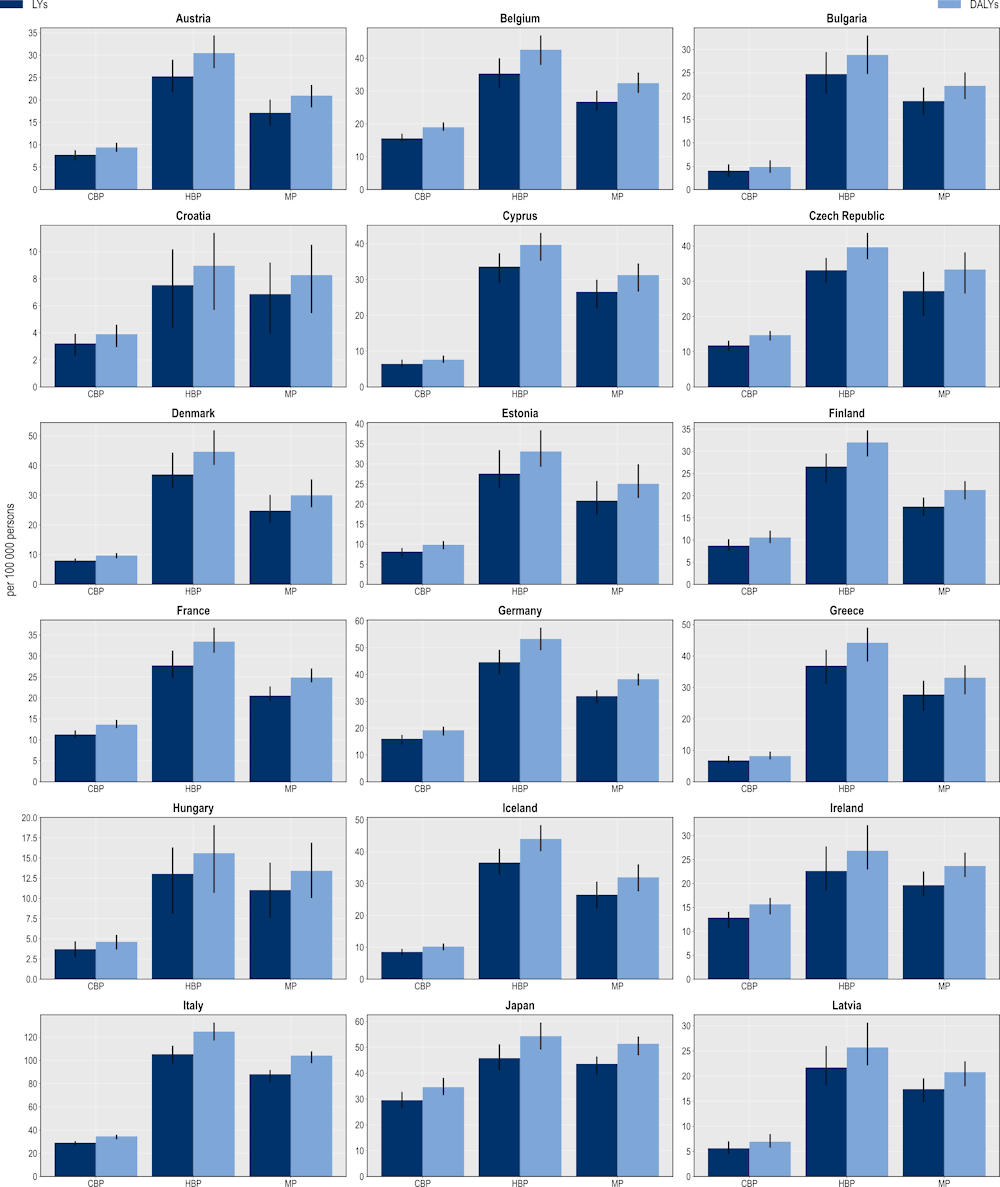
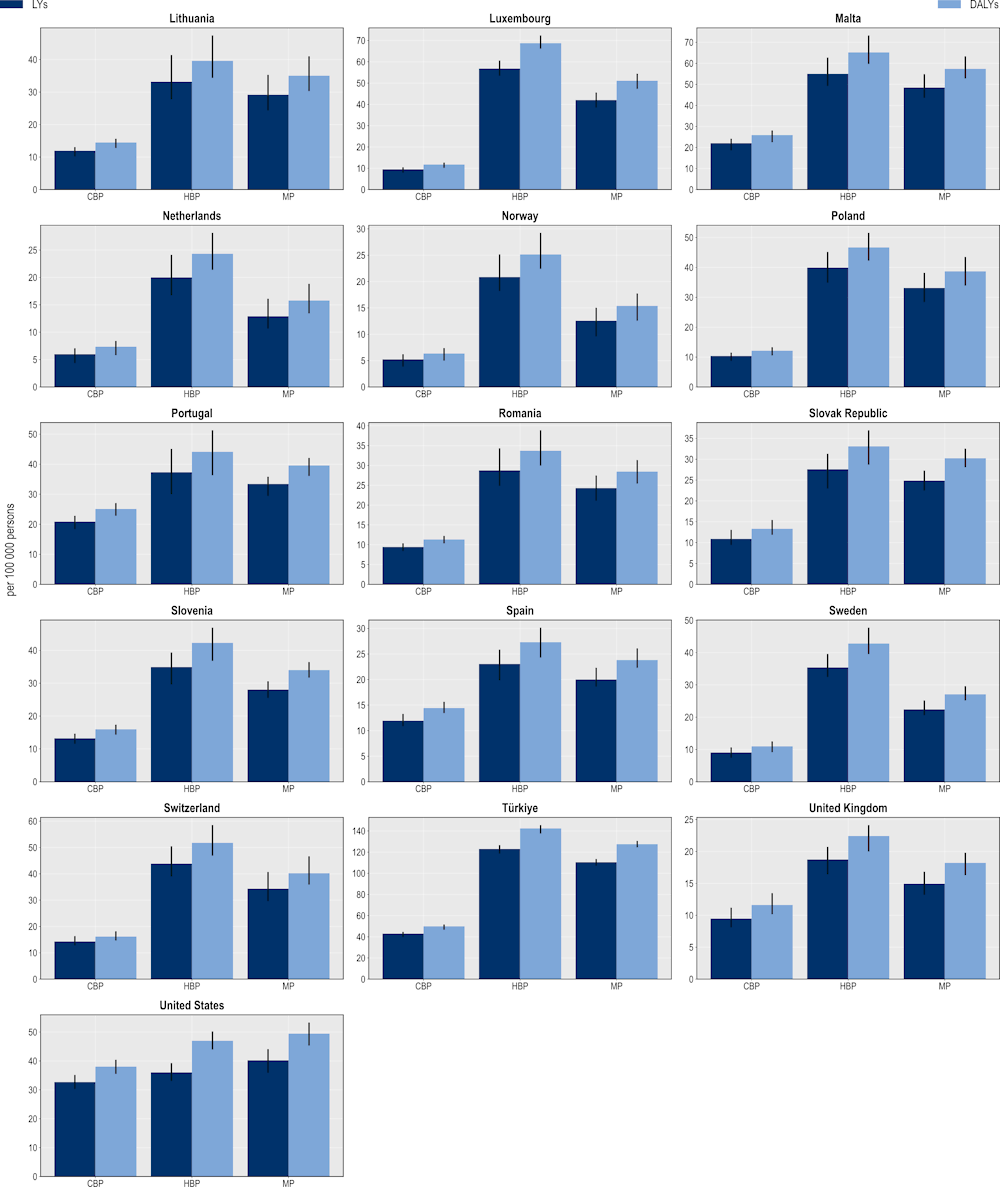
Note: CBP: Community-based package; DALYs: Disability-adjusted life‑years; HBP: Hospital-based package; LYs: Life years; MP: Mixed package.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The hospital-based package is expected to avoid more than 14 million extra days spent in hospital to treat complications due to resistant infections (Figure 6.11). This would be equivalent to freeing up the entire acute bed capacity in the Netherlands in 2020 for an entire year. In comparison, the mixed package can potentially prevent more than 12 million additional hospital days and the community-based package more than 6.7 million additional hospital days. The findings point to considerable cross-country variation. Across the EU/EEA countries included in the analysis, Italy is expected to make the most of gains from scaling up this intervention, with around 2 781 extra hospital days avoided per 100 000 persons, followed by Luxembourg and Germany (1 743 and 1 341 additional hospital days avoided per 100 000 persons respectively). Among the non-EU/EEA member OECD countries, Switzerland and Türkiye can prevent more than 2 383 and 1 918 additional days spent in hospital per 100 000 persons respectively.
