Section 6 introduces plan for bringing the HSPA in the Czech Republic alive, after the framework has been set and populated by the indicators. Naturally, the next phase is the HSPA implementation, which should be finalised by the release of the first HSPA report. However, a health system performance assessment is a continuous process, designed in cycles, providing for possibility to adjust the national HSPA to the evolution in the healthcare sector and the changing health policy priorities and objectives. The HSPA implementation roadmap has been discussed by the principal working group during its last meeting in April 2023, and then by the HLAB in April 2023.
Health System Performance Assessment Framework for the Czech Republic

6. HSPA implementation roadmap
6.1. Key components of the Czech HSPA implementation
In the context of the Czech Republic, the HSPA implementation roadmap includes elements of three areas: governance, data management, and HSPA publication and dissemination.
6.1.1. HSPA governance implementation
HSPA ought to be embedded into existing Ministry of Health and its subsidiary organisations’ institutional structures with clear governance and responsibilities for different layers of the HSPA governance. The stakeholders have agreed to build upon the foundations laid out during this project- for details on designed HSPA governance structure see Section 5.2.
The HSPA governance structure will follow up on existing structures of the current HSPA project; however, to operationalise the HSPA and ensure HSPA continuity, HSPA main governance structures should be formalised by a formal act – such as a Ministerial decree, a common practice used to set up various advisory bodies to the Minister of Health and working groups at the ministry. A discussion among HLAB members was held on the possibility of having a governmental resolution on the HSPA report publishing in a 4‑year cycle; this however was not deemed necessary for the establishing of co‑operation among health data and HSPA indicator custodians and setting up the necessary HSPA co‑ordinating structures. Further discussion may be held on the potential a governmental resolution may have for the visibility and outreach of the HSPA reports.
6.1.2. HSPA data management implementation
The health information infrastructure in the Czech Republic is robust, but lacks some desired data linkages and information sharing (see Annex E). Within the context of HSPA implementation, data collection, flow, and indicator calculation must have a clear timeline. To populate the Czech HSPA framework, stakeholders have chosen mostly indicators that are already existent. That means that for most indicators the periodicity of data collection and availability of time series is already known. This information should be noted for each indicator (see Box 4.1). Furthermore, there should be a date – either one for all indicators or one for each indicator – indicating a deadline for transfer of relevant data between institutions (should that step be necessary); for developing an indicator technical sheet, including the first update of indicator value and contextualisation; for review by the other Technical Groups and the Task Force; and for final indicator calculation, contextualisation, and technical sheet approval.
The list of indicators selected in the comprehensive process during this project of Setting up of the Czech HSPA Framework shall serve as an input for the HSPA implementation phase. Further discussions among HSPA stakeholders, mainly the data custodians and suggested indicator custodians, are foreseen and deemed appropriate to develop detailed technical sheets for each individual indicator (see Box 4.1).
Box 6.1. Indicator technical sheets properties
The Ministry of Health and UZIS reached an agreement in 2022 that some of the HSPA indicators, the calculation and interpretation of which will be the responsibility of UZIS, will be included by the Ministry of Health in co‑operation with UZIS in the MoH’s Decree on Departmental Reference Statistics (Resortní referenční statistiky, RRS). Furthermore, there is agreement that it is essential that each indicator (both in HSPA and in RRS) has its own “birth certificate”, or technical sheet.
This technical sheet will contain at least the name of the indicator, description of the indicator (including the denominator), source of the data and periodicity of data collection, institution responsible for data collection and data processing, method of calculation, indicator disaggregation (e.g. regional comparison, comparison via health insurance companies, age, gender, socio‑economic status, etc.), contextualisation of the indicator (e.g. time development, international comparison), existence of a national or international benchmark.
For indicators of which the custody will not be the responsibility of UZIS but another institution, should have the same structure of their technical sheets.
