This chapter reviews the landscape of national action plans to tackle antimicrobial resistance (AMR-NAPs) developed by OECD member countries, key partners and Group of Twenty (G20) countries. First, the chapter takes stock of the global progress in the development of AMR-NAPs and provides a discussion of factors that enable and/or hinder the implementation of these documents. Then, the chapter provides a comparative assessment of AMR-NAPs from OECD, EU/EEA and G20 countries. The findings are the result of a novel application of natural language processing techniques that make use of text from action plans. In addition, the chapter examines the selected design features of these documents. The chapter concludes by discussing the implications of the findings for the implementation of AMR-NAPs by OECD, EU/EEA and G20 countries.
Embracing a One Health Framework to Fight Antimicrobial Resistance

4. Special focus: Assessing the landscape of national action plans on antimicrobial resistance
Abstract
Key findings
Taking stock of the global progress in the development of national action plans to tackle AMR
The publication of the Global Action Plan on Antimicrobial Resistance (AMR-GAP) in 2015 augured well for the development of national action plans to tackle AMR (AMR-NAPs). Globally, the share of countries with an AMR-NAP more than doubled, reaching 149 in 2021‑22 from 70 in 2017. However, only around 10% (17/166) of countries globally reached the final stage of implementation, which entails including financial provisions for the implementation of AMR‑NAPs in the national action plans and budgets.
In 2021‑22, about 92% (47/51) of OECD countries and key partners, European Union (EU) or European Economic Area (EEA) members and G20 countries finished developing their action plans. However, only 20% (10/51) of these countries advanced to the final stage of the implementation of their AMR-NAPs, which involves including financial provisions for the implementation of AMR-NAPs in national action plans and budgets.
Among OECD countries, key partners, EU/EEA and G20 countries, there are gaps in the implementation of One Health approaches that entail the active involvement of multiple sectors in the development and implementation of AMR-NAPs. In these countries, the animal sector actively contributed to the development and implementation of nearly all of the AMR-NAPs in 2021‑22. This is followed by the food safety and the environmental sectors, which played an active role in the development and implementation of 90% (46/51) and 71% (36/51) of AMR‑NAPs respectively. In comparison, the food production and the plant health sectors were actively engaged in the development and implementation of 75% (38/51) and 59% (30/51) of action plans respectively.
Nearly all OECD countries, EU/EEA and G20 countries put in place AMR-relevant multi-sectoral policies consistent with the AMR-GAP. However, there are notable gaps in the implementation of interventions relevant to: optimising antibiotic use in human and animal health; monitoring antibiotic use and AMR surveillance; scaling up infection prevention and control programmes; scaling up nationwide activities to raise AMR awareness; incorporating AMR in the training and education of human healthcare professionals; and implementing good management and hygiene practices in farms and food establishments.
In 2020, the Group of Seven (G7) and OECD countries remained the primary source of development assistance for health (DAH) allocated to AMR, provided as financial and in-kind contributions transferred through international development institutions. Nonetheless, the current levels of DAH for AMR remain at around 2% and domestic sources of financing for AMR are unlikely to fill the existing funding gaps in low-resource settings.
Assessing the content of AMR-NAPs from 21 OECD countries and key partners, EU/EEA and G20 countries
On average, AMR-NAPs from the 21 OECD countries and key partners, EU/EEA and G20 countries cover a span of five years. Many AMR-NAPs predate the publication of the AMR‑GAP in 2015, while others are nearing the end of the period that they cover.
There is little cross-country standardisation in the ways in which OECD countries assess their progress towards the goals that they state in their AMR-NAPs, making it difficult to compare cross-country performance over time.
Only 12 out of 21 AMR-NAPs from OECD countries and key partners, EU/EEA and G20 countries discuss budgetary considerations and less than half refer to the cost-effectiveness of AMR-relevant interventions.
A high degree of convergence is observed between AMR-NAPs and the AMR-GAP in terms of their strategic objectives. Much like the AMR-GAP, optimising the use of antimicrobials in human and animal health is estimated to be the most frequently featured strategic objective in AMR‑NAPs from OECD countries and key partners, EU/EEA and G20 countries, followed by strengthening AMR surveillance, reducing the incidence of infections and making an economic case for sustainable investments. In comparison, improving awareness and understanding of AMR is the least frequently discussed strategic objective in these documents.
OECD countries and key partners, EU/EEA and G20 countries discuss a wide range of AMR-relevant interventions to achieve their strategic priorities, reflecting the broader historical, socio‑economic and health system-related factors that shape the AMR agenda in each setting.
With respect to strategies to optimise antimicrobial use in human and animal health, OECD countries and key partners, EU/EEA and G20 countries primarily emphasise efforts to strengthen antimicrobial stewardship. Further improvements can be achieved by improving the availability of antibiotic prescribing guidelines beyond hospital and acute care settings, encouraging the use of older antimicrobials and scaling up electronic prescribing programmes.
While the importance of strengthening AMR surveillance is recognised in the AMR-NAPs from 21 OECD countries and key partners, EU/EEA and G20 countries, these countries will benefit from deepening their engagement with global and regional AMR surveillance networks, enhancing laboratory network capacity and integrating information from new data sources into AMR surveillance.
In terms of reducing the incidence of infections, the AMR-NAPs from 21 OECD countries and key partners, EU/EEA and G20 countries most frequently emphasise the importance of improving water, sanitation, hygiene and waste management practices and vaccination coverage in human health. There is a need to put more emphasis on veterinary vaccines and enhancing biosecurity.
In terms of strategies to spur AMR-related research and development (R&D), OECD countries and key partners, EU/EEA and G20 countries primarily focus on a range of incentives aiming to encourage the early stage of drug development, whereas emerging evidence points to the need to supplement these incentives with those that can help improve expectations around future revenues.
With respect to strategies to enhance AMR awareness and understanding, the selected AMR-NAPs frequently highlight interventions targeting medical professionals and the general public while less emphasis is given to interventions targeting young children.
Antimicrobial resistance (AMR) is a well-recognised global health challenge
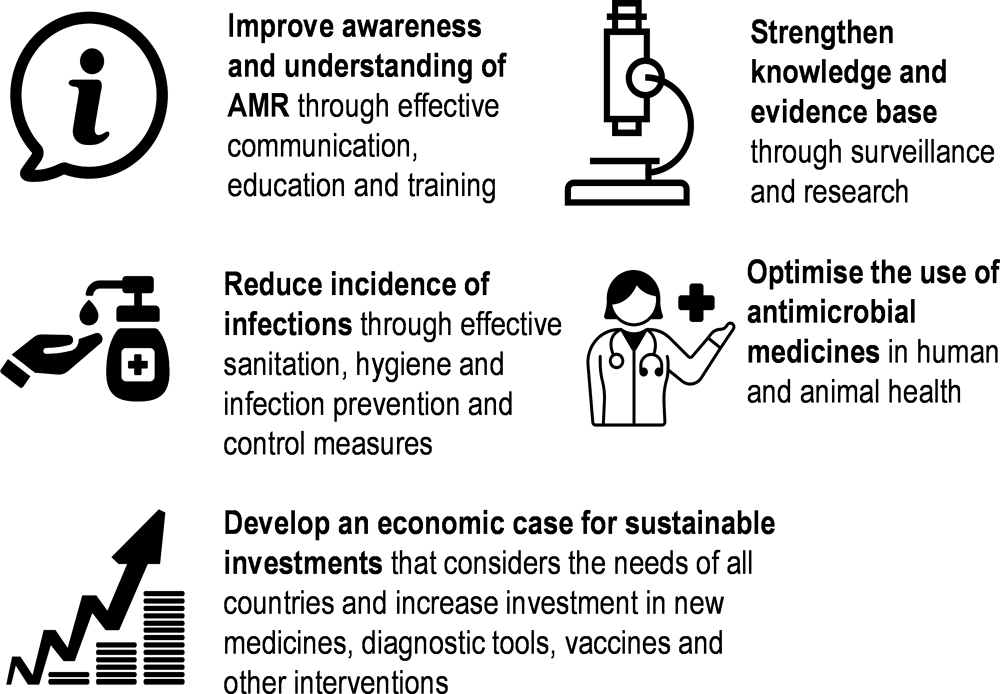
In recent years, the global community made important strides to tackle AMR. In May 2015, all members of the World Health Organization (WHO) made a commitment to tackling AMR by adopting the Global Action Plan (AMR-GAP) (WHO, 2015[1]). The AMR-GAP articulated five strategic objectives (Box 4.1) and urged countries to develop their own AMR national action plans (AMR-NAPs) in line with these strategic objectives, as well as the standards and guidelines championed by other intergovernmental bodies like the Food and Agriculture Organization of the United Nations (FAO), the World Organisation for Animal Health (WOAH) and the Codex Alimentarius Commission.
Box 4.1. A multi-pronged approach to curtailing AMR highlighted in the AMR-GAP involves five strategic objectives
Source: WHO (2015[1]), Global Action Plan on Antimicrobial Resistance, https://apps.who.int/iris/handle/10665/193736.
Following the publication of the AMR-GAP, global efforts to tackle AMR gained momentum. In 2016, the members of the United Nations (UN) reaffirmed their commitment to the vision laid out in the AMR-GAP in the UN Political Declaration on AMR (UN, 2016[2]). The following year, G20 countries endorsed the AMR‑GAP and called for the development of a global AMR R&D collaboration hub, which was launched in 2018. In 2019, the UN Ad Hoc Interagency Co‑ordination Group on Antimicrobial Resistance (ICGAR) issued an urgent call to establish a One Health Global Leaders Group on Antimicrobial Resistance (WHO, 2019[3]). In 2020, AMR was highlighted as one of the five priority areas for global action in the WHO Thirteenth General Program of Work (2019‑23) to improve population health and well-being (WHO, 2020[4]). This programme of work included one indicator – the proportion of bloodstream infections due to resistant organisms – as part of 46 key performance indicators to track progress by 2023 (WHO, 2020[4]). Importantly, many of these efforts have fostered collective, multi-sectoral action widely referred to as the One Health framework (Box 4.2). In 2022, the WHO, FAO, WOAH and United Nations Environment Programme (UNEP) published a new framework which describes the background and context of collaboration to tackle AMR and presents a theory of change associated with collaboration across agencies, including goals and objectives, desired country-level impact, intermediate outcomes, assumptions and risks, and implementation arrangements (WHO et al., 2022[5]).
Box 4.2. The COVID‑19 pandemic brought renewed attention to the One Health approach
A key principle embedded in the AMR-GAP is a collaborative, interdisciplinary, multi-sectoral action, referred to as the One Health approach. This approach recognises that many of the antimicrobial threats to human health are the same as those afflicting the health of animals and plants that share the same ecosystem (FAO and WHO, 2021[6]). It underscores the importance of pairing policies in the human health sector with those that are targeting the drivers of AMR in the animal and plant population, agricultural production, food safety and security, and environmental sectors.
Prior to the advent of the COVID‑19 pandemic, the importance of the One Health framework was demonstrated time and again, with the emergence and spread of zoonotic viruses. In 2003, severe acute respiratory syndrome (SARS), caused by a novel coronavirus, spread rapidly across 29 countries in the Americas, Asia and Europe. Following the 2003 SARS epidemic, other zoonotic viruses undermined the performance of health systems across the globe, including the re‑emergence and rapid spread of the highly pathogenic avian influenza H5N1 in 2013, the Ebola outbreak in West Africa, Zika in 2014‑17 in the Americas and the Pacific regions, and the Middle East Respiratory Syndrome that emerged in 2012. Despite these experiences, the Independent Panel, which was convened at the request of the WHO World Health Assembly, concluded that the One Health framework has been overlooked in efforts to prepare for future health crises (The Independent Panel, 2021[7]).
Today, a new reality is at hand. At the writing of this chapter, the global COVID‑19 infections surpassed 770 million confirmed cases and the attributable death toll reached nearly 7 million (Our World in Data, 2023[8]). Much like past pandemics, the COVID‑19 outbreak put additional strain on health systems across the globe. Mounting evidence pointing to delays and disruptions in non-COVID19 healthcare provision (Chmielewska et al., 2021[9]; Harris et al., 2021[10]) and interruptions in the implementation of antimicrobial stewardship policies and AMR surveillance (Tomczyk et al., 2021[11]). Echoing calls from numerous international bodies, the Independent Panel strongly urged countries to embed the One Health framework as an integral part of their efforts to plan and prepare for future health emergencies (The Independent Panel, 2021[7]).
Source: Chmielewska, B. et al. (2021[9]), “Effects of the COVID‑19 pandemic on maternal and perinatal outcomes: A systematic review and meta‑analysis”, https://doi.org/10.1016/s2214-109x(21)00079-6; Harris, R. et al. (2021[10]), “Impact of COVID‑19 on routine immunisation in South-East Asia and Western Pacific: Disruptions and solutions”, https://doi.org/10.1016/j.lanwpc.2021.100140; The Independent Panel (2021[7]), COVID‑19: Make It the Last Pandemic, https://theindependentpanel.org/wp-content/uploads/2021/05/COVID-19-Make-it-the-Last-Pandemic_final.pdf; Our World in Data (2023[8]), Coronavirus Pandemic (COVID‑19), https://ourworldindata.org/COVID-deaths (accessed on 15 January 2022); Tomczyk, S. et al. (2021[11]), “Impact of the COVID‑19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey”, https://doi.org/10.1093/jac/dkab300.
The goal of this chapter is to review the AMR-NAP landscape in OECD members, key partners and G20 countries. As the first of the two policy chapters included in this publication, it starts by documenting the global progress in the development of AMR-NAPs and describes the factors that enable or hinder the implementation of these documents. Data used for this analysis come primarily from the most recent wave of the Tripartite AMR Country Self-Assessment Surveys conducted by the WHO, WOAH and FAO in 2020‑21 (WHO/FAO/WOAH, 2022[12]). Next, the chapter takes a deep dive into the content of 21 AMR-NAPs selected among OECD and G20 countries. To do this, the chapter provides new evidence on the selected design features of the AMR-NAPs that may influence the effectiveness of the implementation of the vision set out in these documents. Next, the chapter assesses the level of alignment between the AMR-NAPs and the AMR-GAP. Complementing this analysis, Chapter 5 then provides an overview of emerging evidence on the effectiveness of selected AMR interventions.
Global progress in the development and implementation of AMR-NAPs
Key messages
Globally, the number of countries with AMR-NAPs more than doubled since 2016‑17, reaching 149 countries in 2021‑22. Yet only 17 out of 166 countries indicated that they included financial provisions for the implementation of their AMR-NAPs in the national action plans and budgets.
Forty-seven out of 51 OECD countries and key partners, EU/EEA and G20 countries developed AMR-NAPs, but only 20% (10/51) of these countries indicated that they advanced to the final stage of implementation, which involves including financial provisions for the implementation of AMR-NAPs in national action plans and budgets.
In line with the One Health approach, in all OECD members, EU/EEA and G20 countries, the animal sector was actively involved in the development and implementation of AMR-NAPs in 2021‑22. However, there are gaps in multi-sectoral action. In 2021‑22, the development and implementation of around 90% (46/51) of action plans from these countries involved the active participation of the food safety sector, whereas 71% (36/51) involved the environmental sector. In this period, the food production and plant health sectors actively contributed to the development and implementation of around 75% (38/51) and 59% (30/51) of the AMR-NAPs respectively.
Globally, the implementation of AMR-NAPs is marked by a socio‑economic development gradient. The current level of development assistance for health devoted to AMR is unlikely to make up for insufficient funding from domestic resources in resource‑constrained settings.
Globally, there has been notable progress in the development and implementation of AMR-NAPs but gaps remain in the existing arrangements for including financial provisions for the implementation of AMR-NAPs in national action plans and budgets
The launch of the AMR-GAP in 2015 augured well for the development of AMR-NAPs across the globe, though many countries grapple with challenges in the execution of their action plans. The number of countries with AMR-NAPs more than doubled since 2016‑17, reaching 149 countries in 2021‑22 (WHO/FAO/WOAH, 2022[12]). Despite this, only 10% (17/166) of action plans proceeded to the most advanced stage of implementation in 2020‑21, which involves the inclusion of financial provision for AMR-NAPs in the national plans and budgets (WHO/FAO/WOAH, 2022[12]). These findings are consistent with a recent discussion paper by the UN ICGAR, which showed that most countries face challenges in the execution of their AMR‑NAPs rather than the development of these documents (ICGAR, 2018[13]).
AMR remains prominent in the public health agenda in OECD , EU/EEA and G20 countries but challenges persist (Figure 4.1). In 2021‑22, about 92% (47/51) of OECD countries, key partners, EU/EEA and G20 countries finished developing their AMR-NAPs. However, the majority of these countries have not yet proceeded to the most advanced stage of implementation. In 2021‑22, only around 20% (10/51) of these countries proceeded to the final stage of implementation, where financial provisions for the implementation of AMR-NAPs were included in the national plans and budgets (WHO/FAO/WOAH, 2022[12]). Importantly, many OECD countries reported that the AMR-relevant activities that they highlighted in their AMR-NAPs have been adversely impacted by the COVID‑19 pandemic (Box 4.3).
Figure 4.1. Most countries developed an AMR-NAP but further progress is needed to strengthen financial provisions to support implementation
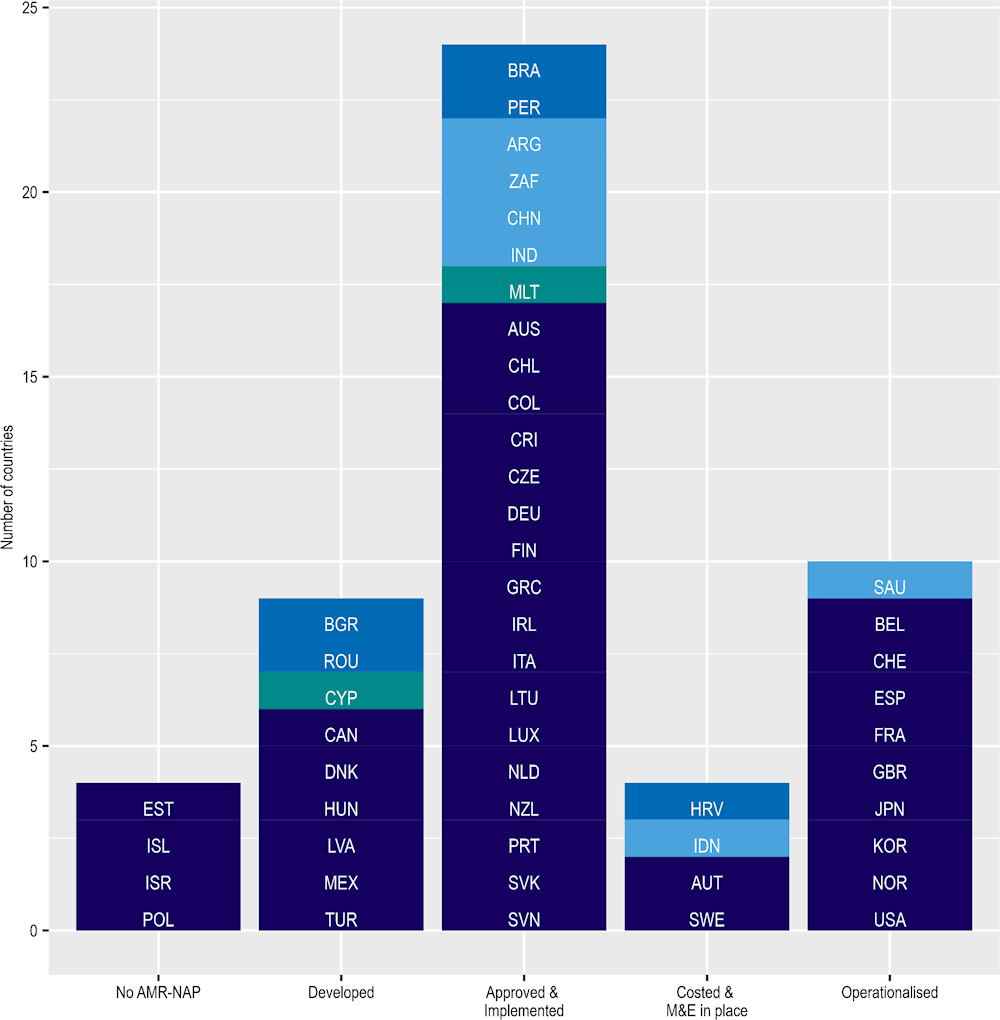
Note: Dark blue = OECD countries; medium blue = OECD accession countries; light blue = non-OECD G20 countries; green = non-OECD EU/EEA countries. The figure above describes the stages of the development of AMR-NAPs in five steps: i) No AMR-NAP: No AMR-NAP action plan; ii) Developed: AMR-NAP has been developed; iii) Approved& Implemented: AMR-NAP approved by government and is being implemented; iv) Costed & M&E in place: AMR-NAP has a costed and budgeted operational plan and has monitoring mechanism in place; v) Operationalised: Financial provision for the National AMR action plan implementation is included in the national plans and budgets.
