This chapter provides an overview of the burden of resistant infections on population health and the economy. The results presented in the chapter are generated using the OECD Strategic Public Health Planning for Antimicrobial Resistance (SPHeP-AMR) model based on data gathered for 34 countries, including 29 European Union (EU)/European Economic Area (EEA) countries as well as Japan, Switzerland, Türkiye, the United Kingdom and the United States. The burden of AMR on population health is assessed through measures of mortality, life expectancy and morbidity. The impact on the economy is examined through the use of healthcare resources, health expenditure, participation in the labour force and workforce productivity.
Embracing a One Health Framework to Fight Antimicrobial Resistance

3. Health and economic burden of antimicrobial resistance
Abstract
Key findings
AMR continues to pose great risks to population health in OECD and EU/EEA countries
Nearly 4.3 million resistant infections occur each year in the 34 OECD countries and EU/EEA countries included in the analysis.
While around 35% of resistant infections are acquired in healthcare settings each year across all countries included in the OECD analysis, these infections account for about 62% to 73% of AMR-related deaths.
Around three-quarters of all deaths that occur due to AMR each year are caused by 3 pathogens – Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae) and Staphylococcus aureus (S. aureus) – across the 34 countries included in the analysis.
A geographic gradient persists in the mortality burden due to AMR. Generally, countries in southern Europe such as Italy, Malta and Portugal are estimated to have the greatest mortality rates each year due to AMR by 2050 whereas the Netherlands and Norway are expected to face the smallest mortality burden. Across non-EU/EEA member OECD countries, Türkiye is estimated to face the highest mortality rate attributable to resistant infections.
Complete elimination of AMR would produce a gain of, on average, about 133 life years (LYs) per 100 000 persons per year up to 2050 across the 34 countries included in the analysis. This figure stands at around 40 LYs gained per 100 000 persons if resistant infections were to be replaced by susceptible infections. Under both scenarios, the greatest gains per capita in LYs are estimated to be experienced in Italy and Malta across EU/EEA countries and in Türkiye across non-EU/EEA member OECD countries. If resistant infections are not eliminated, the reduction in life expectancy due to AMR can be around one‑third of the reduction in life expectancy due to COVID‑19 between 2019‑20.
Impact on quality of life, as measured in gains in disability-adjusted life‑years (DALYs) is estimated to be around 18% greater than the estimated gains in LYs if AMR was entirely eliminated and around the same magnitude if resistant infections were replaced by susceptible infections.
Infections caused by resistant organisms are estimated to have deleterious effects on the budget of health systems across the OECD and EU/EEA countries included in the analysis
AMR will put substantial pressure on hospital resources that were already stretched throughout the COVID‑19 pandemic. Using the elimination scenario, an additional 32.5 million extra days are estimated to be spent in hospitals to treat resistant infections each year up to 2050 across the 34 countries included in the analysis. This figure stands at around 6.9 million extra hospital days using the replacement scenario. This would be equivalent to using the entire acute bed capacity in Spain in 2020 for nearly one year under the elimination scenario and around two months under the replacement scenario.
If resistance proportions continue to follow the expected growth rates, the OECD and EU/EEA countries included in the analysis are estimated to spend around USD 28.9 billion each year up to 2050 adjusting for purchasing power parity (PPP) for treating resistant infections using the elimination scenario, which corresponds to USD PPP 25.6 per capita. Using the replacement scenario, the annual healthcare spending on AMR is estimated to reach around USD PPP 5.9 billion up to 2050, corresponding to about USD PPP 5.2 per capita.
The economic burden of healthcare expenditures attributable to resistant infections is marked by important differences across countries. Using both scenarios, the greatest amount of financial resources per capita each year up to 2050 are estimated to be spent by Italy and Luxembourg across the EU/EEA countries and by Switzerland and the United States across non-EU/EEA member OECD countries.
The cost of inaction to tackle AMR in the next three decades will exceed treatment costs due to COVID‑19 in 2020. In 17 countries for which data are available, the average annual health expenditure incurred each year due to AMR is about 19% of the total health expenditure due to treating COVID‑19 patients in 2020 using the elimination scenario and 4% using the replacement scenario. This means that the cost incurred around every five years for treating patients with resistant infections will be equivalent to the treatment costs due to COVID‑19 in 2020 using the elimination scenario.
Resistant infections have substantial consequences for workforce participation and productivity and the overall macroeconomic performance
Resistant infections are associated with notable declines in participation in the labour force and reductions in productivity, estimated to exceed 734 000 full-time workers annually up to 2050 across the 34 countries included in the analysis.
Across the 34 countries included in the analysis, the total annual losses in the labour market output attributable to AMR averages around USD PPP 36.9 billion, corresponding to USD PPP 32.7 per capita using the elimination scenario. This corresponds to roughly one-fifth of the gross domestic product (GDP) in Portugal in 2020. Annual losses in labour market output are estimated to average around USD PPP 6.6 billion each year up to 2050 using the replacement scenario, corresponding to USD PPP 5.9 per capita. These losses are driven primarily by the declines in employment as well as reductions in workforce productivity particularly due to considerable increases in absenteeism from work.
The fight against antimicrobial resistance is far from over
It is widely recognized that AMR remains one of the leading challenges to human health, jeopardising the effectiveness of many medical and public health advances made in the 20th century. Resistance to readily available, affordable treatments often means that providers must treat such infections with medicines that are more costly, less effective, unavailable or unaffordable in many settings (WHO, 2015[1]).
Results from Chapter 2 suggest that between 2019 and 2035, the average resistance proportions in OECD across 12 priority antibiotic-bacterium combinations are projected to remain largely stable. This new round of analyses revises a previous projection concluding that, between 2015 and 2030, there would have been a 1 percentage point increase in the average resistance proportions, assuming that no new policy actions are implemented beyond those already in place (OECD, 2018[2]). Policies put in place since the previous OECD analysis may have contributed to curbing the growth rate of resistance proportions (see Chapter 4). In recent years, the recognition of AMR as a global health priority led to the proliferation of various policy initiatives with many OECD countries developing their national action plans to tackle AMR in accordance with their own priorities and rolling out a wide range of multi-sectoral programmes with aims ranging from optimising the use of antimicrobials in human and animal health, improving infection prevention and control practices to increasing AMR awareness and understanding in the general public.
Despite some progress, the OECD analysis revealed alarming differences in the resistance proportions across countries, with a tenfold difference between countries with the highest and lowest estimated proportion of resistant infections (see Chapter 2). The results also pointed to worrisome variations in resistance proportions across antibiotic-bacterium combinations, across countries within antibiotic-bacterium combinations as well as antibiotic-bacterium combinations within countries. Exacerbating these challenges are the uncertainties surrounding the COVID‑19 pandemic and its impact on AMR. As new data and analyses continue to emerge, it is crucial to provide accurate estimates of the health and economic burden of AMR to inform the design and implementation of policies and regulations to limit its consequences.
The analyses presented in this chapter builds on the previous OECD analysis on AMR (OECD, 2018[2]), using the most recent data on the incidence of infections and the prevalence of AMR provided by national governmental agencies or by intergovernmental organisations such as the European Centre for Disease Prevention and Control (ECDC). Data provided to the OECD are collected by national surveillance systems and generally reflect the national official statistics. This approach has many advantages. For example, the data used in the OECD analysis are consistent with information presented by countries in their own national reports and evaluations produced by the ECDC. The data obtained from the ECDC are gathered from laboratories and hospitals in countries using procedures that aim to harmonise data collection and management methodologies across countries (ECDC, 2022[3]).
The results presented in this chapter should be considered as conservative. While many OECD countries and EU/EEA countries made efforts in recent years to strengthen AMR surveillance, detection and reporting capacity, notable cross-country differences persist (see Chapter 4). It is important to note that these differences can mean that countries with more accurate reporting systems may show a higher AMR burden because they face a lower risk of under-reporting. Considering this, OECD estimates, both in terms of the overall AMR burden and cross-country comparisons, may differ from estimates generated by other analyses using different data sources. For example, one recent academic analysis that combined data from various sources (e.g. data that are not publicly available, literature reviews and other sources) suggested a significantly higher incidence of infections and the order of countries in terms of their AMR burden differed from those presented in this chapter (Mestrovic et al., 2022[4]).
The chapter starts by providing a brief overview of the current evidence describing the burden of AMR from a One Health perspective and it highlights major knowledge gaps in the current literature. Next, it describes the OECD Strategic Public Health Planning for AMR (SPHeP-AMR) model and presents results across four main areas: i) human population health; ii) healthcare resources and expenditure; iii) labour markets and workforce productivity; and iv) national GDP and fiscal pressure. Next, the chapter discusses how estimates from the OECD compare against other major modelling-based analyses. The final section summarises key takeaways and discusses their policy implications.
In line with the One Health approach, a growing body of evidence emerging from multiple sectors sheds light on the health and economic burden of AMR
Estimating the AMR burden requires acknowledging and measuring the impacts of relationships between human, animal and environmental sectors with respect to AMR emergence and spread. Increasingly understood to be a multi-sectoral problem with resistant organisms evolving and spreading through a complex ecosystem of shared human, animal and environmental habitats, recommended approaches to estimating the burden of AMR have shifted towards a One Health perspective (Hernando-Amado et al., 2019[5]; Thakur and Gray, 2019[6]; Prestinaci, Pezzotti and Pantosti, 2015[7]). This perspective recognises the interconnectedness and emphasises unified efforts across human, animal and environmental sectors to combat the spread of resistant bacteria and resistance determinants across borders (FAO and WHO, 2021[8]). One positive fact is that the literature that examines the health and economic burden of resistant infections has grown since previous OECD analysis. However, most of these studies focused on human health, estimating the AMR burden from the patient, healthcare system or economic perspectives. However, there remains a dearth of evidence that examines the complex relationships between human, animal and plant health, as well as the role of the environment in the emergence and spread of AMR.
In the human health sector, several studies emerged since the 2018 OECD analysis that provided estimates on the health and economic burden of AMR across the globe and among OECD and EU/EEA countries. One study attempted to estimate the global burden of AMR for 23 pathogens and 88 pathogen-drug combinations across 204 countries and territories in 2019 (Murray et al., 2022[9]). By gathering data from systematic literature reviews, hospital systems, surveillance systems and other sources, this study found that in a scenario that assumes susceptible infections would replace resistant infections, an estimated 1.27 million (95% uncertainty interval [UI] 0.911‑1.71) deaths would be attributable to AMR. In an alternative scenario in which resistant infections are eliminated entirely, about 4.95 million (95% UI 3.62‑6.57) deaths would be associated with resistant infections. The study pointed to substantial cross-country variation in deaths due to AMR. This study also concluded that 6 pathogens, combined, accounted for nearly three‑quarters of deaths attributable to bacterial AMR, including E. coli, S. aureus, K. pneumoniae, Streptococcus pneumoniae (S. pneumoniae), Acinetobacter baumannii (A. baumannii) and Pseudomonas aeruginosa (P. aeruginosa).
Several studies with a focus on the EU/EEA countries also emerged. In 2019, one population-level modelling analysis estimated the incidence of infections with 16 antibiotic-bacterium combinations across the EU/EEA countries, using data gathered from the European Antimicrobial Resistance Surveillance Network (EARS-Net) in 2015 (Cassini et al., 2019[10]). This study found that the estimated number of resistant infections reached 671 689 (95% UI 583 148‑763 966) in 2015. These infections are estimated to lead to around 33 110 (95% UI 28 480‑38 430) deaths and around 874 541 (95% UI 768 837‑989 068) DALYs. Similar to earlier OECD work, this study also found that the highest burden was among children under the age of 1 and the elderly population aged 65 and more. The highest AMR burden was estimated to be in Greece and Italy both in terms of deaths and DALYs attributable to resistant infections.
Subsequently, the European Antibiotic Resistance Collaborators estimated deaths and DALYs attributable to and associated with AMR in 2019 (Mestrovic et al., 2022[4]). This study replicated the modelling framework used in the 2019 global burden of AMR study (Murray et al., 2022[9]) and provided estimates for two separate counterfactual scenarios. In a scenario where resistant infections are replaced with susceptible ones, the number of deaths attributable to resist infections was estimated to reach around 133 000 (95% UI 90 100‑188 000). In an alternative scenario, where resistant infections would be eliminated entirely, about 541 000 deaths (95% UI 370 000‑763 000) were estimated to be associated with resistant infections. This study also concluded that around 84% of deaths were due to seven bacteria including E. coli, S. aureus, K. pneumoniae, P. aeruginosa, Enterococcus faecium (E. faecium), S. pneumoniae and A. baumannii.
Most recently, the ECDC provided new estimates for infections with 12 bacterium-antibiotic resistance combinations in the EU/EEA countries between 2016 and 2020 (ECDC, 2022[11]). This analysis found that the number of resistance infections ranged from around 685 433 (95% UI 589 451‑792 873) in 2016 to 865 767 (95% UI 742 802‑1 003 591) in 2019. Coupled with this trend were deaths due to resistant infections ranging from 30 730 (95% UI 26 935‑34 836) in 2016 to 38 710 (95% UI 34 053‑43 748) in 2019. The ECDC analysis highlighted that there were slight declines in the number of infections, attributable deaths and DALYs between 2019 and 2020, though this study also noted that the observed changes in health outcomes may be explained partly by the changes in surveillance practices and the provision of healthcare services in part driven by the COVID‑19 pandemic. Moreover, some of the declines in the observed trends between 2019 and 2020 may be attributable to the measures put in place to curb the COVID‑19 pandemic, including infection prevention and control (IPC) measures, and changes in the mix of patients treated in hospitals throughout the outbreak. Much like earlier studies, the ECDC analysis also showed that the age‑specific burden was the greatest in infants and older adults. The overall burden of infections was estimated to be the highest in Greece, Italy and Romania after adjusting for differences in population size.