Figure 6.11. More than 14 million days spent in hospital can be avoided through the hospital-based package
Number of additional days spent in hospital avoided per 100 000 persons annually up to 2050
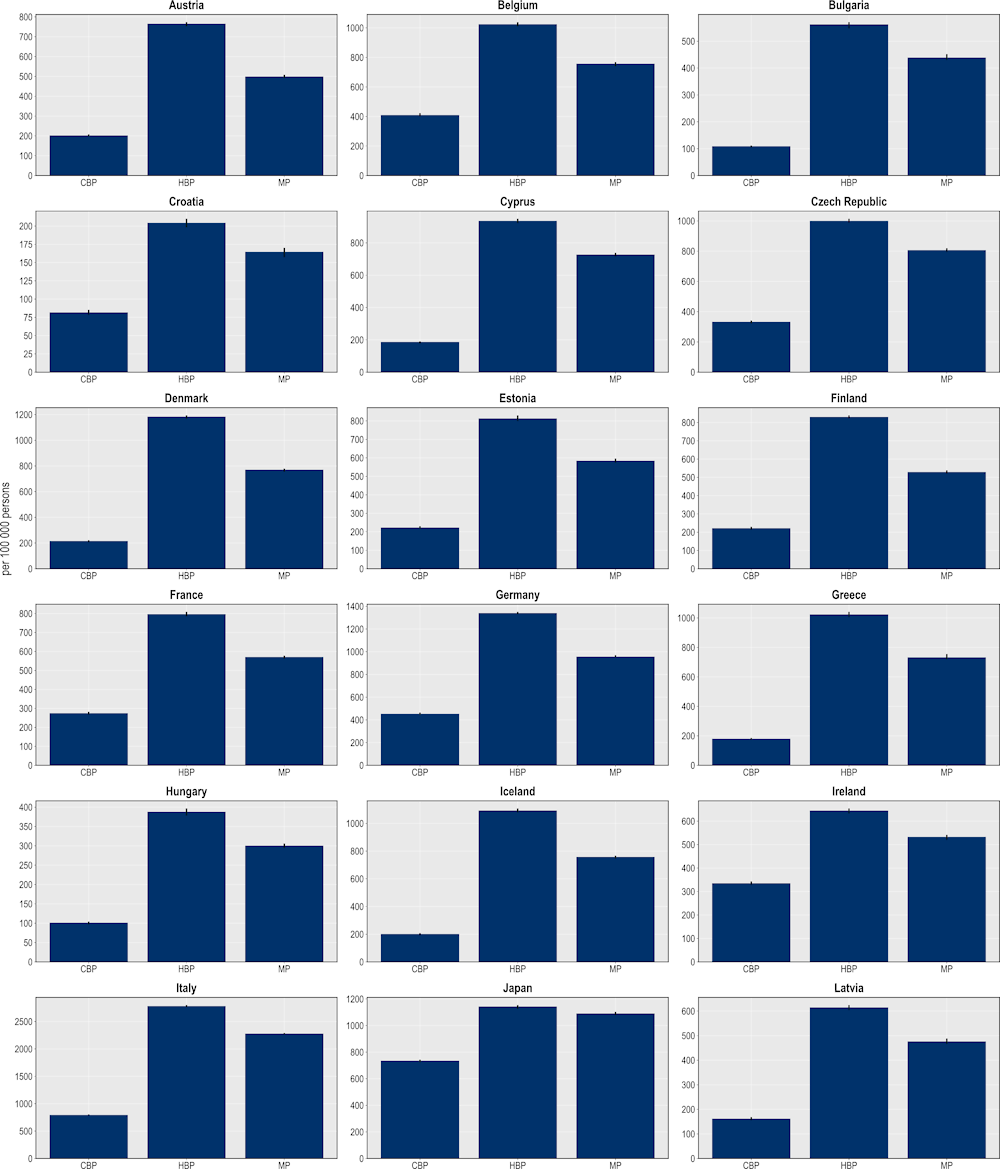
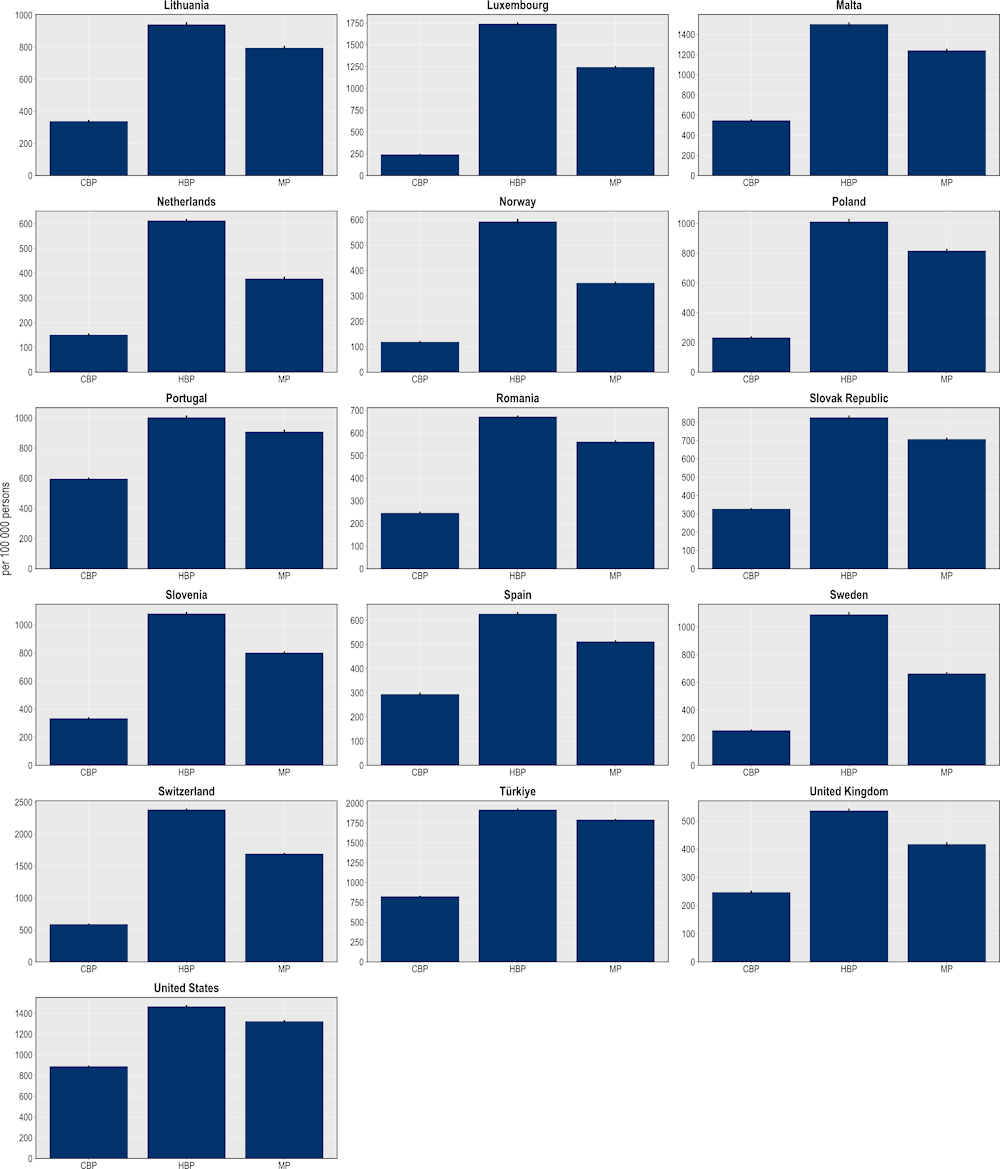
Note: CBP: Community-based package; HBP: Hospital-based package; MP: Mixed package.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The hospital-based package is also estimated to have the greatest impact on health expenditures attributable to treating complications due to resistant infections (Figure 6.12). On average, this package is predicted to save more than USD PPP 11 billion each year (corresponding to USD PPP 9.8 per capita), compared to USD PPP 9.4 billion by the mixed package (corresponding to USD PPP 8.3 per capita) and USD PPP 5.3 billion (corresponding to USD PPP 4.7 per capita) by the community-based package. Savings that can be achieved by scaling up the hospital-based package can be roughly equivalent to half of all health spending in the Czech Republic in 2020. The estimated reductions in attributable health expenditures vary across countries. Luxembourg and Italy are poised to achieve the greatest savings in health expenditure across EU/EEA countries (USD PPP 38.7 per capita and USD PPP 20 per capita) and Switzerland across the non-EU/EEA member OECD countries (USD PPP 28.5 per capita).
Across the three packages evaluated, the hospital-based package is predicted to yield the greatest productivity gains – a measure that combines improvements in participation in the workforce and workforce productivity. On average, this package can bring productivity gains exceeding USD PPP 14.9 billion (corresponding to USD PPP 13.2 per capita) annually across the 34 countries included in the analysis and nearly USD PPP 3.3 billion (corresponding to USD PPP 6.6 per capita) across the 29 EU/EEA countries. In comparison, the mixed package is expected to produce productivity gains to the tune of USD PPP 13.8 billion (corresponding to USD PPP 12 per capita) every year across all countries included in the analysis and around USD PPP 2.8 billion across EU/EEA countries (corresponding to USD PPP 5.6 per capita).
The estimated benefits that can be accrued by upscaling the policy packages exceed the cost of implementing these packages. For example, across the 34 countries included in the OECD analysis, the annual average cost of implementing the mixed package is around 5 times lower than the estimated benefits accrued through the reduction in health expenditure and productivity gains. Each year, the potential benefits that can be achieved through scaling up the hospital-based package are expected to be, on average, around 4.7 times the costs associated with the implementation of this package. In comparison, the potential benefits that can be reaped by implementing the community-based package are 2.5 times that of the cost of scaling up this package.
Figure 6.12. The hospital-based package can help reduce the pressure on healthcare budgets
Cost of interventions and their impact on savings in health expenditure and productivity gains
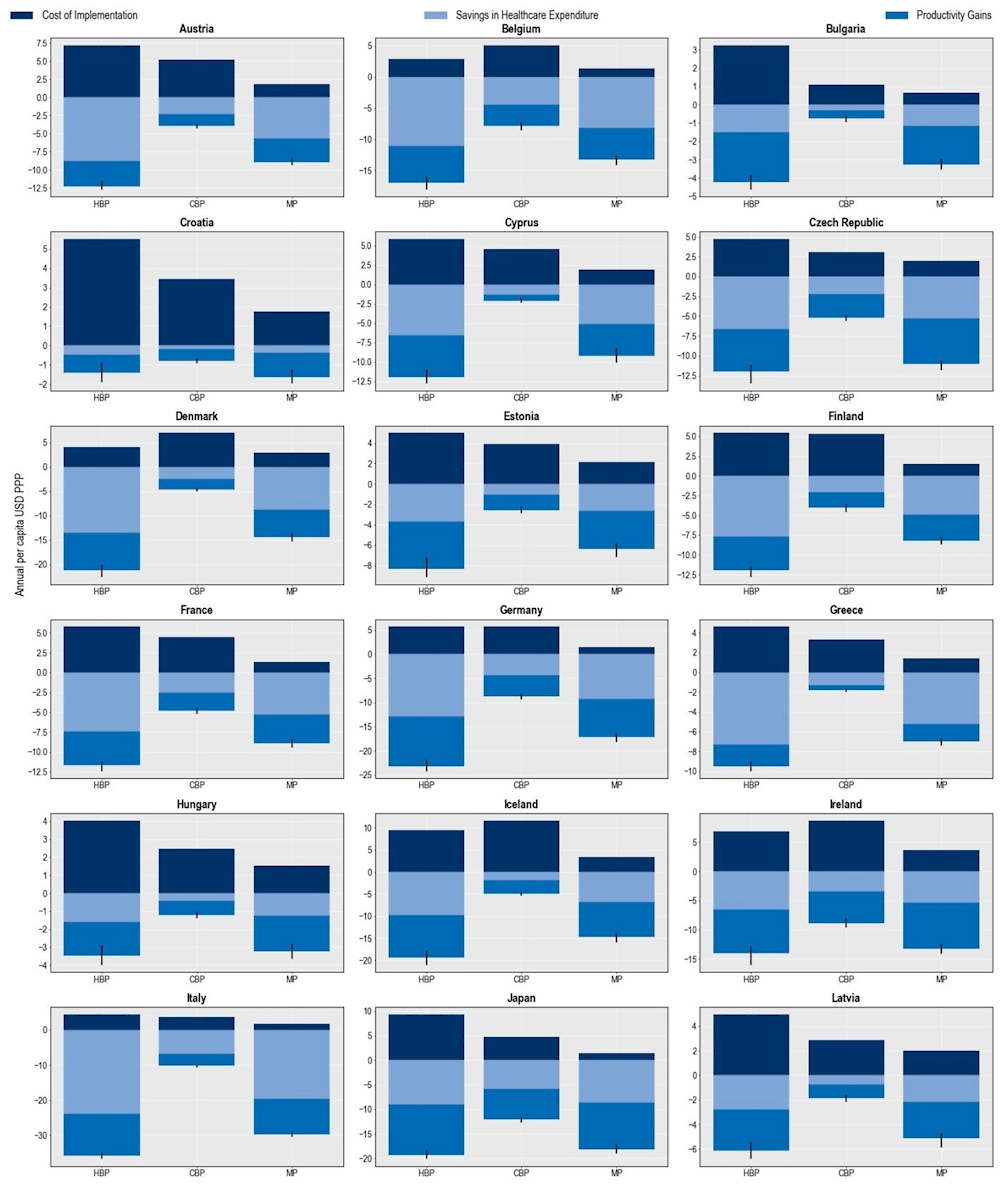
Note: CBP: Community-based package; HBP: Hospital-based package; MP: Mixed package.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Cost-effectiveness of policy packages to tackle AMR
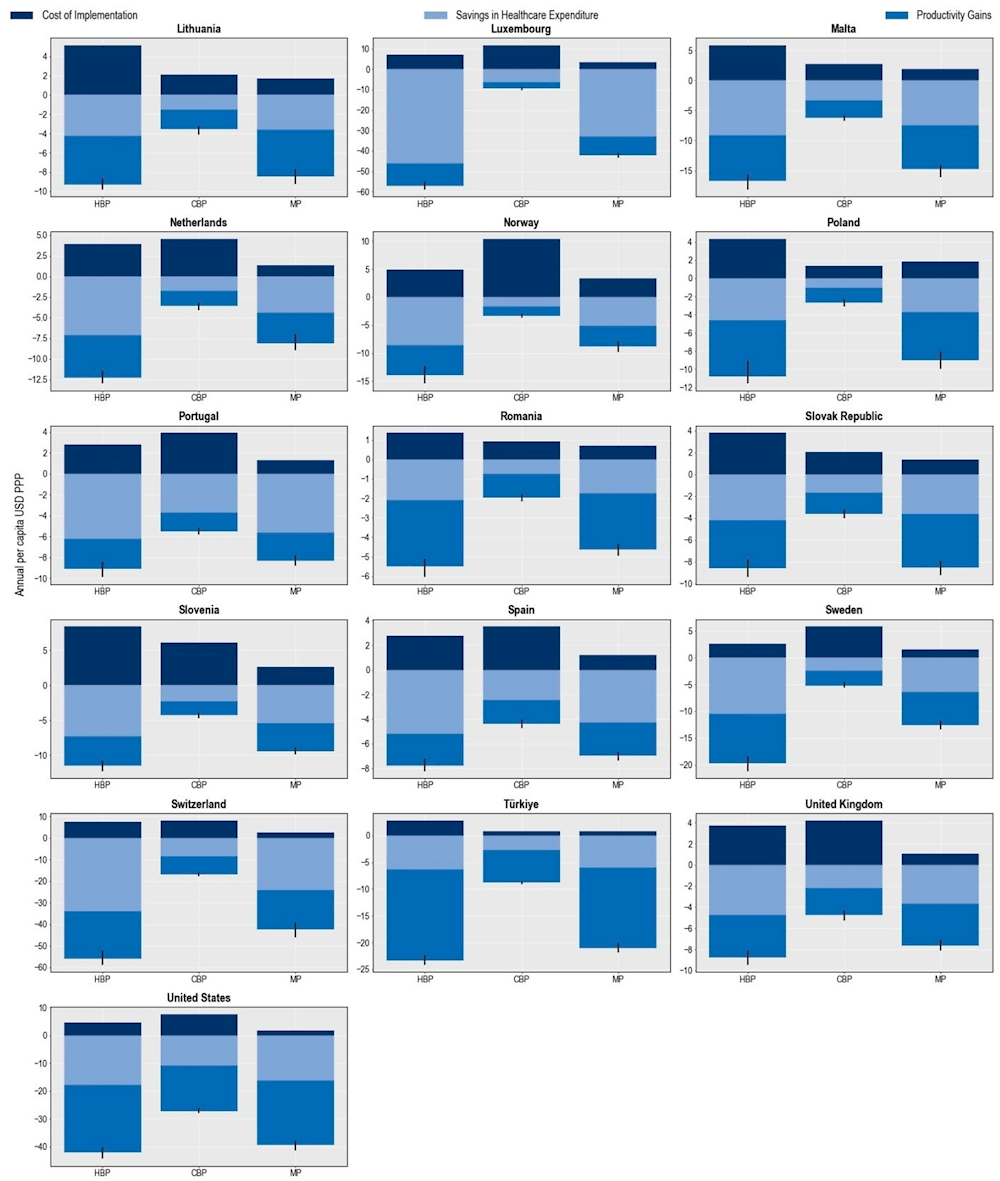
All three policy packages can be considered “best buys” given their favourable cost-effectiveness profiles (Figure 6.13). The mixed package is estimated to produce the most favourable cost-effectiveness profile. Across the 34 countries included in the analysis, the probability that the mixed package would be a cost‑saving option was estimated to be around 98%, suggesting that this package would be the most efficient approach with a very high probability that its implementation will save lives. The hospital-based package also offers high value for money, with the probability that this intervention would be cost-saving reaching 94%. The community-based package also offers a valuable strategy to tackle AMR. The probability that this intervention could be a cost-saving strategy is estimated to be around 52% and that it would be cost-effective is around 39%. The finding that the community-package has a relatively less favourable cost-effective profile compared to the other two policy packages is not surprising. The community-based package is comprised of interventions that are meant to tackle resistant infections occurring in community settings, which have been shown to have a lower risk of mortality compared to resistant infections acquired in healthcare settings.