Source: WHO/FAO/WOAH (2022[12]), Tripartite AMR Country Self‑Assessment Survey (TrACSS) 2021‑2022, https://amrcountryprogress.org/ (accessed on 4 December 2022).
Box 4.3. In many OECD countries, the implementation of AMR-relevant programmes and activities has been impacted by the COVID‑19 pandemic
In many OECD countries, the implementation of many AMR-relevant initiatives featured in national action plans has been disrupted by the COVID‑19 pandemic
As shown in Figure 4.2, the implementation of a wide range of activities highlighted in AMR-NAPs of many OECD countries has been disrupted by the COVID‑19 pandemic. Evidence emerging from OECD countries suggests that the re‑prioritisation of resources to address the COVID‑19 pandemic may have adversely impacted the implementation of the AMR agenda. Of the 26 OECD countries that participated in the OECD Resilience of Health Systems questionnaire (OECD, 2023[14]), 11 countries indicated that activities to improve AMR awareness and understanding in the general public and educational programmes for antibiotic prescribers were disrupted due to the pandemic. Further, disruptions were reported by nine OECD countries in terms of monitoring antibiotic prescribing behaviours in healthcare facilities. In addition, eight OECD countries reported interruptions in AMR surveillance in line with the One Health framework, as well as disruptions in vaccine campaigns for non-COVID‑19 related health conditions. OECD countries also reported interruptions in the rapid testing of patients and disruptions in the compliance of health workers with the existing hand hygiene and environmental hygiene guidelines in healthcare facilities. In addition, many OECD countries indicated that they were forced to delay revising/updating their AMR-NAPs due to the COVID‑19 pandemic while others suggested that final approval and budget allocation of the full implementation of AMR-NAPs were impacted.
Figure 4.2. AMR-relevant activities and programmes were adversely impacted by COVID‑19
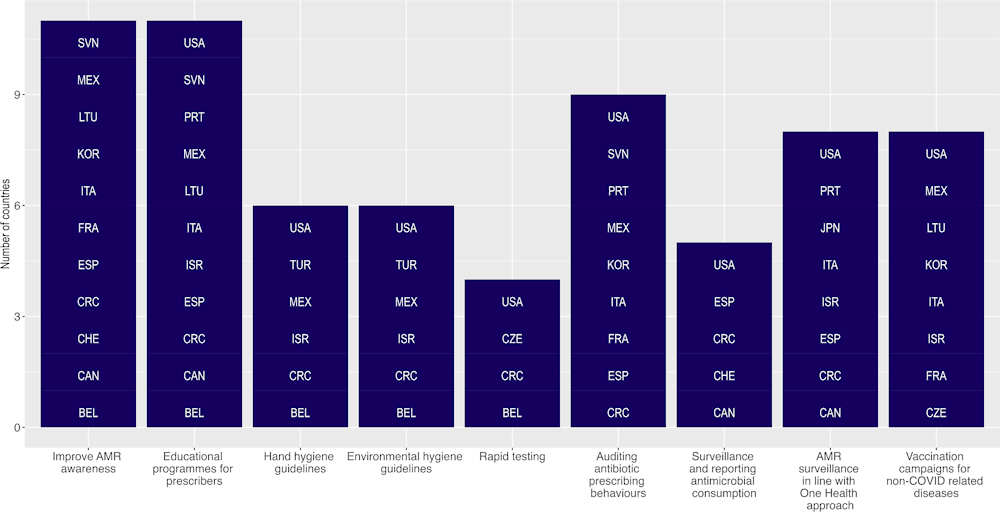
Source: Analysis of OECD (2023[14]), Ready for the Next Crisis? Investing in Health System Resilience, https://doi.org/10.1787/1e53cf80-en.
OECD countries have been actively pursuing approaches to limit the impact of the COVID‑19 pandemic on the implementation of their AMR-NAPs
Many OECD countries employed strategies to limit the impact of the COVID‑19 pandemic on the implementation of their AMR-NAPs. For example, in Belgium, hospitals were given extra financial resources to support their ongoing antimicrobial stewardship programmes and infection prevention and control interventions. In Portugal, regional AMR teams maintained close contact with local hospitals to avoid major disruptions in the already existing AMR measures. In Korea, online educational programmes were used to support the management of AMR policies. In the United States, the Centers for Disease Control and Prevention, one of the federal agencies leading the AMR agenda, continued to highlight AMR as a top priority by continuing investments in various prevention strategies, including early detection and containment, and infection prevention and control.
Source: Analysis of OECD (2023[14]), Ready for the Next Crisis? Investing in Health System Resilience, https://doi.org/10.1787/1e53cf80-en.
Globally, socio‑economic disparities exist in the implementation of AMR-NAPs
A socio‑economic development gradient emerges in the implementation of AMR-NAPs. In 2021‑22, among high-income countries (HICs), about 20% of AMR-NAPs advanced to the final stage of implementation, compared to 7% in upper-middle‑income countries (UMICs) and in lower-middle‑income countries (LMICs) and none of the low-income countries (LICs) (WHO/FAO/WOAH, 2022[12]). While the evidence base that can help explain these discrepancies remains limited, previous works from low-resource settings point to the deficits in technical capacity and staffing, institutional bottlenecks that hinder efforts to scale up local efforts (ICGAR, 2018[13]) and the differences in the governance approach to managing AMR (Birgand et al., 2018[15]).
Insufficient funding devoted to AMR is another bottleneck. A recent Wellcome Trust analysis concluded that financial limitations present a major impediment to implementing AMR-NAPs in many LMICs. Even when these countries identify funding to support the implementation of their AMR-NAPs, the level of funding may be insufficient to cover all of the intended activities (Wellcome, 2020[16]). In addition, access to high-quality drugs remains a challenge in many LMICs (Hauk et al., 2020[17]), which can exacerbate the emergence of drug-resistant pathogens. In recognition, new pooled funding mechanisms have emerged in recent years to overcome these financial constraints (Box 4.4).
Box 4.4. In the last two decades, the Global Drug Facility (GDF) of the Stop Tuberculosis (TB) Partnership contributed to improving access to quality-assured TB drugs across the globe
In 2020, an estimated 10 million people globally suffered from TB, a substantial proportion of which are multidrug resistant (WHO, 2021[18]). LMICs bear a considerable share of the TB burden worldwide (WHO, 2021[18]). Despite this, about 10% of all medicines in LMICs are considered poor-quality or counterfeit (Hauk et al., 2020[17]). Reliance on poor-quality anti-TB drugs not only decreases the likelihood of successful recovery but also promotes the emergence of drug-resistant TB pathogens. Exacerbating these challenges, many LMICs alone have limited negotiation power to reduce the price of TB drugs, even though patents for many of these drugs have already expired (Arinaminpathy et al., 2013[19]).
Recognising these challenges, the GDF was founded in 2001 as an alternative procurement model to help address issues around low-quality anti-TB drugs. The GDF was designed to ensure countries have equitable and uninterrupted access to high-quality medicines, and pools funds from donors and national governments. By consolidating demand for TB drugs from different countries, it negotiates the price of quality-assured TB medicines directly with drug suppliers (Arinaminpathy et al., 2013[19]). It also provides technical assistance, supply management tools and capacity-building tools to accelerate access to and take up of new TB products in resource‑constrained settings.
Today, the GDF is the world’s largest provider of TB-related drugs and diagnostics used in a variety of government-administered TB programmes, as well as TB programmes administered by international agencies (Hauk et al., 2020[17]). Since its launch, the GDF services were utilised by 151 countries to increase access to quality-assured TB diagnostics and treatments (Stop TB Partnership, 2021[20]). In 2020, the value of first-line and second-line medicines procured by the GDF reached USD 108 million and USD 141 million respectively, whereas the value of diagnostics reached USD 57.9 million (Stop TB Partnership, 2021[20]).
Source: Arinaminpathy, N. et al. (2013[19]), “The Global Drug Facility and its role in the market for tuberculosis drugs”, https://doi.org/10.1016/s0140-6736(13)60896-x; Hauk, C. et al. (2020[17]), “Quality assurance in anti-tuberculosis drug procurement by the Stop TB Partnership – Global Drug Facility: Procedures, costs, time requirements, and comparison of assay and dissolution results by manufacturers and by external analysis”, https://doi.org/10.1371/journal.pone.0243428; Stop TB Partnership (2021[20]), GDF Results, https://www.stoptb.org/mission/gdfs-results (accessed on 30 March 2022); WHO (2021[18]), Tuberculosis – Factsheet, https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 30 March 2022).
G7 and OECD countries are committed to mobilising development assistance for health (DAH) allocated to AMR but the current level of financial assistance is unlikely to address the existing gaps in domestic funding in resource‑constrained settings
In recent years, AMR has been reframed as a public health issue with important consequences for socio‑economic development in resource‑constrained settings. In 2018, the ICGAR suggested that AMR is not perceived as a priority issue in many LMICs (2018[13]). The publication analysis indicated that this perception may limit access to development funding and projects. The following year, the World Bank highlighted the need for reframing AMR not only as a public health challenge but also as a global development issue by arguing that AMR has far-reaching implications for human capital in developing countries, and failing to curb the AMR burden may impede progress towards United Nations Sustainable Development Goals (2019[21]).
G7 and OECD countries remain steadfast in their commitment to financing AMR-related activities across the globe but the current level of development funding allocated to AMR is unlikely to fill the existing gaps in domestic funding (Figure 4.3). In 2020, G7 and OECD countries were the leading sources of DAH allocated to AMR, including Australia, France, Germany, the United Kingdom and the United States (IHME, 2021[22]). Yet, the current level of DAH allocated to AMR remains low, with AMR receiving close to around 2% of DAH dedicated to communicable diseases in 2019. Considering that many countries across the globe are marshalling financial resources to address the COVID‑19 pandemic, the current level of DAH for AMR is unlikely to make up for the funding gap in low-resource settings.
Figure 4.3. G7 and OECD countries are still committed to financing AMR activities across the globe, 2019
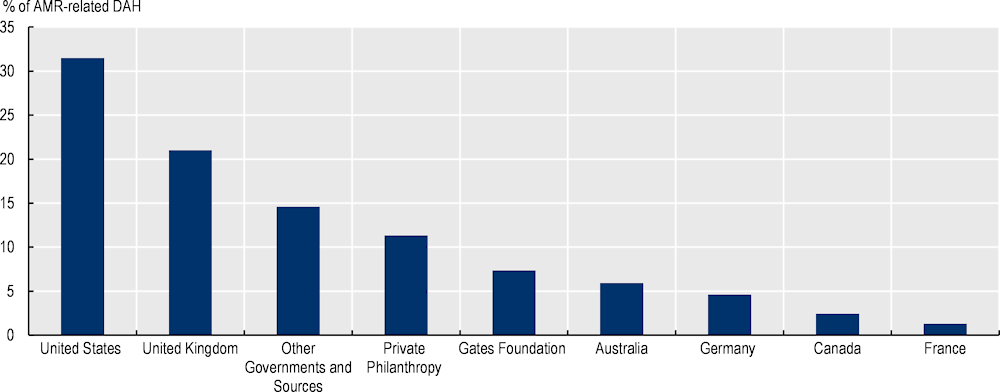
Source: IHME (2021[22]), Flows of Development Assistance for Health, https://vizhub.healthdata.org/fgh/ (accessed on 15 September 2021).
While the animal health sector is actively involved in the development and implementation of AMR-NAPs in most countries, further advancements are needed to incorporate input from other sectors
The period following the publication of the AMR-GAP has seen improvements in the number of countries that sought multi-sectoral feedback while developing their AMR-NAPs. In 2021‑22, globally, more than 1 sector was actively involved in the development and implementation of AMR-NAPs in about 98% (162/166) of countries (WHO/FAO/WOAH, 2022[12]). Similarly, in all OECD countries, EU/EEA and G20 countries, at least two sectors actively participated in the development and implementation of action plans in 2021‑22 (WHO/FAO/WOAH, 2022[12]). This finding is in congruence with earlier studies which showed that most countries across the globe adopted some form of multi-sectoral approach in the development and implementation of their AMR-NAPs (Munkholm et al., 2021[23]).
Yet, the development and implementation of AMR-NAPs do not always entail the active involvement of all relevant sectors (Figure 4.4). The linkages between human and animal health appears to be well recognised. In 2021‑22, in nearly all (165/166) of countries that reported data to the Tripartite AMR Country Self-Assessment Survey, the process to develop and implement AMR-NAPs actively involved the terrestrial animal health sector and, in 96% (158/166) of countries, this process involved the health of aquatic animals. Yet only a fraction of AMR-NAPs, globally, were developed and implemented with the active involvement of the other sectors. In 2021‑22, the development and implementation of about 79% (131/166) of AMR-NAPs involved the food safety sector, whereas 65% (108/166) involved the environment, 55% (90/166) food production and only 51% (84/166) reflected the active involvement of the plant health sector (WHO/FAO/WOAH, 2022[12]).
Across OECD countries and key partners, EU/EEA and G20 countries, stakeholders from the animal health sector most commonly take an active role in the development and implementation of AMR‑NAPs, whereas stakeholders representing food safety and security, the transmission of AMR in the environment and plant health are less involved. In 2021‑22, animal health was nearly universally acknowledged in the AMR-NAPs by OECD members and key partners, EU/EEA and G20 countries. In comparison, in around 90% (46/51) of these countries, the development and implementation of action plans involved the active participation of the food safety sector (WHO/FAO/WOAH, 2022[12]). Similarly, the environment sector was actively involved in the development and implementation of around 71% (36/51) of action plans. In the same period, the food production and plant health sectors were actively involved in the development and implementation of 75% (38/51) and 59% (30/51) of AMR-NAPs respectively.
Figure 4.4. The animal sector is the main non-human health sector routinely involved in the development and implementation of AMR-NAPs
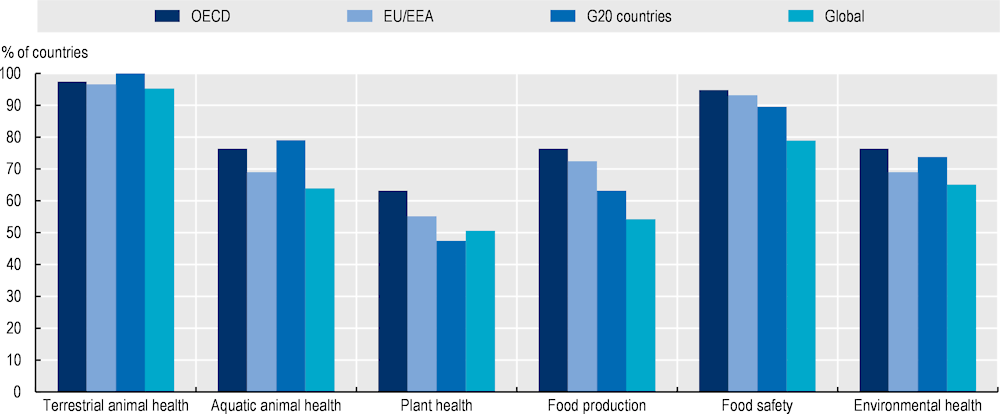
Note: EU: European Union; EEA : European Economic Area; G20: Group of Twenty.
Source: WHO/FAO/OEI (2022[12]), Tripartite AMR Country Self‑Assessment Survey (TrACSS) 2020‑2021, https://amrcountryprogress.org (accessed on 4 December 2022).
Globally, important strides have been made in building multi-sectoral co‑ordination mechanisms to support multi-sectoral approaches to tackling AMR (Figure 4.5). Establishing multi-sectoral co‑ordination mechanisms is an important first step towards facilitating multi-sectoral AMR response (Box 4.5). Globally, 87% (144/166) of countries established some form of a formal multi-sectoral governance or co‑ordination mechanism on AMR 2021‑22 (WHO/FAO/WOAH, 2022[12]). In 2021‑22, nearly all OECD countries, key partners, EU/EEA and G20 countries (49/51) put in place some form of multi-sectoral co‑ordination mechanism (i.e. working groups/co‑ordination committees) to promote multi-sectoral AMR‑relevant policy development and implementation. Importantly, new multinational initiatives emerged to promote multi-sectoral action across countries (Box 4.6). While relatively little is known about the factors that influence the effectiveness of multi-sectoral co‑ordination mechanisms, limited evidence suggests that various factors may influence the co‑ordination of multi-sectoral action including political will, administrative and financial support, as well as the dearth of available AMR data that can be used to facilitate dialogue and varying priorities across stakeholders (Joshi et al., 2021[24]).
Figure 4.5. Many OECD countries rely on integrated-approaches to implement their AMR-NAPs
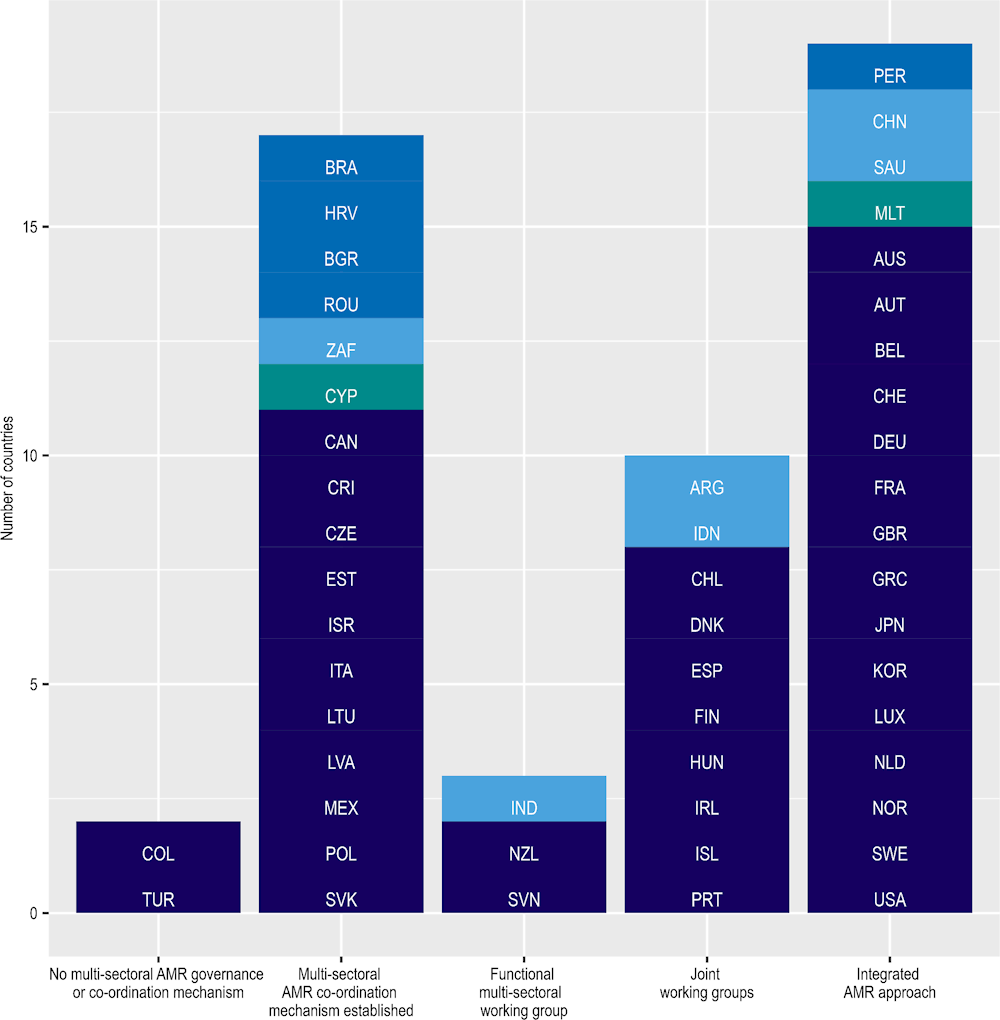
Note: Dark blue = OECD countries; medium blue = OECD accession countries; light blue = non-OECD G20 countries; green = non-OECD EU/EEA countries. The figure above describes the stages of multi-sectoral collaboration: i) No multi-sectoral AMR governance or co‑ordination mechanism; ii) Multi-sectoral AMR co‑ordination mechanism established: Multi-sectoral AMR co‑ordination mechanisms are established with government leadership; iii) Functional multi-sectoral working group: Formalised multi-sector co‑ordination mechanism with technical working groups established with clear terms of reference, regular meetings and funding for working group(s) with activities and reporting/accountability arrangements defined; iv) Joint working groups: Joint work on issues including agreement on common objectives; v) Integrated AMR approaches: Integrated approaches are used to implement the AMR-NAP with relevant data and lessons learnt across sectors used to adapt implementation of AMR-NAP.