In the United States, the Centers for Disease Control and Prevention (CDC) have been regularly publishing technical reports that assess the impact of AMR in the country. In 2013, one CDC analysis quantified that the overall cost of AMR to the US economy was estimated to be around USD 20 billion in direct healthcare costs and an additional USD 35 billion in additional costs due to loss of productivity (CDC, 2013[12]). Following this analysis, one 2019 CDC analysis focused on 18 pathogens and found that around 2.8 million resistant infections occurred annually in the United States and the number of deaths due to AMR exceeded 35 000 each year (CDC, 2019[13]). This analysis also showed that deaths due to AMR declined by 18% between 2012 and 2017, with the size of this reduction reaching around 28% for deaths occurring in hospital settings. In 2022, the CDC published a special report that estimated the impact of COVID‑19 on AMR (CDC, 2022[14]). Despite a number of challenges that may have hindered the precision of estimates, the CDC analysis found a 15% increase in the number of resistant infections between 2019 and 2020, with particularly alarming rising trends in infections due to carbapenem-resistant Acinetobacter (78%), antifungal-resistant Candidate auris (60%) and carbapenem-resistant Enterobacterales (35%). This analysis also noted that the COVID‑19 pandemic led to a rise in antibiotic use and challenges in adhering to IPC guidelines. Combined, these factors led to an increase in healthcare‑acquired infections (HAIs) and resistant infections in hospitals across the United States (CDC, 2022[14]).
In Canada, the Council of Canadian Academies (CCA) looked at the impact of AMR on population health and the economy (CCA, 2019[15]). This work estimated that AMR was associated with a reduction in labour productivity amounting to CAD 2 billion in 2018, with pronounced effects across industries including hospitality, transportation and education, as well as agriculture. Further, this study concluded that AMR was associated with longer hospital stays, prolonged courses of treatment and a rise in other expenses to the healthcare system. Combined, these costs amounted to about CAD 1.4 billion in 2018. This report also estimated that if resistance to first-line antimicrobials stayed constant at around 26% or rose to 40% by 2050, the cost of AMR to the Canadian economy would range between CAD 13 billion to CAD 21 billion every year and the annual cost to the Canadian health system would range from CAD 6 billion to CAD 8 billion respectively (CCA, 2019[15]).
In the animal sector, the emerging evidence suggests that the use of veterinary antimicrobials imposes significant health and economic costs on society. As discussed in more detail in Chapters 2 and 5, several studies showed that restrictions on antimicrobial use in animals have been associated with decreases in resistant bacteria in humans (Innes et al., 2019[16]; Scott et al., 2018[17]; Tang et al., 2017[18]). New evidence has also been emerging to provide estimates of how much AMR in animals contributes to AMR in humans. For instance, one recent analysis carried out jointly by the ECDC, European Food Safety Authority and European Medicines Agency explored the potential associations between antibiotic consumption and AMR in humans and food-producing animals, using data from various surveillance and monitoring networks (ECDC/EFSA/EMA, 2021[19]). Results from this work suggested, for instance, the consumption of fluoroquinolones and other quinolones in food-producing animals like pigs and veal indirectly contributed to resistance to fluoroquinolones in invasive E. coli from humans. Other studies suggested, however, that reducing the consumption of veterinary antimicrobials alone is unlikely to be sufficient to limit the burden of AMR in humans (van Bunnik and Woolhouse, 2017[20]; Booton et al., 2021[21]).
With respect to the environmental sector, resistant pathogens that contaminate natural ecosystems have been suggested to have an impact on human health. Such pathogens are primarily introduced to the environment by the release of human or animal waste (Hernando-Amado et al., 2019[5]; Karkman, Pärnänen and Larsson, 2019[22]). Research has identified contaminated drinking water to be an important vehicle of spread from the environment to humans (Hernando-Amado et al., 2019[5]; Yang et al., 2017[23]). Because of the role of waterways and water systems in the transfer of resistant pathogens from the environment to humans, efforts have focused on different types of wastewater treatment programmes to reduce the number of pathogens in water systems (Rodríguez-Chueca et al., 2019[24]; Jojoa-Sierra et al., 2017[25]; Paulus et al., 2019[26]). However, their downstream effects on human health and the burden of AMR remain largely unknown.
The OECD SPHeP-AMR model
The OECD SPHeP-AMR model is a microsimulation model that simulates the emergence and spread of AMR by replicating transmission dynamics in the health sector and the community in 34 countries using a One Health framework (Box 3.1). Broadly, the OECD model aims to:
Build a business-as-usual model to quantify the health and economic impact of AMR.
Forecast these impacts in the long term and generate a cost-effectiveness model that evaluates the potential impact of AMR-relevant interventions by comparing the effectiveness of scaling up these interventions against the business-as-usual scenario.
The remainder of this chapter focuses on the first objective whereas methodologies and results related to the second objective are presented in Chapter 6.
Box 3.1. The OECD SPHeP-AMR model
In line with the One Health approach, the OECD model includes an expanded list of priority antibiotic-bacterium pairs, covering infections with significant environmental and zoonotic reservoirs (Table 3.1). The selection of infective agents reflects expert advice based on the disease burden, policy priorities and data availability in the 34 countries included in the analysis. Some infections considered in the model can be both hospital- and community-acquired and they can be resistant to multiple antibiotics.
Table 3.1. Pathogens included in the model
|
Pathogens |
Strain characteristics |
Setting |
|
|---|---|---|---|
|
Healthcare |
Community |
||
|
Acinetobacter spp. |
Acinetobacter spp. excluding isolates with resistance to carbapenem and/or fluoroquinolones |
x |
|
|
Acinetobacter spp. with resistance to carbapenem |
x |
||
|
Acinetobacter spp. with multidrug resistance (i.e. three or more of piperacillin ± tazobactam, fluoroquinolones, ceftazidime and aminoglycosides) excluding carbapenem |
x |
||
|
Campylobacter jejuni (C. jejuni) & Campylobacter coli (C. coli) |
C. jejuni and C. coli excluding isolates with resistance to fluoroquinolones and macrolides |
x |
|
|
C. jejuni and C. coli with resistance to fluoroquinolones |
x |
||
|
C. jejuni and C. coli with resistance to macrolides |
x |
||
|
Enterococcus faecalis (E. faecalis) & Enterococcus faecium (E. faecium) |
E. faecalis and E. faecium excluding vancomycin-resistant isolates |
x |
|
|
E. faecalis and E. faecium resistant to Vancomycin |
x |
||
|
Escherichia coli (E. coli) |
E. coli excluding isolates with resistance to third-generation cephalosporins and/or carbapenems |
x |
x |
|
E. coli. with resistance to carbapenem |
x |
x |
|
|
E. coli with resistance to third-generation cephalosporins excluding carbapenem |
x |
x |
|
|
Klebsiella pneumoniae (K. pneumoniae) |
K. pneumoniae excluding isolates with resistance to third-generation cephalosporins and/or carbapenems |
x |
x |
|
K. pneumoniae with resistance to third-generation cephalosporins excluding carbapenem |
x |
x |
|
|
K. pneumoniae with carbapenem resistance |
x |
||
|
Mycobacterium tuberculosis (M. tuberculosis) |
M. tuberculosis excluding isolates with multidrug resistance (i.e. at least isoniazid and rifampin) and extensive drug resistance (i.e. isoniazid, rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs: amikacin, kanamycin or capreomycin) |
x |
|
|
M. tuberculosis with multidrug resistance (i.e. at least isoniazid and rifampin) excluding extensive drug resistance |
x |
||
|
M. tuberculosis with extensive drug resistance (i.e. isoniazid, rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs: amikacin, kanamycin or capreomycin) |
x |
||
|
Pseudomonas aeruginosa (P. aeruginosa) |
P. aeruginosa excluding isolates with carbapenem resistance and/or resistance to three or more of piperacillin ± tazobactam, fluoroquinolones, ceftazidime and aminoglycosides |
x |
|
|
P. aeruginosa with carbapenem resistance |
x |
||
|
P. aeruginosa with multidrug resistance (i.e. three or more of piperacillin ± tazobactam, fluoroquinolones, ceftazidime and aminoglycosides) excluding carbapenem |
x |
||
|
Salmonella spp. |
Salmonella spp. excluding isolates with resistance to fluoroquinolones, cephalosporins and resistance to three or more of ampicillin, chloramphenicol, streptomycin, sulphonamides and/or tetracycline |
x |
|
|
Salmonella spp. with resistance to fluoroquinolones |
x |
||
|
Salmonella spp. with multidrug resistance (i.e. three or more of ampicillin, chloramphenicol, streptomycin, sulphonamides and/or tetracycline, and/or cephalosporins) excluding fluoroquinolones |
x |
||
|
Staphylococcus aureus (S. aureus) |
S. aureus excluding methicillin-resistant Staphylococcus aureus (MRSA) isolates |
x |
x |
|
Methicillin-resistant S. aureus (MRSA) |
x |
x |
|
|
Streptococcus pneumoniae (S. pneumoniae) |
S. pneumoniae excluding isolates with single penicillin resistance and combined resistance to penicillins and macrolides |
x |
|
|
Penicillin-resistant S. pneumoniae excluding macrolide‑resistant isolates |
x |
||
|
S. pneumoniae with combined penicillin and macrolide resistance |
x |
||
Individual models were run separately for 34 countries: 29 EU/EEA countries and Japan, Switzerland, Türkiye, the United Kingdom and the United States. For each country, the OECD SPHeP-AMR model was initialised using country-aggregated data collated across multiple datasets available in the public domain and national datasets:
Demographic characteristics of populations: The SPHeP-AMR model simulates dynamic populations based on historical and projected birth, migration and mortality rates available by sex and age. Data informing the composition of these populations for each country examined in the model were obtained from the Human Mortality Database maintained by researchers in Germany and the United States with the support of collaborators from around the world.
Infection epidemiology for each antibiotic-bacterium pair: The ECDC provided epidemiological data on the antibiotic-bacterium pairs analysed in 29 EU/EEA countries (ECDC, 2022[11]). Data for Japan, Switzerland and the United States was provided by local experts contacted via national delegates to the OECD Expert Group on the Economics of Public Health. Data for Türkiye were provided by the World Health Organization (WHO) Regional Office for Europe.
Hospital resource use: Statistics on the use of hospital resources and related expenditure were sourced from OECD.Stat and other international datasets such as Eurostat. The likelihood of hospitalisation by age and gender was derived from an OECD analysis.
Labour market and productivity indicators: Data on employment and related labour market features were obtained from Eurostat and the International Labour Organization (ILO) databases. Data informing measures of absenteeism and presenteeism to compute changes in labour productivity were obtained based on a review of the published academic literature.
Analyses were conducted from a societal perspective, considering direct and indirect healthcare costs as well as costs arising from losses in labour productivity among infected individuals.
Health impacts capture mortality and morbidity due to infections caused by resistant organisms and are measured through the number of deaths due to AMR, losses in life expectancy (LE) and healthy life expectancy (HALE), LYs and DALYs lost due to AMR. Economic impacts quantify the number of additional days spent in hospital, attributable health expenditure, employment rate, rate of absenteeism and presenteeism, and GDP.
Little evidence exists to assess the extent to which resistant infections could be eliminated or replaced by infections that are susceptible to antibiotics. Recognising this, and after extensive expert consultation, the OECD SPHeP-AMR model makes use of two scenarios to assess the health and economic impact of AMR:
In a first scenario, the replacement scenario, the total incidence of infections (i.e. incidence of susceptible infections and incidence of resistant infections) is maintained constant, while the prevalence of resistant infections is set to zero. In practice, this means that all resistant infections are completely replaced by susceptible infections. This scenario assumes that bacteria can no longer develop resistance and people that were infected by resistant bacteria continue being infected by bacteria that are susceptible to antibiotics. Outputs from this scenario are more conservative because susceptible bacteria increase the risk of complications and deaths but less than resistant bacteria.
In a second scenario, the elimination scenario, the incidence of susceptible infections is maintained constant while the incidence of resistant infections is set to zero which results in a complete elimination of all resistant infections. This scenario uses the classical burden of disease approach and assumes that antibiotic-resistant bacteria no longer exist. In practical terms, the scenario evaluates how assessed outcomes change resulting from a fictitious elimination of the risk factor and, consequently, of all its consequences.
The elimination scenario can be considered as an optimistic option whereas the replacement scenario is a pessimistic alternative. The results generated using these two scenarios are significantly different from one another due to the lower but still significant burden caused by susceptible infections. Both scenarios are considered plausible given the lack of any concluding evidence in the literature on which scenario is more likely to occur in case of elimination of AMR. By adopting a multiple-scenario modelling approach, the OECD analysis aims to improve the usefulness of results for identifying policy options and interventions that offer the most cost-effective means to tackling AMR. The elimination scenario might be more useful to policy makers when evaluating the impact of interventions that would prevent resistant infections from emerging in the first place, such as improving vaccination coverage, and IPC measures like improving hand hygiene and enhancing environmental hygiene. Whereas the replacement scenario may be more useful when evaluating the effectiveness of interventions whose scale-up might result in a swap of susceptible and resistant bacteria rather than the elimination of resistant bacteria. These interventions may include antimicrobial stewardship programmes in healthcare settings, financial incentives that promote the prudent use of antimicrobials, provider training and education and others.
Propagating uncertainty
The simulation uncertainty is calculated on independent runs of 20 random subsamples for both business-as-usual and the two counterfactuals (i.e. elimination scenario and replacement scenario).