Within the first 1.5 years following the completion of the current project, it is suggested for the Czech Republic to aim to establish the HSPA structures and publish its first HSPA Report at a Stakeholder Conference in January 2025.
6.1.3. Implementing structures for HSPA dissemination
Stakeholders have agreed that the Czech HSPA should be made public via a website, so that indicators can be updated whenever new data are available. This would complement the 4‑year cycle of publishing a full HSPA report.
A dedicated website for the online version of the HSPA was preferred by the principal working group. This could potentially also become part of existing platforms, such as the National Health Information Portal www.nzip.cz, managed by the UZIS.
During the HSPA implementation phase, discussions should also touch on the division of responsibilities for regular indicator data updates on the dedicated HSPA website. This has direct technical consequences on the design of the platform: decisions must be taken such as whether indicator custodians should have a direct access to the HSPA platform and publish their indicator updates independently. Alternatively, indicator technical sheets, i.e. the calculated indicators with its full description, context, and benchmarking, can be collected by a single institution that would be then responsible for making it public, also on the website.
During the principal working group’s 6th workshop, held in January 2023, stakeholders have agreed that the published HSPA indicator technical sheets should have the following properties:
All HSPA indicators and related information should be available at one website on the internet.
Indicators should be updated whenever new data is available.
The website should provide links to other more detailed information sources with relevant institutions (stakeholders).
It is preferable to include visualisation of indicators and as many of their various dimensions and disaggregation as possible.
The full HSPA Report should be published regularly, and the period of every 4 years is considered a reasonable frequency. Meanwhile, annual Stakeholder Conferences should be held to provide feedback on existing indicator calculation and context, also within the health policy priorities and objectives, on placeholder indicator development, and to provide input for new indicators and/or framework (sub)domains. The Stakeholders Conference represents a good opportunity for the professional public (including the representatives of patients, healthcare managers, healthcare policy makers, healthcare providers, and health insurance representatives) to gather and to hear updates on the HSPA development and provide feedback.
It has been noted by the Czech stakeholders that having communication experts as part of the HSPA co‑ordinating structures is highly advisable. It is recommended the HSPA report is well developed not only in terms of the data it presents, but also in terms of the data visualisation, i.e. how the HSPA indicators are presented and communicated. The latter is even more important for getting a good HSPA outreach to general and expert public and health policy makers. The infographics gathered in Annex D as an example of existing indicator visualisation may serve as a starting point for HSPA visualisation.
6.2. Key steps of the Czech HSPA implementation
Following the end of the current project, on setting up the framework for health system performance assessment in the Czech Republic, there are several necessary steps to be taken and a sequence of actions to follow to reach the first HSPA report publication within a year and half, i.e. around January 2025. These are depicted in Figure 6.1.
Figure 6.1. Implementation roadmap: steps leading to the first the Czech HSPA Report within 1.5 years
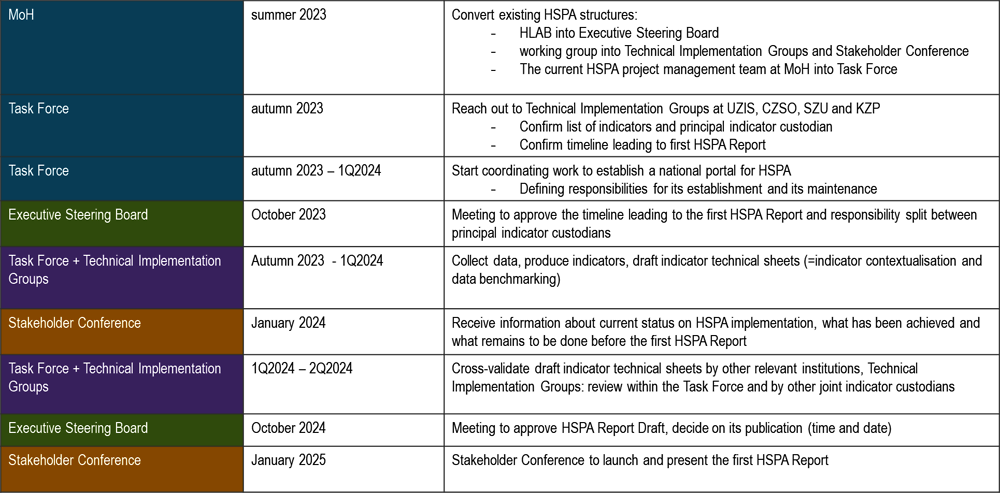
Note: Steps to take to achieve the first HSPA report publication in one and half year after the conclusion of this project were discussed with the principal working group and the High-Level Advisory Board in April 2023.
