The mapping exercise presents scholarly output, including experimental and modelling studies, relating to six selected scenarios of hydrogen-related accidents. The main conclusions from the study of the literature and identified knowledge gaps are also discussed for each scenario.
Risk-based Regulatory Design for the Safe Use of Hydrogen

8. Mapping exercise
Abstract
Scenario 1 – Production: Leakage from the pipe connected to electrolyser
Hydrogen production can be onsite or offsite, with the former more suitable for refuelling stations that are far away from external hydrogen sources, together with a lowered expense for hydrogen transportation; while the later produces hydrogen at large scale that is then delivered through tube trailers, LH2 trucks or hydrogen pipelines (Tian et al., 2021[1]). (Tchouvelev et al., 2006[2]) pointed out that on-site water electrolysis presents a lower societal risk as well as a lowered risk for individual harm exposure as compared with on-site steam methane reforming (SMR). Other technologies (Kalamaras and Efstathiou, 2013[3]) used for hydrogen production include oil/naphtha reforming and coal gasification etc.
Despite the fact that water electrolysis is a greener method1 for hydrogen production if the electricity used is from a renewable source or fossil fuels equipped with carbon capture, utilisation or storage (CCUS) technologies, it only represents a very small proportion of world’s hydrogen production (IEA, 2021[4]). Electrolysers for water electrolysis can operate under either acidic or alkaline conditions. Alkaline electrolyser including anion exchange membranes (AEM) is a more mature technology, with most large-scale plants (up to 165 MW) built between 1920s to 1980s in response to hydrogen demand for ammonia industry2 (Krishnan et al., 2020[5]). Other technologies, including proton exchange membrane (PEM) and solid oxide electrolyser cell (SOEC) are gaining market traction as they expect to be either more flexible or efficient, and hence have a smaller footprint (IRENA, 2018[6]). Nonetheless, alkaline products still dominate the market and Bloomberg estimates them to account for 75-78% of the shipments in 2022. Being cheaper than newer technologies, alkaline electrolysis is also more suitable for large-scale projects, more of which are set to start construction in 2022 (BloombergNEF, 2022[7]).
Table 8.1. The three main types of electrolysers with their characteristic parameters and typical operating conditions
|
Type |
Temperature (OC) |
Pressure (MPa) |
Cold Start (IRENA, 2021) |
|---|---|---|---|
|
Alkaline |
60-220 |
<3.4 |
< 50 minutes |
|
PEM |
40-80 |
<3.4 |
< 20 minutes |
|
SOEC |
600-1 000 |
1 |
> 600 minutes |
Note: Based on key performance metrics of the largest device available of European suppliers.
In some countries, code and standards on hydrogen generators are already in force.3 For example, the Chinese standard defines a safety distance of 2 m between the electrolysers. While determining the minimum distance, the size of the electrolyser and its production rate is of importance as well. This is because the size determines the production rate. Furthermore, international standard ISO 22734:2019 defines the construction, safety and performance requirements of hydrogen generators that use electrochemical reactions to produce hydrogen.
In general, the major risk factors of an electrolysis hydrogen production plant consists of: 1) a chemical component (electrolyser); 2) mechanical component (compressor. etc) and 3) storage component for temporary storage (Zarei, Khan and Yazdi, 2021[9]). Also noteworthy are 4) power electronics, and 5) the energy source. In the next sections, first, a holistic picture of risks associated with hydrogen production sites, quoting results from 3 independent sources, is provided. Then, the major risk contributor (compressor) is identified. Afterwards, a discussion on hydrogen pipeworks4 within a production site focusing on pipes connected to electrolysers, is presented.