Note: More information on the key model assumptions and model structure are available at http://oecdpublichealthexplorer.org/amr-doc/.
The burgeoning burden of AMR on population health
Nearly 4.3 million resistant infections occur each year across OECD countries and EU/EEA countries
Resistant infections present a considerable threat to population health. Nearly 4.3 million infections are estimated to occur each year in the 34 countries included in the OECD analysis due to bacteria that are resistant to antimicrobial treatment (Figure 3.1). Almost 1.7 million of these infections occur across the EU/EEA countries. These aggregate figures, however, mask important cross-country differences. Across the EU/EEA countries, the Czech Republic appears to have the highest estimated burden of resistant infections as measured by resistant infection rates, followed by Italy and Luxembourg. Across non-EU/EEA member OECD countries, Switzerland is estimated to have the greatest annual rate of resistant infections, whereas Japan has the lowest annual rate of resistant infections averaging at nearly 291 per 100 000 persons. However, as discussed earlier, these cross-country differences in the burden of resistant infectious should be interpreted with care, considering that countries with stronger AMR surveillance systems risk a greater likelihood of a lower under-reporting of their true burden.
Figure 3.1. The number of resistant infections reaches nearly 4.3 million each year across the 34 countries included in the OECD analysis
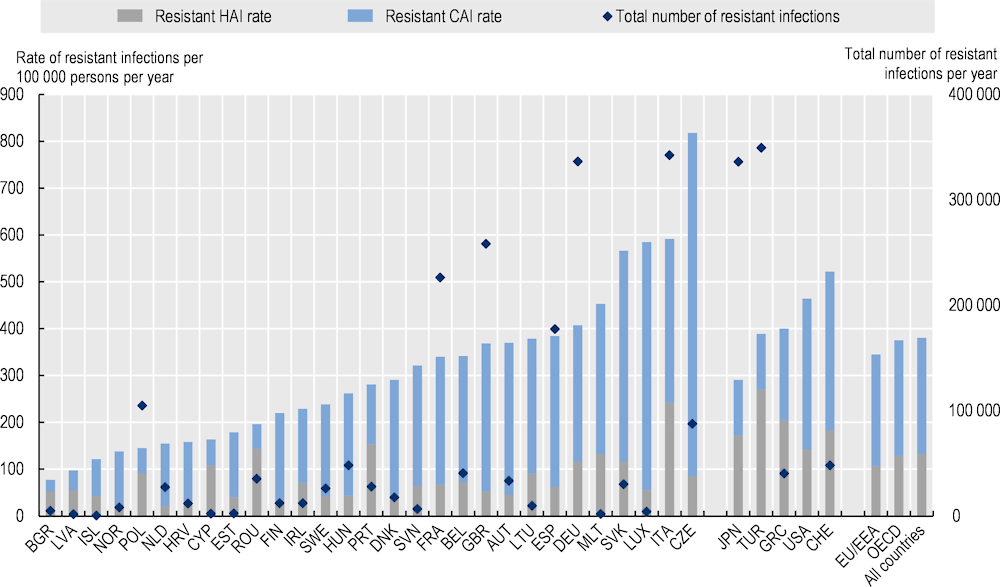
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices. In the USA, the total number of resistant infections is estimated to exceed 1.6 million per year.
CAI: Community-acquired infection; HAI: Hospital-acquired infection.
Source: OECD analysis based on the OECD SPHeP-AMR model.
On average, around two in three resistant infections are acquired in the community (Figure 3.1). The OECD analysis suggests that, on average, around 65% of all resistant infections that occur each year are estimated to be acquired in community settings across all 34 countries whereas the remainder of these infections are acquired in healthcare settings. The share of community-acquired resistant infections appears to be slightly higher across the EU/EEA countries. On average, around 69% of resistant infections are estimated to be acquired in community settings across the EU/EEA countries.
Importantly, the share of resistant infections that occur in healthcare settings varies greatly across countries. Around seven out of every ten resistant infections in Bulgaria and Romania are estimated to occur in healthcare settings whereas, in the Czech Republic and Luxembourg, only around 10% of infections are estimated to be acquired in healthcare settings. In Türkiye, around 70% of resistant infections are estimated to occur in healthcare settings, (the highest across non-EU/EEA member OECD countries) whereas the United States has the lowest share of resistant infections occurring in healthcare settings. Understanding these cross-country differences in terms of the setting in which resistant infections occur is crucial to inform the selection of interventions that are best fit to support the OECD countries and EU/EEA countries in their continued efforts to tackle AMR in accordance with their unique country context.
Resistant infections claim the lives of tens of thousands of citizens in OECD and EU/EEA countries every year
Every year, tens of thousands of lives are lost due to resistant infections across the 34 EU/EEA and OECD countries included in the analysis (Figure 3.2). Using the elimination scenario, resistant infections are estimated to claim the lives of around 79 000 people, on average, each year up to 2050 across all of the countries included in the analysis. Of these deaths, nearly 22 000 are estimated to occur in the EU/EEA countries. The estimated death toll due to resistant infections is lower using the replacement scenario whereby the number of deaths due to resistant infections exceeds 24 000 every year up to 2050 across the 34 countries included in the analysis, with around 6 000 of these deaths occurring across the EU/EEA countries.
The mortality rate due to resistant infections varies substantially across countries, with countries in southern Europe facing a greater AMR burden. Similar to the previous OECD analysis (2018[2]), the new OECD analysis suggests an important geographic gradient in the mortality burden of AMR. Across the EU/EEA countries, Italy and Portugal are generally estimated to have the highest annual mortality burden due to AMR – both in terms of mortality rate and the total number of deaths due to AMR – using both modelling scenarios. For instance, in Italy, the annual mortality rate due to resistant infections is estimated to average nearly 11 deaths per 100 000 inhabitants under the elimination scenario and around 3.4 deaths per 100 000 inhabitants using the replacement scenario. Across non-EU/EEA member OECD countries, Türkiye is estimated to have the highest mortality rate attributable to resistant infections using both modelling scenarios.
Cross-country variation in the mortality estimates should be interpreted with caution. Several factors help explain the differences in estimated mortality rates across countries. First, certain countries have made laudable efforts in recent years to improve AMR surveillance, detection and reporting. As discussed earlier, it is expected that countries that have been investing in strengthening their AMR surveillance systems are able to track the incidence of infections at a higher rate than some of the other countries included in the analysis. For example, Switzerland’s AMR monitoring system encompasses humans, animals, agriculture and the environment. In human health, the Swiss Sentinel Surveillance Network enables the collection of epidemiological data and the use of antibiotics (Bell and Nuzzo, 2021[27]). Similarly, Türkiye has prioritised the strengthening of disease surveillance capacity and healthcare data quality in its large‑scale healthcare reforms since 2003 (WHO, 2022[28]). In terms of AMR surveillance, Türkiye established a national antimicrobial resistance surveillance system that collects data on antimicrobial resistance, antibiotic consumption and HAIs and reports data to the Central Asian and European Surveillance of Antimicrobial Resistance network, which uses the same methodology as the EARS-Net (Bell and Jennifer, 2022[29]). Another important factor that influences the cross-country differences in the mortality rates relates to the relative burden of resistant infections. As shown in Chapter 2, in 2019, resistance proportions in Türkiye were estimated to reach 46.5% and they are projected to remain the highest among OECD countries by 2035. Finally, population ageing in the next decade is expected to exacerbate the mortality burden due to AMR because the elderly population faces an elevated risk of death caused by resistant infections.
Across the 34 countries included in the analysis, the estimated number of yearly deaths due to AMR is comparable to the number of lives lost due to tuberculosis (TB), influenza and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) in 2020 or the nearest year for which information is available. The total number of deaths due to AMR that occur annually in the 34 countries included in the analysis is estimated to be around 2.4 times that of deaths due to TB, influenza and HIV/AIDS in these countries under the elimination scenario and 0.7 times under the replacement scenario. Across the EU/EEA countries, the total number of AMR-related deaths that occur each year is estimated to be 1.5 and 0.4 times that of the number of deaths attributable to TB, influenza and HIV/AIDS using the elimination and replacement scenarios respectively.
Figure 3.2. Annual mortality attributable to resistant infections varies greatly across countries
Annual total number of deaths and mortality rate due to AMR up to 2050
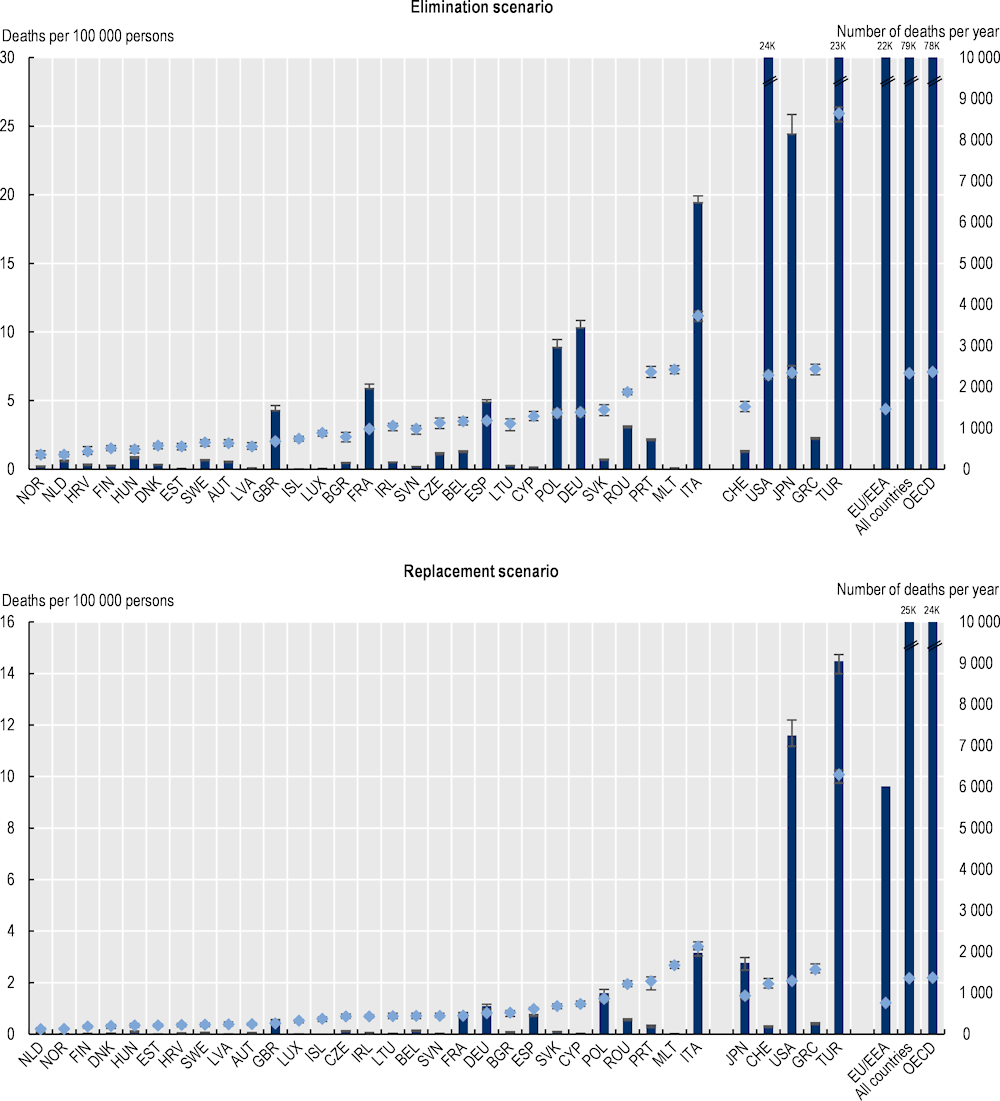
Note: Results for Greece are presented in the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
Source: OECD analysis based on the OECD SPHeP-AMR model.
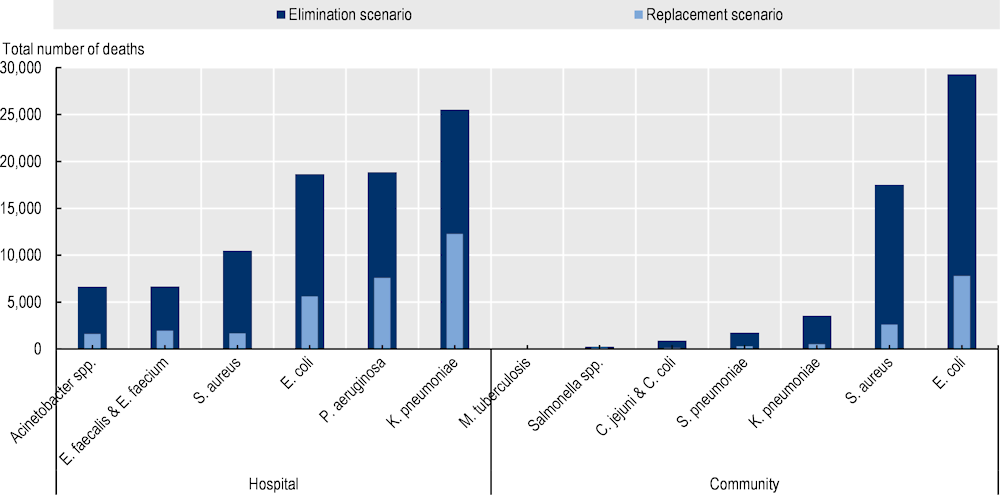
Three pathogens – E. coli, K. pneumoniae and S. aureus – are responsible for around three out of four deaths that occur each year due to resistant infections
Every year, nearly three in four deaths attributable to resistant infections are caused by E. coli, K. pneumoniae and S. aureus across all countries included in the analysis (Figure 3.3). E. coli, K. pneumoniae and S. aureus account for 75% of deaths due to resistant infections using the elimination scenario and 72% using the replacement scenario. In both scenarios, deaths due to E. coli represent about one‑third of all deaths due to resistant infections. In both scenarios, K. pneumoniae follows E. coli as the second leading pathogen that causes mortality, accounting for about 21% and 30% of all deaths due to resistant infections in the elimination and replacement scenarios respectively. In contrast, Salmonella spp., C. jejuni and C. coli represent a very small share of the mortality burden due to resistant infections across the 34 countries included in the analysis.