Source: WHO/FAO/WOAH (2022[12]), Tripartite AMR Country Self‑Assessment Survey (TrACSS) 2021‑2022, https://amrcountryprogress.org/ (accessed on 4 December 2022).
Box 4.5. Co‑ordinating multi-sectoral collaboration and co‑operation for AMR-relevant action in the United States
In the United States, the government’s Interagency Task Force for Combating Antibiotic-Resistant Bacteria (CARB) remains the key driver of multi-sectoral collaboration and co‑ordination and that co‑ordination enabled the development of the updated AMR-NAP, as well as the implementation of CARB work in general (CARB, 2020[25]). Established in 2015, the CARB Task Force is co-chaired by the Secretaries of the US Departments of Health and Human Services, Agriculture and Defence, as well as representatives from the Departments of the Interior, State, and Veterans Affairs, the Environmental Protection Agency, the US Agency for International Development, the National Science Foundation, as well as representatives from the Executive Office of the President (CARB, 2020[25]). The Health and Human Services Office of the Assistant Secretary for Planning and Evaluation is tasked with the co‑ordination of the CARB Task Force, as well as the development of annual progress reports, and led the development of the 2020 AMR-NAP (CARB, 2020[25]). The CARB Task Force meets on a quarterly basis to discuss the ongoing work and reports annually on the progress toward goals stated in the AMR‑NAP.
The CARB Task Force recruited more than 100 federal experts from multiple disciplines to work as part of cross-agency teams that helped develop the goals included in the US AMR-NAP published in 2020. Each cross-agency team was comprised of experts from multiple disciplines (e.g. experts on human health surveillance and experts on animal health surveillance). The process to develop the goals included in the 2020 AMR-NAP entailed several steps. First, reviews of the progress towards each milestone highlighted in the previous AMR-NAP dated 2015 were carried out. These reviews looked at the progress in the implementation of actions associated with each milestone, identified both the challenges that had been experienced and opportunities that had risen since 2015, and pointed out the challenges and opportunities that are anticipated in the future. Subsequently, the cross-agency teams proposed, reiterated and refined new objectives and targets included in the 2020 AMR-NAP.
Source: CARB (2020[25]), National Action Plan for Combating Antibiotic‑Resistant Bacteria 2020‑25, https://www.hhs.gov/sites/default/files/carb-national-action-plan-2020-2025.pdf (accessed on 21 June 2022).
Box 4.6. The EU Strategic Approach to addressing pharmaceuticals in the environment rests on multi-sectoral action
In March 2019, the EU member states adopted a common approach to addressing the presence of pharmaceuticals in the environment, including antimicrobials (European Commission, 2019[26]). The EU approach recognises that the presence of antimicrobials used in human and veterinary medicine found in water and soil systems may contribute to the development, maintenance and spread of resistant pathogens. Aligned explicitly with the objectives of the European One Health Action Plan against Antimicrobial Resistance, the EU approach lays out six strategic priority areas for action that cover the lifecycle of pharmaceuticals:
Increase awareness and promote prudent use of pharmaceuticals, including antimicrobials.
Support the development of pharmaceuticals intrinsically less harmful to the environment and promote greener manufacturing practices.
Improve environmental risk assessment and its review.
Reduce wastage and improve waste management.
Expand environmental monitoring.
Fill other knowledge gaps, including the links between the presence of antimicrobials in the environment and the development and spread of AMR.
Since 2019, notable progress has been made in the implementation of the EU strategic approach. For instance, ministers of health in the EU member states started considering options that can help promote the consideration of the environmental impacts of medicines in the prescription decisions of health professionals (European Commission, 2020[27]). Another initiative led by the ad hoc working group through the Pharmaceutical Committee for human medicines was an agreement towards sharing best practices among health professionals across EU member states to promote environmentally safe disposal of medicinal products and clinical waste, as well as the ways in which pharmaceutical residues can be collected in an environmentally safe manner (European Commission, 2020[27]).
Source: European Commission (2019[26]), European Union Strategic Approach to Pharmaceuticals in the Environment, https://ec.europa.eu/environment/water/water-dangersub/pdf/strategic_approach_pharmaceuticals_env.PDF; European Commission (2020[27]), Update on Progress and Implementation: European Union Strategic Approach to Pharmaceuticals in the Environment, https://ec.europa.eu/environment/water/water-dangersub/pdf/Progress_Overview%20PiE_KH0320727ENN.pdf (accessed on 4 April 2022).
Gaps exist in the implementation of AMR-relevant multi-sectoral policies consistent with the AMR-GAP
Table 4.1 provides a dashboard of AMR-relevant multi-sectoral policies implemented in OECD countries and key partners, EU/EEA and G20 countries in congruence with the AMR-GAP based on responses provided by countries in the latest round of the Tripartite AMR Country Self-Assessment Survey (2021‑22) (WHO/FAO/WOAH, 2022[12]) (Annex 4.A provides more detailed information on the methodology used to develop the dashboard). Findings emerging from this dashboard point to important gaps in implementation:
In nearly all OECD countries and key partners, EU/EEA and G20 countries, there are national policies for antimicrobial governance that pertains to the community and healthcare settings. However, only eight of these countries currently have in place guidelines for optimising antibiotic use in human health for all major syndromes, with data on antibiotic use shared back with prescribers in a systematic manner.
In nearly all OECD countries and key partners, EU/EEA and G20 countries, a national policy or legislation exists to regulate the quality, safety and efficacy of antimicrobial productions used in terrestrial and aquatic animal health, as well as their distribution sale or use. But, only in 18 of these countries, enforcement and control mechanisms are reportedly in place to ensure compliance with the existing policy or legislation.
Nearly all OECD countries and key partners, EU/EEA and G20 countries have a national plan or system for monitoring antimicrobial use in their own settings. But only 26 of these countries regularly collect and report data on antimicrobial sales and consumption at the national level for human use, and data on antibiotic prescribing and appropriate/rational antibiotic use are drawn from a representative sample of health facilities in the public and private sectors.
All OECD countries and key partners, EU/EEA and G20 countries reported having the capacity to: i) generate data on antibiotic susceptibility testing, as well as related clinical and epidemiological data; and ii) report AMR. However, only 14 of these countries have a national AMR surveillance system that links AMR surveillance with antimicrobial consumption and/or use data in the human health sector.
23 OECD countries and key partners, EU/EEA and G20 countries reported that infection prevention and control (IPC) programmes are in place and functioning at the national and health facility levels in line with the WHO IPC core components. In these countries, compliance and effectiveness are regularly evaluated and published, and guidance is updated in accordance with monitoring.
All OECD countries and key partners, EU/EEA and G20 countries promote AMR awareness, but only nine of these countries have in place routine targeted, nationwide, government-supported activities to raise AMR awareness to facilitate behaviour change among priority stakeholders, with regular monitoring of these activities.
All OECD countries and key partners, EU/EEA and G20 countries provide training and professional education opportunities to raise awareness of AMR among health professionals in the human health sector, though only eleven of these countries systematically incorporate AMR in pre‑service training curricula for all relevant human health cadres, and in-service training and other professional education opportunities are taken up by relevant groups for the human health sector in public and private sectors.
All OECD countries and key partners, EU/EEA and G20 countries reported having in place some systematic efforts to improve good animal husbandry and biosecurity practices in terrestrial animal health. But only eight of these countries monitor the implementation of their nationwide plans periodically. Similarly, 43 OECD countries and key partners, EU/EEA and G20 countries make systematic efforts to improve good practices for aquatic animals and only six of these countries monitor the implementation of their nationwide plans regularly.
Forty-eight OECD countries and key partners, EU/EEA and G20 countries reported having in place some mechanisms to improve good practices in food processing. However, only ten of these countries monitor the implementation of their nationwide action plans periodically.
Table 4.1. Dashboard on the implementation of selected AMR-relevant policies in OECD countries and key partners, EU/EEA and G20 countries
|
Country |
Optimising antimicrobial use in human health |
Optimising antimicrobial use in animal health |
National monitoring system for consumption and rational use of antimicrobials in human health |
National surveillance system for AMR in humans |
Strengthening IPC practices in human healthcare |
Raising AMR awareness and understanding |
Training and education on AMR in human health |
Biosecurity and good animal husbandry practices (terrestrial animal production) |
Biosecurity and good animal husbandry practices (aquatic animal production) |
Good management and hygiene practices in food processing |
|---|---|---|---|---|---|---|---|---|---|---|
|
Australia |
||||||||||
|
Austria |
||||||||||
|
Belgium |
||||||||||
|
Canada |
||||||||||
|
Chile |
||||||||||
|
Colombia |
||||||||||
|
Costa Rica |
||||||||||
|
Czech Republic |
||||||||||
|
Denmark |
||||||||||
|
Estonia |
||||||||||
|
Finland |
||||||||||
|
France |
||||||||||
|
Germany |
||||||||||
|
Greece |
||||||||||
|
Hungary |
||||||||||
|
Iceland |
||||||||||
|
Ireland |
||||||||||
|
Israel |
||||||||||
|
Italy |
||||||||||
|
Japan |
||||||||||
|
Korea |
||||||||||
|
Latvia |
||||||||||
|
Lithuania |
||||||||||
|
Luxembourg |
||||||||||
|
Mexico |
||||||||||
|
Netherlands |
||||||||||
|
New Zealand |
||||||||||
|
Norway |
||||||||||
|
Poland |
||||||||||
|
Portugal |
||||||||||
|
Slovak Republic |
||||||||||
|
Slovenia |
||||||||||
|
Spain |
||||||||||
|
Sweden |
||||||||||
|
Switzerland |
||||||||||
|
Türkiye |
||||||||||
|
United Kingdom |
||||||||||
|
United States |
||||||||||
|
Argentina |
||||||||||
|
Brazil |
||||||||||
|
Bulgaria |
||||||||||
|
China |
||||||||||
|
Croatia |
||||||||||
|
Cyprus |
||||||||||
|
India |
||||||||||
|
Indonesia |
||||||||||
|
Malta |
||||||||||
|
Peru |
||||||||||
|
Romania |
||||||||||
|
Saudi Arabia |
||||||||||
|
South Africa |
Note: The methodology used to build the dashboard is available in Annex 4.A. OECD and non-OECD countries are listed in alphabetical order.
 No data
No data
 No implementation
No implementation
 First stage of implementation
First stage of implementation
 Second stage of implementation
Second stage of implementation
 Third stage of implementation
Third stage of implementation
 Most advanced stage of implementation
Most advanced stage of implementation
Source: WHO/FAO/WOAH (2022[12]), Tripartite AMR Country Self‑Assessment Survey (TrACSS) 2021‑2022, https://amrcountryprogress.org/ (accessed on 4 December 2022).
Assessing the key design features of AMR-NAPs
Key messages
OECD countries and key partners, EU/EEA and G20 countries will benefit from keeping their AMR-NAPs up to date while streamlining efforts to measure performance over time.
Deepening engagement with international and regional organisations that facilitate co‑ordinated action in line with the One Health approach is needed.
Many AMR-NAPs can be further improved by incorporating budget considerations and cost‑effectiveness assessments.
The remainder of this chapter presents results from a systematic assessment of the content of AMR-NAPs from selected OECD countries and key partners, EU/EEA and G20 countries based on a natural language processing (NLP) approach (Box 4.7). The OECD analysis first looks at the selected design features of AMR-NAPs, including performance tracking over time, engagement with international and regional bodies, and financial considerations and cost-effectiveness assessments. These features were selected because they were proposed as part of key design aspects of the AMR-NAPs that impact the effectiveness of the vision laid out in these documents (Chua et al., 2021[28]; Ogyu et al., 2020[29]; Anderson et al., 2019[30]). Next, the level of alignment between AMR-NAPs and the AMR-GAP is examined in terms of the strategic objectives and interventions recommended in the AMR-GAP.
Box 4.7. The OECD analysis deploys natural language processing techniques to examine the landscape of AMR-NAPs
Using text from AMR-NAPs as analysable data
The OECD analysis presented here is the first application of NLP guided methods to ascertain the level of alignment between AMR-NAPs and the AMR-GAP in 21 OECD countries and key partners, EU/EEA and G20 countries. Reflecting the advancement made in the application of machine learning techniques, NLP-guided techniques are increasingly being used to explore a variety of public health issues ranging from smoking behaviours (Pearson et al., 2018[31]), alcohol consumption (Rudge et al., 2021[32]) and obesity (Chou, Prestin and Kunath, 2014[33]) to public perception of policies to mitigate the impacts of the COVID‑19 pandemic (Petersen and Gerken, 2021[34]). The methodology used by the OECD analysis was vetted through a peer-review process in a high-impact Journal (Özçelik et al., 2022[35]) (a brief explanation of the methodology and the list of AMR-NAPs from the OECD countries and key partners, EU/EEA and G20 countries included in the analysis are provided in the Annex 4.A).
The OECD analysis makes use of two commonly used NLP metrics to assess the level of alignment between AMR-NAPs and the AMR-GAP. Term frequency (TF) is the first metric used in the analysis. It is interpreted as the relative emphasis on each strategic objective/intervention in a collection of AMR‑NAPs. It is a preferable metric to assess relative emphasis because it enables an analysis that takes into account the differences between the lengths of documents. It quantifies the frequency by which each term is associated with strategic objectives and recommended interventions that occur within an AMR-NAP with respect to the total number of terms in the term dictionary developed by the OECD. The second NLP metric used in the OECD analysis is term frequency-inverse document frequency (TF-IDF). TF-IDF is calculated to enable a comparative analysis of AMR-relevant interventions that occur in a given AMR-NAP in comparison to how frequently these interventions are featured across the collection of action plans. By evaluating TF-IDF scores, interventions that are most distinctly highlighted in each AMR-NAP compared to other documents can be identified.
Source: Chou, W., A. Prestin and S. Kunath (2014[33]), “Obesity in social media: A mixed methods analysis”, https://doi.org/10.1007/s13142-014-0256-1; Gentzkow, M., B. Kelly and M. Taddy (2019[36]), “Text as data”, https://doi.org/10.1257/jel.20181020; Petersen, K. and J. Gerken (2021[34]), “#COVID‑19: An exploratory investigation of hashtag usage on Twitter”, https://doi.org/10.1016/j.healthpol.2021.01.001; Pearson, J. et al. (2018[31]), “Exposure to positive peer sentiment about nicotine replacement therapy in an online smoking cessation community is associated with NRT use”, https://doi.org/10.1016/j.addbeh.2018.06.022; Rudge, A. et al. (2021[32]), “How are the links between alcohol consumption and breast cancer portrayed in Australian newspapers?: A paired thematic and framing media analysis”, https://doi.org/10.3390/ijerph18147657.
Performance tracking toward the objectives stated in the AMR-NAPs can be enhanced across OECD countries and key partners, EU/EEA and G20 countries
OECD countries and key partners, EU/EEA and G20 countries are diverse in terms of the time period of implementation they cover in their AMR-NAPs. Typically, AMR-NAPs are forward-looking documents that set out strategic goals and objectives to be realised in a predetermined period of time. The OECD analysis shows that, on average, the AMR-NAPs from the countries included in the analysis cover a span of nearly five years. But exceptions arise. The AMR-NAP from the Slovak Republic has the narrowest time span covering the two‑year period 2019‑21, whereas the AMR-NAP from Australia sets a 20‑year vision for the years from 2020 to 2040. In addition, the OECD analysis shows that AMR-NAPs from 6 OECD countries and key partners, EU/EEA and G20 countries predate the AMR-GAP and have not yet been updated since their initial publication, while many other AMR-NAPs are approaching the end of their coverage period.
There is a need to streamline the process to track performance relevant to the commitments made in the AMR-NAPs. Once they develop their AMR-NAPs, OECD members rely on different approaches to tracking their performance. For example, following the publication of its AMR-NAP in 2015, Germany regularly published interim reports that describe the national and subnational progress towards the goals stated in its AMR-NAP. France provides annual updates on the country’s progress towards the strategic priorities discussed in its AMR-NAP. Similarly, Australia publishes technical reports and analyses in regular intervals to continue to improve AMR awareness in hospital and community settings (ACSQHC, 2021[37]). While these efforts provide a valuable avenue to assess each country’s performance, there is little cross-country standardisation in the ways in which OECD countries examine their performance, making it difficult to compare cross-country performance over time.
Closer engagement with international organisations can help facilitate co‑ordinated action in line with the One Health approach
The OECD countries and key partners, EU/EEA and G20 countries explicitly recognise that curtailing AMR requires building international alliances and partnerships, but the nature of engagement with international bodies is often left undiscussed. While all 21 AMR-NAPs referred to the WHO as a key partner in tackling AMR, only around 71% (15/21) directly referenced the AMR-GAP. In addition to the WHO, nearly all OECD countries and key partners, EU/EEA and G20 countries made references to the WOAH, reflecting increasing attention to animal health as a pathway to tackle AMR. In comparison, the FAO was mentioned only by two‑thirds of AMR-NAPs (14/21) and UNEP was highlighted in less than 15% (4/21) of these documents. Importantly, even when these documents reference international bodies in their action plans, they do not often provide details on the extent of their engagement.
Globally, a number of regional AMR initiatives proliferated in recent years to tackle AMR (Box 4.8). In 2017, EU member states adopted the 2017 European One Health Action Plan against Antimicrobial Resistance, with the aim of bringing the EU to the forefront of efforts to tackle AMR (European Commission, 2017[38]). Another important regional initiative was initiated when AMR was included in the five‑year work programme of the Association of the Southeast Asian Nations (ASEAN) from 2016 to 2020 (Yam et al., 2019[39]). ASEAN members reiterated their commitment to regional co‑operation in tackling AMR in the 2017 Joint Declaration on Action against AMR and 2018 ASEAN Plus Three Leaders’ Statement on Co‑operation against Antimicrobial Resistance. In 2018, the newly launched Africa Centres for Disease Control and Prevention (Africa CDC) network developed a framework for tackling AMR (Africa CDC, 2018[40]). In this framework, the members of Africa CDC committed to establishing the Antimicrobial Resistance Surveillance Network, which will serve as a platform to foster collaboration across national public health institutions in the region.
Box 4.8. The European One Health Action Plan provides an important platform for cross-country collaboration and co‑operation to tackle AMR
Developed in 2017, the European One Health Action Plan supports the EU and its member states through a three‑pillar strategy of making the EU a best-practice region, boosting research, development and innovation, and shaping the global AMR agenda. Each pillar details actionable, interdependent steps to be pursued concurrently by the EC (European Commission, 2017[38]). The EU action plan stipulates that the member states are primarily responsible for identifying policies in alignment with their own needs and priorities, though the highlighted policies are considered to offer substantial value. Recently, the European Commission’s Directorate‑General for Health and Food Safety published a review of the policy priorities highlighted in the EU member states’ AMR-NAPs (European Commission, 2022[41]).
As shown in Figure 4.6, policies that receive the greatest attention in the EU action plan include supporting AMR-relevant R&D, exploring new economic models and incentives that promote AMR‑relevant innovations, scaling up AMR surveillance and stewardship interventions that promote the prudent use of antibiotics in the EU and beyond. In comparison, policies that promote improved water, sanitation, hygiene, waste and wastewater management practices are featured to a lesser extent, as well as policies to improve AMR awareness in the general public, enhancing food safety and improving food production and standards.