General concerns on hydrogen production site (Electrolysis)
Two risk analyses on hydrogen production site (Zarei, Khan and Yazdi, 2021[9]), (Kasai et al., 2016[10]) suggests that most root events that may lead to accidents can be either eliminated or reduced to low risk following current recommended safety measures, such as use safety valves (pressure relief valve etc) to provide extra security, sensors (Tchouvelev et al., 2021[11]) to monitor reaction conditions, fire wall (Schefer et al., 2009[12]) to reduce eventual loss following an explosion. Nonetheless, there are two scenarios that cannot be eliminated under current risk measures, namely the crashing of an aircraft/helicopter or collapse of a crane into the facility.
Moreover, energy-related Service Accident Database (ENSAD) recorded 43 accidents related to hydrogen production, which represents 25% of all hydrogen-related accidents between 1995 and 2014 (Spada, Burgherr and Rouelle, 2018[13]). None of these accidents recorded any hydrogen release. Calculations by the same authors also suggested the current practice of hydrogen production is associated with a lower normalised risk5 for fire/explosion as compared with traditional energy productions (oil, coal and natural gas). Nonetheless, the accidents cost one life, 56 injuries and property damage at ca. 4.4 million euros.
Analysation of incidents in additional 2 databases (HIAD6 2.0 and H2tools) concludes that the risk associated with electrolysers are small compared to compressors and pressurised storage (FCH2JU, 2020[14]).7 (Skjold et al., 2017[15]) suggest that compressors are the major risk contributor in hydrogen production plants.8
Database H2tools recorded 5 accidents related to compressors Table 8.2, 2 resulted in fire, out of which one accident required emergency shutdown of the plant. It was suggested improving leak detection can prevent escalation and hence reduce the risk.
Table 8.2. Hydrogen compressors-related accidents in H2tools database
|
Accident causation |
Additional lessons learnt |
No. accidents |
|---|---|---|
|
Failed pressure switch |
Stop-valve at Storage vessel to prevent escalation |
2 |
|
Damaged Component |
Check components’ H2 compatibility |
3 |
Pipeworks, focus on those connected to electrolysers
A Sandia report on a hydrogen plant located near nuclear power plants scenario (Glover, Baird and Brooks, 2020[16]) discussed potential hazards and risk associated with the pipeworks connected to the electrolyser. In the setting, a steam pipe enters the electrolyser at 0.5 MPa and the pressure was assumed to be maintained until reaching the separator vessels. After which the purified hydrogen is pressured in 2 steps to reach 2.2 MPa for delivery. A Bayesian statistical model was then developed based on hydrogen data for compressors, cylinders, hoses, joints, pipes and valves, with all other data coming from offshore oil industry. Connecting pipe leaking frequency ranging between 2.99 ∙ 10-9 m-1 year-1 for very small leakage and 3.13 ∙ 10-10 m-1 year-1 for rupture at 95% confidence level. These values fall within the range advertised by the purple book (Uijt de Haag and Ale, 2005[17]), suggesting a low risk of hydrogen leakage from the pipework connecting to the electrolyser.
In addition, analysis on a laboratory accident together with a computational simulation (Ichard et al., 2012[18]) suggests that in a confined space9 with good ventilation, minor release of hydrogen should not cause major safety concerns as long as current safety measures on hydrogen are followed, which include restrictions in the bottle/regulator system, significant distance to the ceiling, limited total gas inventory etc. The study reasoned that hydrogen mixes well with air and upon leakage so that hydrogen concentration drops below its ignition limit quickly. In their simulation, only 9% of the room has a hydrogen concentration above 4% (ignition limit) 30 s after leakage.
Hydrogen accidents data in database HIAD 2.0 suggest that 84% of hydrogen-related accidents are of fire/explosion type (FCH2JU, 2021[19]). This is due to hydrogen’s unique chemical properties such as tendency to escape due to small size, low ignition energy, and wide flammability range. It suggests that simulation work may be optimistic or that minor, unintended releases that caused no harm tend not to be reported. The latter is in line with a report from Air Liquide (Campbell, 2005[20]), which suggests that “small leaks are hard to detect”.