The burden of M. tuberculosis is also low across countries included in the analysis. While infections by these agents are well controlled in OECD countries, they remain top public health challenges elsewhere. For example, in 2019, diarrheal diseases were estimated to cause more than 1.5 million deaths worldwide. Diarrheal diseases were ranked as the third most common cause of death among children under five years of age, claiming more than half a million lives among children in this age group worldwide (Vos et al., 2020[30]). Similarly, it is estimated that, globally, tuberculosis killed 1.6 million people in 2021, with a significant part of these deaths attributable to resistant tuberculosis (WHO, 2022[31]). About two‑thirds of cases of resistant tuberculosis worldwide can be found in seven countries: China, India, Indonesia, Pakistan, the Philippines, the Russian Federation and South Africa (WHO, 2022[31]).
Figure 3.3. Around three in four deaths attributable to resistant infections occur annually due to E. coli, K. pneumoniae and S. aureus
Annual total number of deaths due to AMR up to 2050, by pathogen
HAIs are linked with the majority of annual deaths due to AMR
The OECD analysis further demonstrates that resistant HAIs account for the majority of annual mortality due to resistant infections across the 34 countries included in the analysis (Figure 3.3). Using the elimination scenario, infections acquired in hospital settings are estimated to represent around 62% of mortality due to resistant infections whereas under the replacement scenario, this figure reaches around 73%. This finding should be interpreted with care. In general, a greater proportion of infections acquired in healthcare settings are reported compared to those acquired in the community. This challenge data reporting is likely to influence the precision of the estimates.
In particular, K. pneumoniae, P. aeruginosa and E. coli acquired in healthcare settings pose a substantially greater risk of death compared to main community-acquired infections (Figure 3.4). For instance, K. pneumoniae acquired in healthcare settings is estimated to cause around only around 4% of all resistant infections each year and is estimated to account for 18‑29% of AMR-related deaths in the elimination and replacement scenarios respectively. Similarly, E. coli and P. aeruginosa combined account for less than one-fifth of infections but represent 27‑31% of deaths (in elimination and replacement scenarios respectively). Conversely, C. jejuni and C. coli occurring in community settings are estimated to cause more than 36% of all resistant infections (corresponding to more than 1.5 million infections) every year but account for less than 1% of all AMR-related annual deaths. Combined, these findings underline the importance of IPC measures to reduce the burden of HAIs.
Figure 3.4. K. pneumoniae, E. coli and P. aeruginosa in healthcare settings account for less than a quarter of all resistant infections but represent nearly 45‑60% of deaths due to AMR
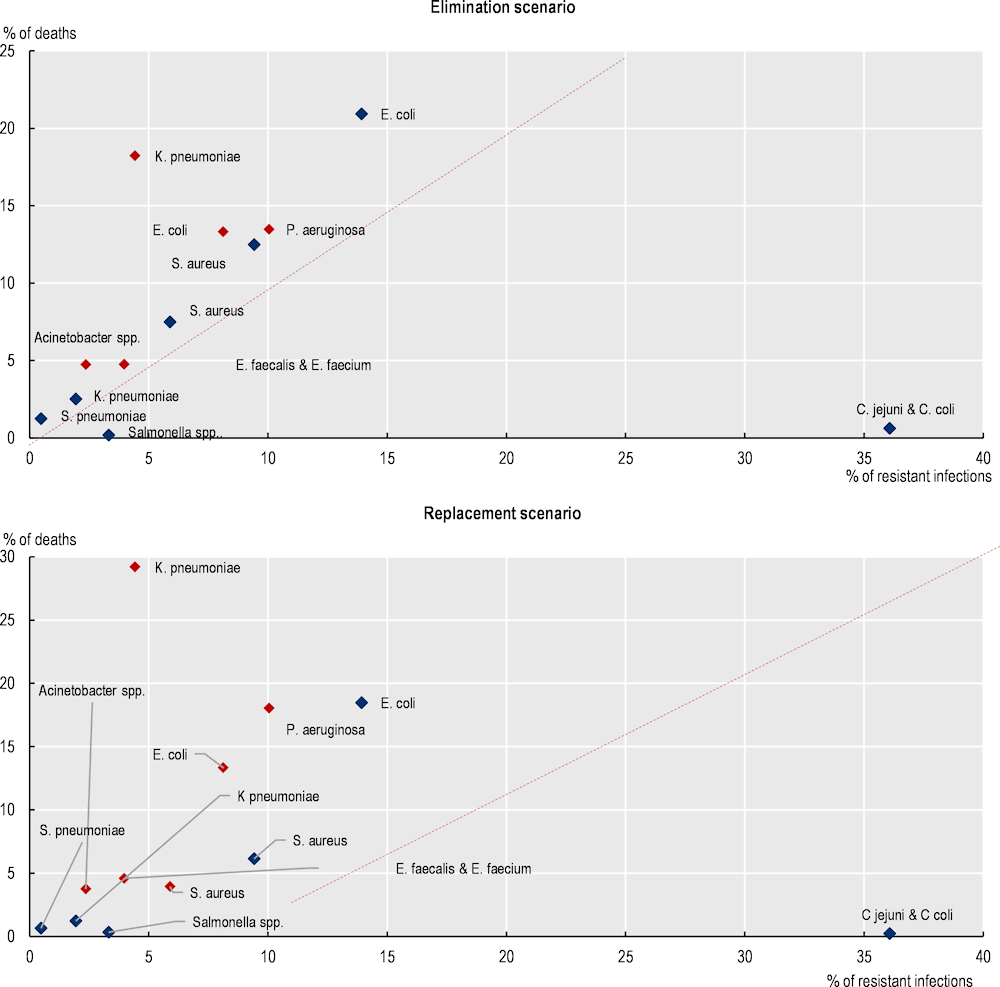
Notes: The colour red denotes HAIs and dark blue denotes CAIs.
Source: OECD analysis based on the OECD SPHeP-AMR model.
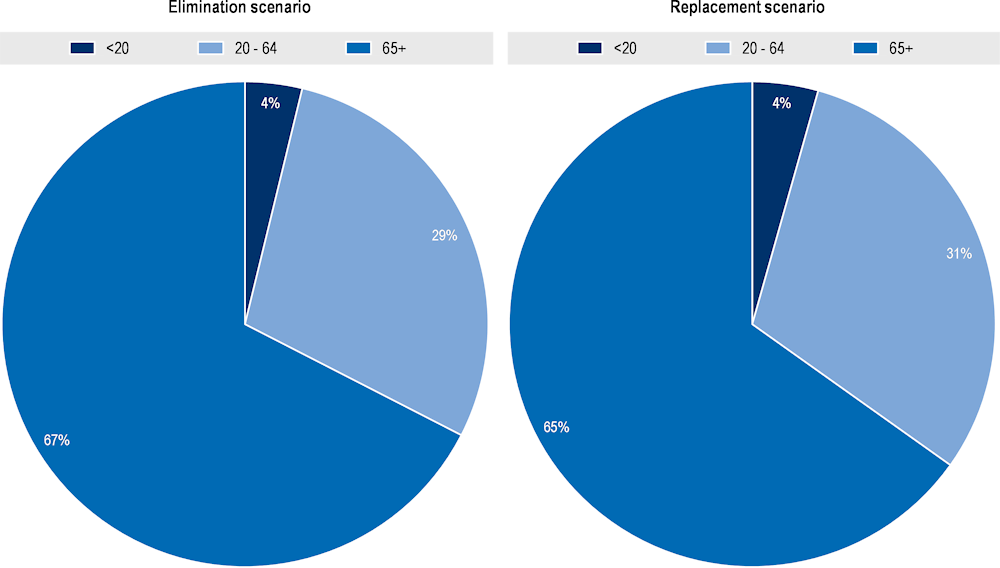
The elderly population face the greatest risk of death due to resistant infections
Deaths due to AMR are concentrated among the elderly populations across the 34 countries included in the analysis (Figure 3.5). The OECD analysis suggests that around 65‑67% of annual deaths due to AMR occur among people above 65 years of age in the replacement and elimination scenarios respectively. Working-age population between 20 and 64 years of age also face mortality risks due to AMR, with approximately 29‑30% of deaths occurring across people in this age group. About 4% of deaths due to AMR occur among people under 20 years of age. Findings from the analysis on the burden of AMR by age group are in line with several earlier analyses. For example, a 2022 study by the European Antimicrobial Resistance Collaborators provided age‑specific mortality rates in 2019 for each country in the WHO European region (Mestrovic et al., 2022[4]). Similar to the OECD analysis, this study also found that the majority of deaths due to AMR occurred in older age groups, with people above the age of 60 facing a greater risk of mortality compared to people below 60 years of age.
Figure 3.5. Annual number of deaths due to AMR by age groups, per year, up to 2050
The elevated risk of mortality due to resistant infections among the elderly is driven by several factors. Previous studies showed that immune response declines with age. Older adults do not respond to pathogens as efficiently as younger individuals even if they were exposed to the same pathogen before (Montecino-Rodriguez, Berent-Maoz and Dorshkind, 2013[32]; Keilich, Bartley and Haynes, 2019[33]). Coupled with weakened immune response, co-morbidities commonly observed among the older populations are linked to increased risk of infection (e.g. pulmonary diseases are associated with increased risk of pneumonia) while the presence of foreign materials (e.g. pacemakers, prostheses, etc.) can make them more vulnerable to medical device-associated infections (Beckett, Harbarth and Huttner, 2015[34]). It has been shown that diagnostic uncertainty is another pressing challenge for elderly patients. Previous studies demonstrated that the sensitivity of certain diagnostic procedures was lower for older adults than for younger individuals (Gavazzi and Krause, 2002[35]). Moreover, many infections have different clinical presentations among elderly adults compared to younger individuals (Beckett, Harbarth and Huttner, 2015[34]).
Preventing deaths due to AMR can translate into gains in life expectancy and healthy life expectancy in the next three decades
AMR is linked with reductions in life expectancy at birth (Figure 3.6). Using the elimination scenario, on average, life expectancy across the 34 OECD and EU/EEA countries included in the analysis is estimated to be 2.6 months lower due to AMR. Across the EU/EEA countries, the loss in life expectancy due to AMR is estimated to average around 1.6 months over the course of the projection period. Using the replacement scenario, life expectancy is 0.8 months lower due to AMR across the 34 countries included in the analysis and 0.4 months lower across the EU/EEA countries over the next 3 decades. Under both scenarios, Italy and Portugal are estimated to risk the greatest reductions in life expectancy across the EU/EEA countries. In contrast, the Netherlands and Norway are estimated to risk the smallest declines. Among non-EU/EEA member OECD countries, the largest predicted declines in life expectancy are estimated to be experienced by Türkiye.
These estimated losses in life expectancy due to AMR are not negligible, especially when compared to declines in life expectancy due to COVID‑19. Partly driven by the adverse shock of the COVID‑19 pandemic, life expectancy at birth declined by 7.5 months across the 34 countries included in the analysis and by 8 months across the EU/EEA countries between 2019 and 2020. This can potentially mean that, under the elimination scenario, loss of life due to AMR can reach as high as almost 35% of the reduction in life expectancy due to COVID‑19 across 34 countries included in the analysis and about 20% of potential losses across the EU/EEA countries. As expected, the potential losses in life expectancy due to AMR compared to the COVID‑19 pandemic is relatively modest using the replacement scenario, with these losses averaging around 11% for the 34 countries included in the analysis and around 6% for the EU/EEA countries.
AMR also lowers healthy life expectancy (HALE) – a key measure of population health that quantifies the average number of years each person is expected to live in full health by considering the number of years lived in less than full health due to disease or/and injury (Figure 3.6). Based on the elimination scenario, on average, AMR is estimated to lower HALE by 2.84 months by 2050 across the 34 countries included in the OECD analysis and by 1.7 months across the EU/EEA countries. Using the replacement scenario, AMR lowers HALE by 0.8 months across the 34 OECD and EU/EEA countries included in the analysis and 0.4 months lower across the EU/EEA countries over the next 3 decades. Across the EU/EEA countries, the greatest declines in HALE are expected to be observed in Italy and Portugal. Among non-EU/EEA member OECD countries, Türkiye is expected to experience the largest predicted reductions in HALE.
AMR is associated with reductions in years of life and the quality of life
Under the elimination scenario, a total of 1.5 million LYs are estimated to be lost due to AMR every year up to 2050 across the 34 countries included in the analysis, corresponding to around 133 LYs per 100 000 persons (Figure 3.7). Using the replacement scenario, about 454 000 LYs are estimated to be lost due to AMR annually up to 2050 across all 34 countries included in the analysis, corresponding to about 40 LYs per 100 000 persons. Using both scenarios, the deleterious impact of AMR on mortality is estimated to be lower across EU/EEA countries than the mortality estimates for all countries included in the analysis.
Resistant infections have important implications for quality of life as measured by the number of years lived with disability. Using the elimination scenario, across the 34 countries included in the analysis, on average, 1.8 million DALYs are estimated to be lost due to AMR every year up by 2050, corresponding to around 157 DALYs per 100 000 persons (Figure 3.7). Using the replacement scenario, all countries included in the analysis lose, on average, around 455 000 DALYs in total annually due to AMR, corresponding to around 40 DALYs per 100 000 persons.