Figure 6.13. Probability of cost-effectiveness of the modelled policy packages vs. business-as-usual scenario
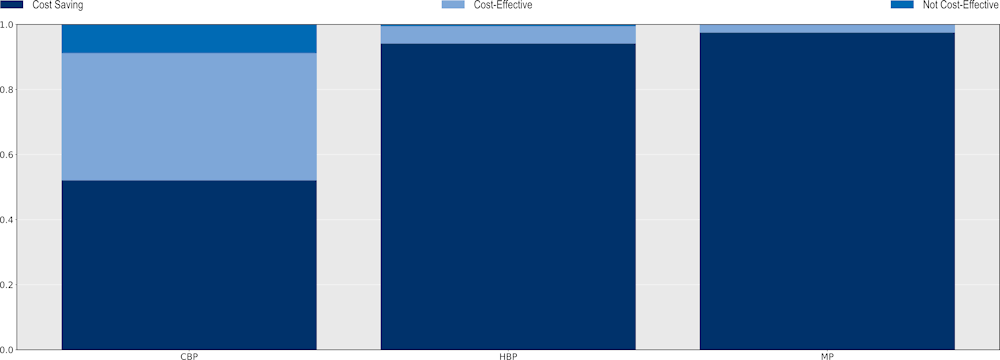
Note: CBP: Community-based package; HBP: Hospital-based package; MP: Mixed package.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The cost-effectiveness estimates presented in this chapter align with previous evidence
The findings presented in this chapter suggest that, compared to the 2018 OECD analysis, the OECD and EU/EEA countries included in the analysis stand to make relatively more conservative health and economic benefits by investing in policy intervention to tackle AMR. For example, the current chapter and the 2018 OECD publication concur that ASPs offer the greatest potential to reduce resistant infections, though the estimated number of deaths that can be avoided by scaling up ASPs is lower than the estimates provided in the 2018 OECD publication (OECD, 2018[14]).
These differences between the two iterations of the OECD analyses are driven primarily by two factors:
Increased level of business-as-usual coverage of the modelled interventions: An analysis of the 2016‑17 and 2019‑20 Tripartite AMR Self-Assessments suggests that the implementation of many of the interventions modelled in this chapter improved over time. Beyond a significant improvement in the development of the national action plans for AMR, many OECD countries and EU/EEA countries assess that they experienced improvements in the implementation of all the interventions. For example, regulatory frameworks for the implementation of ASPs improved substantially with the share of countries indicating that they had no/weak policies for appropriate antibiotic use declining from 21% (7/34) in 2016‑17 to 6% (2/34) in 2019‑20.
Lower predicted rates of resistance proportion (Figure 6.14): The new round of OECD projections suggest that AMR is likely to growth at a slower pace than predicted in the previous OECD analysis. The previous OECD analysis predicted that across the countries included in the analysis, resistance to antibiotics was expected to grow by 13% between 2020 and 2050, compared to a 9% growth rate in the most recent forecast. Beyond methodological differences between the two rounds of analysis, these findings are also suggestive of the recent advancements in the scale-up of AMR policies across considered countries.
Figure 6.14. The new OECD analysis suggests that AMR will grow at a slower pace than projected in the previous OECD work study
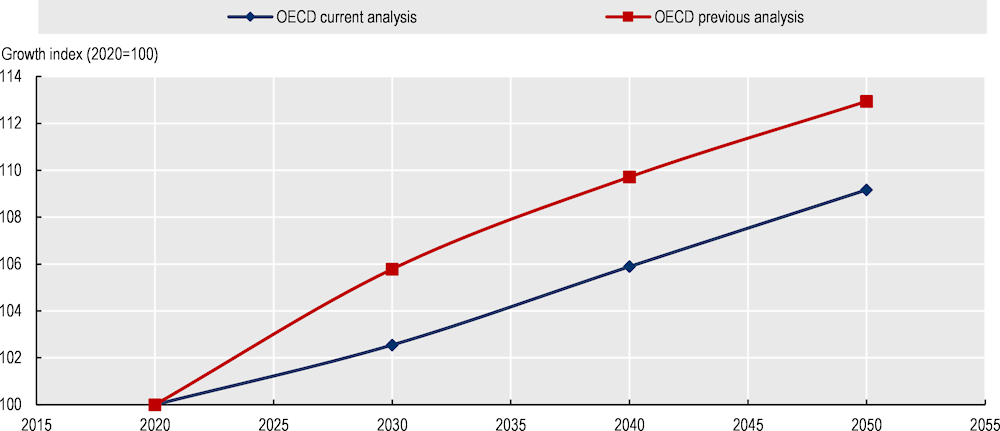
Note: Data on AMR growth rates were normalised to average AMR in 2020 (equal to 100). For example, a value of 113 for resistance in 2050 means that resistance is 13% higher than it was in 2020 in the countries included in the OECD analysis.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The OECD analysis on the cost-effectiveness of the policy interventions to tackle AMR is in line with evidence generated at the country level. For example, the finding that ASPs are among the most cost-effective options to tackle AMR aligns with evidence generated in EU/EEA countries and OECD countries:
In the Netherlands, one multi-modal ASP that was implemented in the Canisius Wilhelmina Hospital in Nijmegen involved building multi-professional stewardship teams, strengthening the monitoring of antibiotic use and developing feedback mechanisms. This intervention resulted in hospital-wide savings to the tune of EUR 40 000 over the first year of the implementation while reducing antimicrobial consumption by 10% (Oberjé, Tanke and Jeurissen, 2017[15]).
In the United States, one ASP introduced procalcitonin-guided decision algorithms to improve antibiotic use for hospitalised patients with sepsis and lower respiratory tract infections. This intervention resulted in average per capita cost savings of USD 25 611 for sepsis and USD 3 630 for lower respiratory tract infections while reducing the length of hospital stays, reliance on antibiotics and reducing days spent in mechanical ventilation (Voermans et al., 2019[16]).
In Canada, an ASP aimed to improve prudent antibiotic use in four intensive care units by providing in-person coaching by pharmacists and physicians combined with performance reports (Morris et al., 2019[17]). An evaluation of this ASP concluded that it was associated with reductions in antibacterial use to the tune of about 12.12 defined daily doses per 100 patient days while the monthly costs associated with antibiotic use declined by CAD 642.
The findings reported in this chapter are broadly aligned with results generated by previous multi-country studies. For example, the two IPC interventions modelled in this chapter – enhancing environmental hygiene and improving hand hygiene – are shown to be amongst the most effective means of reducing the burden of AMR on population health. This finding broadly aligns with the key messages of the first global IPC report published by the WHO (2022[9]). Building on global evidence as well as results from previous OECD analyses, this report highlighted that investments in hand hygiene and environmental hygiene are particularly valuable means to limit the impact of resistant infections. This report highlighted global evidence that showed that these interventions are cost-effective options for tackling infections acquired in healthcare settings in general and resistant infections in particular.
The OECD estimates on the potential benefits of vaccines in the fight against AMR are also in line with previous works. A growing body of evidence demonstrates that increasing vaccination coverage can help tackle AMR through multiple pathways (Vekemans et al., 2021[18]; Jit, Anderson and Cooper, 2020[19]) (e.g. lowering the rate with which populations are infected, lowering infection severity, etc.). Congruent with previous works, the OECD analysis showed that scaling up the coverage of PVV23 across the 34 countries included in the analysis could help reduce the incidence of resistant infections and deaths caused by these infections.
The OECD estimates presented in this chapter should be considered conservative estimates for many of the modelled interventions given that the positive implications of scaling up the assessed interventions may be greater than the impact accounted for in the model. For example:
Previous studies demonstrated that IPC interventions in human health can indirectly contribute to efforts to optimise the use of antibiotics by preventing infections from occurring in the first place (Okubo et al., 2023[20]).
Similar to the IPC interventions discussed above, the OECD model did not capture all potential pathways through which PVV23 can safeguard population health. For example, OECD estimates do not consider the potential reductions in the likelihood of disease transmission across people in the community attributable to vaccines, a concept referred to as heard immunity. However, vaccines have been shown to offer considerable health benefits through herd effects, even shortly after their rollout (Shiri et al., 2017[21]).
Limited by the evidence gap on the complex pathways through which AMR is transmitted between and across humans, animals and the environment, the OECD analysis does not consider many of the potential pathways through which the One Health interventions can reduce the number of resistant infections. For example, the OECD analysis assumes that improving farm biosecurity can disrupt AMR transmission between people and animal populations. But the analysis does not consider that increased use of PPE can influence the spread of AMR through other channels (e.g. through reduced exposure to antibiotic residues during manure applications and in the soil (He et al., 2020[22]), reduced antibiotic use in human and animal populations by preventing infection from occurring in the first place, etc.).
Similarly, the analyses of interventions outside of the human health sector do not consider all of the potential benefits of these interventions. For example, while modelling the effectiveness and cost-effectiveness of the intervention to improve farm biosecurity, the OECD analysis considers only the beneficial impacts mediated through declines in AMR in the human population. However, as discussed in more detail in Chapter 5, earlier works suggest that biosecurity measures could potentially help improve farm productivity and livestock production (Renault et al., 2019[23]; Postma et al., 2016[24]), generating greater economic benefits than quantified in the current analysis.