For “worst-case scenario”, a computational simulation of large-scale hydrogen jet fire10 from pipework (Jang and Jung, 2016[21]) shows a rapid fire expansion from ignition to 3 s, and later on reaches equilibrium at 22 s. In addition to the fire, the simulation indicates radiation heat also causes critical consequences for humans as well as facilities. Another simulation11 (Matthijsen and Kooi, 2006[22]) calculated the individual risk (IR) 10-6 contour (purple book) at 4.5 m for in-plant pipeworks i.e. a distance of 4.5 metres should be maintained to keep the risk of personal injury lower than 10-6 per year.
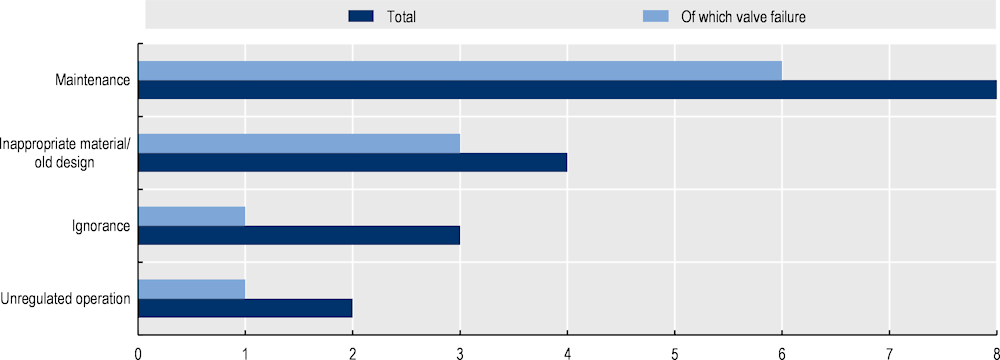
Finally, incident data relating to pipework failure from database H2tools are summarised in Figure 8.1. In 17 accidents reported, 11 are related to valve failure highlighting the importance of regular preventive maintenance. All accidents (4) that involve fire/explosion and injuries were dated before the 1990s when modern valve design and safety regulations were not available. The only accident involving death (1992) was in a laboratory setting which lacked hydrogen detection sensors.
Figure 8.1. Analysis of hydrogen pipework-related accidents in H2tools database
Conclusions and knowledge gaps
The main conclusions based on the literature review related to Scenario 1 are:
Alkaline water electrolysis represents a mature technology with most large plants built between 1920s-1980s.
Risk analysis on hydrogen production plants and suggested that most initiating events can be reduced/eliminated following existing risk mitigation measures.
Current hydrogen production presents a lower normalised risk for fire and explosion as compared to the production of oil, coal and natural gas.
The Chinese standard requires a safety distance of 2 m between the electrolysers.
Three accident databases (ENSAD, HIAD 2.0 and H2tools) were analysed: in ENSAD, no hydrogen release was reported for production site accidents; HIAD 2.0 data suggest the risk associated with electrolysers are small compared to compressors and pressurised storage; For H2tools, no accidents that can relate to Scenario 1 were reported after 1990. However, these databases do not provide complete coverage and so any observations should be taken with some caution.
A Sandia report used Bayesian statistics to estimate the risk for leakage from pipelines connected to the electrolyser using hydrogen-specific leakage data; the estimated risk is within the boundary set by the Purple book (5 ∙ 10-6 m-1 year -1).
Scientific studies suggest that minor hydrogen release should not cause safety concerns. Computational simulation calculated the IR 10-6 contour (distance for a 10-6 probability of injury each year) to be 4.5 m for in-plant pipeworks.
Gaps
Based on the above remarks, it can be concluded that current research identifies that risk associated with Scenario 1 is within the boundary set by the purple book. Nonetheless, we recommend an up-to-date review of available hydrogen accident databases to follow the current development and hence complement the literature review.