Figure 4.6. Top 10 interventions highlighted most frequently in the EU One Health Action Plan
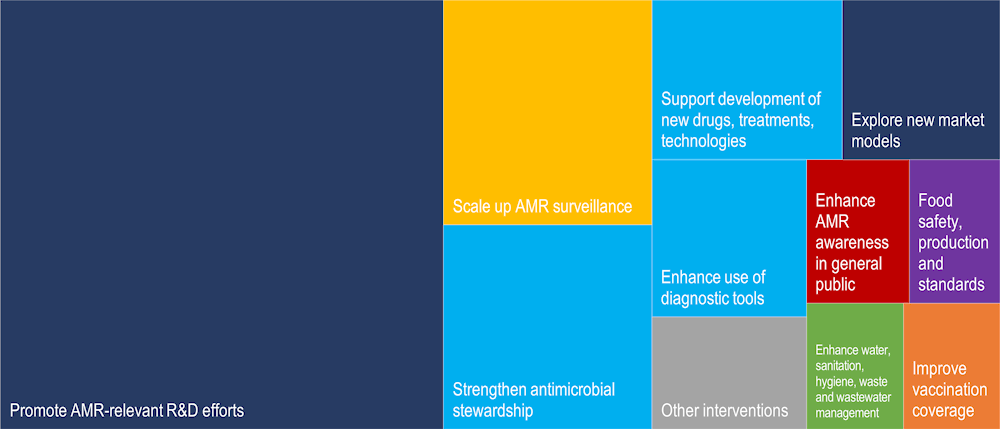
Note: The colour dark blue denotes interventions that can help develop an economic case for sustainable investment; the light blue depicts interventions that aim to optimise antimicrobial use in human and animal health; the colour yellow denotes interventions to strengthen knowledge and evidence base through surveillance and research; the colour red represents interventions that can help improve awareness and understanding of AMR; orange denotes interventions to improve vaccination coverage and the colour grey denotes other interventions.
Source: OECD analysis focusing on the content of the EU One Action Plan in terms of the relative emphasis of policy interventions linked to strategic objectives highlighted in the WHO-GAP. Emphasis on each policy is measured as a function of the frequency of terms associated with that policy relative to the frequency of terms linked to all of the strategic objectives.
Source: European Commission (2017[38]), A European One Health Action Plan against Antimicrobial Resistance (AMR), https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf; European Commission (2022[41]), Overview Report: Member States’ One Health National Action Plans against Antimicrobial Resistance, https://health.ec.europa.eu/system/files/2022-11/amr_onehealth_naps_rep_en.pdf.
OECD countries and key partners, EU/EEA and G20 countries can further improve their action plans by integrating financial considerations and cost-effectiveness assessments in these documents
Most AMR-NAPs lack detailed discussions around financial resources allocated to supporting the AMR agenda. The AMR-GAP underscores that countries need to make financial commitments to ensure advancements towards the policy vision laid out in their action plans (WHO, 2015[1]). Further, the WHO, FAO and WOAH recommend that countries perform regular assessments and reviews of the existing financial commitments in order to ascertain whether funds are dispersed in a timely fashion and in accordance with the priorities discussed in the AMR-NAPs (WHO/FAO/WOAH, 2019[42]). Despite this, only 57% (12/21) of the AMR-NAPs from OECD countries and key partners, EU/EEA and G20 countries discuss financial considerations and, even when financial considerations are mentioned, the level of financial resources committed to the AMR agenda often remains unclear.
Return on AMR-relevant investments can be better understood by utilising evidence generated by cost‑effectiveness assessments of interventions highlighted in AMR-NAPs. The OECD analysis shows that only around 43% (9/21) of OECD countries and key partners, EU/EEA and G20 countries refer to the cost-effectiveness of AMR-relevant investments that they consider in their action plans. For instance, the AMR-NAP from Switzerland highlights that research efforts focusing on the development of new diagnostic products are considered to be a cost-effective measure to facilitate the rapid detection of AMR. The AMR-NAP from the United Kingdom also alludes to the cost-effectiveness of diagnostic tools and suggests that evidence generated by cost-effectiveness models that demonstrate the value of diagnostic tools can be used to spur behaviour change among prescribers and health commissioners and encourage greater use of diagnostic tools. The AMR-NAP from Canada highlights that establishing a fast-track process to license antimicrobial drugs, alternatives to antimicrobials and new diagnostics is a cost-effective strategy to scale up investments in the development of pharmaceuticals. In the AMR-NAP from Malta, raising the awareness of employers on the benefits of extending options for home rest for employees who recover from mild infections is highlighted as a cost-effective strategy to interrupt the transmission of diseases in the workplace.
Assessing the alignment between AMR-NAPs and the AMR-GAP
Key messages
OECD countries and key partners, EU/EEA and G20 countries are consistent with the AMR-GAP in terms of the strategic objectives that they adopt in their action plans. There is a diversity of approaches across countries in terms of the range of interventions highlighted in AMR-NAPs to achieve these strategic objectives.
Optimising the use of antimicrobial medicines in human and animal health is the most prominently featured strategic priority but further improvements can be achieved:
Even though some older classes of antibiotics can still be used to treat certain indications, only about 24% of AMR-NAPs (5/21) reference older antimicrobials.
Less than half of AMR-NAPs (10/21) include discussions around AMR among the elderly populations, even though providers frequently prescribe antibiotics to their older patients as part of their treatment.
There are notable gaps in the monitoring of antibiotic consumption, with only around 19% of AMR-NAPs (4/21) referring to having at least one indicator based on a measure of defined daily doses or days of therapy.
In animal health, about one‑third of AMR-NAPs (6/21) lack any references to the antibiotics critically important to human health altogether.
While OECD countries and key partners, EU/EEA and G20 countries well recognise the centrality of strengthening AMR surveillance, deficits exist in engagement with global and regional AMR surveillance networks, enhancing laboratory network capacity and collecting information from new data sources.
The existing infection prevention and control programmes can be further advanced by incorporating strategies that promote food security and safety, and enhance biosecurity:
In 2021‑22, 49 out of 51 OECD, EU/EEA and G20 countries had in place national and facility-level IPC programmes in line with the WHO IPC core components, but only 23 out of 51 of these countries reported having IPC programmes that operate at the national and facility levels, where compliance and effectiveness are monitored and evaluated regularly.
Only a handful of AMR-NAPs mention IPC measures like decolonisation (i.e. the eradication or the reduction in the asymptomatic carriage of bacteria) and environmental hygiene and only 12 out of 21 AMR-NAPs stress the importance of hand hygiene practices.
Only 3 out of 21 AMR-NAPs refer to biosecurity measures in farm settings.
The OECD countries remain the largest financers of AMR-related R&D but there is room for new commitments to push incentives and harness public-private partnerships.
With respect to strategies to improve AMR awareness and understanding, OECD countries and key partners, EU/EEA and G20 countries put greater emphasis on interventions targeting medical professionals and the general community, whereas interventions targeting young children receive less attention.
The OECD countries and key partners, EU/EEA and G20 countries are consistent with the AMR‑GAP in terms of strategic objectives that they adopt in their action plans (Figure 4.7). Much like the AMR-GAP, the most frequently emphasised strategic objective by OECD, EU/EEA and G20 countries relates to interventions aiming to optimise the use of antimicrobial medicines in human and animal health, followed by strengthening AMR surveillance, enhancing sanitation, hygiene and waste management practices and spurring investments in AMR technologies. In comparison, increasing AMR awareness and education is the least frequently discussed strategic priority by countries and key partners, EU/EEA and G20 countries, as well as the AMR-GAP.
Figure 4.7. AMR-NAPs in most countries are well-aligned with the AMR-GAP in terms of the five strategic priorities
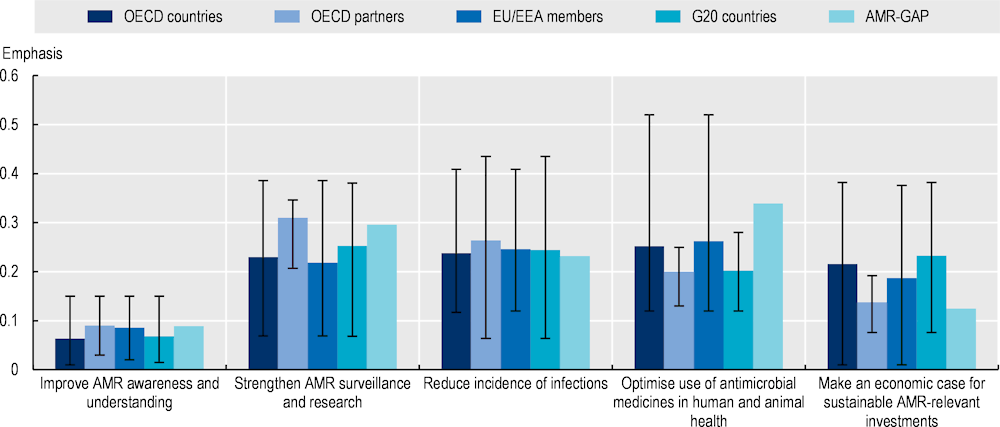
Note: The five strategic objectives displayed in the graph above are adapted from those discussed in the AMR-GAP. Emphasis on each strategic objective is quantified as a function of the total number of terms associated with that strategic objective relative to the total number of terms included in the term dictionary. Strategic objectives with greater term frequency are discussed more frequently in the text compared to those with lower term frequency. The whiskers represent the lowest and highest emphasis given to each strategic objective across the collection of AMR-NAPs.
Countries included in the analysis: Australia, Canada, China, Denmark, Finland, France, Germany, India, Indonesia, Ireland, Japan, Malta, New Zealand, Norway, South Africa, Saudi Arabia, the Slovak Republic, Sweden, Switzerland, the United Kingdom and the United States.
AMR-GAP: Global Action Plan on Antimicrobial Resistance; EU: European Union; EEA: European Economic Area; G20: Group of Twenty.
Source: Özçelik, E.A. et al. (2022[35]), “A comparative assessment of action plans on antimicrobial resistance from OECD and G20 countries using natural language processing”, https://doi.org/10.1016/j.healthpol.2022.03.011.
OECD countries and key partners, EU/EEA and G20 countries are highly diverse in the interventions that they distinctly highlight in their action plans
Different AMR-relevant interventions receive varying levels of attention across AMR-NAPs from the OECD countries and key partners, EU/EEA and G20 countries. For instance, with respect to interventions aiming to raise AMR awareness and understanding, Denmark and France stand out as countries that more frequently emphasise strategies to improve public awareness of AMR compared to others. Integrating AMR in professional education and training is more frequently highlighted in the action plans from Germany and the Slovak Republic. In terms of strengthening AMR knowledge and surveillance, Japan, New Zealand and the United States more frequently emphasise considerations around integrating new data sources into AMR surveillance, compared to the other countries included in the analysis. With respect to interventions to optimise antimicrobial use, Denmark, France and Norway more frequently discuss efforts to monitor antimicrobial consumption compared to other countries. Discussions around the importance of vaccines are more distinctly highlighted by Norway compared to other countries. In comparison, Finland, France and Japan more frequently discuss concerns related to enhancing biosecurity, and food safety and security. With respect to initiatives that aim to make an economic case for AMR investments, Switzerland and the United Kingdom more distinctly include discussions around exploring new market models and economic incentives, whereas Australia and France more distinctly highlight promoting public-private partnerships (PPP).
Several factors help explain the diverging patterns in interventions that countries emphasise in their AMR‑NAPs. A greater emphasis on one intervention does not imply that countries neglect other interventions. Instead, these diverging patterns may reflect the range of challenges that influence health system performance in each country at the time that policy makers develop these guiding documents. For example, India – one of the global AMR hotspots – is among the countries that explicitly highlight the importance of restricting the sale of antimicrobials without proper prescription. This pronounced emphasis may be partly due to the high prevalence of informal healthcare providers with no formal medical training in prescribing antimicrobials without prescription (Das et al., 2016[43]).
Alternatively, countries may choose to highlight strategies that they aspire to implement in the future rather than discussing strategies that they already put in place. For instance, Denmark does not provide in-depth discussions on the use of antimicrobials as growth promoters in its action plan even though this practice has been outlawed in the country in 1995. Similarly, in the United States, the AMR action plan does not specifically refer to electronic prescribing (e‑prescribing) because this practice is considered to be well-integrated into the health system. Another alternative explanation relates to the socio‑economic, historical and political factors, as well as the broader health system governance arrangements that may influence which interventions are ultimately featured in the AMR-NAPs. For instance, in Denmark and Sweden, veterinarians, farmers and regulatory authorities have a long history of co‑operation and collaboration, which has been shown to affect the ways in which the AMR agenda has developed in these countries (Björkman et al., 2021[44]; FAO, 2020[45]).
OECD countries and key partners, EU/EEA and G20 countries highlight a wide range of interventions in their AMR-NAPs to optimise the use of antimicrobial medicines in human and animal health
Most policies that aim to optimise the use of antibiotics recognise that the choices made by individuals are an important part of antibiotic use. Health professionals’ prescription behaviours are influenced by a range of factors including their medical training, the availability of systems that support clinical decision-making, provider compensation methods, professional and social preferences, and norms. Similarly, patient knowledge, preferences and attitudes play an important role in antibiotic use. Patient behaviours such as self-medication and non-compliance with the recommended course of treatment undermine efforts to curb AMR. Interactions between healthcare providers and patients have also been shown to influence behaviours around antibiotics.
All 21 AMR-NAPs explicitly recognise the importance of optimising the use of antibiotics, though these documents lay out a wide array of approaches to achieve this goal (Figure 4.8). Much like the AMR-GAP, AMR-NAPs from OECD countries and key partners, EU/EEA and G20 countries most frequently highlight efforts to strengthen antimicrobial stewardship programmes (ASPs) to promote the prudent use of antimicrobials. ASPs typically refer to a set of complex programmes that involve the implementation of multiple interventions designed to improve the ways in which antibiotics are prescribed by health professionals and used by patients. In addition to ASPs, enhancing the use of diagnostic tools is another frequently mentioned strategy in action plans, as well as monitoring the consumption of antimicrobials. In comparison to other interventions to optimise antibiotic use, OECD countries and key partners, EU/EEA and G20 countries less frequently mention efforts to limit the sale of antibiotics without a prescription and counterfeit or substandard antimicrobial sales, optimise animal feeding practices and restrict the use of antibiotics as growth promoters.
Figure 4.8. Antimicrobial stewardship programmes in human and animal health are the most highlighted interventions in AMR-NAPs
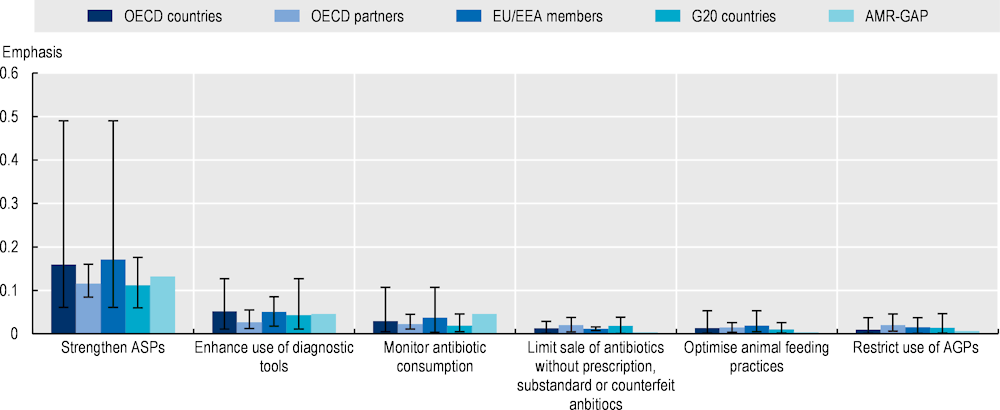
Note: The graph above displays a set of interventions selected from those recommended by the WHO to optimise antimicrobial use in human and animal health. Emphasis on each AMR-relevant intervention is quantified as a function of the total number of terms associated with that intervention relative to the total number of terms included in the term dictionary. Interventions with greater term frequency are discussed more frequently compared to interventions with lower term frequency. The whiskers represent the lowest and highest emphasis given to each intervention across the collection of AMR-NAPs.
Countries included in the analysis: Australia, Canada, China, Denmark, Finland, France, Germany, India, Indonesia, Ireland, Japan, Malta, New Zealand, Norway, South Africa, Saudi Arabia, the Slovak Republic, Sweden, Switzerland, the United Kingdom and the United States.
AGPs = antimicrobials as growth promoters; AMR-GAP: Global Action Plan on Antimicrobial Resistance; ASPs = antimicrobial stewardship programmes; EU: European Union; EEA: European Economic Area; G20: Group of Twenty.
Source: Özçelik, E.A. et al. (2022[35]), “A comparative assessment of action plans on antimicrobial resistance from OECD and G20 countries using natural language processing”, https://doi.org/10.1016/j.healthpol.2022.03.011.
Support antimicrobial stewardship programmes in human health
OECD countries rely on ASPs with varying design features to optimise antibiotic use to reflect the needs and priorities in their own settings. For example, in its AMR-NAP, Denmark considers a range of national and local measures to reduce the overall consumption of antibiotics in primary healthcare, with a recognition that different regions may require different measures to achieve the desired reductions in antibiotic consumption. The Danish approach has an explicit focus on the treatment of specific target groups like respiratory infections in children, coughs in adults or urinary tract infections in women. In addition, the Danish AMR-NAP encourages delayed prescribing practices, co‑operation with regional consultants and promotion of tools that can provide electronic overviews and comparisons of prescribing practices. In comparison, in its AMR-NAP, Sweden aims to promote the responsible use of antibiotics rather than an overall reduction in antibiotic consumption. To achieve this goal, Sweden relies on a multi‑modal approach that includes a continued focus on antibiotics prescriptions by authorised professionals, continued measurement of data on compliance with treatment guidelines both in human healthcare and veterinary medicine, adequate access to new and older antibiotics, and an emphasis on quality assured and adequate diagnostics, as well as the management of common infections. Importantly, Sweden combines interventions focusing on prescribing behaviours in the human health sector with efforts to promote responsible antibiotic manufacturing, safe disposal of antibiotics and waste management to promote responsible use in the lifetime of the antibiotics, as well as efforts to optimise antibiotic prescribing in veterinary medicine.
Recent WHO guidance points out that course corrections may be needed in the implementation of activities carried out under the overall organisation of ASPs over time. These modifications may be introduced either by altering the ways in which the interventions are implemented on the ground or by introducing new interventions to reflect the evolving needs in a given context of care (WHO, 2019[46]). The WHO guidance notes that the ease of implementation of each type of ASP will largely correlate with the availability of resources and competencies in health facilities and recommends the prioritisation of interventions in accordance with resource availability in a given context.
The effectiveness of many ASPs can be enhanced by extending antibiotic guidance to healthcare settings beyond hospital and acute care. The OECD analysis shows that hospitals and acute care facilities constitute around 75% of different types of healthcare settings discussed in AMR-NAPs from OECD countries and key partners, EU/EEA and G20 countries, followed by primary healthcare, and community settings (14%) and long‑term care (11%). Moreover, none of the AMR-NAPs makes any references to developing guidance for telemedicine, even though this mode of healthcare delivery had already been on the rise even before the COVID‑19 pandemic (Oliveira Hashiguchi, 2020[47]).
Only a handful of OECD countries adopt a comprehensive approach to tackling AMR in older populations. For example, Japan is considering options to incorporate materials concerning AMR, IPC and antimicrobial stewardship into the undergraduate curriculum and training guidelines for professionals deployed in nursing care. In addition, the national qualification examinations for nursing care staff will expand their focus on these topics. Japan also aims to strengthen AMR surveillance in nursing care, while conducting research to establish the current status of AMR in nursing care facilities. Complementing these efforts, Japan aims to establish clinical reference centres for AMR at the local level. These centres will be responsible for developing AMR-relevant educational materials to be used in a variety of settings, including nursing homes. These interventions will be supported by revising the IPC guidelines and manuals, which will introduce AMR and AMR screening components.
The Access, Watch and Reserve (AWaRe) framework offers another important avenue for OECD countries to support their local, national and global efforts to strengthen ASPs. The WHO developed the AWaRe framework in 2017 as part of the Essential Medicines List (EML) (WHO, 2021[48]). The AWaRe classifications provide a valuable framework for monitoring the use of antibiotics, setting targets and evaluating the effectiveness of ASPs (WHO, 2021[48]). It also provides a list of drugs that are is considered essential for the provision of basic healthcare services. In 2021, the AWaRe framework classified a total of 258 antibiotics across three groups:
Access: Broadly, Access antibiotics are comprised of lower-spectrum drugs used primarily as first- or second-line therapies. The WHO recommends that Access antibiotics constitute at least 60% of total consumption at the national level by 2023.