Conclusions
This chapter provides an assessment of the return on investment in 11 AMR policies spanning human health, animal health and food safety sectors. Findings from the chapter highlight that the modelled interventions could yield a substantial protective impact on population health and reduce the economic burden of AMR, with the magnitude of the impact varying across interventions. Of the 11 interventions modelled, upscaling stewardship programmes that promote prudent use of antibiotics were estimated to yield the greatest health and economic benefits. Beyond the human health sector, enhancing food safety measures and improving biosecurity in farm settings can be effective means to limit the impact of AMR though the findings also suggest that these two policies will not be sufficient on their own to eliminate AMR. Importantly, the chapter showed that the effectiveness of AMR policies is more pronounced when they are rolled out as part of a package of interventions, compared to a situation where they are rolled out individually.
References
[33] Aabenhus, R. et al. (2014), “Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care”, Cochrane Database of Systematic Reviews, Wiley, https://doi.org/10.1002/14651858.cd010130.pub2.
[11] Bahrs, C. et al. (2021), “A longitudinal analysis of pneumococcal vaccine serotypes in pneumonia patients in Germany”, European Respiratory Journal, Vol. 59/2, p. 2102432, https://doi.org/10.1183/13993003.02432-2021.
[54] Banach, D. (ed.) (2019), “Government policy interventions to reduce human antimicrobial use: A systematic review and evidence map”, PLOS Medicine, Vol. 16/6, p. e1002819, https://doi.org/10.1371/journal.pmed.1002819.
[37] Bou-Antoun, S. et al. (2018), “Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the Quality Premium) for health commissioners to reduce use of antibiotics in the community: An interrupted time series analysis”, Journal of Antimicrobial Chemotherapy, Vol. 73/10, pp. 2883-2892, https://doi.org/10.1093/jac/dky237.
[30] Carpenter, C. (ed.) (2020), “Accuracy of biomarkers for the diagnosis of adult community‐acquired pneumonia: A meta‐analysis”, Academic Emergency Medicine, Vol. 27/3, pp. 195-206, https://doi.org/10.1111/acem.13889.
[48] CDC (2019), Drug Resistant Streptococcus Pneumoniae, Centers for Disease Control and Prevention, https://www.cdc.gov/drugresistance/pdf/threats-report/strep-pneumoniae-508.pdf.
[42] Chou, R. et al. (2022), “Update Alert 10: Epidemiology of and risk factors for coronavirus infection in health care workers”, Annals of Internal Medicine, Vol. 175/1, pp. W8-W9, https://doi.org/10.7326/m21-4294.
[53] Coxeter, P. et al. (2015), “Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care”, Cochrane Database of Systematic Reviews, Vol. 2017/2, https://doi.org/10.1002/14651858.cd010907.pub2.
[25] Davey, P. et al. (2017), “Interventions to improve antibiotic prescribing practices for hospital inpatients”, Cochrane Database of Systematic Reviews, Vol. 2017/2, https://doi.org/10.1002/14651858.cd003543.pub4.
[3] de Kraker, M. et al. (2022), “Implementation of hand hygiene in health-care facilities: Results from the WHO Hand Hygiene Self-Assessment Framework global survey 2019”, The Lancet Infectious Diseases, Vol. 22/6, pp. 835-844, https://doi.org/10.1016/s1473-3099(21)00618-6.
[45] Donskey, C. (2013), “Does improving surface cleaning and disinfection reduce health care-associated infections?”, American Journal of Infection Control, Vol. 41/5, pp. S12-S19, https://doi.org/10.1016/j.ajic.2012.12.010.
[52] ECDC (2022), Vaccine Scheduler: Pneumococcal Disease: Recommended Vaccinations, European Centre for Disease Prevention and Control, https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1 (accessed on 24 June 2022).
[46] ECDC/WHO (2022), Antimicrobial Resistance Surveillance in Europe, European Centre for Disease Prevention and Control and World Health Organization, https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-WHO-AMR-report.pdf.
[36] Ellegård, L., J. Dietrichson and A. Anell (2017), “Can pay-for-performance to primary care providers stimulate appropriate use of antibiotics?”, Health Economics, Vol. 27/1, pp. e39-e54, https://doi.org/10.1002/hec.3535.
[28] Falk, G. and T. Fahey (2008), “C-reactive protein and community-acquired pneumonia in ambulatory care: Systematic review of diagnostic accuracy studies”, Family Practice, Vol. 26/1, pp. 10-21, https://doi.org/10.1093/fampra/cmn095.
[63] FDA (1997), HACCP Principles & Application Guidelines, United States Food and Drug Administration, https://www.fda.gov/food/hazard-analysis-critical-control-point-haccp/haccp-principles-application-guidelines (accessed on 2022 April 18).
[8] FiRe Network (2004), “Effect of macrolide consumption on erythromycin resistance in Streptococcus pyogenes in Finland in 1997-2001”, Clinical Infectious Diseases, Vol. 38/9, pp. 1251-1256, https://doi.org/10.1086/383309.
[55] Formoso, G. et al. (2013), “Feasibility and effectiveness of a low cost campaign on antibiotic prescribing in Italy: Community level, controlled, non-randomised trial”, BMJ, Vol. 347, p. f5391, https://doi.org/10.1136/bmj.f5391.
[66] Gillison, F. et al. (2018), “A meta-analysis of techniques to promote motivation for health behaviour change from a self-determination theory perspective”, Health Psychology Review, Vol. 13/1, pp. 110-130, https://doi.org/10.1080/17437199.2018.1534071.
[44] Gould, D. et al. (2017), “Interventions to improve hand hygiene compliance in patient care”, Cochrane Database of Systematic Reviews, Vol. 2017/9, https://doi.org/10.1002/14651858.cd005186.pub4.
[22] He, Y. et al. (2020), “Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment”, npj Clean Water, Vol. 3/1, https://doi.org/10.1038/s41545-020-0051-0.
[51] Ho, P. (ed.) (2017), “Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: Systematic review and meta-analysis”, PLOS ONE, Vol. 12/1, p. e0169368, https://doi.org/10.1371/journal.pone.0169368.
[32] Huang, Y. et al. (2013), “Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: A systematic review and meta-analysis of primary care studies”, British Journal of General Practice, Vol. 63/616, pp. e787-e794, https://doi.org/10.3399/bjgp13x674477.
[60] ILO (2011), Code of Practice on Safety and Health in Agriculture, International Labour Organization, Geneva, http://www.ilo.org/wcmsp5/groups/public/@dgreports/@dcomm/@publ/documents/publication/wcms_159457.pdf.
[40] Jefferson, T. et al. (2020), “Physical interventions to interrupt or reduce the spread of respiratory viruses”, Cochrane Database of Systematic Reviews, Wiley, https://doi.org/10.1002/14651858.cd006207.pub5.
[41] Jefferson, T. et al. (2011), “Physical interventions to interrupt or reduce the spread of respiratory viruses”, Cochrane Database of Systematic Reviews, Wiley, https://doi.org/10.1002/14651858.cd006207.pub4.
[19] Jit, M., M. Anderson and B. Cooper (2020), “Quantifying the benefits of vaccines in combating antimicrobial resistance”, Eurohealth, Vol. 26/1, pp. 16 - 19, https://apps.who.int/iris/handle/10665/332480.
[7] Kaier, K., U. Frank and E. Meyer (2011), “Economic incentives for the (over-)prescription of broad-spectrum antimicrobials in German ambulatory care”, Journal of Antimicrobial Chemotherapy, Vol. 66/7, pp. 1656-1658, https://doi.org/10.1093/jac/dkr134.
[56] Lambert, M., G. Masters and S. Brent (2007), “Can mass media campaigns change antimicrobial prescribing? A regional evaluation study”, Journal of Antimicrobial Chemotherapy, Vol. 59/3, pp. 537-543, https://doi.org/10.1093/jac/dkl511.
[6] Lee, C. et al. (2013), “Strategies to minimize antibiotic resistance”, International Journal of Environmental Research and Public Health, Vol. 10/9, pp. 4274-4305, https://doi.org/10.3390/ijerph10094274.
[67] Levy, N., T. Cravo Oliveira Hashiguchi and M. Cecchini (2022), “Food safety policies and their effectiveness to prevent foodborne diseases in catering establishments: A systematic review and meta-analysis”, Food Research International, Vol. 156, p. 111076, https://doi.org/10.1016/j.foodres.2022.111076.
[43] Luangasanatip, N. et al. (2015), “Comparative efficacy of interventions to promote hand hygiene in hospital: Systematic review and network meta-analysis”, BMJ, p. h3728, https://doi.org/10.1136/bmj.h3728.
[31] Martínez-González, N. et al. (2020), “Point-of-care C-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: Systematic review and meta-analysis of randomised controlled trials”, Antibiotics, Vol. 9/9, p. 610, https://doi.org/10.3390/antibiotics9090610.
[17] Morris, A. et al. (2019), “Long-term effects of phased implementation of antimicrobial stewardship in academic ICUs”, Critical Care Medicine, Vol. 47/2, pp. 159-166, https://doi.org/10.1097/ccm.0000000000003514.
[38] Mullen, K., R. Frank and M. Rosenthal (2010), “Can you get what you pay for? Pay-for-performance and the quality of healthcare providers”, The RAND Journal of Economics, Vol. 41/1, pp. 64-91, https://doi.org/10.1111/j.1756-2171.2009.00090.x.
[49] Naito, T. et al. (2020), “The estimated impact of the 5-year national vaccination program on the trend of 23-valent pneumococcal polysaccharide vaccine vaccination rates in the elderly in Japan, 2009-2018”, Journal of Infection and Chemotherapy, Vol. 26/4, pp. 407-410, https://doi.org/10.1016/j.jiac.2019.12.011.
[13] Nelson, R. et al. (2021), “Mortality, length of stay, and healthcare costs associated with multidrug-resistant bacterial infections among elderly hospitalized patients in the United States”, Clinical Infectious Diseases, Vol. 74/6, pp. 1070-1080, https://doi.org/10.1093/cid/ciab696.
[15] Oberjé, E., M. Tanke and P. Jeurissen (2017), “Antimicrobial stewardship initiatives throughout Europe: Proven value for money”, Infectious Disease Reports, Vol. 9/1, p. 6800, https://doi.org/10.4081/idr.2017.6800.
[14] OECD (2018), Stemming the Superbug Tide: Just A Few Dollars More, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/9789264307599-en.
[20] Okubo, Y. et al. (2023), “Financial incentives for infection prevention and antimicrobial stewardship to reduce antibiotic use: Japan’s nationwide observational study”, Journal of Hospital Infection, Vol. 131, pp. 89-98, https://doi.org/10.1016/j.jhin.2022.09.027.
[50] Papagiannis, D. et al. (2020), “Vaccination coverage of the elderly in Greece: A cross-sectional nationwide study”, Canadian Journal of Infectious Diseases and Medical Microbiology, Vol. 2020, pp. 1-5, https://doi.org/10.1155/2020/5459793.
[47] Pavia, M. et al. (2009), “Efficacy of pneumococcal vaccination in children younger than 24 months: A meta-analysis”, Pediatrics, Vol. 123/6, pp. e1103-e1110, https://doi.org/10.1542/peds.2008-3422.
[57] Perz, J. (2002), “Changes in antibiotic prescribing for children after a community-wide campaign”, JAMA, Vol. 287/23, p. 3103, https://doi.org/10.1001/jama.287.23.3103.
[24] Postma, M. et al. (2016), “Reducing antimicrobial usage in pig production without jeopardizing production parameters”, Zoonoses and Public Health, Vol. 64/1, pp. 63-74, https://doi.org/10.1111/zph.12283.
[23] Renault, V. et al. (2019), “Pilot study assessing the possible benefits of a higher level of implementation of biosecurity measures on farm productivity and health status in Belgian cattle farms”, Transboundary and Emerging Diseases, Vol. 67/2, pp. 769-777, https://doi.org/10.1111/tbed.13396.