Source: Czech HSPA framework project, HLAB meeting in April 2023.
Once the first HSPA Report is published, the key activities should stay in place and happen regularly, on an annual basis and within a 4‑year cycle for the full HSPA report, ideally at the same time of year. These activities and workflows are depicted in Figure 6.2.
Figure 6.2. Recommended workflows for the collection and analysis of HSPA data – the annual cycle
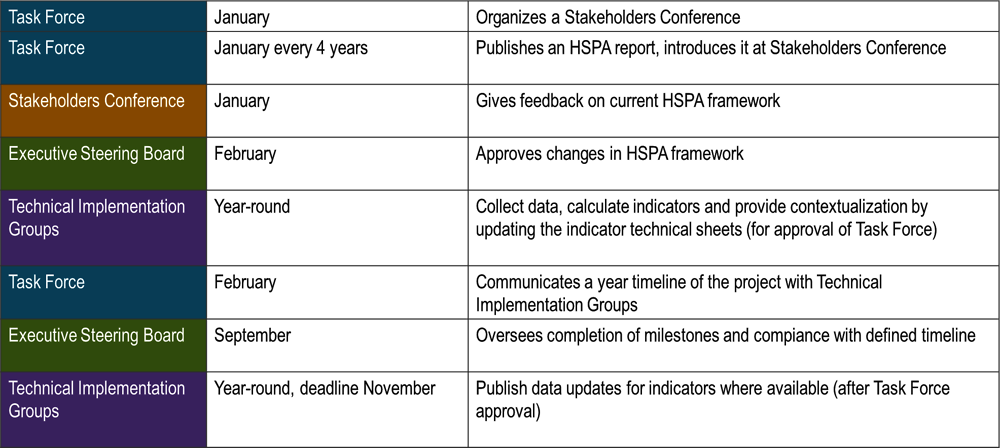
Source: Czech HSPA framework project, HLAB meeting in April 2023.
The date for publication of the first full HSPA Report was discussed at the Principal Working Group as well as with the HLAB members during the two meetings that took place in April 2023. The January 2025 first publication date corresponds to the political cycle as well and stakeholders agreed to have the report available in January of the year of parliamentary elections. Generally, parliamentary elections take place in October every 4 years and the next election is scheduled for fall 2025.
January precedes the date of election by almost 9 months, meaning that it will be still relevant and up-to date when the new government is being formed and when new health priorities are set and policies are drafted. At the same time, the HSPA report would be released early enough before the general elections not to directly impact the close‑to-the‑election political debates.
The question of specific workflows was also discussed at April 2023 Principal Working Group meeting. The group confirmed that there was no need for a special workflow for data collection and indicator calculation as the current legislation is sufficient to support an activity such as the one of the HSPA. Stakeholders have also agreed that no special procedure is needed in order to introduce HSPA Report to Parliament, as the parliament can ask for any material from the MoH and has the freedom to discuss it or to ask the MoH for further information. Stakeholders have however suggested that in the future it might be beneficial to send the HSPA Report to the official meeting of the government. This is simply done by the MoH and again no special procedure is needed. However, a governmental resolution may have the potential for the visibility and outreach of the HSPA reports.