Figure 3.6. AMR lowers life expectancy and healthy life expectancy
Total reduction in LE and HALE at birth due to AMR up to 2050
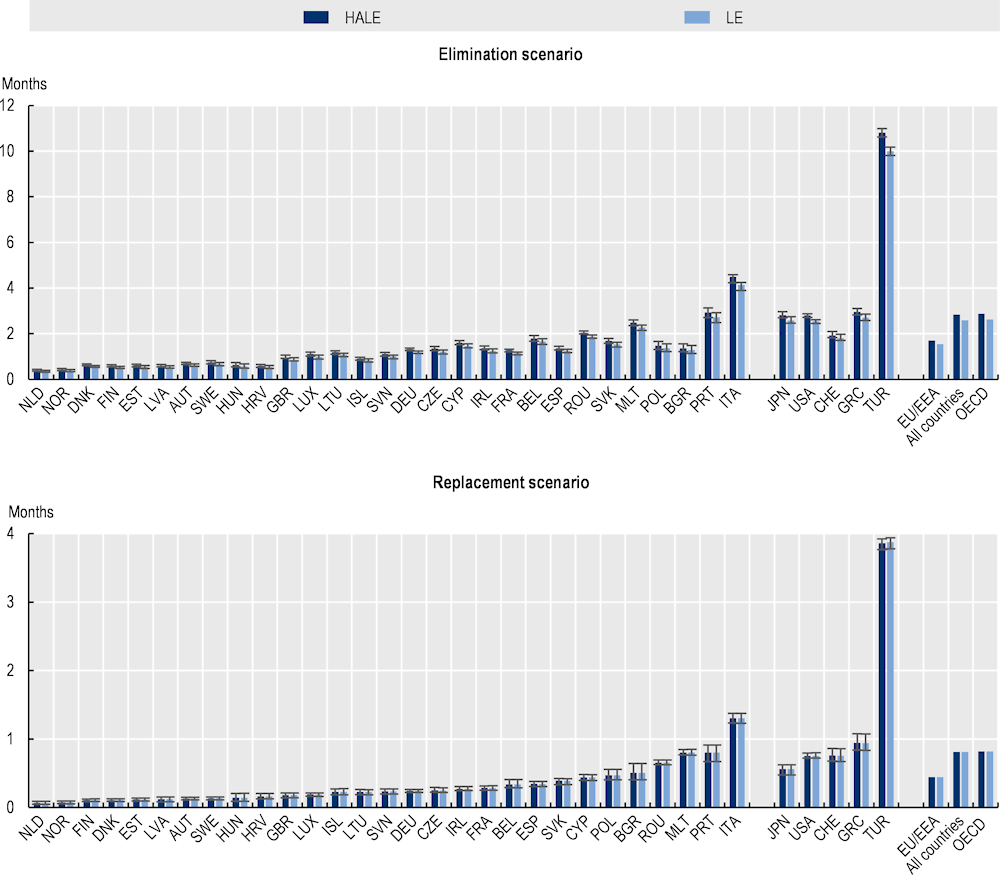
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
HALE: Healthy life expectancy; LE: Life expectancy.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Figure 3.7. AMR is associated with years of life lost and disability-adjusted life years each year up to 2050 across the 34 countries included in the OECD analysis
Average annual number of DALYS and LYs lost due to AMR up to 2050
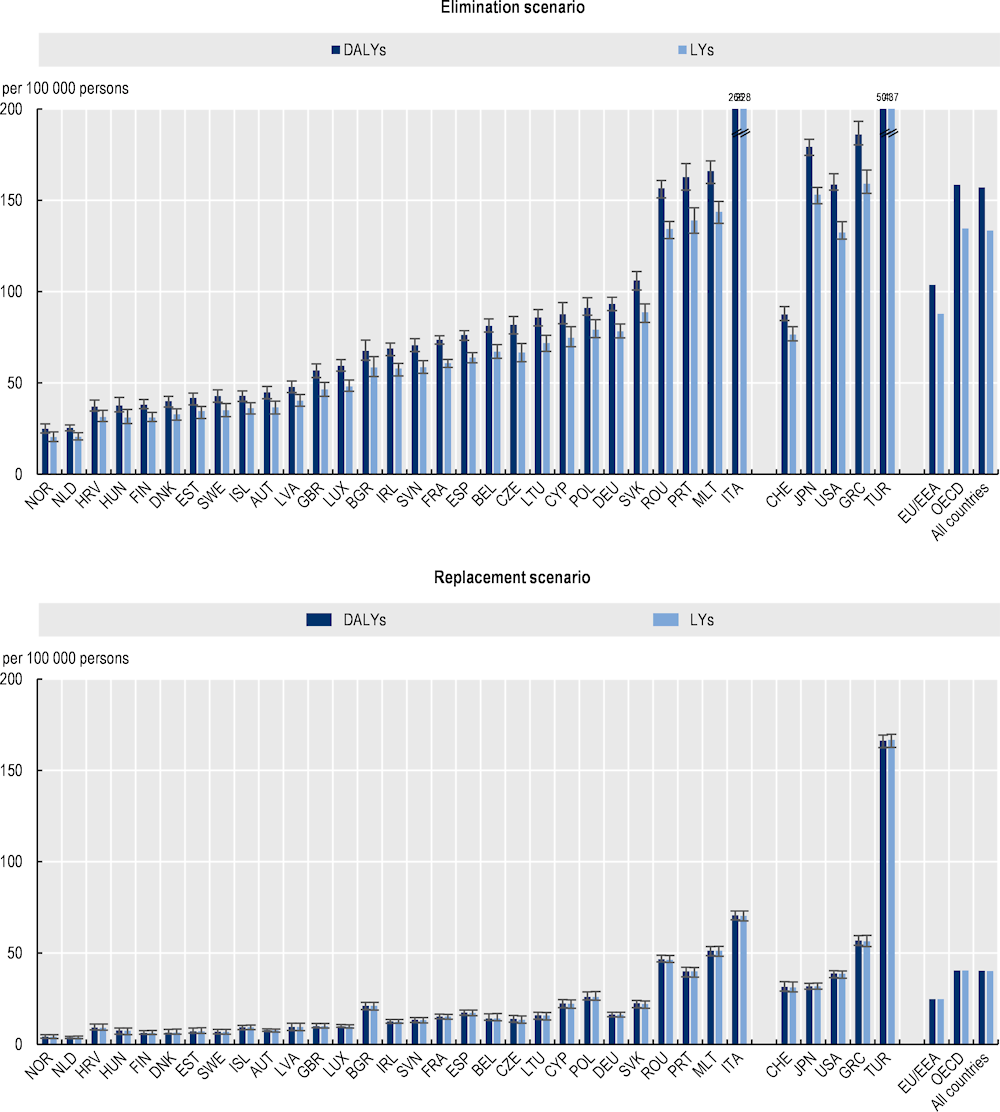
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
DALY: Disability-adjusted life‑years; LYs = Life years.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Impacts of AMR on healthcare resources and expenditure
Treating infections caused by resistant organisms translates into increased pressure on the use of hospital resources
Patients developing resistant infections typically require more intensive healthcare and are more likely to develop complications and spend a longer hospital stay if they require hospital care. The OECD analysis shows that resistant infections are estimated to result in nearly 32.5 million extra days spent in hospitals every year up to 2050 under the elimination scenario and 6.9 million extra days under the replacement scenario across the 34 countries included in the analysis (Figure 3.8). Across the EU/EEA countries, this figure stands at more than 9.5 million extra hospital days under the elimination scenario and about 2 million extra hospital days under the replacement scenario. These estimates suggest that extra days spent in hospitals annually for treating complications due to AMR across all 34 countries included in the analysis would be equivalent to using the entire acute bed capacity in Spain in 2020 for nearly 1 year under the elimination scenario and around 2 months under the replacement scenario.
Moreover, there are important cross-country differences in the estimates of use of hospital resources for treating resistant infections. Across the EU/EEA countries, Italy is expected to face the greatest pressure on its hospital resources in both modelling scenarios, as measured by the annual rate of hospital days per 100 000 persons up to 2050. On average, in Italy, about 4 608 additional days are estimated to be spent in hospitals per 100 000 persons annually for treating complications due to AMR up to 2050 using the elimination scenario and almost 1 040 additional days per 100 000 persons using the replacement scenario. Across non-EU/EEA member OECD countries, Türkiye is estimated to risk the highest annual rate of extra days spent in hospital due to AMR: by 2050, in Türkiye, the number of additional days spent in hospital each year is estimated to average around 6 018 days per 100 000 persons using the elimination scenario and 1 618 days per 100 000 persons under the replacement scenario. Variation in the incidence of infections across countries is the primary driver of these differences in varying levels of pressure caused by AMR on hospital resources, though other factors like variation in clinical practices in treating patients with resistant infections also play a role.
Figure 3.8. AMR puts additional pressure on hospital resources that were already overstretched over the course of the COVID‑19 pandemic
Annual total number of extra days spent in hospital due to AMR up to 2050
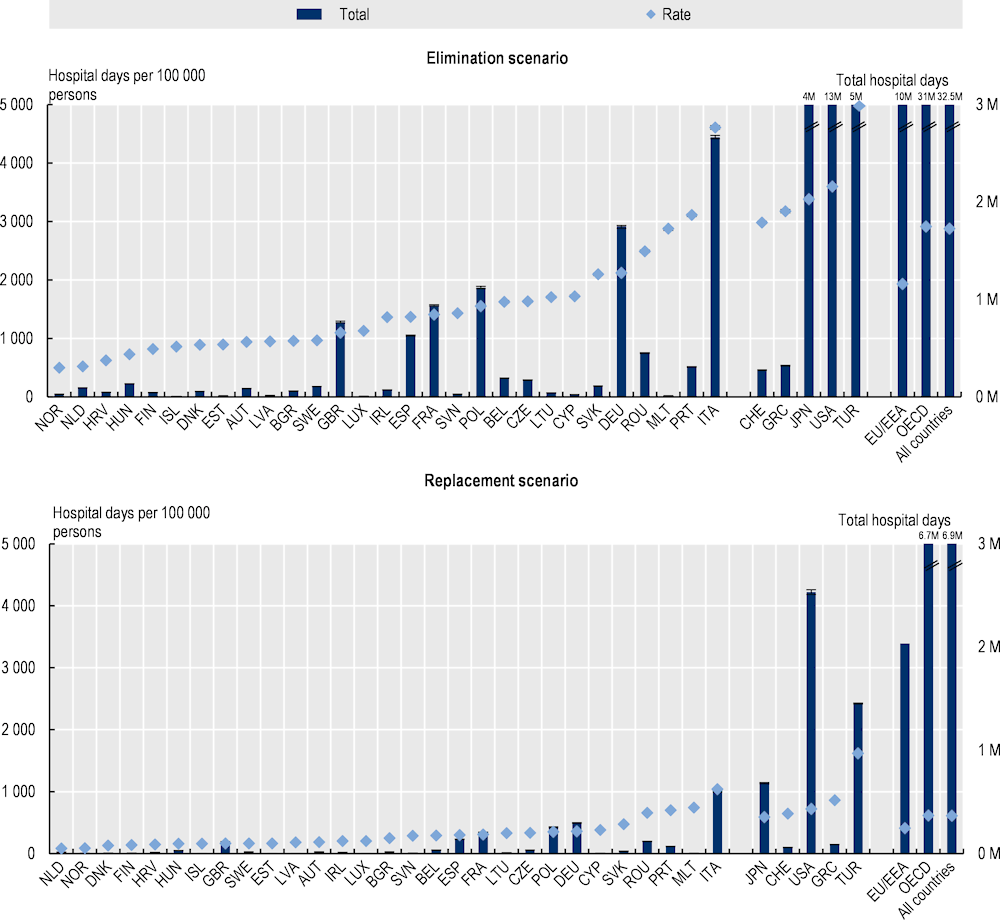
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
M: Millions.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Health care budgets in OECD and EU/EEA countries will face a substantial burden
Infections caused by antimicrobial-resistant pathogens are considerably more costly to treat compared to susceptible infections. Typically, treating complications caused by resistant infections necessitate a greater reliance on more intensive medical procedures, requires additional investigations such as advanced laboratory tests and prolonged stays in hospital. Health providers may need to rely on more expensive and aggressive therapies involving the use of second-line treatment or various combinations of antimicrobials to treat resistant infections.
Under the elimination scenario, the annual cost of treating complications caused by resistant infections is estimated to average more than USD 28.9 billion up to 2050 adjusting for PPP across all the countries included in the analysis, corresponding to nearly USD PPP 26 per capita (Figure 3.9). Across the EU/EEA countries, the cost of AMR to the health systems is estimated to reach around USD PPP 7.5 billion every year up to 2050 (see Annex 3.A for total hospital expenditure by country). This figure corresponds to around USD PPP 15.3 per capita. Using the replacement scenario, the annual cost of AMR is estimated to average around USD PPP 5.9 billion each year by 2050 across the 34 countries included in the analysis, corresponding to around USD PPP 5.2 per capita. Across the EU/EEA countries, the total spending on AMR averages around USD PPP 1.6 billion annually up to 2050, which is about USD PPP 3.2 per capita.
In both modelling scenarios, across the EU/EEA countries included in the analysis, Italy is estimated to spend the greatest amount of financial resources to treat complications each year due to resistant infections, both in terms of the total annual spending on AMR and per capita spending. Across non-EU/EEA member OECD countries, Switzerland and the United States are estimated to allocate the highest amount of financial resources each year to treating complications caused by resistant infections. These cross-country differences are driven both by the incidence of resistant infections and the cost of medical treatment.