Watch: The Watch antibiotics contain broad-spectrum antibiotics and they pose a greater risk for AMR. The WHO recommends that Watch antibiotics are used only for treating specific indications.
Reserve: The Reserve antibiotics should be considered as a last resort, with their use being monitored closely and targeting multidrug resistant infections.
Emerging evidence suggests that, without urgent policy action, the WHO national-level targets for Access antibiotics is unlikely to be met. In its General Programme of Work 2019‑23, the WHO adopted a country-level target of the Access antibiotics accounting for at least 60% of the total consumption by 2023 (WHO, 2020[4]). Recent trends in antibiotic use across the globe suggest that this goal is unlikely to be met. Between 2000 and 2015, global antibiotic consumption increased by 39% between 2000 and 2015 (Klein et al., 2018[49]). While a rise in antibiotic use does not necessarily imply a rise in the imprudent use of antibiotics, this period has seen an alarming rise in the use of Watch antibiotics, especially in LMICs. The consumption of Watch antibiotics rose as much as 91% from 2000 to 2015, as measured by an increase from 3.3 to 6.3 defined daily doses (DDD) per 1 000 inhabitants per day (Klein et al., 2021[50]). At the same time, per-capita consumption of Access antibiotics as a share of total antibiotic consumption has seen an increase of 26% from 2000 to 2015 (Klein et al., 2021[50]). Compared to the Access and Watch antibiotics, the consumption of the Reserve group remains low. The rapid rise of Watch antibiotics points to challenges in the execution of ASPs, particularly in LMICs, and makes it difficult to achieve the WHO target for the use of Access antibiotics by 2023 (Klein et al., 2021[50]; Roberts and Zembower, 2021[51]).
The OECD countries and key partners, EU/EEA and G20 countries should also consider increasing access to older antibiotics. As discussed earlier, some older classes of antibiotics like tetracyclines and temocillin can still be used to treat certain indications. Despite this, only about 24% of AMR-NAPs (5/21) included in the analysis reference older antimicrobials. Different OECD countries put forward different motivations for promoting the use of older antibiotics in their AMR-NAPs. For instance, Sweden indicates that the use of older antibiotics, combined with access to newer antibiotics, is one strategy to increase the availability and use of antibiotics in the drug market in order to provide the best possible care. The United States also highlights the need for identifying new avenues for using older agents. In its AMR-NAP, the United States aims to make progress towards this goal by supporting data collection and evaluation, and by supporting the establishment/revision of antibiotic susceptibility testing standards.
E‑prescribing offers another promising avenue to improve monitoring antimicrobial use. Only 4 out of 21 AMR-NAPs included in the OECD analysis reference e‑prescribing. E‑prescribing practices are often featured in the AMR-NAPs as a way to improve the existing arrangements for monitoring antimicrobial use in healthcare settings. For example, the AMR-NAP from the United Kingdom indicates that, in Scotland, unique patient identifiers are used across primary and secondary care to track patients and monitor changes in antimicrobial use over time. In Finland, the option to use e‑prescribing is explored as an option to improve the surveillance of the consumption of antimicrobials. In Malta, e‑prescribing is considered as one option to measure antibiotic use at the farm level and information gathered through e‑prescribing can be used to link clinical indication, microbiological and consumption data.
Enhance the use of diagnostics
Enhancing the use of diagnostics is another highly emphasised strategy by OECD countries and key partners, EU/EEA and G20 countries. New diagnostic technologies like rapid diagnostic tests can aid providers in their decisions in the course of medical treatment by helping to obtain information about their patients rapidly, thereby curbing the unnecessary use of antibiotics. In recent years, OECD countries have made important strides to improve the availability of rapid diagnostics. For instance, the United Kingdom established the Longitude Prize in 2014 – an innovation fund aimed at incentivising the development of novel rapid tests to help reduce the overuse and misuse of antibiotics in human health. In the United States, the Antimicrobial Resistance Diagnostic Challenge – a federal prize competition – seeks to incentivise the development of novel rapid point-of-care and in-vitro laboratory diagnostic tests that can help identify and categorise resistant bacteria and/or discriminate between viral and bacterial infections (NIH, 2020[52]). These continued investments in the development of new diagnostic technologies have been instrumental in the development of a new point-of-care Clostridioides difficile diagnostic assay in 2017 and a diagnostic test for gonorrhoea in 2020 (Trevas et al., 2020[53]).
Monitor antibiotic consumption
Most AMR-NAPs do not include indicators to monitor the consumption of antimicrobials. The AMR-GAP and subsequent guidance from the WHO, FAO and WOAH underscore the importance of tracking patterns in the consumption of antimicrobials to assess the performance of antimicrobial stewardship efforts (WHO/FAO/WOAH, 2019[42]; WHO, 2015[1]). Despite this, only around 19% of AMR-NAPs (4/21) refer to having at least one indicator based on a measure of DDDs or days of therapy. Moreover, none of the countries included in the OECD analysis refer to performance indicators that can help track changes in the fraction of bloodstream infections due to selected AMR organisms as recommended by the WHO (2020[4]).
OECD countries vary substantially in terms of the quantifiable performance targets that they adopt (Table 4.2). For example, in its AMR-NAP dating October 2020, the United States sets out a 20% reduction in the number of healthcare‑associated resistant infections by 2025 and a 10% decline in community-acquired resistant infections. In comparison, the AMR-NAP from Denmark describes three related goals for optimising antibiotic consumption from 2016 to 2020. In the primary healthcare sector, a 24% reduction is set in the number of antibiotic prescriptions redeemed from 450 to 350 per 1 000 inhabitants from 2016 to 2020. In this period, a 10% reduction is aimed at the consumption of critically important antibiotics, while increasing its reliance on narrow-spectrum antibiotics like penicillin V. Norway also sets a comprehensive list of targets in optimising antibiotic use in its AMR-NAP. For instance, Norway aims to become one of the European countries with the lowest levels of antibiotic consumption. To achieve this goal, a 30% reduction in antibiotic use is set from 2012 to 2020, as measured in DDD per 1 000 inhabitants per day. In addition, Norway aims to reduce the use of antibiotics prescribed for respiratory infections by 20% in the same period. These targets in the human health sector are supplemented with quantifiable targets in animal health. For instance, for food-producing animals and household pets, Norway aims to achieve at least a 10% and 30% respective reduction in antimicrobial use from 2013 to 2020.
Table 4.2. Example quantifiable performance targets used in the AMR-NAPs from OECD countries
|
Country |
Target |
|---|---|
|
Denmark |
24% reduction in the number of antibiotic prescriptions redeemed from 450 to 350 per 1 000 inhabitants between 2016 to 2020 10% reduction in the consumption of critically important antibiotics between 2016 to 2020 |
|
Norway |
30% reduction in antibiotic use between 2012 to 2020 as measured in DDD per 1 000 inhabitants per day 20% reduction in antibiotic use for respiratory infections from 2012 to 2020 as measured in DDD per 1 000 inhabitants per day 10% reduction in antibiotic use for food-producing animals from 2013 to 2020 30% reduction in antibiotic use for food-producing animals from 2013 to 2020 |
|
United States |
Decrease healthcare‑associated antibiotic-resistant infections by 20% from 2020 to 2025 Reduce community-acquired antibiotic-resistant infections by 10% from 2020 to 2025 |
Optimise antimicrobial use in animal health
Providers in the human and animal health sectors often rely on the same or highly related antibiotics for treatment (WHO, 2017[54]). The WHO systematically groups antimicrobials into separate categories in accordance with their importance to human health: important, highly important or critically important for human medicine. This classification system underpins the WHO List of Critically Important Antimicrobials for Human Medicine (CIA List). CIAs are antibiotic classes that are used either: i) as the sole or among the limited therapies to treat serious bacterial infections in humans; or ii) to treat infections in humans caused by either bacteria that may be spread from non-human sources, or bacteria that may attain resistance genes from non-human sources (WHO, 2017[54]).
The WHO urges countries to consider the list of CIAs in the development and implementation of interventions to manage risks associated with antimicrobial use in food animals (WHO, 2017[54]). Yet, the OECD analysis suggests that gaps remain in the available antibiotic guidance in veterinary medicine, with about one‑third of AMR-NAPs (6/21) from 21 countries included in the analysis lacking any references to the CIAs altogether. Moreover, none of the action plans include a performance measure to track the volume of CIAs sold, even though this is one of the indicators recommended by the WHO to assess progress in the implementation of the AMR-GAP (WHO, 2017[55]).
Efforts to provide guidance on the veterinary use of antibiotics are often supplemented with regulatory measures to limit the use of antimicrobials as growth enhancers on otherwise healthy animals to accelerate weight gain and improve feed efficiency as recommended by the WHO (2017[54]). Currently, regulatory frameworks that restrict the use of antimicrobials for growth promotion remain uneven across geographic regions. In 2018, around 23% (35/153) of countries that participated in the most recent WOAH global survey indicated that they currently allowed the use of antimicrobials for growth promotion (WOAH, 2020[56]). Most countries that allowed antibiotics to be used for growth promotion were located in the Americas region (17/30), followed by the Asia, Far East and Oceania regions (9/25) and the Africa region (8/44). In contrast, in Europe, only 1 out of 48 countries in the region allowed antimicrobials to be used as growth promoters (WOAH, 2020[56]).
Most OECD countries and EU/EEA members have regulations in place that restrict access to veterinary antimicrobials (e.g. purchases only through authorised pharmacies, veterinarians and wholesalers and based on prescription). For instance, in early 2022, the Veterinary Medicinal Products Regulation (i.e. Regulation EU 2019/6) became applicable (EMA, 2022[57]). This regulation contains measures which outlaw the use of antimicrobial medicinal products, including designated antimicrobials, for prophylactic purposes with certain exceptions and enforce new restrictions for metaphylactic use (EMA, 2018[58]). Moreover, the new regulations include measures for imports from third parties outside the EU area. Specifically, with the new regulations, third county operators that export animals and animal products to the EU area are required to abide by the bans on the use of antimicrobials for growth enhancement purposes (Article 107(2)) and the use of antimicrobials for treating certain infections in human health (Article 37(5)) (EMA, 2018[58]). This is in stark contrast to many LMICs where the over-the‑counter purchase of veterinary antimicrobials without the need for a prescription remains the norm and access to veterinary antimicrobials is largely unchecked due to the existing regulatory gaps and difficulties around enforcing existing regulations (Sulis et al., 2020[59]).
Enhancing animal feeding practices is another strategy to optimise the veterinary use of antimicrobials. The FAO indicates that improving animal feeding practices can help reduce the need to use antibiotics by enhancing gut health, fortifying the immune system and building resistance against the pathogens that exist in the environment (FAO, 2012[60]). OECD analysis has showed that OECD countries and key partners, EU/EEA and G20 countries do not consistently refer to options to improve animal feeding practices in their own settings, with only around 52% (11/21) of action plans referring to this strategy.
Some OECD countries are making headways in improving animal feeding practices in their own settings. In 2019, the EC adopted a new regulation in 2019 (i.e. Regulation EU 2019/4), which regulates the use of medicated feed in animal populations (European Commission, 2022[61]). With the introduction of the new regulatory framework, the EU banned the use of antimicrobials in medicated feed for prophylaxis and growth enhancement purposes, established common limits for including antimicrobials in ordinary feed and set common standards for manufacturing safe medicated feed (European Commission, 2022[61]). In addition, the new regulations serve as a legal framework for manufacturing and distributing medicated feeds used for pets.
The centrality of strengthening AMR surveillance is acknowledged by all OECD countries and key partners, EU/EEA and G20 countries, though substantial efforts are needed to improve AMR surveillance
Strengthening AMR surveillance is key to addressing the AMR burden. Data gathered through surveillance provide the basis for developing and revising clinical treatment guidelines and inform the design and implementation of many ASPs and IPC guidelines, as well as the implementation of public health initiatives like vaccination programmes. Similar to the AMR-GAP, all OECD countries and key partners, EU/EEA and G20 countries included in the OECD analysis refer to the importance of strengthening AMR surveillance. These countries most frequently discuss strategies to enhance AMR surveillance capacity, while options to deepen the level of engagement with global and regional AMR surveillance networks and to promote new data sources in AMR surveillance are discussed to a lesser extent (Figure 4.9).
Figure 4.9. AMR-NAPs are generally well-aligned with the AMR-GAP on actions to enhance surveillance capacity
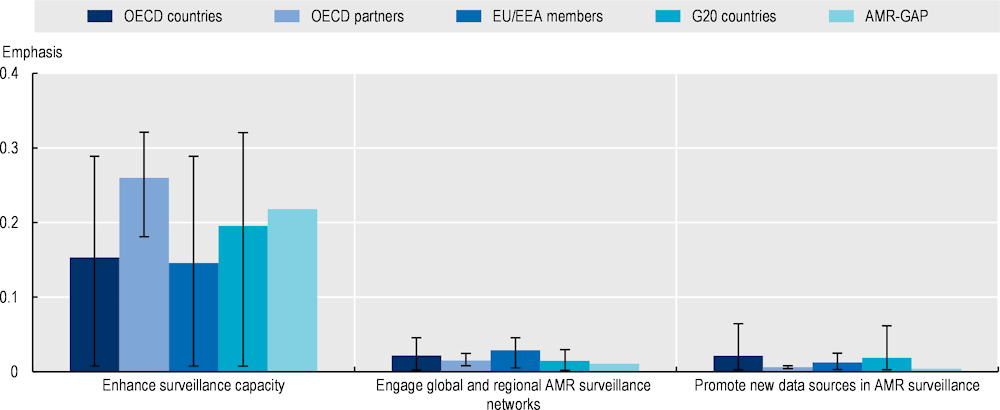
Note: The graph above displays a set of interventions selected from those recommended by the WHO to strengthen AMR surveillance. Emphasis on each AMR-relevant intervention is quantified as a function of the total number of terms associated with that intervention relative to the total number of terms included in the term dictionary. Interventions with greater term frequency are discussed more frequently compared to interventions with lower term frequency. The whiskers represent the lowest and highest emphasis given to each intervention across the collection of AMR-NAPs.
Countries included in the analysis: Australia, Canada, China, Denmark, Finland, France, Germany, India, Indonesia, Ireland, Japan, Malta, New Zealand, Norway, South Africa, Saudi Arabia, the Slovak Republic, Sweden, Switzerland, the United Kingdom and the United States.
AMR-GAP: Global Action Plan on Antimicrobial Resistance; EU: European Union; EEA: European Economic Area; G20: Group of Twenty.
Source: Özçelik, E.A. et al. (2022[35]), “A comparative assessment of action plans on antimicrobial resistance from OECD and G20 countries using natural language processing”, https://doi.org/10.1016/j.healthpol.2022.03.011.
Enhance AMR surveillance capacity
While OECD countries and key partners, EU/EEA and G20 countries universally acknowledge the centrality of AMR surveillance in their action plans, further advancements can be made by harmonising methodological approaches in data collection. A lack of harmonisation in the standardisation of epidemiological definitions of AMR, coupled with the variations in data and sample collection approaches, and microbial testing methods and data sharing policies hinder reliable and collaborative AMR surveillance. For instance, one recent study found that only one‑third of EU/EEA member states with AMR surveillance networks provide a clear definition of AMR in their technical guidelines and close to half do not indicate whether the definitions that they use are consistent with the definition used by European Committee on Antimicrobial Susceptibility Testing or Clinical and Laboratory Standards Institute guidelines (Tacconelli et al., 2018[62]).
Expanding the laboratory network capacity can help enhance rapid detection of AMR, identify new threats and inform the development of strategies to prevent the emergence of infections. Efforts to expand the laboratory network capacity are not often discussed in the AMR-NAPs, though there are notable exceptions. For instance, in its AMR-NAP, the United States refers to the Antibiotic Resistance Laboratory Network, which was established in 2016 as a network of laboratories across 50 states, including 7 regional laboratories and the National TB Molecular Surveillance Centre. In Europe, 29 EU countries participate in the European Antimicrobial Resistance Surveillance Network (EARS-Net), which is the largest publicly funded AMR surveillance platform. Other OECD countries are also taking steps to improve the existing standards around laboratory testing. As part of an effort to establish national minimum standards for laboratory testing and reporting antimicrobial susceptibility, New Zealand aims to establish a committee that will be tasked with providing expert guidance for laboratories and other stakeholders, with a specific focus on human susceptibility testing and reporting. This move will be supplemented with efforts to standardise the methodology and reporting of AMR data from human health laboratories.
Engage with global and regional AMR surveillance networks
OECD countries, EU/EEA and G20 countries will benefit from clarifying the ways in which they engage with the existing global and regional surveillance networks. Since 2000, more than 72 supranational networks have been developed to monitor AMR in bacteria, fungi, human immunodeficiency virus (HIV), TB and malaria (Ashley et al., 2018[63]). Yet earlier studies suggest that many local and national AMR surveillance systems have very little co‑ordination, harmonisation and information sharing with international surveillance frameworks (Tacconelli et al., 2018[62]). Consistent with this study, the OECD analysis shows that 16 out of 21 OECD countries and key partners, EU/EEA and G20 countries make references to global and regional AMR surveillance networks like the Central Asian and European Surveillance of Antimicrobial Resistance (CAESAR), EARS-Net, the Global Antimicrobial Resistance and Use Surveillance System (GLASS) and the Global Antibiotic Research and Development Partnership (GARDP). However, even when countries refer to these networks, they do not consistently describe the ways in which they engage with these networks, nor do they always provide a vision for future engagement.
Further progress is needed to scale up international and national-level surveillance systems for AMR in animal populations and in the food chain. While some OECD countries lack surveillance systems to monitor AMR in animals, others have made efforts in recent years to build their own systems, including the Czech Republic, Denmark, Finland, France, Germany, Norway, Sweden and the United Kingdom (EU-JAMRAI, 2021[64]). While this is good news, previous studies highlight that the existing surveillance networks are highly fragmented, with countries monitoring different animal specials, antimicrobials and bacterial species (EU-JAMRAI, 2021[64]). Moreover, cross-country comparison of available data is often not possible due to methodological differences in data collection efforts.
Integrate data from new sources in the AMR surveillance
Investing in timely and targeted dissemination of surveillance data is another vital strategy to strengthen AMR surveillance. Currently, point-prevalence surveys and laboratory-based surveillance are the primary sources of AMR-related information in many countries (Tacconelli et al., 2018[62]). Data collected through these means often take time to publish and disseminate, which limits the usefulness of these data for informing clinical and regulatory decision making. Many OECD countries continue to rely primarily on point-prevalence surveys and laboratory-based surveillance. For instance, in Europe, only about 3% of AMR surveillance systems are equipped to provide access to real-time data (Tacconelli et al., 2018[62]).
Integrating data from novel sources can help enhance AMR surveillance and help generate more accurate estimates of the true AMR burden. A growing body of evidence demonstrates the potential of new data sources and technologies like whole‑genome sequencing and whole‑metagenome sequencing to study the genetic determinants of AMR (Boolchandani, D’Souza and Dantas, 2019[65]). Some OECD countries refer to these technologies in their AMR-NAPs. For instance, in its action plan, the United States sets goals to improve the data infrastructure, data collection and analysis methods. As part of these efforts, it aims to build a new accelerator programme that will progress the implementation of whole‑genome sequencing, metagenomics and other molecular testing for resistant pathogens in human, animal and plant populations, food as well as in the environment.
Integrating data from novel sources will require co‑ordination across multiple stakeholders. For instance, in the United States, the Genomics for Food Safety (Gen-FS) consortium is one body that co‑ordinates efforts to facilitate whole‑genome sequencing among federal and state partners, with a focus on crosscutting priorities for molecular sequencing of foodborne and other zoonotic pathogens causing human illness, including the emergence and spread of the genetic determinants of antibiotic resistance, and using this information to support surveillance and outbreak investigation activities. The Gen-FS includes the U.S. Department of Agriculture, Food Safety and Inspection Service, as well as the Food and Drug Administration, the US Centers for Disease Control and Prevention (CDC), the Agricultural Research Service (ARS), the Animal and Plant Health Inspection Service (USDA/APHIS) and the National Center for Biotechnology Information (NIH/NLM/NCBI).