[29] Sanders, S. et al. (2008), “Systematic review of the diagnostic accuracy of C-reactive protein to detect bacterial infection in nonhospitalized infants and children with fever”, The Journal of Pediatrics, Vol. 153/4, pp. 570-574.e3, https://doi.org/10.1016/j.jpeds.2008.04.023.
[62] Schimmer, B. et al. (2014), “Coxiella burnetii seroprevalence and risk for humans on dairy cattle farms, the Netherlands, 2010-2011”, Emerging Infectious Diseases, Vol. 20/3, https://doi.org/10.3201/eid2003.131111.
[21] Shiri, T. et al. (2017), “Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: A systematic review and meta-analysis”, The Lancet Global Health, Vol. 5/1, pp. e51-e59, https://doi.org/10.1016/s2214-109x(16)30306-0.
[27] Spurling, G. et al. (2017), “Delayed antibiotic prescriptions for respiratory infections”, Cochrane Database of Systematic Reviews, Wiley, https://doi.org/10.1002/14651858.cd004417.pub5.
[26] Stuart, B. et al. (2021), “Delayed antibiotic prescribing for respiratory tract infections: Individual patient data meta-analysis”, BMJ, p. n808, https://doi.org/10.1136/bmj.n808.
[4] Tomczyk, S. et al. (2022), “The first WHO global survey on infection prevention and control in health-care facilities”, The Lancet Infectious Diseases, Vol. 22/6, pp. 845-856, https://doi.org/10.1016/s1473-3099(21)00809-4.
[18] Vekemans, J. et al. (2021), “Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: A World Health Organization action framework”, Clinical Infectious Diseases, Vol. 73/4, pp. e1011-e1017, https://doi.org/10.1093/cid/ciab062.
[34] Verbakel, J. et al. (2019), “Impact of point-of-care C-reactive protein in ambulatory care: A systematic review and meta-analysis”, BMJ Open, Vol. 9/1, p. e025036, https://doi.org/10.1136/bmjopen-2018-025036.
[16] Voermans, A. et al. (2019), “Cost-effectiveness analysis of a procalcitonin-guided decision algorithm for antibiotic stewardship using real-world U.S. hospital data”, OMICS: A Journal of Integrative Biology, Vol. 23/10, pp. 508-515, https://doi.org/10.1089/omi.2019.0113.
[12] Wahl, B. et al. (2018), “Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000-15”, The Lancet Global Health, Vol. 6/7, pp. e744-e757, https://doi.org/10.1016/s2214-109x(18)30247-x.
[9] WHO (2022), Global Report on Infection Prevention and Control, World Health Organization, https://apps.who.int/iris/handle/10665/354489.
[10] WHO (2016), Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level, World Health Organization, https://apps.who.int/iris/handle/10665/251730.
[1] WHO (2015), Global Action Plan on Antimicrobial Resistance, World Health Organization, https://apps.who.int/iris/handle/10665/193736.
[5] WHO (2003), Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis, World Health Organization, https://apps.who.int/iris/handle/10665/42699.
[2] WHO/FAO/OIE (2021), Tripartite AMR Country Self-Assessment Survey (TrACSS) 2020-2021, World Health Organization, Food and Agriculture Organization of the United Nations & World Organisation for Animal Health, https://www.who.int/publications/m/item/tripartite-amr-country-self-assessment-survey-(tracss)-2020-2021.
[39] Yip, W. et al. (2014), “Capitation combined with pay-for-performance improves antibiotic prescribing practices in rural China”, Health Affairs, Vol. 33/3, pp. 502-510, https://doi.org/10.1377/hlthaff.2013.0702.
[35] Yoshikawa, Y. et al. (2021), “Financial strategies targeting healthcare providers to promote the prudent use of antibiotics: A systematic review of the evidence”, International Journal of Antimicrobial Agents, Vol. 58/6, p. 106446, https://doi.org/10.1016/j.ijantimicag.2021.106446.
[64] Young, I. et al. (2019), “Effectiveness of food handler training and education interventions: A systematic review and meta-analysis”, Journal of Food Protection, Vol. 82/10, pp. 1714-1728, https://doi.org/10.4315/0362-028x.jfp-19-108.
[65] Young, I. et al. (2020), “A systematic review and meta-regression of single group, pre-post studies evaluating food safety education and training interventions for food handlers”, Food Research International, Vol. 128, p. 108711, https://doi.org/10.1016/j.foodres.2019.108711.
[59] Youssef, D. et al. (2021), “The effectiveness of biosecurity interventions in reducing the transmission of bacteria from livestock to humans at the farm level: A systematic literature review”, Zoonoses and Public Health, Vol. 68/6, pp. 549-562, https://doi.org/10.1111/zph.12807.
[61] Zamboni, D. (ed.) (2012), “Seroprevalence and risk factors for Coxiella burnetii (Q Fever) seropositivity in dairy goat farmers’ households in the Netherlands, 2009-2010”, PLoS ONE, Vol. 7/7, p. e42364, https://doi.org/10.1371/journal.pone.0042364.
[58] Zucco, R. et al. (2018), “Internet and social media use for antibiotic-related information seeking: Findings from a survey among adult population in Italy”, International Journal of Medical Informatics, Vol. 111, pp. 131-139, https://doi.org/10.1016/j.ijmedinf.2017.12.005.
Annex 6.A. Modelling policy interventions to tackle AMR
This annex provides detailed descriptions of the design features of the 11 interventions modelled in the scope of the OECD analysis.
Policies to optimise the use of antibiotics in human health
Strengthen antimicrobial stewardship programmes (ASPs)
Modelled intervention
The modelled intervention entails scaling up a hospital-based programme that involves the creation of multi-disciplinary teams that provide antibiotic stewardship and the scale‑up of monitoring and surveillance systems.
The beneficial effects are assumed to become observable immediately and sustained over the analysis period.
The modelled intervention is a hospital-based intervention with two main components. The first involves the creation of multi-disciplinary ASP teams comprised of infectious disease specialists, pharmacists and microbiologists who are tasked with providing antibiotic guidance in each health facility. The ASP teams would develop and disseminate antibiotic prescribing guidelines in line with national and international guidelines and deliver training sessions once a year on best practices in antibiotic prescribing behaviours. The second component entails the development of a monitoring and surveillance system which tracks antibiotic use and AMR burden in healthcare facilities. Results generated through this system would be used subsequently to assess whether course corrections are needed in the implementation of the intervention (e.g. revising treatment guidelines) and to provide feedback to prescribers in health facilities about their prescribing behaviours.
The individual-level effectiveness estimates were extracted from the Cochrane review conducted by Davey et al. (2017[25]). Based on evidence gathered through randomised controlled trials (RCTs), ASPs were associated with reductions in the risk of antibiotic consumption (risk difference = ‑0.25 [‑0.37, ‑0.13]). Importantly, this review found that the majority of evidence suggested that the beneficial effects of ASPs were sustained 12 months after the initial rollout of the intervention.
The business-as-usual coverage was determined separately for each country using the 2020‑21 Tripartite AMR Self-Assessment Survey, based on a survey question that examines the stage of implementation of ASPs to optimise antibiotic use in human health. This question characterises ASPs by identifying different stages of implementation ranging from having no guidelines on optimised antibiotic use to having in place antibiotic guidelines and data for all major syndromes (see Question 1 in Annex 6.B). The target coverage is set at 80%, such that the intervention is assumed to be rolled out in 80% of all hospitals in each country. The rollout of the intervention is assumed to affect only infections acquired in healthcare settings. The beneficial effects are assumed to become measurable immediately and remain at the same level over the simulation period.
The per capita cost of implementing such a programme was estimated to range from USD PPP 0.53 to USD PPP 6 per capita, reflecting the specific characteristics of the country. The most expensive cost component was those associated with building multi-disciplinary stewardship teams, which includes both salaries and training expenses. The remaining costs covered administrative costs at the national and local levels and monitoring and evaluation arrangements at the local level. Implementation costs varied quite substantially across countries, driven primarily by the cross-country differences in wages as well as the differences in the number of healthcare facilities across countries.
Delayed antibiotic prescribing

Modelled intervention
The model intervention is the rollout of antimicrobial prescribing guidelines that promote delayed prescription in primary healthcare settings.
The modelled intervention is assumed to result in a 60% reduction in antibiotic use.
The effectiveness is assumed to be sustained over course of the simulation.
Delayed antibiotic prescribing is another means to reduce unnecessary use of antibiotics. A delayed prescription is similar to a typical prescription except that the patient is instructed to redeem the prescription only if symptoms remain unresolved in a time period determined by their prescriber. Since the last OECD publication, the evidence base on the effectiveness of delayed prescribing grew. In 2021, Stuart and colleagues showed that, compared to immediate antibiotic prescription, delayed prescriptions were associated with reductions in complications attributable to hospital admissions and deaths (Stuart et al., 2021[26]), while there were no detectable differences in follow-up symptom severity. This study further pointed out that delayed prescribing was correlated with significant reductions in re‑consultation rates and increases in patient satisfaction in comparison to no antibiotic treatment.
The modelled intervention is conceptualised as a community-based intervention with multiple components. The first component aims to ensure that prescribers have access to clinical guidelines, which can help in their decisions related to whether they should recommend delayed use of antibiotics. The second component involves a training and education programme for prescribers in order to improve their awareness and understanding of best practices in delayed prescribing. A one‑hour training programme is assumed to be conducted at regular intervals across the simulation period. The third component is the rollout of a feedback programme that will help assess prescriber performance over time and provide feedback. The final component involves the development and distribution of information materials targeting prescribers, like brochures and posters, to serve as best practice reminders.
The effectiveness of the intervention is calculated based on a Cochrane review by Spurling et al. (2017[27]). By pooling evidence from RCTs included in this review, it was calculated that a delayed antibiotic prescribing programme similar to the one included in the OECD analysis would lead to a 60% decline in antibiotic use in comparison to immediate antibiotic prescription (relative risk [RR] = 0.40, 95% confidence interval [CI]: 0.30‑0.53). The intervention is assumed to target 40% of all prescribers. The effect of the intervention is assumed to become observable immediately after the programme rollout and sustained over the simulation period.
The estimated per capita cost of the intervention varied between USD PPP 0.06 and USD PPP 1.26. Costs are calculated as a composite of administrative expenses at the national and district levels, cost of trainings for prescribers and cost of producing informational materials and prescriber feedback programme. One‑off costs of developing the guidelines are also included. The cost analysis does not consider any economic impact caused by a decrease in the sales of antimicrobials or the extra time needed for doctors to provide a prescription during the second visit, under the assumption that the prescription would be prepared during the first visit.
Scale up the use of rapid diagnostic tests (C-reactive protein [CRP] testing)
Modelled intervention
A novel programme aims to increase the use of rapid diagnostic tests by increasing the availability of point-of-care (POC) CRP in ambulatory care settings in combination with antibiotic treatment guidelines depending on the CRP levels.
The modelled increase in the availability of CRP is assumed to reduce immediate antibiotic prescribing by 32% in adults and 46% in children under 18 years of age.
The intervention is assumed to yield immediate effects on antibiotic prescribing behaviours.
Increasingly, many healthcare providers are relying on POC CRP tests to reduce unnecessary antibiotic prescribing (Falk and Fahey, 2008[28]; Sanders et al., 2008[29]; Ebell et al., 2020[30]). The POC CRP tests can offer a relatively faster option to guide antibiotic prescribing decisions, as these tests can produce results in minutes (Martínez-González et al., 2020[31]), compared to traditional diagnostic testing methods that typically require 48‑72 hours. Previous studies suggest that there is an inverse relationship between the use of POC CRP tests and antibiotic prescription rates. For instance, one recent systematic review and meta‑analysis found that the use of POC CRP testing was linked with a 25% reduction in antibiotic prescription in general practice during first consultation for patients with respiratory tract infections (Huang et al., 2013[32]). Subsequently, a Cochrane review examined data from six RCTs and concluded that the use of CRP at the POC was associated with a 22% reduction in antibiotic prescribing in adults that suffered from acute respiratory tract infections (Aabenhus et al., 2014[33]).