The cost of inaction to tackle AMR in the next three decades will exceed treatment costs due to COVID‑19 in 2020. For the 17 OECD countries and EU/EEA countries for which data were available, the total health expenditure incurred each year due to AMR is about 19% of the total health expenditure due to treating COVID‑19 patients in 2020 using the elimination scenario and 4% using the replacement scenario. In effect, this means the cost of treating complications due to AMR incurred about every six years up to 2050 is estimated to be equivalent to the treatment costs associated with the COVID‑19 patients in 2020 using the elimination scenario and around 32 years using the replacement scenario.
Figure 3.9. AMR poses a substantial burden on the healthcare budgets
Annual per capita hospital expenditure incurred due to AMR up to 2050, USD PPP
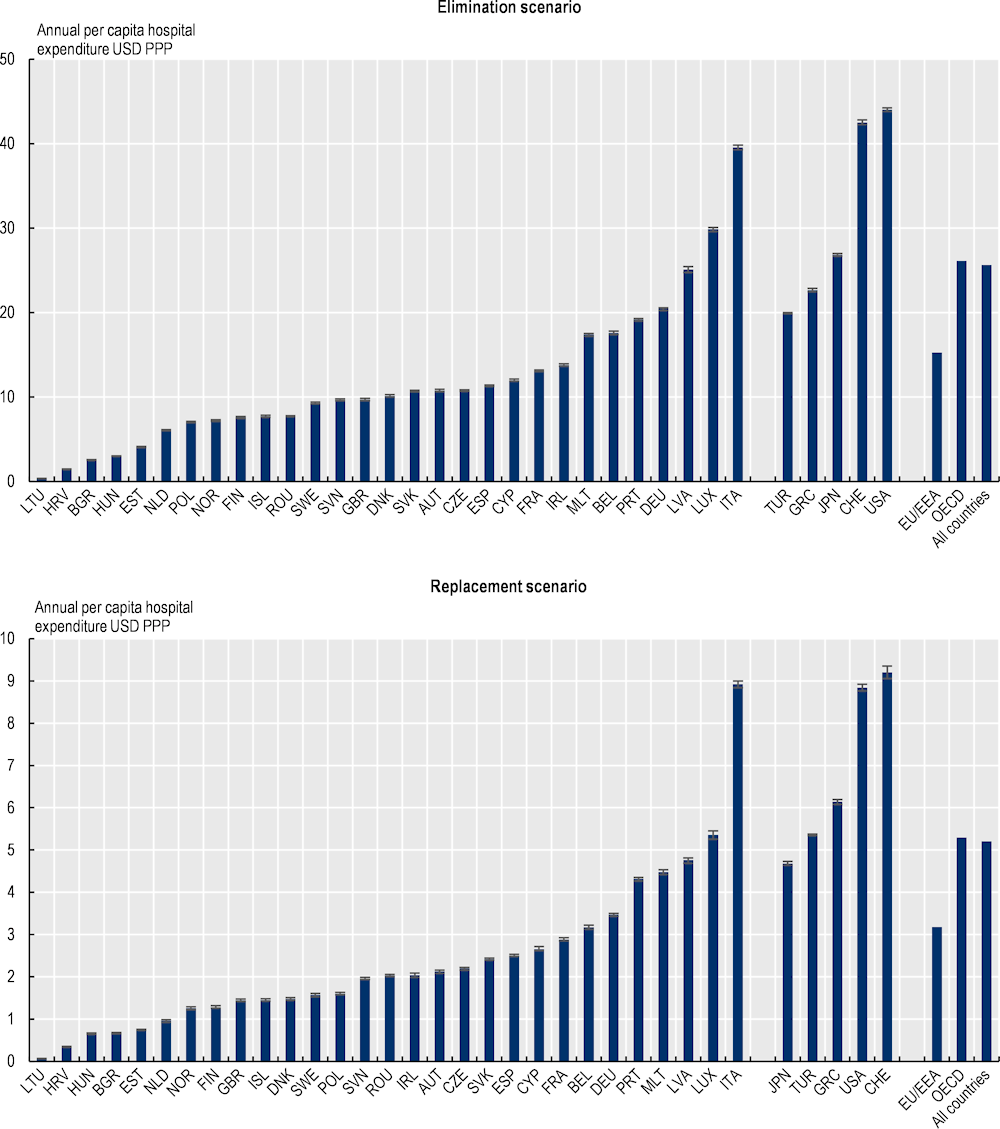
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Impact of AMR on participation in the workforce and productivity
The OECD SPHeP-AMR model also quantifies the impact of AMR on labour market output by assessing participation in the workforce and workforce productivity. Participation in the workforce is assessed through employment rate – an economic measure of labour supply which reflects the proportion of working-age population age that is employed. Workforce productivity refers to the extent to which labour output may be lost due to AMR either through absence from work due to ill health (i.e. absenteeism) or reduced productivity even though the employee is present at work (i.e. presenteeism). Combined, declines in participation in the workforce and productivity can have important consequences for the economy. Declines in the workforce mean productive workers are no longer in the workforce, resulting in lost economic output. Reductions in productivity – either through absenteeism or presenteeism – mean that wages are paid with no return in terms of productivity. In the OECD analysis, changes in labour supply and workforce productivity are translated to monetary losses using the human capital approach, whereby the duration of work foregone is multiplied by the national average wage.
Resistant infections have a deleterious impact on the labour markets and workforce productivity
Using the elimination scenario, the average labour market output (i.e. combination of participation in the workforce and productivity) is estimated to decline by 116 full-time equivalents (FTEs) per 100 000 working population every year due to infections caused by resistant pathogens across the 34 countries included in the OECD analysis, corresponding to about 0.12% decline in labour market output (Figure 3.10). Across the EU/EEA countries, the average yearly loss in labour market output stands at around 58 FTEs per 100 000 working population. This figure corresponds to about 0.06% decline in labour market output. Using the replacement scenario, the annual losses in labour market productivity are estimated to average around 18.7 FTEs per 100 000 working population across the 34 countries included in the analysis and 9.6 FTEs per 100 000 working population across EU/EEA countries. These figures correspond to around 0.02% and 0.01% decline in labour market output respectively. Much like previous results, the OECD analysis point to substantial cross-country variation in the labour market output lost due to AMR. Using both modelling scenarios, across EU/EEA countries, Italy and Romania are estimated to face the largest losses. Across other countries, the greatest losses to labour market output are estimated to take place in Türkiye and the United States.
Translating these figures at the population level underlines the urgency of continued action to stem the AMR tide. Using the elimination scenario, the total shrinkage in the workforce due to AMR is estimated to be equivalent to 734 000 FTEs each year across the 34 countries included in the analysis and 161 000 FTEs across the EU/EEA countries. Using the replacement scenario, on average, nearly 119 000 FTEs are estimated to be lost due to AMR each year across all countries included in the analysis and nearly 27 000 across the EU/EEA countries.
Job losses are the primary driver of the declines in labour market outputs across the OECD and EU/EEA countries (Figure 3.10). Across these countries, on average, AMR is estimated to reduce the employment rate by 97 per 100 000 working population each year up to 2050 using the elimination scenario and 17 per 100 000 working population annually using the replacement scenario. Similar reductions are observed across the EU/EEA countries whereby the employment rate is estimated to reduce by 45 per 100 000 working population annually using the elimination scenario and 8 per 100 000 working population each year using the replacement scenario. The magnitude of the reductions in employment rates is estimated to be the highest in Italy, Portugal and Romania across the EU/EEA countries and in Türkiye and the United States across non-EU/EEA member OECD countries.
Figure 3.10. AMR has negative consequences in the labour market by reducing employment and propagating absenteeism and presenteeism
Annual job losses, absenteeism and presenteeism up to 2050
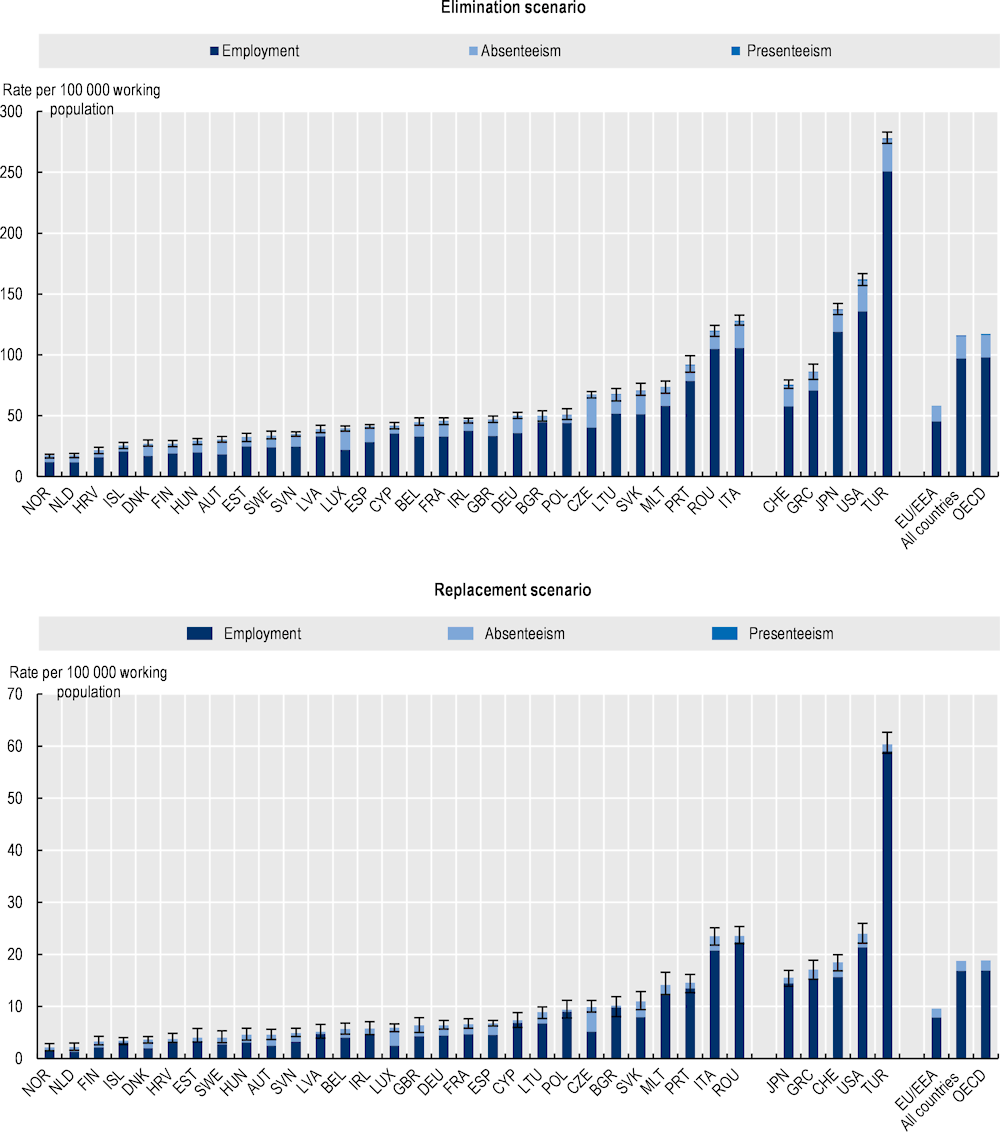
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The OECD analysis further suggests that declines in productivity due to AMR are predominantly due to absenteeism (Figure 3.10). The annual absenteeism rate across the 34 countries included in the analysis averages around 18 per 100 000 working population under the elimination scenario and 2 per 100 000 working population using the replacement scenario respectively. Across the EU/EEA countries, annual absenteeism rates are estimated to reach around 12 and 2 per 100 000 working population under the elimination and replacement scenarios respectively. Across all countries included in the analysis, these estimated average annual absenteeism rates are consistently larger than the estimated rates of presenteeism at work due to AMR under both modelling scenarios.
The estimated reductions in labour market outputs translate into considerable financial losses
As shown in Figure 3.11, OECD analysis suggests that the estimated declines in participation in the workforce and productivity translate into considerable financial losses (see Annex 3.A for annual average labour market output per worker by country). Using the elimination scenario, the 34 countries included in the analysis are estimated to lose a total of around USD PPP 36.9 billion each year up to 2050 in labour market output due to AMR, corresponding to around USD PPP 32.7 per capita. This corresponds to roughly one-fifth of GDP in Portugal in 2020. In the same period, the magnitude of the estimated total annual losses in labour market outputs across the EU/EEA countries is estimated to average around USD PPP 5.8 billion, corresponding to around USD PPP 11.8 per capita. The magnitude of the estimated losses in labour market outputs due to AMR is more modest under the replacement scenario. Using the replacement scenario, the total annual losses in labour market outputs due to AMR are estimated to exceed USD PPP 6.6 billion up to the year 2050 across all countries included in the analysis, corresponding to USD PPP 5.9 per capita. Across the EU/EEA countries, AMR is estimated to result in total annual losses in labour market outputs amounting to nearly USD PPP 960 million by 2050, corresponding to USD PPP 1.9 per capita.