Interventions to improve infection prevention and control in human and animal health are key to tackling AMR in human and animal health sectors
Efforts to reduce the incidence of infections through improved IPC measures are vital to tackling AMR. While all 21 AMR-NAPs from OECD countries and partners, EU/EEA and G20 countries are consistent with the AMR-GAP in that they all highlight the importance of IPC measures, they differ from the AMR-GAP in terms of the interventions that they most frequently emphasise (Figure 4.10). For instance, the AMR-GAP most frequently highlights the need for improving vaccination coverage. In comparison, the OECD countries and partners, EU/EEA and G20 countries most frequently focus on supporting their IPC programmes and practices. Both in the AMR-GAP and the AMR-NAPs from OECD countries and partners, EU/EEA and G20 countries, options to promote food safety and security and enhance biosecurity are given less emphasis compared to the other IPC measures.
Support IPC programmes
The AMR-GAP underscores the importance of IPC programmes and guidelines to creating a robust framework to tackle AMR. Despite this, in 2021‑22, about 9% of countries (14/163), globally, lacked a national IPC programme or operational plan, compared to 11.4% (18/158) in 2017 (WHO/FAO/WOAH, 2022[12]). The first Global Report on the status of IPC also pointed to important deficits in the implementation of IPC programmes across countries different levels of socio-economic development (WHO, 2022[66]).
Figure 4.10. AMR-NAPs place the highest emphasis on infection prevention and control policies in human health
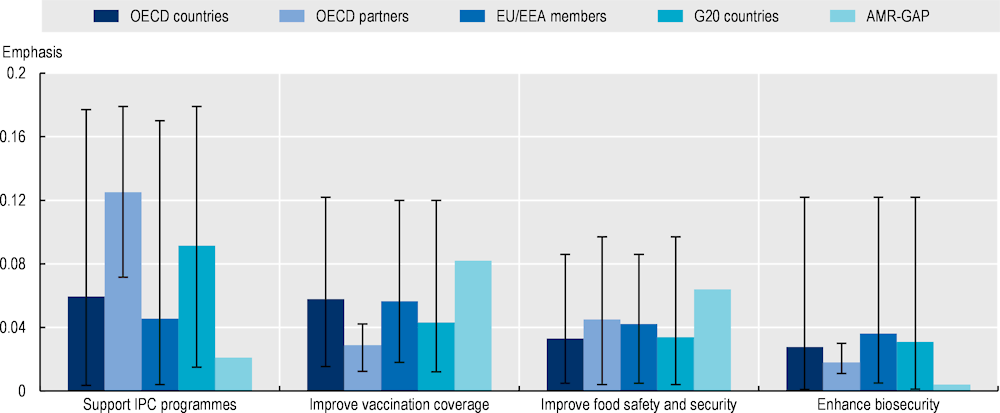
Note: The graph above displays a set of interventions selected from those recommended by the WHO to reduce the incidence of infections by strengthening IPC measures. Emphasis on each AMR-relevant intervention is quantified as a function of the total number of terms associated with that intervention relative to the total number of terms included in the term dictionary. Interventions with greater term frequency are discussed more frequently compared to interventions with lower term frequency. The whiskers represent the lowest and highest emphasis given to each intervention across the collection of AMR-NAPs. OECD, EU/EEA and G20 countries included in the analysis include: Australia, Canada, China, Denmark, Finland, France, Germany, India, Indonesia, Ireland, Japan, Malta, New Zealand, Norway, South Africa, Saudi Arabia, the Slovak Republic, Sweden, Switzerland, the United Kingdom and the United States. AMR-GAP: Global Action Plan on Antimicrobial Resistance; EU: European Union; EEA: European Economic Area;; G20: Group of Twenty; IPC = Infection prevention and control.
Source: Özçelik, E.A. et al. (2022[35]), “A comparative assessment of action plans on antimicrobial resistance from OECD and G20 countries using natural language processing”, https://doi.org/10.1016/j.healthpol.2022.03.011.
The OECD analysis reveals that all AMR-NAPs from the selected OECD countries and key partners, EU/EEA and G20 countries explicitly reference the importance of IPC programmes in healthcare settings but only a fraction of these countries have mechanisms in place to monitor these programmes. The results from the OECD analysis are in line with the latest AMR Country Self-Assessment Survey. In 2021‑22, nearly all OECD members, key partners and G20 countries (49/51) indicated that had in place national and facility-level IPC programmes in accordance with the WHO Guidelines on Core Components of IPC but only 45% (23/51) reported having IPC programmes that function both at the national and health facility levels consistent with the WHO IPC core components guidelines, where compliance and effectiveness are monitored and evaluated on a regular basis and guidance is updated in accordance with the results from monitoring (WHO/FAO/WOAH, 2022[12]). While the OECD countries and key partners, EU/EEA and G20 countries frequently discuss IPC interventions, they do not always describe efforts to improve the existing IPC practices. For instance, only a handful of AMR-NAPs mention IPC measures like decolonisation and environmental hygiene, and only around 57% (12/21) AMR-NAPs highlight the importance of hand hygiene practices.
Even when these documents refer to IPC measures, specific actions to enhance the existing IPC practices are not always described. Some exceptions emerge. For example, South Africa indicates in its AMR-NAP that it aims to scale up community outreach to enhance hand hygiene practices and aspires to supplement this activity with changes in legislation and national guidelines to include core IPC requirements and facilities for improved hand hygiene practices, whereas Ireland integrates compliance with the WHO’s My 5 Moments for Hand Hygiene approach into its monitoring and evaluation framework by tracking the level of compliance among hospital staff as a key performance measure of the performance of the overall health system. In Australia, the National Hand Hygiene Initiative was launched in 2008, which relies on a multi-model strategy involving the use of alcohol-based hand rubs at the point of care, provision of IPC education and training, monitoring of hand hygiene compliance and feedback, and encouraging culture change around hand hygiene practices (ACSQHC, 2008[67]).
Improve human and veterinary vaccination coverage
Increasing vaccination coverage is another widely recognised strategy to curb the spread of infections. All AMR-NAPs refer to vaccines as part of efforts to prevent the spread of resistant infections in human health. Moreover, OECD countries like Norway, the United Kingdom and the United States indicate in their action plans to continue supporting vaccination campaigns in other countries not only through bilateral contributions but also by funding contributions to international initiatives like Gavi, the Vaccine Alliance. The widespread recognition of the value of vaccines is also reflected in the high vaccination coverage among OECD members, though some countries are facing challenges in maintaining the vaccination rates high (Chapter 5 provides more detailed information on a wide range of strategies to improve vaccination coverage).
Less attention has been paid to improving the coverage of veterinary vaccines. Norway is among the OECD countries that place veterinary vaccines at the centre of efforts to curb the spread of infections. In its action plan, Norway attributes the near elimination of antimicrobial use in aquaculture production since 1987 to the expansion of access to and use of veterinary vaccines. The country highlights that the scale‑up of veterinary vaccines over the last three decades coincided with a 20‑fold increase in national fish production, allaying potential concerns over agricultural production capacity. Building on its own experience, Norway stresses that the advancements in the development and application of veterinary vaccines remain to be the most prominent strategy to avoid the need for using antibiotics in aquaculture production.
Enhance biosecurity in farm settings
Biosecurity measures can help curb the emergence and spread of infections among animals that share the same environment. Broadly, biosecurity measures can be classified into two groups (Alarcón, Alberto and Mateu, 2021[68]): external and internal. Combined, these measures are meant to reduce the transmission of pathogens between and within farms. External biosecurity covers the range of strategies that aim to prevent the emergence of pathogens within the farm (e.g. test livestock and feed before their purchase; develop a list of health requirements for incoming animals that group diseases in accordance with risks they present to the farm, and identify verification tests that will be routinely performed; protect feed from contact with wildlife; practice safe animal transport) (Alarcón, Alberto and Mateu, 2021[68]). In comparison, internal biosecurity relates to strategies that can help reduce the spread of pathogens once they are already detected on the farm. Internal biosecurity measures can be grouped as: those that relate to herd management (e.g. strict application of an all-in/all-out system); improvements in sanitary measures (e.g. separate infected animals from the rest of the animals; avoid reusing bedding from infected animals); cleaning and disinfection (e.g. cleaning and disinfecting facilities before a new batch of animals enters into the farm); and farm personnel strategies (e.g. use gloves; practice routine hand washing and foot baths) (Alarcón, Alberto and Mateu, 2021[68]).
Despite its potential benefits, enhancing biosecurity in agricultural production is not a frequently mentioned strategy by OECD countries and key partners, EU/EEA and G20 countries in their AMR-NAPs. Only 3 out of 21 countries included in the OECD analysis discuss biosecurity measures in their AMR‑NAPs. Among OECD countries that discuss biosecurity in their action plans, different approaches are pursued. For instance, France highlights that biosecurity measures will focus on strengthening stockbreeding conditions. Whereas Ireland underscores the need to adopt a holistic approach to biosecurity and animal husbandry, which involves actions to scale up of national guidelines and standards on biosecurity and hygiene practices. In comparison, the United Kingdom highlights the importance of raising awareness around the centrality of disease prevention and co‑ordinating with the livestock industry and animal keepers.
The OECD countries play a crucial role in promoting AMR-related R&D across the globe
Curtailing the AMR burden will require new developments in new antimicrobial drugs, treatments and diagnostic tools. Currently, 50 antibiotics are in different stages of clinical trials, 32 of which target pathogens identified in the WHO’s priority list (WHO, 2020[69]). However, the vast majority of these antibiotics offer only marginal benefits in comparison to already existing antibiotics. Recognising this, the AMR-GAP acknowledges that the existing deficits in investments for AMR-related R&D partly reflect the deteriorating market conditions that limit the potential revenue streams and concerns over lower expected return on investment compared to other therapeutic fields. To address these concerns, the AMR-GAP stresses the importance of spurring AMR-related R&D activities through incentives and public-private partnerships.
Incentivise AMR-related R&D
Broadly, countries have in their arsenal two types of incentives to spur AMR-related R&D: pull and push (Table 4.3). Push incentives typically refer to those that aim to reduce entry barriers by reducing costs associated with developing new drugs (Renwick, Simpkin and Mossialos, 2016[70]). In comparison, pull incentives are those that aim to spur the development of new drugs by increasing the expected future revenues. Previous works note that both types of incentives come with certain advantages and caveats and that countries may benefit from adapting a mixed strategy that combined these incentives to spur AMR-related innovation (Simpkin et al., 2017[71]; Outterson, 2021[72]).
Table 4.3. Example push and pull incentives to spur AMR-related R&D
|
Incentive type |
Example |
|---|---|
|
Push incentives |
|
|
Outcome‑based pull incentives |
|
|
Lego-regulatory pull incentives |
|
Source: Adapted from Renwick, M., V. Simpkin and E. Mossialos (2016[70]), Targeting Innovation in Antibiotic Drug Discovery and Development: The need for a One Health – One Europe – One World Framework, https://pubmed.ncbi.nlm.nih.gov/28806044/.
OECD countries will benefit from putting greater emphasis on the pull incentives to spur AMR-related innovations without scaling back on their current comments for push incentives (Box 4.9). With respect to strategies to spur sustainable AMR investments, 21 countries included in the OECD analysis primarily highlight push incentives in their action plans such as direct funding, product development partnerships, scientific grants dedicated to AMR-related research projects and increasing engagement with domestic and international scientific research communities and collaborations. This finding is in congruence with earlier works showing that the major international R&D funding programmes, as well as those funded by the EU, the United Kingdom and the United States prioritise early-stage push incentives (Simpkin et al., 2017[71]). While a robust commitment to push incentives is welcomed, recent modelling studies underscore the need to supplement push incentives with additional commitments to pull incentives (Outterson, 2021[72]).
Box 4.9. OECD countries remain the leading source of financing for R&D relevant to AMR but the overall financing for R&D has been on a decline
Globally, resources allocated to R&D relevant to AMR is shrinking
Between 2017 and 2020, the total spending on R&D for AMR remained relatively stable, with a slight decline from USD 1.67 billion in 2017 to USD 1.92 billion in 2020 (Global AMR Hub, 2023[73]). In 2020, G7 and OECD countries, including Germany, the United Kingdom and the United States, as well as EU/EEA member states, were the lead source of financing for R&D allocated to AMR (Figure 4.11).
Figure 4.11. In 2020, G7 and OECD countries remained the main source of funding for AMR innovations
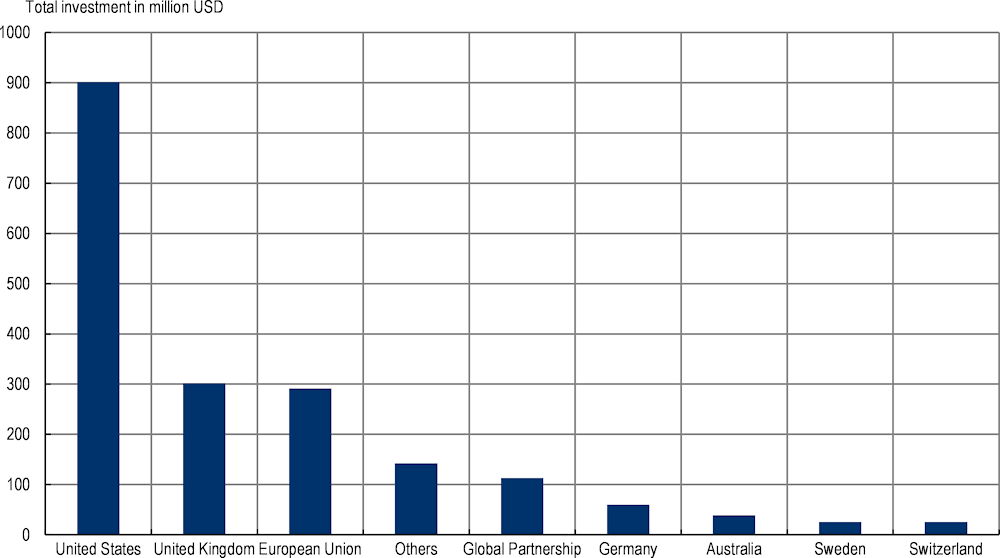
Note: The “Others” category includes all other countries listed in the Dynamic Dashboard of the Global AMR Hub.
Source: Global AMR Hub (2023[73]), Dynamic Dashboard: Investing in AMR R&D, https://dashboard.globalamrhub.org/reports/investments/overview (accessed on 12 June 2023).
Increasing funding dedicated to the later stages of antimicrobial development is crucial
It is crucial to supplement funding allocated to the earlier stages of clinical development with additional funding directed towards the later stages to incentivise market access and attract private investment. In 2020, most R&D funding for AMR was allocated to funding basic research, development of therapeutics, operational and implementation research that can help support decision-making and management strategies, and diagnostics and capacity-building activities (Global AMR Hub, 2023[73]). This finding is consistent with studies that examined earlier periods, which concluded that the majority of R&D funding for AMR is allocated to supporting basic research and preclinical trials (Simpkin et al., 2017[71]). While this emphasis on the early stage of antimicrobial development is essential, increasing financial resources available for the later stages of clinical development can offer an important incentive that facilitates timely access to pharmaceutical markets in newly developed antibiotics. Moreover, increasing late‑stage incentives can help attract greater private investments.
Sources: Simpkin, V. et al. (2017[71]), “Incentivising innovation in antibiotic drug discovery and development: Progress, challenges and next steps”, https://doi.org/10.1038/ja.2017.124; Global AMR Hub (2023[73]), Dynamic Dashboard: Investing in AMR R&D, https://dashboard.globalamrhub.org/reports/investments/overview (accessed on 23 July 2020).
A handful of OECD countries highlight in their AMR-NAPs a range of pull incentives and pilot initiatives to encourage AMR-related innovations. For example, in Japan, new regulatory approval processes have been introduced, including priority reviews for new antimicrobials that can be used for treating resistant infections. The United States is considering obtaining antibiotic products directly through Public Health and National Security purchases to encourage commercialisation. Some OECD countries are also enacting pull incentives for AMR-related innovations. Since publishing its AMR-NAP, the United Kingdom embarked on a new pilot project in 2019 that aims to interrupt the link between sales volume and sales revenues. Through this programme, the National Health Service committed to paying an annual subscription fee of up to GBP 10 million per each new antibiotic covering WHO-priority pathogens regardless of the consumption volume of the antibiotic.
There is a need to build a measurement framework that can help track cross-country progress in bolstering the different stages of AMR-relevant R&D activities over time. Currently, only a handful of OECD countries use measurable performance indicators to track performance in spurring AMR-relevant R&D over time, with a particular emphasis on the earlier stage of clinical development. For example, the United States – the leading funder of AMR-relevant R&D – measures performance using three indicators: i) support the publication of at least 1 000 publications focusing on basic, traditional, and clinical AMR research by 2021; ii) support the training of at least 60 new/early career researchers whose research is applicable to AMR; and iii) build at least two collaborations between human health and agriculture sectors via agreements across agencies. Japan is another country that uses evaluation indices for measuring progress in supporting R&D innovations. These indices include: i) the number of publications applicable to AMR funded through national grants; and ii) the number of genomes accumulated in the genome database to promote AMR genome surveillance.
Foster PPPs
Fostering PPPs to garner AMR innovations is an overlooked area, though notable examples are emerging. PPPs offer an important means to harness the comparative advantage of public and private organisations. Around 67% (14/21) of OECD and G20 countries explicitly reference considerations around PPPs. In recent years, examples of PPPs relevant to AMR emerged. For example, in 2016, the Combating Antibiotic-Resistant Bacteria X (CARB-X) was launched as a global partnership to help finance the preclinical development of drug candidates to prevent and treat resistant infections (CARB-X, 2021[74]). Today, CARB-X has become the world’s largest PPP that funds the early development pipeline of new antibiotics, diagnostics and relevant products, with contributions from the United Kingdom and the United States. In Europe, the Innovative Medicines Initiative (IMI) was formed in 2007 as a PPP between the European Union and the European pharmaceutical industry. Considered to be the largest life sciences PPP globally, the IMI aims to enhance the efficiency and effectiveness of drug development processes. In 2012, the IMI created the New Drugs for Bad Bugs project that funded eight projects costing EUR 650 (IMI, 2017[75]). In 2018, the IMI launched the AMR Accelerator which aims to develop new medicines for preventing and treating resistant infections with Mycobacterium tuberculosis, nontuberculous mycobacteria and Gram-negative bacteria.
Compared to the other strategic objectives highlighted in the AMR-GAP, the AMR-NAPs included in the OECD analysis put the least emphasis on improving AMR awareness and understanding
Raising awareness and understanding around AMR is paramount to promoting behaviour change among antibiotic prescribers and users. In recognition, the AMR-GAP urges countries to scale up programmes targeting a variety of stakeholders in the human and animal health sectors, including prescribers, pharmacists, veterinarians, farmers and consumers (WHO, 2015[1]). Much like the AMR-GAP, OECD countries and key partners, EU/EEA and G20 countries primarily emphasise interventions that aim to raise AMR awareness and understanding among healthcare professionals and the general public, whereas school-based interventions for young children are less frequently discussed (Figure 4.12).
Integrate AMR in professional education and training
OECD countries and key partners, EU/EEA and G20 countries pursue a range of interventions to integrate AMR-relevant materials in the education and training of health professionals across different stages of their professional development. Many countries rely on revising/updating the curriculum in undergraduate and postgraduate training to include materials relevant to infection and disease prevention and AMR. For example, in Ireland, undergraduate and postgraduate core curricula and examinations involve academic materials on disease prevention, AMR as well as prudent antibiotic use. In Switzerland, infectious disease specialists undergo extensive training and education on AMR as part of their specialisation requirements. In Germany, health professionals have access to advanced training programmes on rational antibiotic therapy, as well as training courses on the prevention of nosocomial infections in hospital settings. AMR-related materials are also integrated into the licensing and accreditation examinations. This is the case in Japan, where the national examinations for obtaining the required qualifications as a professional in human and veterinary medicine, nursing care and public welfare are aimed at including a more expanded focus on AMR, IPC and antimicrobial stewardship.
In addition, OECD countries provide new avenues for continuous professional education (e.g. organising training workshops, websites, e‑learning initiatives). For instance, France recently launched new webpages on AMR and the prudent use of antibiotics for both healthcare professionals as well as the general public. In the United Kingdom, a new Animal Medicines Best Practice training course, a set of online courses targeting farmers and veterinary surgeons, was kicked off in 2018 in order to promote the prudent use of antibiotics in farm settings.