Since the last OECD analysis, the literature on the use of rapid diagnostic tests grew. A recent systematic review and meta‑analysis conducted by Verbakel et al. examined evidence from randomised trials and found that, in ambulatory care settings, the use of POC CRP testing is associated with a reduction in immediate antibiotic prescribing in the first consultation (RR = 0.81, 95% CI: 0.71‑0.92) in comparison to usual care (Verbakel et al., 2019[34]). Importantly, this study showed that when guidelines focusing on when to start antibiotic treatment depending on CRP levels were available, the beneficial effects were more pronounced, with an overall 32% (RR = 0.68, 95% CI: 0.63‑0.74) reduction in antibiotic prescribing for adults and 46% for children under 18 years of age (RR = 0.54, 95% CI: 0.33‑0.95) (Verbakel et al., 2019[34]). Similarly, another meta‑analysis conducted by Martínez-González et al. found that, in primary healthcare settings, the use of POC CRP testing was associated with reductions in immediate antibiotic prescribing (RR = 0.79, 95% CI: 0.70‑0.90) compared to usual care (Martínez-González et al., 2020[31]), though this study did not examine whether the effect size varied depending on the availability of antibiotic treatment guidance in accordance with CPR levels.
The modelled intervention is conceptualised as a novel programme that aims to scale up the use of POC CRP in primary care facilities. It is assumed that the increased use of POC CRP testing is paired with increasing the availability of antibiotic prescribing guidelines on when to initiate antibiotic treatment in relation to the level of CRP. It is assumed that health workers would be provided with a one‑hour training session at regular intervals throughout the simulation period to increase awareness of the benefits of using POC CRP testing and to highlight that antibiotic prescribing guidelines are available. In addition, informational materials (e.g. flyers and leaflets) would be disseminated. Finally, monitoring and evaluation arrangements are assumed to be put in place to ensure compliance with antibiotic prescribing guidelines that include the use of POC CRP testing.
The individual-level estimates for the intervention effectiveness reflect those generated by Verbakel et al. (2019[34]). The business-as-usual coverage is set at 0 because this is considered to be a novel intervention and the scale-up of the intervention is assumed to cover 70% of primary healthcare facilities. The intervention is assumed to have an immediate impact on antibiotic prescribing behaviours. The beneficial effects are assumed to persist throughout the simulation based on findings generated by Martínez-González et al., which showed that the beneficial impact of POC CRP tests was sustained one year after their rollout (Martínez-González et al., 2020[31]). The incidence of HAIs and susceptible infections acquired in the community are assumed to remain unchanged.
The per capita cost of the intervention is estimated to vary between USD PPP 0.53‑2.15 across countries. The estimated costs take into account the cost of buying the POC CRP tests, costs related to training the prescribers on the clinical guidelines related to the use of POC CRP tests and informational materials. The total intervention cost also includes some administrative expenses and expenses covering monitoring and evaluation activities at the national and local levels. No additional costs were included in these estimates to account for any additional time that prescribers may spend to perform the POC CRP tests because the tests are assumed to be carried out during the standard period that the prescriber spends with the patient to make a diagnosis.
Financial incentives to optimise antimicrobial use
Modelled intervention
A nationwide pay-for-performance (P4P) programme that aims to optimise antimicrobial use in community settings by rewarding bonuses to prescribers for achieving pre‑set antibiotic prescribing targets.
The modelled P4P programme is assumed to result in an 8% decrease in antibiotic prescribing.
The effectiveness is assumed to be sustained over the course of the simulation.
A growing strand of literature demonstrates that financial incentives targeting prescribers can be an effective means to promote the prudent use of antimicrobials. For example, one recent systematic review conducted by the OECD found that financial incentives are associated with measurable changes in prescribing behaviours, with the magnitude of the desired effects varying across different provider payment methods (Yoshikawa et al., 2021[35]). Capitation payments and pay-for-performance schemes that reward prescribers for achieving pre‑specified antibiotic prescribing targets are found to be among the most successful financial instruments in terms of promoting prudent antibiotic prescribing behaviours.
Building on findings from this review, the modelled intervention involves a nationwide P4P programme that aims to encourage the prudent use of antimicrobials in primary healthcare settings. The modelled intervention rests on a two‑pronged approach. The first prong entails the rollout of a nationwide pay-for-performance programme that aims to incentivise more prudent antibiotic prescribing practices. To this end, the programme is assumed to ensure that prescribers have access to clinical guidelines which delineate the classes of antimicrobials that should be used across different cases. In countries where clinical guidelines are already available, the existing guidelines will be linked to set performance targets for antibiotic prescription. New clinical guidelines will be developed only if a country does not already have clinical guidelines for antibiotic prescriptions. This is assumed to be supplemented with a monitoring mechanism to assess whether prescribers are meeting their prescribing targets. To this end, primary healthcare centres will provide self-reported prescriber-level data on relevant antibiotic prescription indicators.
Each month, approximately 10% of the self-reported data will be selected at random by the authorities responsible for monitoring and evaluation of the programme. Data from the selected primary healthcare centres will, then, be audited by the existing authorities that are responsible for monitoring and evaluation arrangements to verify the self-reported data through reviews of patient records and patient interviews. The volume of the bonus payment will be based on the performance of the primary healthcare centre as a whole, and account for 1‑5% of the total reimbursement of each healthcare centre. This choice is similar to the design of P4P programmes from OECD countries that have been shown successful in promoting the appropriate use of antibiotics (Ellegård, Dietrichson and Anell, 2017[36]). Prescribers who meet their performance targets will be rewarded lump-sum bonus payments corresponding to about 1% of their base salary, in addition to their base salaries. No financial penalties will be administered for primary healthcare centres that do not meet their performance targets.
It is assumed that the modelled P4P programme is associated with an 8% decline in antibiotic prescribing calculated as an average of the effect size reported in three studies that were determined to have with relatively lower/moderate risk of bias by (Yoshikawa et al., 2021[35]). One study examined the impact of the 2015/16 NHS England Quality Premium programme, which provided financial rewards to Clinical Commissioning Groups for meeting antibiotic prescribing targets like a 1% reduction in total antibiotic prescribing in primary care and a 10% decline in the share of broad-spectrum antibiotics like co‑amoxiclav, cephalosporins and quinolones. This study found a 3% decline in the antibiotic prescribing rate (Bou-Antoun et al., 2018[37]). Another study from the State of California in the United States showed that two P4P programmes that provided performance bonuses to providers that meet a set of quality measures that included targets on antibiotic prescriptions. This study found that the introduction of the P4P programmes was associated with a 6% decline in preferred antibiotic use (Mullen, Frank and Rosenthal, 2010[38]). Finally, another study conducted a matched-pair cluster-randomised experiment in China between 2009 and 2012. In this study, a new P4P programme awarded bonus payments to primary care providers for meeting their performance targets that included antibiotic prescription rates, in combination with capitation payments. This intervention was associated with an approximately 15% reduction in antibiotic prescription (Yip et al., 2014[39]).
The intervention is assumed to cover 70% of prescribers who work in outpatient care settings. Based on identified evidence, it is assumed that there is a perfectly elastic relationship between antibiotic consumption and AMR rate (Kaier, Frank and Meyer, 2011[7]). This means an 8% decline in antibiotic prescribing is assumed to yield an 8% decline in AMR rate for community-based resistant infections. The beneficial effects are modelled to be sustained over the course of the simulation, which is consistent with the findings generated by Bou-Antoun and colleagues, which showed that the observed effects of the interventions remained at the same Level 2 years after the rollout of the intervention (Bou-Antoun et al., 2018[37]).
The per capita cost associated with this intervention ranges from USD PPP 0.15‑8.01. The cost estimated takes into account the ingredient costs of the two streams of work. The running costs – i.e. the spending related to the bonus payment – account for most of the total intervention costs. Costs related to the strengthening of the monitoring mechanism are also included in the analysis, as well as one‑off costs of developing or updating guidelines. Planning at the central and local levels as well as checks at the local level are also included in the costs.
Policies in human health to reduce the incidence of infections
Enhance hand hygiene practices
Modelled intervention
A facility-based intervention that aims to enhance hand hygiene practices among health workers.
The effectiveness of the modelled intervention is assumed to be associated with a 33% reduction in the incidence of healthcare-acquired infections.
The beneficial effects of the modelled intervention are assumed to become observable immediately and persist over the course of the simulation.
A growing strand of literature points to the importance of enhancing hand hygiene for reducing the AMR burden. The protective effects of hand hygiene practices have been demonstrated again in the context of COVID‑19 (Jefferson et al., 2020[40]) and previous outbreaks including SARS-CoV‑1 during the 2003 epidemic and MERS-CoV (Jefferson et al., 2011[41]; Chou et al., 2022[42]). Further, one systematic review and meta‑analysis of evidence generated through RCTs, cluster RCTs and quasi‑experimental study designs demonstrates that hand hygiene interventions that incorporate multiple components in line with the WHO‑5 moments approach achieve a greater level of compliance among health workers in comparison to interventions that rely on a single component (Luangasanatip et al., 2015[43]). Though, evidence is not yet sufficient to determine the precise combination of components within the WHO‑5 moment approach that generates the highest compliance rates (Gould et al., 2017[44]).
Consistent with the emerging literature, the modelled intervention aims to enhance hand hygiene practices in healthcare settings through multiple components. The first component aims to ensure that alcohol dispensers are available throughout health facilities. In addition, in each health facility, one trained health worker per 250 beds is assumed to take up the responsibility of the IPC focal point, as recommended by the WHO. The IPC focal point will be responsible for organising and co‑ordinating all educational activities around improving hand hygiene practices like developing the training curriculum and logistical materials like identifying the physical space in which the training session will take place. The selection of the IPC focal point will depend on the availability and skillset of the health workers deployed in each health facility to avoid any potential disruption in healthcare service provision.
Hand hygiene training sessions will be carried out on a regular basis throughout the simulation period, with each session lasting two‑hours. Teaching materials will be consistent with the open-access IPC training course developed by the WHO, as well as the IPC Core Components. Training sessions will prioritise participation and include simulation exercises, as these teaching methods have been shown to produce greater teaching outcomes. The learning outcomes will combine both improvements in theoretical knowledge around best practices in communication with patients and practical applications. Each training session will be evaluated and updated based on feedback collected from participating health workers. The training and education programme will be supported by the distribution of informative materials (e.g. posters and brochures) in visible spaces across health facilities as a reminder of best practices. Finally, compliance with hand hygiene guidelines will be monitored and feedback will be provided.
The effectiveness of the modelled intervention is based on a systematic review of the literature conducted by the OECD. In congruence with WHO guidance, evidence generated through these studies suggests that enhancing hand hygiene practices is linked with declines in the incidence of resistant and susceptible infections. Specifically, it is assumed that the modelled intervention is associated with a 33% decline in the incidence of healthcare-acquired infections, with the estimated relative risk equivalent to 0.67 (95%CI: 0.64‑0.71). It is also assumed that the scale-up of the modelled intervention does not affect the incidence of community-acquired resistant infections.
The level of coverage in the business-as-usual scenario was determined based on data extracted from the 2019 Hand Hygiene Self-Assessment Framework Survey conducted by the WHO (de Kraker et al., 2022[3]). The intervention is assumed to be scaled up at 70% of health facilities in each country. It is assumed that the beneficial effects become observable as soon as the intervention is scaled up and remain unchanged throughout the simulation period.
The implementation costs associated with this intervention range from USD PPP 0.02‑1.06 per capita. Costs associated with this intervention include the purchase of supplies to ensure the handwashing practices comply with the WHO guidelines (e.g. sufficient supplies of alcohol-based hand rub or liquid soap), training on best practices in hand hygiene, expenses associated with developing informational materials, as well as administrative costs at the national and local levels to support the implementation of the intervention across healthcare services. The estimated costs also include monitoring and evaluation arrangements to ensure smooth implementation of the programme at the healthcare facility level.
Enhance environmental hygiene practices
Modelled intervention
A bundled intervention that aims to enhance environmental hygiene practices in hospitals.
The modelled intervention is assumed to reduce the risk of infection by 26% in hospital settings.
The beneficial effects are assumed to become observable immediately and persist over the course of the simulation.