There is substantial cross-country variation in the magnitude of the estimated losses in labour market output due to AMR. Under the elimination scenario, Italy, Ireland and Malta are estimated to incur the greatest losses in per capita labour market output each year across the EU/EEA countries, with the magnitude of these losses ranging from around USD PPP 16.5 in Malta to USD PPP 23.8 in Italy. Across non-EU/EEA member OECD countries, the greatest losses in per capita labour market output are estimated to occur in Türkiye and the United States, with the estimated per capita losses reaching around USD PPP 61.8 in the United States and USD PPP 56.9 in Türkiye. Under the replacement scenario, Malta and Romania follow Italy as the 2 countries that risk the greatest losses in labour market output due to AMR, with estimated per capita losses ranging from USD PPP 3.4 in Malta and USD PPP 3.2 in Romania. Across non-EU/EEA member OECD countries, the United States is estimated to incur the greatest amount of losses in per capita labour market output under the elimination scenario, whereas Türkiye is estimated to risk the greatest amount of losses in labour market output per capita using the replacement scenario. These cross-country differences in the magnitude of losses in labour market output reflect the differences in the burden of resistant infections and wages.
Figure 3.11. The impact of AMR on labour market outputs is considerable
Annual average labour market output lost due to AMR based on average wages up to 2050
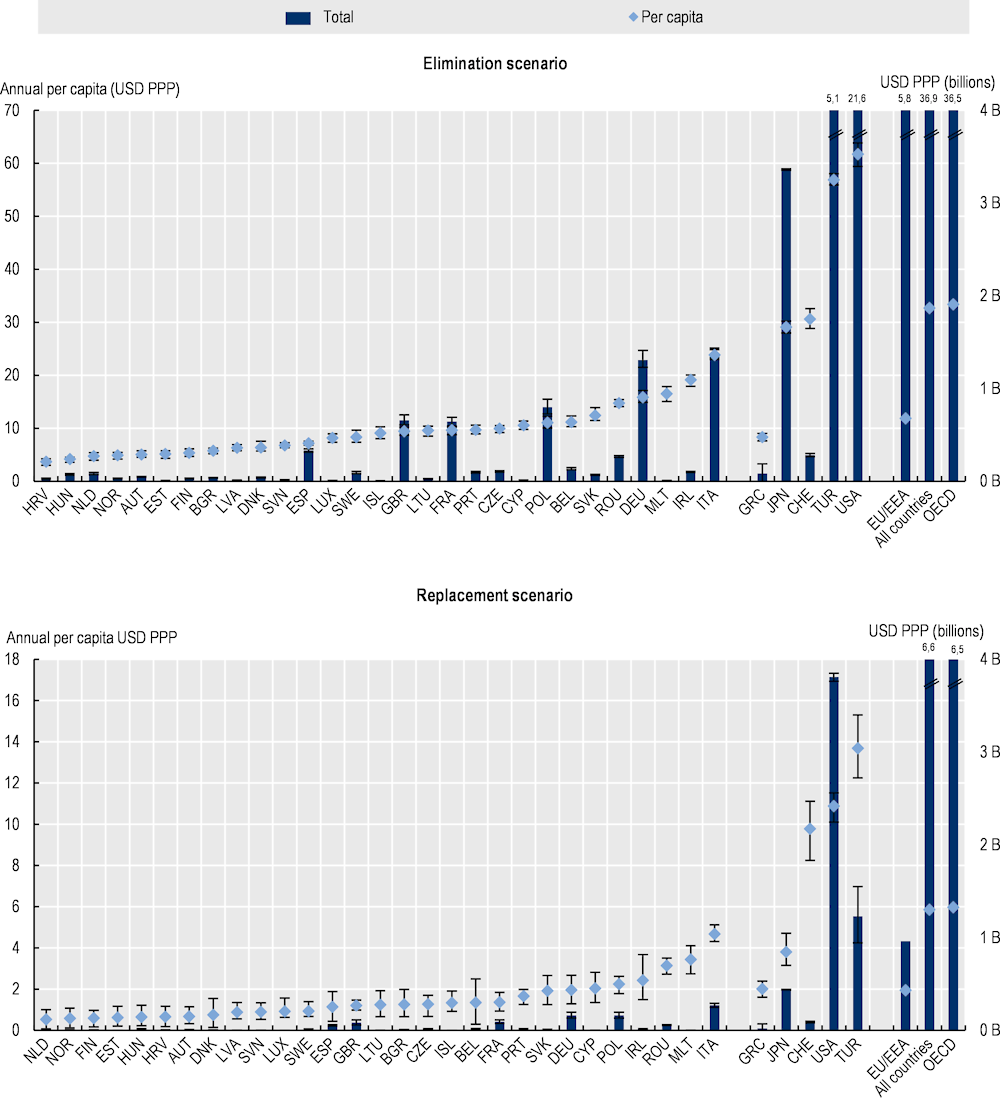
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
B: Billion; PPP: Purchasing power parity.
Source: OECD analysis based on the OECD SPHeP-AMR model.
The OECD analysis broadly aligns with previous studies that estimate the health and economic burden of AMR
The results presented in this chapter suggest that around 79 000 deaths can occur across the 34 countries included in the analysis each year up to 2050. Nearly 22 000 of these deaths are estimated to take place across the EU/EEA countries. In comparison, the 2018 OECD publication estimated that, between 2015 and 2050, the annual number of deaths due to AMR is estimated to average around 63 000 deaths across the 33 countries included in the analysis, with around 33 000 of these deaths occurring across the EU/EEA countries (OECD, 2018[2]). The observed differences between the two rounds of OECD analysis are driven primarily by the incorporation of new data collected since the 2018 OECD analysis into the modelling framework, as well as the differences in the sample of countries included in the analysis. The estimated reduction in the number of AMR-related deaths across the EU/EEA countries also suggests that the wide range of policies and interventions put in place across these countries in line with the WHO Global Action Plan on AMR (2015[1]) may have been contributing to the observed reduction in the health impact of AMR.
The results presented in this chapter are broadly consistent with estimates generated by earlier studies that used data sources and methodologies similar to the OECD analysis (see 0 for more detailed descriptions). For example, the 2022 ECDC analysis of the mortality burden of AMR used data from countries that reported them to the EARS-Net network for a similar set of antibiotic-bacterium combinations and showed that, in 2019, the estimated number of deaths due to AMR was 38 710 (95% UI 24 053‑43 710) using assumptions and inputs similar to the OECD’s elimination scenario. The observed differences in the magnitude of the estimated mortality due to AMR are partly explained by methodological differences. The OECD model opts for a dynamic approach given the modelling period that spans nearly 30 years. In effect, this means that the OECD model takes into account other causes of mortality that compete with AMR, such that it assumes that a person that is recovered from a resistant infection dies due to other illnesses. This assumption was used to avoid implausibly high population estimates by 2050.
In comparison, the results presented in this chapter provide much more conservative estimates compared to those recently generated by the European Antibiotic Resistance Collaborators (Mestrovic et al., 2022[4]). In this study, deaths due to AMR in 2019 are estimated to average around 541 000 under a scenario similar to the OECD’s elimination scenario and around 133 000 using a scenario similar to the replacement scenario, suggesting around 25‑to‑21‑fold difference in mortality estimates compared to the OECD estimates. These large differences between the two analyses are driven primarily by the differences in the analytical scope, methodologies and data sources. For example, the study by Mestrovic and colleagues (2022[4]) covered 23 pathogens and 88 antibiotic-bacterium combinations. Whereas consistent with the analyses carried out by the ECDC (ECDC, 2022[11]) and other studies carried out at the national level (CDC, 2022[14]; CCA, 2019[15]), the OECD model focuses on a more limited set of 10 pathogens and 18 antibiotic-bacterium combinations that are regarded as a priority across the OECD and EU/EEA countries. This study provides estimates for 53 countries included in the WHO European region, many of which are shown to have a substantially high AMR burden. In comparison, the OECD analysis focuses on 34 OECD and EU/EEA countries that are generally estimated to have a lower AMR burden. A final significant driver of the difference between the two studies is the number of infections used as input data to feed the two models. The OECD model uses estimates on the number of infections provided by the ECDC, national surveillance systems and the WHO. In comparison, the European Antibiotic Resistance Collaborators use a variety of sources combined through a meta‑analytical approach. A direct comparison between the inputs of the two models is not currently possible because data on the number of infections are not publicly available. However, it should be noted that, particularly for some specific antibiotic-bacterium combinations, the number of deaths estimated by the European Antibiotic Resistance Collaborators is significantly higher than the number of infections officially reported by countries included in the OECD analysis.
Conclusion: There is no room for complacency in the fight against AMR
This chapter provided an analysis of the health and economic impact of resistant infections in 34 OECD and EU/EEA countries. Broadly, findings from the chapter demonstrated that AMR has a deleterious impact on population health by exacerbating mortality and morbidity while reducing the life span and the number of years lived in good health. It showed that three pathogens – E. coli, K. pneumoniae and S. aureus – account for around three‑quarters of deaths each year that occur due to resistant infections. Further, the chapter showed that while the majority of resistant infections were acquired in community settings, infections acquired in hospital settings account for the majority of deaths caused by resistant infections.
The chapter also demonstrated that AMR imposes a considerable economic burden on the 34 countries included in the analysis. Depending on the modelling scenario, AMR is estimated to result in 6.9‑32.5 million extra days spent in hospital every year. Resistance to readily available, affordable treatments will also mean that treatment of these infections will resort to medicines that are more costly, less effective, unavailable or unaffordable in many settings. The annual health expenditure due to AMR is estimated to amount to USD PPP 5.9 to USD PPP 28.9 billion, corresponding to per capita spending of around USD PPP 5.2 to USD PPP 25.6. AMR also has important consequences for participation in the workforce and productivity.
The chapter showed that worrisome discrepancies persisted in the health and economic burden of AMR across countries, with many southern European countries bearing a heavy cost. This cross-country variation reflects the differences in the epidemiology of AMR, health system characteristics and workforce policies in place in each country. Addressing these differences will require countries with heavier AMR burdens to take further steps in line with the priorities highlighted in the WHO Global Action Plan.
Findings from the chapter suggest that the health and economic burden of AMR is comparable to the COVID‑19 pandemic. The chapter showed that the estimated loss of life due to AMR can reach up to one‑third of the reduction in life expectancy attributable to COVID‑19 between 2019 and 2020. Without robust policy action, the estimated costs associated with treating resistant infections can be equivalent to having a COVID‑19 pandemic in nearly every five years. Much like the COVID‑19 pandemic, the silent pandemic of AMR has important consequences for long-term macroeconomic performance and imposes considerable fiscal pressure. Similar to SARS‑CoV‑2, resistant bacteria can also evolve over time and adapt to the existing antibiotic treatments, and changes in the epidemiology of bacteria are difficult to predict ahead of time. Combined, these factors make it challenging to quantify the impact of AMR on population health and the economy.
The results presented in this chapter should be interpreted with care. The OECD analysis has several limitations. The OECD model considers priority antibiotic-bacterium combinations that are considered to pose the greatest threat to population health rather than attempting to account for all types of resistant infections. Differences in the data collection methods between countries that report data to the ECDC, the WHO and national AMR surveillance networks impact the comparability of results across countries. The model makes use of several assumptions which may influence the precision of its estimates. For example, the model assumes that resistant infections are not transferred across countries. This means that the potential impacts of the rising AMR trends in countries where the resistant proportions are estimated to be concerningly high are not directly accounted for in the model. Results presented here should be considered conservative because the model does not include the long-term sequelae of AMR, nor does it quantify their costs.
Combined, the results provided in this chapter underline the importance of continuing to invest in multi-sectoral policies to curb AMR. As discussed in detail in Chapter 4, OECD and EU/EEA countries have made important strides in recent years in developing and implementing their national action plans to tackle AMR, which put forward a wide range of policies and interventions to tackle AMR not only in the health sector but also in agricultural practices concerning animal and plant health, and in the environment. As shown in Chapter 5, many of these interventions have been shown effective though the precise magnitude of their effectiveness is influenced by a variety of contextual factors. Chapter 6 examines the cost‑effectiveness of 11 interventions in line with the One Health approach to ascertain their impact of population and economy using the OECD SPHeP-AMR model.
References
[34] Beckett, C., S. Harbarth and B. Huttner (2015), “Special considerations of antibiotic prescription in the geriatric population”, Clinical Microbiology and Infection, Vol. 21/1, pp. 3-9, https://doi.org/10.1016/j.cmi.2014.08.018.
[29] Bell, J. and N. Jennifer (2022), Global Health Security Index: Advancing Collective Action and Accountability Amid Global Crisis: Turkey, https://www.ghsindex.org/wp-content/uploads/2021/12/Turkey.pdf (accessed on 3 February 2023).
[27] Bell, J. and J. Nuzzo (2021), Global Health Security Index: Advancing Collective Action and Accountability Amid Global Crisis: Switzerland, https://www.ghsindex.org/wp-content/uploads/2021/12/2021_GHSindexFullReport_Final.pdf (accessed on 3 February 2023).
[21] Booton, R. et al. (2021), “One Health drivers of antibacterial resistance: Quantifying the relative impacts of human, animal and environmental use and transmission”, One Health, Vol. 12, p. 100220, https://doi.org/10.1016/j.onehlt.2021.100220.
[10] Cassini, A. et al. (2019), “Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis”, The Lancet Infectious Diseases, Vol. 19/1, pp. 56-66, https://doi.org/10.1016/s1473-3099(18)30605-4.
[15] CCA (2019), When Antibiotics Fail: The Expert Panel on the Potential Socio-Economic Impacts of Antimicrobial Resistance in Canada, Council of Canadian Academies, https://cca-reports.ca/wp-content/uploads/2018/10/When-Antibiotics-Fail-1.pdf.
[14] CDC (2022), COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022, Centers for Disease Control and Prevention, https://doi.org/10.15620/cdc:117915.
[13] CDC (2019), Antibiotic Resistance Threats in the United States, 2019, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, https://doi.org/10.15620/cdc:82532.