Figure 4.12. Similar to the AMR-GAP, improving AMR awareness in the public and among health professionals is frequently emphasized in AMR-NAPs
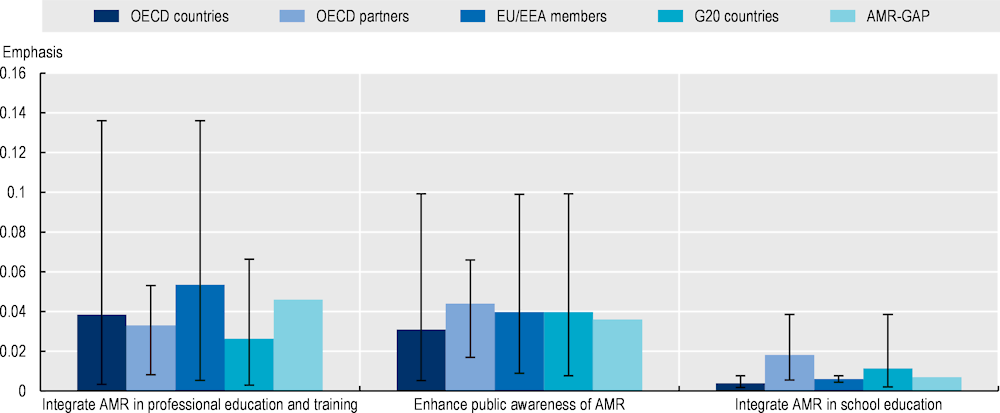
Note: The graph above displays a set of interventions selected from those recommended by the WHO to improve awareness and understanding of AMR. Emphasis on each AMR-relevant intervention is quantified as a function of the total number of terms associated with that intervention relative to the total number of terms included in the term dictionary. Interventions with greater term frequency are discussed more frequently compared to interventions with lower term frequency. The whiskers represent the lowest and highest emphasis given to each intervention across the collection of AMR-NAPs.
Countries included in the analysis: Australia, Canada, China, Denmark, Finland, France, Germany, India, Indonesia, Ireland, Japan, Malta, New Zealand, Norway, South Africa, Saudi Arabia, the Slovak Republic, Sweden, Switzerland, the United Kingdom and the United States.
AMR-GAP: Global Action Plan on Antimicrobial Resistance; EU: European Union; EEA: European Economic Area; G20: Group of Twenty.
Source: Özçelik, E.A. et al. (2022[35]), “A comparative assessment of action plans on antimicrobial resistance from OECD and G20 countries using natural language processing”, https://doi.org/10.1016/j.healthpol.2022.03.011.
Improve antibiotic awareness and understanding in the public
The OECD countries and key partners, EU/EEA and G20 countries often rely on antibiotic awareness campaigns to raise awareness and education in the general public. Awareness campaigns offer a tempting option for governments, as they can help disseminate valuable information to large audiences at a relatively low cost (Huttner et al., 2019[76]). They are typically organised by public health authorities and target the general community and healthcare professionals at the same time. They tend to rely on communication and educational materials disseminated through print materials, television, radio and online platforms.
The OECD analysis shows that 16 out of 21 OECD countries and key partners, EU/EEA and G20 countries explicitly discuss activities related to some version of an antibiotic awareness campaign (e.g. World Antibiotic Awareness Week) in their own setting. For instance, across EU/EEA members included in the analysis, five out of nine explicitly highlighted the European Antibiotic Awareness Day.
Translating AMR knowledge into changes in attitudes and behaviours around antibiotics remains an important public health challenge. Echoing earlier works, the 2018 Eurobarometer survey found that 85% of respondents were aware of the adverse effects of unnecessary use of antibiotics and 85% indicated that they knew that compliance with prescribed antibiotic dosage was important (Eurobarometer, 2018[77]; Paget et al., 2017[78]). But the same survey also showed that having AMR knowledge did not guarantee changes in attitudes towards antibiotics and behaviours. Only 29% of respondents indicated that AMR information changed their views on the misuse of antibiotics and 7% indicated that they used antibiotics in the last 12 months without a prescription.
Integrate AMR in the education of young children
The OECD analysis suggests that integrating AMR into the education of young children is not a consistently used strategy among OECD countries and key partners, EU/EEA and G20 countries. Broadly, interventions that attempt to incorporate AMR in the education of school-aged children aim at improving the knowledge and understanding of antimicrobials among future users. A promising body of evidence suggests that these interventions may decrease infections among children and reduce school absenteeism (Willmott et al., 2015[79]). Despite this, the OECD analysis shows that only a handful of AMR-NAPs from OECD countries and key partners, EU/EEA and G20 countries explicitly mention the potential options to introduce and scale up educational initiatives targeting young children.
In recent years, the e‑Bug programme has emerged as one international education initiative aiming to enhance hygiene and AMR knowledge among young children by providing free educational materials. Launched in 2006, e‑Bug has been adopted by 29 countries by 2019 (Hayes et al., 2020[80]). Emerging evidence, primarily from OECD countries such as the Czech Republic, France and the United Kingdom shows that the e‑Bug programme can be effective in improving awareness and understanding of antibiotics and hygiene among young children (e-Bug Working Group, 2010[81]; Farrell et al., 2011[82]; Hawking et al., 2013[83]). Some OECD countries explicitly refer to the programme in their AMR-NAPs. For instance, in its AMR-NAP, Ireland indicates that first- and secondary-level school students are among the primary target of activities to improve AMR knowledge and awareness, and the e‑Bug initiative should be adopted in primary and post-primary curricula. The United Kingdom also refers to a recent e‑Bug initiative, which was specifically designed in collaboration with Farming and Countryside Education and farmers to improve young school children’s understanding of farm hygiene.
Conclusion
This chapter provides an overview of the global progress made in the implementation of action plans to tackle AMR. Findings from the chapter demonstrate that there have been important advancements in the development of action plans to tackle AMR across the globe. However, the implementation of AMR-NAPs globally is characterised by a socio‑economic development gradient, with LMICs lagging in terms of advancing the implementation and financing of AMR-NAPs.
Among OECD countries and key partners, EU/EEA and G20 countries, there has been notable progress in the uptake of multi-sectoral approaches, with the animal sector being involved in the development of all action plans. Yet, further progress is needed to expand multi-sectoral action to include plant health and the AMR transition in the environment. Having developed their action plans, many of these countries are now grappling with the implementation of their AMR-NAPs. Rigorous monitoring and evaluation of the implementation of AMR-NAPs are paramount to ensure course corrections can be made based on lessons learned from the execution of these documents on the ground.
Further, the OECD analysis pointed to the importance of keeping the AMR-NAPs up to date. It showed that some OECD countries have not updated their action plans since their initial publication, while others are nearing to the end of their coverage period. At a time when health systems across the world are grappling with the COVID‑19 pandemic, updating and/or revising AMR-NAPs is key to ensuring that these documents reflect the lessons learned from implementation, filling the gaps in the existing guidance and incorporating new guidance that considers the evolving health financing and delivery needs.
The analysis also suggested little cross-country standardisation in measuring performance over time in terms of the goals stated in AMR-NAPs, which hinders efforts to benchmark cross-country performance. Another important finding showed that funding considerations and cost-effectiveness of interventions to tackle AMR are often left undiscussed. Addressing these gaps in the design of the action plans helps improve the effectiveness of the vision laid out in these documents.
Finally, the chapter presented a systematic assessment of the strategic priorities and interventions adapted by OECD countries and key partners, EU/EEA and G20 countries in action plans using the AMR‑GAP as a blueprint. The results suggested a high degree of alignment between countries included in the OECD analysis and the AMR-GAP in terms of their strategic objectives. Coupled with this, there is a diversity of interventions countries consider to achieve their strategic objectives. The diverging patterns in terms of the preferred interventions are likely a reflection of the health system challenges, as well as the broader historical, socio‑economic and political factors that shape policy design and implementation in each setting. Combined, evidence generated by this chapter suggests that countries that are considering developing new action plans and/or revising the existing ones will benefit from examining the main drivers of AMR in their own settings and identify interventions to address these challenges in congruence with the strategic objectives and interventions recommended by the WHO.
References
[37] ACSQHC (2021), Fourth Australian report on antimicrobial use and resistance in human health, Australian Commission on Safety and Quality in Health Care, https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-2021 (accessed on 4 November 2022).
[67] ACSQHC (2008), National Hand Hygiene Initiative, Australian Commission on Safety and Quality in Health Care, https://www.safetyandquality.gov.au/our-work/infection-prevention-and-control/national-hand-hygiene-initiative (accessed on 4 November 2022).
[40] Africa CDC (2018), Africa CDC Framework for Antimicrobial Resistance, Africa Centres for Disease Control and Prevention, https://africacdc.org/download/africa-cdc-framework-for-antimicrobial-resistance/ (accessed on 23 July 2020).
[68] Alarcón, L., A. Alberto and E. Mateu (2021), “Biosecurity in pig farms: A review”, Porcine Health Management, Vol. 7/1, https://doi.org/10.1186/s40813-020-00181-z.
[30] Anderson, M. et al. (2019), “A governance framework for development and assessment of national action plans on antimicrobial resistance”, The Lancet Infectious Diseases, Vol. 19/11, pp. e371-e384, https://doi.org/10.1016/s1473-3099(19)30415-3.
[19] Arinaminpathy, N. et al. (2013), “The Global Drug Facility and its role in the market for tuberculosis drugs”, The Lancet, Vol. 382/9901, pp. 1373-1379, https://doi.org/10.1016/s0140-6736(13)60896-x.
[63] Ashley, E. et al. (2018), “An inventory of supranational antimicrobial resistance surveillance networks involving low- and middle-income countries since 2000”, Journal of Antimicrobial Chemotherapy, Vol. 73/7, pp. 1737-1749, https://doi.org/10.1093/jac/dky026.
[15] Birgand, G. et al. (2018), “Comparison of governance approaches for the control of antimicrobial resistance: Analysis of three European countries”, Antimicrobial Resistance & Infection Control, Vol. 7/1, https://doi.org/10.1186/s13756-018-0321-5.
[44] Björkman, I. et al. (2021), “Animal production with restrictive use of antibiotics to contain antimicrobial resistance in Sweden – A qualitative study”, Frontiers in Veterinary Science, Vol. 7, https://doi.org/10.3389/fvets.2020.619030.
[65] Boolchandani, M., A. D’Souza and G. Dantas (2019), “Sequencing-based methods and resources to study antimicrobial resistance”, Nature Reviews Genetics, https://doi.org/10.1038/s41576-019-0108-4.
[25] CARB (2020), National Action Plan for Combating Antibiotic-Resistant Bacteria 2020-2025, Federal Task Force on Combating Antibiotic-Resistant Bacteria, https://www.hhs.gov/sites/default/files/carb-national-action-plan-2020-2025.pdf.
[74] CARB-X (2021), Stewardship & Access Plan (SAP), Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator, https://carb-x.org/wp-content/uploads/2021/03/Stewardship__Access_DevGuide_2021.pdf.
[9] Chmielewska, B. et al. (2021), “Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis”, The Lancet Global Health, Vol. 9/6, pp. e759-e772, https://doi.org/10.1016/s2214-109x(21)00079-6.
[33] Chou, W., A. Prestin and S. Kunath (2014), “Obesity in social media: A mixed methods analysis”, Translational Behavioral Medicine, Vol. 4/3, pp. 314-323, https://doi.org/10.1007/s13142-014-0256-1.
[28] Chua, A. et al. (2021), “An analysis of national action plans on antimicrobial resistance in Southeast Asia using a governance framework approach”, The Lancet Regional Health - Western Pacific, Vol. 7, p. 100084, https://doi.org/10.1016/j.lanwpc.2020.100084.
[43] Das, J. et al. (2016), “The impact of training informal health care providers in India: A randomized controlled trial”, Science, Vol. 354/6308, pp. aaf7384-aaf7384, https://doi.org/10.1126/science.aaf7384.
[81] e-Bug Working Group (2010), “Evaluation of e-Bug, an educational pack, teaching about prudent antibiotic use and hygiene, in the Czech Republic, France and England”, Journal of Antimicrobial Chemotherapy, Vol. 65/12, pp. 2674-2684, https://doi.org/10.1093/jac/dkq356.
[57] EMA (2022), Veterinary Medicinal Products Regulation, European Medicines Agency, https://www.ema.europa.eu/en/veterinary-regulatory/overview/veterinary-medicinal-products-regulation (accessed on 15 June 2022).
[58] EMA (2018), Regulation (EU) 2019/6 of the European Parliament and of the Council, Official Journal of the European Union, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0006&from=EN (accessed on 15 June 2022).
[64] EU-JAMRAI (2021), “Building the European Antimicrobial Resistance Surveillance network in veterinary medicine (EARS-Vet)”, Eurosurveillance, Vol. 26/4, https://doi.org/10.2807/1560-7917.es.2021.26.4.2001359.
[77] Eurobarometer (2018), Special Eurobarometer 478 Report on Antimicrobial Resistance, https://ec.europa.eu/commfrontoffice/publicopinion/index.cfm/survey/getsurveydetail/instruments/special/surveyky/2190 (accessed on 30 July 2020).
[61] European Commission (2022), Medicated Feed - Safe and Controlled Oral Treatment, European Commission, https://ec.europa.eu/food/animals/animal-health/vet-meds-med-feed/medicated-feed-safe-and-controlled-oral-treatment_en (accessed on 15 December 2021).
[41] European Commission (2022), Overview Report: Member States’ One Health National Action Plans against Antimicrobial Resistance, European Commission, https://health.ec.europa.eu/system/files/2022-11/amr_onehealth_naps_rep_en.pdf.
[27] European Commission (2020), Update on Progress and Implementation: European Union Strategic Approach to Pharmaceuticals in the Environment, European Commission, https://ec.europa.eu/environment/water/water-dangersub/pdf/Progress_Overview%20PiE_KH0320727ENN.pdf.
[26] European Commission (2019), European Union Strategic Approach to Pharmaceuticals in the Environment, European Commission, https://ec.europa.eu/environment/water/water-dangersub/pdf/strategic_approach_pharmaceuticals_env.PDF.
[38] European Commission (2017), A European One Health Action Plan against Antimicrobial Resistance (AMR), European Commission, https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf.
[45] FAO (2020), Tackling Antimicrobial Use and Resistance in Dairy Cattle, Food and Agriculture Organization of the United Nations, https://doi.org/10.4060/cb2201en.
[60] FAO (2012), Impact of Animal Nutrition on Animal Welfare – Expert Consultation 26−30 September 2011 – FAO Headquarters, Food and Agriculture Organization of the United Nations, https://www.fao.org/3/i3148e/i3148e.pdf.
[6] FAO and WHO (2021), Code of Practice to Minimize and Contain Foodborne Antimicrobial Resistance, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B61-2005%252FCXC_061e.pdf (accessed on 12 June 2022).
[82] Farrell, D. et al. (2011), “Computer games to teach hygiene: An evaluation of the e-Bug junior game”, Journal of Antimicrobial Chemotherapy, Vol. 66/Supplement 5, pp. v39-v44, https://doi.org/10.1093/jac/dkr122.
[36] Gentzkow, M., B. Kelly and M. Taddy (2019), “Text as data”, Journal of Economic Literature, Vol. 57/3, pp. 535-574, https://doi.org/10.1257/jel.20181020.
[73] Global AMR Hub (2023), Dynamic Dashboard: Investing in AMR R&D, Global AMR R&D Hub, https://dashboard.globalamrhub.org/reports/investments/overview (accessed on 23 July 2020).
[10] Harris, R. et al. (2021), “Impact of COVID-19 on routine immunisation in South-East Asia and Western Pacific: Disruptions and solutions”, The Lancet Regional Health - Western Pacific, Vol. 10, p. 100140, https://doi.org/10.1016/j.lanwpc.2021.100140.
[17] Hauk, C. et al. (2020), “Quality assurance in anti-tuberculosis drug procurement by the Stop TB Partnership - Global Drug Facility: Procedures, costs, time requirements, and comparison of assay and dissolution results by manufacturers and by external analysis”, PLoS ONE, Vol. 15/12, p. e0243428, https://doi.org/10.1371/journal.pone.0243428.
[80] Hayes, C. et al. (2020), “International promotion of e-Bug, an infection prevention and control educational intervention: Survey of partners across 14 countries”, JAC-Antimicrobial Resistance, Vol. 2/1, https://doi.org/10.1093/jacamr/dlaa003.
[76] Huttner, B. et al. (2019), “How to improve antibiotic awareness campaigns: Findings of a WHO global survey”, BMJ Global Health, Vol. 4/3, p. e001239, https://doi.org/10.1136/bmjgh-2018-001239.
[13] ICGAR (2018), Antimicrobial Resistance: National Action Plans, World Health Organisation, https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_AMR_National_Action_Plans_110618.pdf?ua=1.
[22] IHME (2021), Flows of Development Assistance for Health, Institute for Health Metrics and Evaluation, https://vizhub.healthdata.org/fgh/ (accessed on 15 September 2021).
[75] IMI (2017), New Drugs for Bad Bugs: The Innovative Medicines Initiative Response to Antimicrobial Resistance, Innovative Medicines Initiative, https://www.imi.europa.eu/sites/default/files/uploads/documents/projects/IMI_AMR_2017_LR.pdf.
[24] Joshi, M. et al. (2021), “Strengthening multisectoral coordination on antimicrobial resistance: A landscape analysis of efforts in 11 countries”, Journal of Pharmaceutical Policy and Practice, Vol. 14/1, https://doi.org/10.1186/s40545-021-00309-8.
[50] Klein, E. et al. (2021), “Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: An analysis of pharmaceutical sales data”, The Lancet Infectious Diseases, Vol. 21/1, pp. 107-115, https://doi.org/10.1016/s1473-3099(20)30332-7.
[49] Klein, E. et al. (2018), “Global increase and geographic convergence in antibiotic consumption between 2000 and 2015”, Proceedings of the National Academy of Sciences, Vol. 115/15, pp. E3463-E3470, https://doi.org/10.1073/pnas.1717295115.
[59] Kruk, M. (ed.) (2020), “Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis”, PLoS Medicine, Vol. 17/6, p. e1003139, https://doi.org/10.1371/journal.pmed.1003139.
[83] Lasmezas, C. (ed.) (2013), “Fun on the farm: Evaluation of a lesson to teach students about the spread of infection on school farm visits”, PLoS ONE, Vol. 8/10, p. e75641, https://doi.org/10.1371/journal.pone.0075641.
[23] Munkholm, L. et al. (2021), “Attention to the Tripartite’s One Health measures in national action plans on antimicrobial resistance”, Journal of Public Health Policy, Vol. 42/2, pp. 236-248, https://doi.org/10.1057/s41271-021-00277-y.
[52] NIH (2020), “Antimicrobial Resistance Diagnostic Challenge”, Division of Program Coordination, Planning, and Strategic Initiatives, National Institutes of Health, Bethesda, Maryland, https://dpcpsi.nih.gov/AMRChallenge#overview (accessed on 15 December 2021).
[14] OECD (2023), Ready for the Next Crisis? Investing in Health System Resilience, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/1e53cf80-en.
[29] Ogyu, A. et al. (2020), “National action to combat AMR: A One Health approach to assess policy priorities in action plans”, BMJ Global Health, Vol. 5/7, p. e002427, https://doi.org/10.1136/bmjgh-2020-002427.
[47] Oliveira Hashiguchi, T. (2020), “Bringing health care to the patient: An overview of the use of telemedicine in OECD countries”, OECD Health Working Papers, No. 116, OECD Publishing, Paris, https://doi.org/10.1787/8e56ede7-en.
[8] Our World in Data (2023), Coronavirus Pandemic (COVID-19), https://ourworldindata.org/covid-deaths (accessed on 5 September 2023).
[72] Outterson, K. (2021), “Estimating the appropriate size of global pull incentives for antibacterial medicines”, Health Affairs, Vol. 40/11, pp. 1758-1765, https://doi.org/10.1377/hlthaff.2021.00688.
[35] Özçelik, E. et al. (2022), “A comparative assessment of action plans on antimicrobial resistance from OECD and G20 countries using natural language processing”, Health Policy, https://doi.org/10.1016/j.healthpol.2022.03.011.