Broadly, environmental hygiene practices can be grouped into three broad categories (Donskey, 2013[45]):
Disinfectant product substitution: Shifting to disinfectants that have shown greater effectiveness against pathogens (e.g. substituting sporicidal products with non-sporicidal products).
No-touch disinfection methods: Using of hydrogen peroxide vapour, aerosol devices and ultraviolet (UV) radiation devices.
Strategies that aim to improve the effectiveness of existing cleaning and disinfection practices: training of cleaning staff members to improve daily and terminal cleaning, and checklists to guide environmental cleaning practices.
The modelled intervention is a hospital-based programme that aims to enhance routine cleaning practices through the introduction of a bundled intervention that combines all three broad categories of environmental hygiene practices. Specifically, the first component of the intervention will entail the substitution of disinfectant products already being used in health facilities with those that have shown greater effectiveness in line with the WHO guidance. The second component will involve the introduction of no-touch disinfection methods as part of the terminal cleaning of rooms/areas in between occupying patients. These two components will be complemented with a one‑hour training programme for staff members who are responsible for environmental cleaning. This training will provide information and recommendations on cleaning techniques, frequency of cleaning and best practices in environmental cleaning. The environmental cleaning activities will be audited by a designated hospital staff member on a regular basis.
For this intervention, the individual-level effectiveness was quantified by the OECD based on a systematic review of the studies that focused on the implementation of the three groups of environmental hygiene categories discussed earlier compared to traditional environmental cleaning practices. Based on this review, it is assumed that enhancing environmental hygiene practices in hospital settings is associated with a 26% reduction in the risk of acquiring hospital-acquired infections, with relative risk equivalent to 0.74 (95%CI: 0.72, 0.76).
The level of coverage in the business-as-usual scenario was calculated using data gathered from studies that use information from the 2019 Infection Prevention and Control Assessment Framework Survey conducted by the WHO (Tomczyk et al., 2022[4]). The intervention is assumed to be adopted by 70% of hospitals in each country. It is assumed that the intervention results in reductions in the incidence of infections acquired in healthcare settings. The impact of the intervention is assumed to become observable immediately and the protective effects are assumed to remain constant over time.
The per capita cost of the modelled intervention varies from USD PPP 0.71‑5.06. The estimated costs are calculated as a combination of national and local level administrative costs, the procurement of disinfectant products in line with WHO guidelines, expenses associated with environmental cleaning training and regular audits.
Improve vaccination coverage
Modelled intervention
Scale up of nationwide campaign of 23‑valent pneumococcal polysaccharide (PVV23) targeting older adults
The modelled intervention is associated with a 64% decline in the incidence of all serotypes of invasive pneumococcal disease and pneumococcal pneumonia
The effectiveness is assumed to be sustained over the course of the simulation
The modelled intervention consists of a nationwide vaccination campaign targeting older adults to provide protection against Streptococcus pneumoniae (S. pneumoniae), an opportunistic pathogen which can result in severe illness and failures in medical treatment. It is estimated that about 30% of infections caused by this pathogen are resistant to at least one antibiotic. Though data on the burden of S. pneumoniae remains limited, data collated from countries in the WHO European Region in 2020 suggested that, compared to previous years, the number of S. pneumonia isolates was lower (ECDC/WHO, 2022[46]). However, the available evidence points to large cross-country variations. For instance, in 2020, in European countries like Austria, the Netherlands and the Czech Republic, the proportion of S. pneumonia isolates that have increased exposure or resistance to penicillin remained at or below 5%, whereas this figure stood at least 25% in countries like Cyprus, France, Iceland, Malta, Romania and Türkiye (ECDC/WHO, 2022[46]).
Pneumococcal vaccines offer one effective strategy to curb the burden of S. pneumonia. To date, the scale‑up of pneumococcal vaccines has been associated with reductions in the incidence of infections and slow the pace with which pneumococcal resistance occurs (Pavia et al., 2009[47]; CDC, 2019[48]). In many OECD countries, various pneumococcal vaccines are recommended for children under 5 years of age, as well as adults with certain health risks and older adults.
Over the last two decades, many OECD countries made important strides in improving pneumococcal conjugate vaccine (PCV) coverage among young children, with the coverage of PCV3 vaccine averaging at 87% in 2020 among OECD countries and key partners, EU/EEA and G‑20 countries. In comparison, the coverage of the vaccine PVV23 vaccine among older adults remains relatively low and varied, with the estimated coverage standing at about 74% in Japan in 2018 (Naito et al., 2020[49])to 24% in Greece in 2019 (Papagiannis et al., 2020[50]).
The vaccination programme is conceptualised to have three components. The first component involves scaling up the availability of PVV23 vaccines in health facilities. The second component involves a vaccination campaign which will entail developing and disseminating informational materials that target the general public’s understanding and knowledge of the benefits associated with PVV23 vaccines. This awareness campaign is assumed to rely heavily on promotional materials disseminated in mass media outlets (e.g. television, radio) and social media platforms. Additionally, the update of the vaccines among the target population will be regularly monitored.
The individual-level effectiveness estimates were extracted from a recent literature review and meta‑analysis conducted by Falkenhorst and colleagues (Falkenhorst et al., 2017[51]). In their review, the authors identified studies conducted primarily in OECD countries to quantify the effectiveness of PVV23 vaccine among adults at the age of 60 and above. Building on this review, it is assumed that the efficacy of the PPV23 vaccine is 64% (95%CI: 35‑80%) against all serotypes of invasive pneumococcal disease and pneumococcal pneumonia.
The level of vaccination coverage in the business-as-usual scenario was determined based on a review of the publicly available data sources whenever possible. For countries for which this information was not available, it was assumed that approximately 50% of adults at the age of 65 and older assumed have already received their PVV23 vaccines, reflecting the average of PVV23 vaccine coverage among countries where data were available. It is assumed that the modelled vaccination campaign will aim to cover 90% of adults at the age of 60 and above, reflecting recommended vaccination schedules in many OECD countries (ECDC, 2022[52]). The intervention is assumed to have immediate beneficial impacts, which are sustained over the simulation period.
The per capita cost of this intervention is calculated to vary between USD 0.03‑0.57. The estimated costs reflect expenses associated with administrating the vaccination campaign at the national and central levels including costs to cover the time of health workers who administer vaccines, costs associated with the purchase of vaccines and the development and dissemination of informational materials. In addition, the estimated costs also include monitoring the uptake of the vaccines among the target population at the local and national levels.
Policies to promote AMR awareness and understanding
Enhance health professional training on enhanced communication skills
Modelled intervention
A training programme for health professionals to improve communication skills during consultations with their patients in outpatient care settings.
The modelled intervention is associated with a 39% reduction in antibiotic prescribing.
The effectiveness of the intervention is assumed to remain unchanged over time.
Several strands of literature focusing on professional education and training programmes suggest that these programmes are linked to improvements in AMR-relevant behaviours, including antibiotic prescribing patterns (Davey et al., 2017[25]), greater compliance with hand hygiene (Gould et al., 2017[44])and IPC guidelines (Chou et al., 2022[42]). In recent years, a growing body of evidence further point to the potential benefits of professional education and training programmes that focus on improving communication between prescribers and patients on antibiotic use. Broadly, these training programmes attempt to improve health professionals’ communication skills relevant to eliciting patient beliefs and expectations; clarifying any misconceptions around antibiotic use; and conveying the potential benefits and harms associated with the use of antibiotics, as well as self-medication (Coxeter et al., 2015[53]). A recent Cochrane review showed that educational interventions aiming to improve communication between prescribers and patients in outpatient care settings can help reduce antibiotic prescribing for acute respiratory infections in the tune of 39% within six weeks of the first consultation, with a risk ratio equivalent to 0.61 (95%CI: 0.55‑0.68) (Coxeter et al., 2015[53]).
The modelled intervention is conceptualised as a training programme, with the aim of improving communication skills among prescribers in outpatient care settings. Two-hour communication training sessions will be carried out in the winter and/or autumn months on a regular basis. Teaching materials will focus on improving proficiency in communication during consultations and skills in building rapport with patients. Teaching sessions will aim to improve providers’ awareness of methods to assess and modify patients’ beliefs and expectations about the use of antibiotics; and teach techniques to communicate with patients on prognosis and treatment options, as well as communicating benefits and harms associated with antibiotic use and self-medication. During each training session, active participation and simulation exercises will be prioritised. Learning outcomes will measure improvements in both theoretical knowledge and practical communication skills. Informative materials such as posters and brochures will be made available in visible areas within health facilities to serve as best practice reminders. A feedback programme will be put in place to ensure the training sessions result in the improvement of communication skills among providers.
The individual-level effectiveness estimates were extracted from the Cochrane review conducted by Coxeter and colleagues (Coxeter et al., 2015[53]). In line with this review, it is assumed that the modelled intervention results in a 39% reduction in antibiotic prescribing. The same Cochrane review found that the beneficial effects of the intervention may wane after 12 months, though these results were not statistically significant (Coxeter et al., 2015[53]). In recognition of these findings, the modelled intervention is assumed to have an immediate beneficial impact on health worker communication skills and the beneficial impacts are modelled to remain the same over the simulation period.
For each country, the level of coverage in the business-as-usual scenario was identified based on data retrieved from the 2020‑21 Tripartite AMR Self-Assessment Survey based on the question that investigates the extent to which AMR training and education opportunities are available in each country (See Question 2 in Annex B). It is assumed that 70% of health facilities will roll out the modelled communication training and all prescribers that work in these facilities will be enrolled in the training sessions.
The per capita cost of this intervention is estimated to range from USD PPP 0.05‑0.86. Intervention costs comprise expenses associated with developing the training at the national level and delivering it at the local level. In addition, the preparation and the printing of the material to be provided to participants are included in the total intervention cost as well as some administrative costs at the national and local levels. Expenses associated with building and administrating the feedback programme are also included. Conversely, the intervention costs do not include the time spent by participants on the training as this is assumed to be part of regular training activities for these workers (e.g. as part of continuing medical education initiatives that are normal practice across many OECD countries.)
Scale up mass media campaigns
Modelled intervention
The modelled intervention is a nationwide mass media campaign involving mass media and social media platforms to raise AMR understanding and awareness across key stakeholders.
The modelled mass media campaign is assumed to result in a 7% decline in antibiotic prescription.
The effectiveness is assumed to be sustained over the course of the simulation.
Among OECD countries, mass media campaigns are a frequently used tool to raise awareness and understanding of AMR among health professionals and the general public (Rogers Van Katwyk et al., 2019[54]). Typically, these campaigns aim to demonstrate the beneficial and harmful effects of antibiotic use and attempt to improve knowledge and understanding of good prescribing practices.
Despite the ubiquity of campaigns to raise AMR awareness and understanding in the general public, the effectiveness of these programmes on behaviours around antibiotic use has not yet been well understood (Rogers Van Katwyk et al., 2019[54]). A handful of studies suggest modest improvements attributable to AMR awareness campaigns, though many of the studies should be interpreted with care due to methodological concerns. To date, one non-randomised controlled trial from Italy showed that a community-level awareness campaign led to a 4.3% decline in antibiotic prescribing in areas that received the intervention in comparison to the areas that did not receive a community-wide awareness campaign (Formoso et al., 2013[55]). Another study from the United Kingdom found a 5.8% decline in antibiotic reduction in areas where two mass media campaigns disseminated information on the appropriate use of antibiotics, in comparison to areas that did not receive any antibiotic-relevant information (Lambert, Masters and Brent, 2007[56]; Formoso et al., 2013[55]). In the United States, a community-wide, year-long AMR awareness campaign in the State of Tennessee led to an 11% decline in antibiotics prescribed to children compared to the communities that did not roll out similar community-wide interventions (Perz, 2002[57]).