[12] CDC (2013), Antibiotic Resistance Threats in the United States, 2013, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
[3] ECDC (2022), Antimicrobial Resistance Surveillance in Europe, European Centre for Disease Prevention and Control, https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf.
[11] ECDC (2022), Assessing the Health Burden of Infections with Antibiotic-Resistant Bacteria in the EU/EEA, 2016-2020, European Centre for Disease Prevention and Control, https://www.ecdc.europa.eu/en/publications-data/health-burden-infections-antibiotic-resistant-bacteria-2016-2020.
[19] ECDC/EFSA/EMA (2021), Antimicrobial Consumption and Resistance in Bacteria from Humans and Animals: Third Joint Interagency Report, European Centre for Disease Prevention and Control, European Food Safety Authority and European Medicines Agency, https://www.ema.europa.eu/en/documents/report/ema/ecdc/efsa-third-joint-report-integrated-analysis-consumption-antimicrobial-agents-occurrence_en.pdf.
[8] FAO and WHO (2021), Code of Practice to Minimize and Contain Foodborne Antimicrobial Resistance, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B61-2005%252FCXC_061e.pdf (accessed on 12 June 2022).
[35] Gavazzi, G. and K. Krause (2002), “Ageing and infection”, The Lancet Infectious Diseases, Vol. 2/11, pp. 659-666, https://doi.org/10.1016/s1473-3099(02)00437-1.
[5] Hernando-Amado, S. et al. (2019), “Defining and combating antibiotic resistance from One Health and Global Health perspectives”, Nature Microbiology, pp. 1432-1442, https://doi.org/10.1038/s41564-019-0503-9.
[16] Innes, G. et al. (2019), “External societal costs of antimicrobial resistance in humans attributable to antimicrobial use in livestock”, Annual Review of Public Health, Vol. 41, pp. 141-157, https://doi.org/10.1146/annurev-publhealth-040218-043954.
[25] Jojoa-Sierra, S. et al. (2017), “Elimination of the antibiotic norfloxacin in municipal wastewater, urine and seawater by electrochemical oxidation on IrO2 anodes”, Science of the Total Environment, Vol. 575, pp. 1228-1238, https://doi.org/10.1016/j.scitotenv.2016.09.201.
[22] Karkman, A., K. Pärnänen and D. Larsson (2019), “Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments”, Nature Communications, Vol. 10/1, https://doi.org/10.1038/s41467-018-07992-3.
[33] Keilich, S., J. Bartley and L. Haynes (2019), “Diminished immune responses with aging predispose older adults to common and uncommon influenza complications”, Cellular Immunology, Vol. 345, p. 103992, https://doi.org/10.1016/j.cellimm.2019.103992.
[37] McEwen, S. and P. Collignon (2018), “Antimicrobial resistance: A One Health perspective”, in Antimicrobial Resistance in Bacteria from Livestock and Companion Animals, American Society of Microbiology, https://doi.org/10.1128/microbiolspec.arba-0009-2017.
[4] Mestrovic, T. et al. (2022), “The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis”, The Lancet Public Health, Vol. 7/11, pp. e897-e913, https://doi.org/10.1016/s2468-2667(22)00225-0.
[32] Montecino-Rodriguez, E., B. Berent-Maoz and K. Dorshkind (2013), “Causes, consequences, and reversal of immune system aging”, Journal of Clinical Investigation, Vol. 123/3, pp. 958-965, https://doi.org/10.1172/jci64096.
[9] Murray, C. et al. (2022), “Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis”, The Lancet, Vol. 399/10325, pp. 629-655, https://doi.org/10.1016/s0140-6736(21)02724-0.
[2] OECD (2018), Stemming the Superbug Tide: Just A Few Dollars More, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/9789264307599-en.
[26] Paulus, G. et al. (2019), “The impact of on-site hospital wastewater treatment on the downstream communal wastewater system in terms of antibiotics and antibiotic resistance genes”, International Journal of Hygiene and Environmental Health, Vol. 222/4, pp. 635-644, https://doi.org/10.1016/j.ijheh.2019.01.004.
[7] Prestinaci, F., P. Pezzotti and A. Pantosti (2015), “Antimicrobial resistance: A global multifaceted phenomenon”, Pathogens and Global Health, Vol. 109/7, pp. 309-318, https://doi.org/10.1179/2047773215Y.0000000030.
[24] Rodríguez-Chueca, J. et al. (2019), “Assessment of full-scale tertiary wastewater treatment by UV-C based-AOPs: Removal or persistence of antibiotics and antibiotic resistance genes?”, Science of the Total Environment, Vol. 652, pp. 1051-1061, https://doi.org/10.1016/j.scitotenv.2018.10.223.
[17] Scott, A. et al. (2018), “Is antimicrobial administration to food animals a direct threat to human health? A rapid systematic review”, International Journal of Antimicrobial Agents, Vol. 52/3, pp. 316-323, https://doi.org/10.1016/j.ijantimicag.2018.04.005.
[18] Tang, K. et al. (2017), “Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis”, The Lancet Planetary Health, Vol. 1/8, pp. e316-e327, https://doi.org/10.1016/S2542-5196(17)30141-9.
[6] Thakur, S. and G. Gray (2019), “The mandate for a global “one health” approach to antimicrobial resistance surveillance”, American Journal of Tropical Medicine and Hygiene, Vol. 100/2, pp. 227-228, https://doi.org/10.4269/ajtmh.18-0973.
[20] van Bunnik, B. and M. Woolhouse (2017), “Modelling the impact of curtailing antibiotic usage in food animals on antibiotic resistance in humans”, Royal Society Open Science, Vol. 4/4, p. 161067, https://doi.org/10.1098/rsos.161067.
[30] Vos, T. et al. (2020), “Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019”, The Lancet, Vol. 396/10258, pp. 1204-1222, https://doi.org/10.1016/s0140-6736(20)30925-9.
[31] WHO (2022), Global Tuberculosis Report 2022, World Health Organization, https://apps.who.int/iris/handle/10665/363752.
[28] WHO (2022), Health Systems in Action: Türkiye: 2022 Edition, European Observatory on Health Systems and Policies and Regional Office for Europe, World Health Organization, https://apps.who.int/iris/handle/10665/362347.
[36] WHO (2020), Antimicrobial Resistance - One Health, Regional Office for Europe, World Health Organization, https://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/policy/one-health.
[1] WHO (2015), Global Action Plan on Antimicrobial Resistance, World Health Organization, https://apps.who.int/iris/handle/10665/193736.
[23] Yang, Y. et al. (2017), “Antibiotic resistance genes in surface water of eutrophic urban lakes are related to heavy metals, antibiotics, lake morphology and anthropic impact”, Ecotoxicology, Vol. 26/6, pp. 831-840, https://doi.org/10.1007/s10646-017-1814-3.
Annex 3.A. Impact of AMR on health expenditure and labour market outputs
Annex Figure 3.A.1. Total annual hospital expenditure due to AMR up to 2050
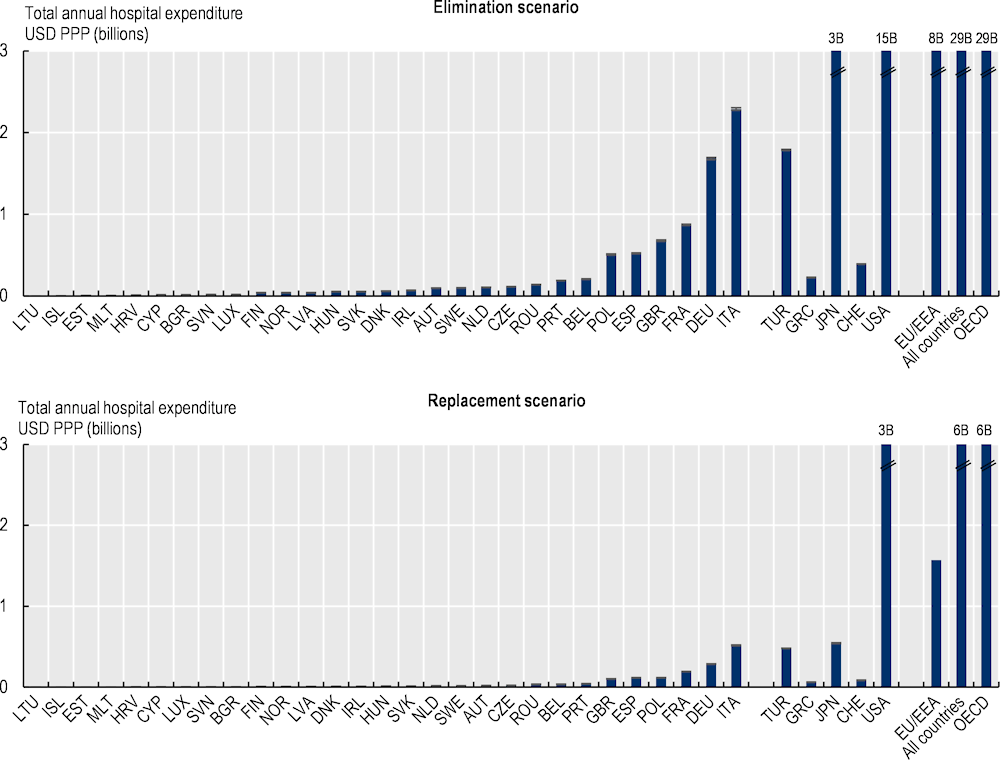
Note: Results for Greece are presented on the right-hand side of the panel because data for S. pneumoniae are not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right-hand sides of the panel due to the methodological differences in data collection and data extraction practices.
Source: OECD analysis based on the OECD SPHeP-AMR model.
Annex Figure 3.A.2. Annual average labour market output per worker lost due to AMR based on average wages up to 2050
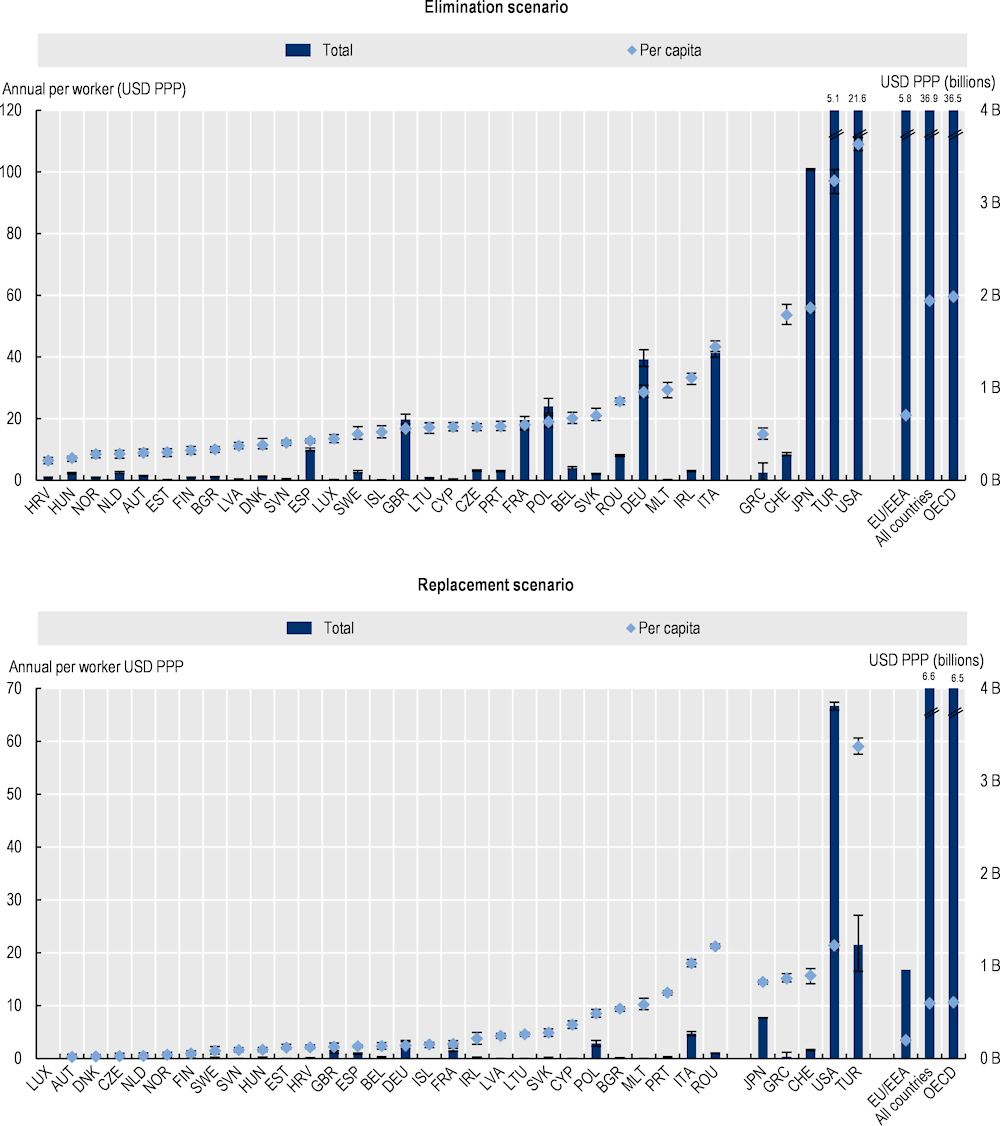
Note: Results for Greece are presented in the right hand-side of the panel because data for S. pneumoniae is not available. Results are presented based on the sources of input data, with data for countries in the group on the left that are all from the same source and calculated with a comparable methodology. Results are not directly comparable for countries on the left- and right hand-side of the panel due to the methodological differences in data collection and data extraction practices. B: Billion; PPP: Purchasing power parity
Source: OECD analysis based on the OECD SPHeP-AMR model.