[78] Paget, J. et al. (2017), Antimicrobial resistance and causes of non-prudent use of antibiotics in human medicine in the EU, European Commission Directorate General for Health and Food Safety, Brussels, https://data.europa.eu/doi/10.2875/326847.
[31] Pearson, J. et al. (2018), “Exposure to positive peer sentiment about nicotine replacement therapy in an online smoking cessation community is associated with NRT use”, Addictive Behaviors, Vol. 87, pp. 39-45, https://doi.org/10.1016/j.addbeh.2018.06.022.
[34] Petersen, K. and J. Gerken (2021), “#Covid-19: An exploratory investigation of hashtag usage on Twitter”, Health Policy, Vol. 125/4, pp. 541-547, https://doi.org/10.1016/j.healthpol.2021.01.001.
[70] Renwick, M., V. Simpkin and E. Mossialos (2016), Targeting Innovation in Antibiotic Drug Discovery and Development: The need for a One Health - One Europe - One World Framework, European Observatory Health Policy Series, European Observatory on Health Systems and Policies, https://pubmed.ncbi.nlm.nih.gov/28806044/.
[51] Roberts, S. and T. Zembower (2021), “Global increases in antibiotic consumption: A concerning trend for WHO targets”, The Lancet Infectious Diseases, Vol. 21/1, pp. 10-11, https://doi.org/10.1016/s1473-3099(20)30456-4.
[32] Rudge, A. et al. (2021), “How are the links between alcohol consumption and breast cancer portrayed in Australian newspapers?: A paired thematic and framing media analysis”, International Journal of Environmental Research and Public Health, Vol. 18/14, p. 7657, https://doi.org/10.3390/ijerph18147657.
[71] Simpkin, V. et al. (2017), “Incentivising innovation in antibiotic drug discovery and development: Progress, challenges and next steps”, Journal of Antibiotics, Vol. 70/12, pp. 1087-1096, https://doi.org/10.1038/ja.2017.124.
[20] Stop TB Partnership (2021), GDF Results, https://www.stoptb.org/mission/gdfs-results (accessed on 30 March 2022).
[62] Tacconelli, E. et al. (2018), “Surveillance for control of antimicrobial resistance”, The Lancet Infectious Diseases, Vol. 18/3, pp. e99-e106, https://doi.org/10.1016/s1473-3099(17)30485-1.
[7] The Independent Panel (2021), COVID-19: Make It the Last Pandemic, https://theindependentpanel.org/wp-content/uploads/2021/05/COVID-19-Make-it-the-Last-Pandemic_final.pdf.
[11] Tomczyk, S. et al. (2021), “Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey”, Journal of Antimicrobial Chemotherapy, https://doi.org/10.1093/jac/dkab300.
[53] Trevas, D. et al. (2020), “Diagnostic tests can stem the threat of antimicrobial resistance: Infectious Disease professionals can help”, Clinical Infectious Diseases, Vol. 72/11, pp. e893-e900, https://doi.org/10.1093/cid/ciaa1527.
[2] UN (2016), Political Declaration of the High-level Meeting of the General Assembly on Antimicrobial Resistance, United Nations.
[16] Wellcome (2020), The Global Response to AMR: Momentum, Success and Critical Gaps, Wellcome Trust, https://cdn.eventsforce.net/files/ef-lpifs4q56r2a/website/785/wellcome-global-response-amr-report.pdf.
[66] WHO (2022), Global report on infection prevention and control, World Health Organization, https://apps.who.int/iris/handle/10665/354489.
[18] WHO (2021), Tuberculosis - Factsheet, World Health Organization, https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 30 March 2022).
[48] WHO (2021), WHO Access, Watch, Reserve, Classification of Antibiotics for Evaluation and Monitoring of Use: 2021 AWaRe Classification, World Health Organization, https://apps.who.int/iris/handle/10665/345555.
[69] WHO (2020), “Lack of new antibiotics threatens global efforts to contain drug-resistant infections”, World Health Organization, https://www.who.int/news-room/detail/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections (accessed on 24 July 2020).
[4] WHO (2020), Thirteenth General Programme of Work (GPW13) Methods for Impact Measurement, World Health Organization, https://apps.who.int/iris/handle/10665/341371.
[46] WHO (2019), Antimicrobial Stewardship Programmes in Health-care Facilities in Low- and Middle-income Countries: A WHO Practical Toolkit, World Health Organization, https://apps.who.int/iris/handle/10665/329404.
[3] WHO (2019), Global Leaders Group on Antimicrobial Resistance, World Health Organization, https://www.who.int/groups/one-health-global-leaders-group-on-antimicrobial-resistance (accessed on 15 July 2020).
[55] WHO (2017), Monitoring and Evaluation of the Global ActioN Plan on AMR: Regional Expert Consultation on Monitoring and Evaluation of AMR Interventions, World Health Organization, https://www.paho.org/hq/dmdocuments/2017/2017-cha-monit-eval-gapar-meeting-report.pdf.
[54] WHO (2017), WHO Guidelines on Use of Medically Important Antimicrobials in Food-producing Animals, World Health Organization, https://apps.who.int/iris/handle/10665/258970.
[1] WHO (2015), Global Action Plan on Antimicrobial Resistance, World Health Organization, https://apps.who.int/iris/handle/10665/193736.
[12] WHO/FAO/WOAH (2022), Tripartite AMR Country Self-Assessment Survey (TrACSS) 2021-2022, World Health Organization, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health, https://amrcountryprogress.org/ (accessed on 23 March 2022).
[42] WHO/FAO/WOAH (2019), Monitoring and evaluation of the Global Action Plan on Antimicrobial Resistance: Framework and Recommended Indicators, World Health Organization, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health, https://apps.who.int/iris/handle/10665/325006.
[5] WHO et al. (2022), Strategic Framework for Collaboration on Antimicrobial Resistance: Together for One Health, World Health Organization, Food and Agriculture Organization of the United Nations, World Organisation forAnimal Health and United Nations Environment Programme, https://apps.who.int/iris/handle/10665/352625.
[79] Willmott, M. et al. (2015), “Effectiveness of hand hygiene interventions in reducing illness absence among children in educational settings: A systematic review and meta-analysis”, Archives of Disease in Childhood, Vol. 101/1, pp. 42-50, https://doi.org/10.1136/archdischild-2015-308875.
[56] WOAH (2020), Annual Report on Antimicrobial Agents Intended for Use in Animals: Better Understanding of the Global Situation, World Organisation for Animal Health, https://www.woah.org/app/uploads/2022/06/a-sixth-annual-report-amu-final.pdf.
[21] World Bank (2019), Pulling Together to Beat Superbugs: Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance, World Bank Group, https://documents1.worldbank.org/curated/en/430051570735014540/pdf/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf.
[39] Yam, E. et al. (2019), “Antimicrobial resistance in the Asia Pacific region: A meeting report”, Antimicrobial Resistance & Infection Control, Vol. 8/1, https://doi.org/10.1186/s13756-019-0654-8.
Annex 4.A. National language processing (NLP) techniques used in the OECD analysis
NLP techniques used in the analysis of national action plans to tackle antimicrobial resistance (AMR-NAPs)
In this chapter, a combination of NLP-guided techniques is deployed to systematically examine the content included in the AMR-NAPs from OECD countries and key partners, EU/EEA and G20 countries. First, a unique, text-based dataset was assembled. This was done by identifying the AMR-NAPs that were developed after the publication of the Global Action Plan on Antimicrobial Resistance (AMR-GAP) and extracting them from the World Health Organization (WHO) AMR-NAP repository, the European Centre for Disease Prevention and Control (ECDC) AMR-NAP library and publicly available websites. In occasions when more than one AMR-NAP was published by a country in the analysis period, only the most recent document was included. Supplementary materials (e.g. progress reports, commentaries, complementary operational sectoral plans) were excluded. Only documents written in English were included. These steps resulted in the inclusion of 21 AMR-NAPs in the final assessment. A list of countries included in the OECD analysis is provided.
Building an analysable dataset using AMR-NAPs
The text from 21 AMR-NAPs was transformed into an analysable dataset in several steps. First, the pages that may not include substantive information were discarded (e.g. acknowledgements, cover pages). Next, each document was split into smaller units referred to as tokens (e.g. words, web links, punctuations) and tokens that may contain little analytical value were removed. Once this procedure was completed, the entirety of the sample was converted to lowercase characters. The next step entailed the removal of stop words: high-frequency terms that contribute no substantive information (e.g. and, also, a, etc.). Next, all terms were stemmed such that different variations of the same term are recorded as the same entry with the same root. Finally, the pre‑processed data were transformed into a document term matrix, which enabled to count the number of times each term occurred in each AMR-NAP.
Dictionary-based analysis
Dictionary-based NLP techniques were deployed to assess the level of alignment between the AMR-NAPs and the AMR-GAP. Dictionary-based methods offer a suitable option for textual analysis when reliable information is already available to help guide the development of a term dictionary, with limited availability of datasets that can be used to train text-based models (Gentzkow, Kelly and Taddy, 2019[36]). An AMR term dictionary was developed using a two‑step approach. As the first step, the AMR-GAP was reviewed to extract terms that were used to describe each strategic objective and recommended intervention. Next, the other AMR publications were reviewed, with the aim of identifying additional terms. The process of building the term dictionary was iterative. Multiple labels were assigned to interventions that were relevant to more than one strategic objective.
Methods that rely on simply counting the number of times that terms occur in a document are not sufficient to consider the differences in the length of documents. Recognising this, the OECD analysis makes use of two commonly used NLP metrics to assess the level of alignment between AMR-NAPs and the AMR-GAP: i) term frequency (TF); and ii) term frequency-inverse document frequency (TF-IDF). TF is a measure of the frequency with which each term occurs within an AMR-NAP with respect to the entire length of that document. Quantifying TF associated with each strategic objective/intervention facilitates a comparative assessment of the relative prominence of each strategic objective/intervention in the collection of AMR‑NAPs. TF-IDF facilitates a comparative analysis of the interventions to tackle AMR that occur in a given AMR-NAP in comparison to how frequently it features across the collection of documents. By deriving TF-IDF scores, the interventions that are most distinctly highlighted in each AMR-NAP compared to the others are identified.
Annex Table 4.A.1. National action plans on AMR included in the OECD analysis
|
Country |
National action plan |
Period |
|---|---|---|
|
Australia |
Australia’s National Antimicrobial Resistance Strategy 2020 & Beyond |
2020‑40 |
|
Canada |
Tackling Antimicrobial Resistance Use: A Pan-Canadian Framework for Action |
2017‑onwards |
|
China |
National Action Plan to Contain Antimicrobial Resistance (2016‑20) |
2016‑20 |
|
Denmark |
National Action Plan on Antibiotics in Human Healthcare: Three Measurable Goals for a Reduction of Antibiotic Consumption Towards 2020 |
2017‑20 |
|
Finland |
National Action Plan on Antimicrobial Resistance 2017‑2 021 |
2017‑21 |
|
France |
Interministerial Roadmap for Controlling Antimicrobial Resistance – 13 Overarching Interministerial Measures 40 Actions |
2016‑onwards |
|
Germany |
DART 2020 – Fighting antibiotic resistance for the good of both humans and animals |
2015‑20 |
|
India |
National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017‑2 021 |
2017‑21 |
|
Indonesia |
National Action Plan on Antimicrobial Resistance Indonesia 2017‑2 019 |
2017‑19 |
|
Ireland |
Ireland’s National Action Plan on Antimicrobial Resistance (2017‑20) |
2017‑20 |
|
Japan |
National Action Plan on Antimicrobial Resistance (AMR) 2016‑2 020 |
2016‑20 |
|
Malta |
A Strategy and Action Plan for the Prevention and Containment of Antimicrobial Resistance in Malta 2020‑2 028 |
2020‑28 |
|
New Zealand |
New Zealand Antimicrobial Resistance Action Plan |
2017‑21 |
|
Norway |
National Strategy against Antibiotic Resistance 2015‑2 020 |
2015‑20 |
|
South Africa |
South African Antimicrobial Resistance National Strategy Framework; A One Health Approach 2018‑2 024 |
2018‑24 |
|
Saudi Arabia |
Kingdom Saudi Arabia National Action Plan on Combating Antimicrobial Resistance |
2017‑onwards |
|
Slovak Republic |
National Action Plan on Antimicrobial Resistance in the Slovak Republic 2019‑2 021 |
2019‑21 |
|
Sweden |
Swedish Strategy to Combat Antibiotic Resistance 2020‑2 023 |
2020‑23 |
|
Switzerland |
Strategy on Antibiotic Resistance Switzerland |
2015‑onwards |
|
United Kingdom |
Tackling Antimicrobial Resistance 2019‑24: The UK Five‑Year National Action Plan |
2019‑24 |
|
United States |
National Action Plan for Combating Antimicrobial-Resistant Bacteria 2020‑2 025 |
2020‑25 |
Methodology used to generate the dashboard on the implementation of selected AMR-relevant policies in OECD countries, EU/EEA and G20 countries
The OECD analysis relies on the self-reported responses recorded in the WHO Tripartite AMR Country Self-Assessment Survey (2021‑22) to characterise the implementation of selected AMR-relevant multi-sectoral policies in each country. All questions extracted from this survey include a five‑point rating scaling (from A to E) to summarise a country’s progress. In the OECD analysis, countries that reported an “A” rating for any question were categorised as having no implementation with respect to that specific intervention whereas countries that reported an “E” rating were grouped as achieving the most advanced stage of implementation.
The questions and response categories from the Tripartite AMR Country Self-Assessment Survey (2021‑22) used to build the dashboard are as follows:
Annex Table 4.A.2. Questions and response categories extracted from the Tripartite AMR Country Self-Assessment Survey (2021‑22)
|
Questions |
Response categories |
|---|---|
|
Optimising antimicrobial use in human health (Question 3.6.) |
A. No/weak national policies for appropriate antimicrobial use including availability, quality and disposal of antimicrobials. B. National policies promoting appropriate antimicrobial use/antimicrobial stewardship activities developed for the community and healthcare settings. C. National guidelines for appropriate use of antimicrobials are available and antimicrobial stewardship programmes are being implemented in some healthcare facilities. D. National guidelines for appropriate use of antimicrobials are available and antimicrobial stewardship programmes are being implemented in most healthcare facilities nationwide. Monitoring and surveillance results are used to inform action to update treatment guidelines and essential medicines lists. E. National guidelines on optimising antibiotic use are implemented for all major syndromes and data on use are systematically fed back to prescribers. |
|
Optimising antimicrobial use in animal health (terrestrial and aquatic) (Question 4.11.) |
A. No national policy or legislation regarding the quality, safety and efficacy of antimicrobial products and their distribution, sale or use. B. National legislation covers some aspects of national manufacture, import, marketing authorisation, control of safety, quality and efficacy and distribution of antimicrobial products. C. National legislation covers all aspects of national manufacture, import, marketing authorisation, control of safety, quality and efficacy and distribution of antimicrobial products. D. The national regulatory framework for antimicrobial products incorporates all of the elements included in the related international standards on responsible and prudent use of antimicrobials (e.g. WOAH Terrestrial Animal Health Codes, Codex Alimentarius) according to animal species and/or production sector). E. Enforcement processes and control are in place to ensure compliance with legislation. |
|
National monitoring system for consumption and rational use of antimicrobials in human health (Question 3.2.) |
A. No national plan or system for monitoring the use of antimicrobials. B. System designed for surveillance of antimicrobial use that includes monitoring national-level sales or consumption of antibiotics in health services. C. Total sales of antimicrobials are monitored at the national level and/or some monitoring of antibiotic use at the subnational level. D. Prescribing practices and appropriate antibiotic use are monitored in a national sample of healthcare settings. E. On a regular basis (every year/two years), data are collected and reported on: a) antimicrobial sales or consumption at the national level for human use; and b) antibiotic prescribing and appropriate/rational use, in a representative sample of healthcare facilities, public and private. |
|
National surveillance system for AMR in humans (Question 3.3.) |
A. No capacity for generating data (antibiotic susceptibility testing and accompanying clinical and epidemiological data) and reporting on antibiotic resistance. B. AMR data are collated locally for common bacterial infections in hospitalised and community patients, but data collection may not use a standardised approach and lacks national co‑ordination and/or quality management. C. AMR data are collated nationally for common bacterial infections in hospitalised and community patients, but national co‑ordination and standardisation are lacking. D. There is a standardised national AMR surveillance system collecting data on common bacterial infections in hospitalised and community patients, with established network of surveillance sites, a designated national reference laboratory for AMR and a national co‑ordinating centre producing reports on AMR. E. The national AMR surveillance system links AMR surveillance with antimicrobial consumption and/or use data for human health. |
|
IPC practices in human healthcare (Question 3.5.) |
A. No national IPC programme or operational plan is available. B. A national IPC programme or operational plan is available. National IPC and water, sanitation and hygiene (WASH) and environmental health standards exist but are not fully implemented. C. A national IPC programme and operational plan are available and national guidelines for healthcare IPC are available and disseminated. Selected healthcare facilities are implementing the guidelines, with monitoring and feedback in place. D. National IPC programme available according to the WHO IPC core components guidelines and IPC plans and guidelines implemented nationwide. All healthcare facilities have a functional built environment (including water and sanitation) and the necessary materials and equipment to perform IPC, as per national standards. E. IPC programmes are in place and functioning at the national and healthcare facility levels according to the WHO IPC core components guidelines. Compliance and effectiveness are regularly evaluated and published. Plans and guidance are updated in response to monitoring. |
|
Raising awareness and understanding of AMR risks and response (Question 2.9.) |
A. No awareness-raising activities on risks of antimicrobial resistance. B. Some activities to raise awareness about the risks of antimicrobial resistance and actions that address it. C. Some awareness activities at the local and/or sub-national levels about risks of antimicrobial resistance and actions to address it, targeting some but not all relevant stakeholders, based on stakeholder analysis. D. Nationwide, government-supported antimicrobial resistance awareness-raising campaign targeting all or the majority of priority stakeholder groups, utilising targeted messaging accordingly within sectors. E. Targeted, nationwide government-supported activities regularly implemented to change the behaviour of key stakeholders within sectors, with monitoring undertaken over the last two to five years. |
|
Training and professional education on AMR in the human health sector (Question 3.1.) |
A. No training for human health workers on AMR. B. Ad hoc AMR training courses in some human health-related disciplines. C. AMR is covered in: i) some pre‑service training; and ii) some in-service training or other continuing professional development (CPD) for human health workers. D. AMR is covered in pre‑service training for all relevant cadres. In-service training or other CPD covering AMR is available for all types of human health workers nationwide. E. AMR is systematically and formally incorporated in pre‑service training curricula for all relevant human health cadres. In-service training or other CPD on AMR is taken up by relevant groups for human health nationwide, in public and private sectors. |
|
Biosecurity and good animal husbandry practices (terrestrial animal production) (Question 4.9.) |
A. No systematic efforts to improve good production practices. B. Some activities in place to develop and promote good production practices. C. National plan agreed to ensure good production practices in line with international standards (e.g. WOAH Terrestrial, Codex Alimentarius). Nationally agreed guidance for good production practices developed, adapted for implementation at the local farm and food production levels. D. Nationwide implementation of a plan to ensure good production practices and national guidance published and disseminated. E. Implementation of the nationwide plan is monitored periodically. |
|
Biosecurity and good animal husbandry practices (aquatic animal production) (Question 4.10.) |
A. No systematic efforts to improve good production practices. B. Some activities in place to develop and promote good production practices. C. National plan agreed to ensure good production practices in line with international standards (e.g. WOAH Aquatic, Codex Alimentarius). Nationally agreed guidance for good production practices developed, adapted for implementation at the local farm and food production levels. D. Nationwide implementation of plan to ensure good production practices and national guidance published and disseminated. E. Implementation of the nationwide plan is monitored periodically. |
|
Good management and hygiene practices to reduce the development and transmission of AMR in food processing (Question 5.5.) |
A. No systematic efforts to improve good management and hygiene practices. B. Some activities in place to develop and promote good management and hygiene practices. C. National plan agreed to ensure good management and hygiene practices in line with international standards (e.g. Codex Alimentarius). Nationally agreed guidance for good practices developed and adapted for implementation according to local food processing approaches. D. Nationwide implementation of a plan to ensure good management and hygiene practices and national guidance published and disseminated. E. Implementation of the nationwide plan is monitored periodically. |