The modelled intervention is designed as a nationwide mass media campaign that aims to improve AMR awareness and understanding in the general population. It is conceptualised as an AMR awareness campaign carried out annually, though the majority of the activities are assumed to take place in winter/autumn months when there is a peak in antibiotic consumption often for influenza-like illnesses that do not require antibiotic use. The awareness campaign entails developing several toolkits that contain communication tools and key messages tailored for various target audiences like health professionals in human health, pharmacists and to the general public. Materials targeting the general public will focus primarily on considerations around self-medication and the safe disposal of antibiotics. The modelled intervention will rely not only on mass media outlets (e.g. television, radio, print media etc.) but also on social media platforms). The use of social media platforms reflects the growing evidence from OECD countries that the Internet and social media platforms are widely used by people who are seeking antibiotic-related information (Zucco et al., 2018[58]). In addition, public health agencies will make guidelines and recommendations around the appropriate use of antibiotics available online through their websites.
The national-wide mass media campaigns are assumed to lead to a modest reduction in antibiotic prescription in the tune of 7% based on the average effect size emerging from existing studies (Perz, 2002[57]; Lambert, Masters and Brent, 2007[56]). The intervention is assumed to be scaled up nationally, reaching about 70% of all relevant stakeholders in the human health sector including health professionals and the general public. Under the assumption that the relationship between AMR rate and antibiotic consumption is perfectly elastic (Kaier, Frank and Meyer, 2011[7]), the estimated decline in antibiotic prescription is modelled to result in a 7% decline in AMR rate for community-based infections. The modelled intervention is assumed not to influence the incidence of healthcare-acquired infections (HAIs) and susceptible infections acquired in the community.
For each country, the 2020‑21 Tripartite AMR Self-Assessment Survey was used to set the level of coverage in the business-as-usual scenario based on the question that investigates the stage of implementation of activities to raise awareness and understanding of AMR risks and response (see Question 3 in Annex 6.B). It is assumed that the beneficial effects are realised as soon as the campaign is launched and remain unchanged over time.
The per capita cost of this intervention is estimated to vary from USD PPP 0.40 to USD PPP 1.36. The estimated costs are driven primarily by buying advertising space on mass media outlets, followed by the costs of devising and planning the campaign at the local and national levels.
Policies outside of human health sector to reduce the incidence of infections
Enhance farm hygiene
Modelled intervention
The modelled intervention is a procurement programme that facilitates the purchase of PPE in farm settings by farmers and professional visitors like veterinarians.
The modelled intervention is associated with a 12% reduction in the risk of infection.
The protective effects are assumed to become observable immediately and remain unchanged over time.
In recent years, a strand of literature increasingly examined the role of farm-level biosecurity measures in preventing the emergence and spread of infections in farm settings. For instance, one systematic review demonstrated that a range of biosecurity measures, including handwashing, sanitisation and hygienic measures, scaling up the use of PPE, animal vaccines and other specific interventions like using automatic milking, can yield protective effects against infections (Youssef et al., 2021[59]).
The modelled intervention is conceptualised as the introduction of a regulatory framework that aims to improve the availability of PPE in farm settings in line with the International Labour Organization (ILO) Code of Practice on Safety and Health in Agriculture (ILO, 2011[60]). This intervention involves a novel programme which will help facilitate the purchase of PPE that can be used by farmers and technical visitors like veterinarians. Reflecting the evidence from the literature, the PPE includes farm boots, work clothes worn upon arrival to the farm and outfits for professional visitors. The use of the PPE will be complemented by the development and dissemination of brochures and posters that will be distributed to each farm to serve as a reminder of the importance of wearing PPE in the farm setting. Compliance with the intervention will be monitored through yearly audits, where 10% of farms will be selected for in-person visits by auditors.
It is assumed that the modelled intervention is associated with a 12% reduction in the risk of infection (RR = 0.88, 95% CI: 0.81‑0.96) among farmers and professional visitors like veterinarians. This estimate was calculated by the OECD as a combination of two studies included in a systematic review conducted by Youssef et al. (2021[59]). In one study from dairy goat farms in the Netherlands, Schimmer et al. found that consistently wearing boots and protective clothing by farm staff was associated with a 16% reduction in the risk of Coxiella burnettii infection (RR = 0.84, 95% CI: 0.72‑0.98) (Schimmer et al., 2012[61]). In a subsequent study, Schimmer et al. (2014[62]) also showed that, in dairy cattle farms in the Netherlands, the use of farm boots and work clothes by professional visitors was associated with a 9% reduction in the risk of Coxiella burnettii infection (RR = 0.91, 95% CI: 0.80‑1.03) and wearing work clothes among farm workers was associated with a 12% reduction in the risk of the same infection (RR = 0.88, 95% CI: 0.72‑0.98). In addition, several scenarios that rest on different assumptions of spill-over effects of this intervention beyond farmers and professional visitors will be conducted.
The business-as-usual coverage was identified based on data retrieved from the 2020‑21 Tripartite AMR Self-Assessment Survey and on the question that assesses the stage of implementation of good health, management, and hygiene practices in animal production (see Question 4 in Annex 6.B). The target coverage is set at 70%. It is assumed that the protective effects of enhancing hygiene practices in farm settings become observable immediately and remain unchanged over time.
The per capita costs associated with this intervention are estimated to vary between USD PPP 0.02 and USD PPP 0.73. The estimated costs reflect expenses associated with the administration of the programme at the national and local levels and the cost of developing, printing and disseminating information materials. In addition, costs also cover expenses due to developing monitoring and evaluation arrangements at the national and local levels to ensure a high degree of compliance with the intervention. Conversely, costs associated with the purchase of PPE are excluded because these are considered to be purchased directly by farms.
Enhance food handling practices
Modelled intervention
A food safety control training programme targes food service workers in food establishments, coupled with visual reminders and regular audits based on checklists.
The rollout of the modelled intervention results in a 28.6% reduction in microbial count.
The protective effects are assumed to become observable immediately and sustained over time.
Food safety policies are an effective option to help prevent foodborne illnesses in food establishments. A growing body of literature suggests that a range of food safety and hygiene policies, referred to as hazard analysis and critical control points (HACCP)-based interventions, can help reduce the emergence of foodborne illnesses throughout the food supply chain (FDA, 1997[63]). In particular, interventions focusing on meal preparation have become the focus of many studies in recent years (Young et al., 2019[64]; 2020[65]). These studies demonstrated that educational interventions targeting food handlers were associated with improvements in self-reported knowledge, attitudes and practices, though substantial variation exists in the effect size of these interventions (Young et al., 2020[65]). Importantly, emerging studies broadly concur that multi-faceted interventions yield greater beneficial impacts than interventions that rely on a single component (Gillison et al., 2018[66]).
The modelled intervention is conceptualised as a HACCP-based food safety control training programme targeting food service workers in food establishments. The intervention entails providing one hour of training on a regular basis by a trainer who has expertise in HACC systems. The training will focus on personal hygiene, food preparation and storage. In addition to the training programme, posters and reminders would be made available to the food service workers in order to reinforce the lessons learned during the training sessions. The third component involves regular audits, where a trained person visits food establishments for visual inspection of the tasks performed by food service workers. These inspections will also include a checklist of questions related to facilities (e.g. availability of soap dispensers, paper towels, waste management), staff hygiene and preparation and storage of food (e.g. measures taken to avoid cross contamination), prerequisites and control activities (e.g. pest control) and administrative matters (e.g. recording of activities).
In a systematic review and meta‑analysis recently published in a peer-reviewed journal, the OECD found that, in a sample of OECD countries, the rollout of food safety interventions in catering establishments was associated with a 28.6% reduction in microbial count, with relative risk equivalent to 0.71 (95% CI: 0.69‑0.73) (Levy, Cravo Oliveira Hashiguchi and Cecchini, 2022[67]). Importantly, findings from this analysis further concluded that microbial reductions were consistently observed across different types of microorganisms screened, sample origin and type of food establishment.
The business-as-usual coverage was determined through the 2020‑21 Tripartite Country Self-Assessment Survey based on each country’s response to the question that inquires whether there are good management and hygiene practices in place to reduce the development and transmission of AMR in food processing (see Question 5 in Annex 6.B). It is assumed that the intervention will be taken up by 70% of food establishments. The protective effect of the modelled intervention is assumed to become observable immediately and sustained over the simulation period. This assumption was made based on the finding from Levy, Cravo Oliveira Hashiguchi and Cecchini that showed that microbial reductions continued to be observed regardless of the time elapsed between the introduction of the intervention and sample collection (Levy, Cravo Oliveira Hashiguchi and Cecchini, 2022[67]).
The per capita cost of this training ranges from USD PPP 0.08 to USD PPP 0.78. Intervention costs include all three components of the intervention, namely costs related to providing training to food service workers, the preparation and printing of the educational material as well as administrative and enforcement costs at the national and local levels.
Annex 6.B. Calculating the level of coverage in the business-as-usual scenarios
The questions and response categories from the 2020‑21 Tripartite AMR Country Self-Assessment Survey used to determine the level of coverage in the business-as-usual scenarios of modelled interventions are as follows (Annex Table 6.B.1):
Annex Table 6.B.1. Questions from the 2020-21 Tripartite AMR Country Self-Assessment Survey used to inform the OECD analysis
|
Modelled intervention |
Survey questions |
Response categories |
|---|---|---|
|
Question 1: Strengthen ASPs |
Optimising antimicrobial use in human health |
A. No/weak national policies for appropriate use. B. National policies for antimicrobial governance developed for the community and healthcare settings. C. Practices to ensure appropriate antimicrobial use are being implemented in some healthcare facilities and guidelines for the appropriate use of antimicrobials are available. D. Guidelines and other practices to enable appropriate use are implemented in most health facilities nationwide. Monitoring and surveillance results are used to inform action and to update treatment guidelines and essential medicines lists. E. Guidelines on optimising antibiotic use are implemented for all major syndromes and data on use are systematically fed back to prescribers. |
|
Question 2: Enhance health professional training on enhanced communication skills |
Training and professional education on AMR in the human health sector |
A. No training for human health workers on AMR. B. Ad hoc AMR training courses in some human health-related disciplines. C. AMR is covered in: i) some pre‑service training; and ii) some in-service training or other continuing professional development (CPD) for human health workers. D. AMR is covered in pre‑service training for all relevant cadres. In-service training or other continuing professional development covering AMR is available for all types of human health workers nationwide. E. AMR is systematically and formally incorporated in pre‑service training curricula for all relevant human health cadres. In-service training or other CPD on AMR is taken up by relevant groups for human health nationwide, in public and private sectors. |
|
Question 3: Scale up mass media campaigns |
Raising awareness and understanding of AMR risks and response |
A. No significant awareness-raising activities on relevant aspects of risks of antimicrobial resistance. B. Some activities in parts of the country to raise awareness about the risks of antimicrobial resistance and actions that can be taken to address it. C. Limited or small-scale antimicrobial resistance awareness campaigns targeting some but not all relevant stakeholders. D. Nationwide, government-supported antimicrobial resistance awareness campaign targeting all or the majority of priority stakeholder groups, based on stakeholder analysis, utilising targeted messaging accordingly within sectors. E. Targeted, nationwide government-supported activities regularly implemented to change the behaviour of key stakeholders within sectors, with monitoring undertaken over the last 2‑5 years. |
|
Question 4: Enhance farm hygiene |
Good health, management and hygiene practices in animal production |
A. No systematic efforts to improve good production practices. B. Some activities in place to develop and promote good production practices. C. National plan agreed to ensure good production practices in line with international standards (e.g. World Organisation for Animal Health Terrestrial and Aquatic Animal Health Codes, Codex Alimentarius). Nationally agreed guidance for good production practices developed, adapted for implementation at the local farm and food production levels. D. Nationwide implementation of a plan to ensure good production practices and national guidance published and disseminated. E. Implementation of the nationwide plan is monitored periodically. |
|
Question 5: Enhance food handling practices |
Good management and hygiene practices to reduce the development and transmission of AMR in food processing |
A. No systematic efforts to improve good management and hygiene practices. B. Some activities in place to develop and promote good management and hygiene practices. C. The national plan agreed to ensure good management and hygiene practices in line with international standards (e.g. Codex Alimentarius). Nationally agreed guidance for good practices developed, and adapted for implementation according to local food processing approaches. D. Nationwide implementation of a plan to ensure good management and hygiene practices and national guidance published and disseminated. E. Implementation of the nationwide plan is monitored periodically. |