Scenario 2 – Pipeline transport: leakage from high pressure pipeline
The studies included in this scenario analyse the transport of gaseous hydrogen through high-pressure pipeline and the safety measures that should be taken into account. The research focuses on ignition, leakage and explosion likelihood, the potential damages on buildings, people and the necessary safety distance to prevent these hazards. Quantitative research and experiments, alongside models verify the impact of the consistency and material of the tube, the ground soil, the internal flow and the position of the pipeline, whether buried or over ground, on the aforementioned hazards.
Hydrogen by nature is lighter than natural gas and air and its leakage from pipelines is approximately 1.3 to 2.8 times larger than methane leakage and four-times than air under the same conditions (Rigas and P., 2013[23]).
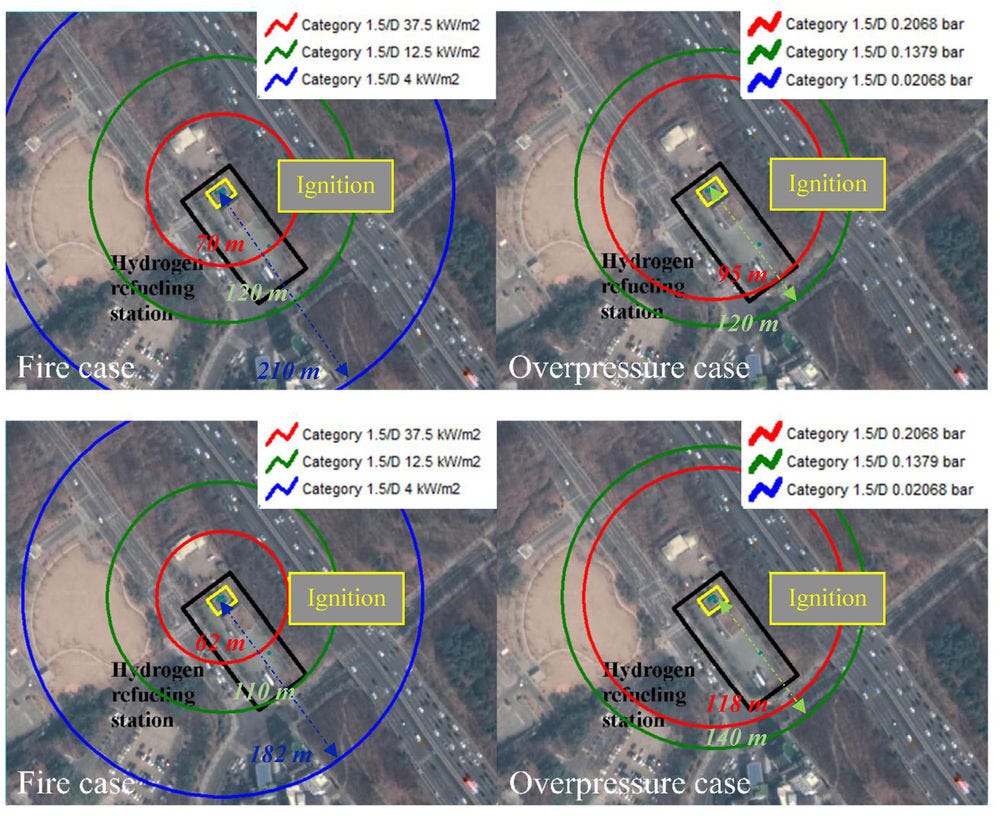
As evidence from Figure 8.2, hazards occurring on a hydrogen transport high pressure pipeline -likewise similar pipelines with flammable gases can lead to major consequences.
When analysing leakage rate and dispersion, it has to be considered that hydrogen diffusion in air is larger than natural gas. It presents a higher diffusion coefficient and greater volumetric flow rate compared to methane for the same pressure and leak size (Lowesmith et al., 2009[24]). Liquefied hydrogen confined, for instance, in a pipe between two valves, will eventually warm to ambient temperature, resulting in a significant pressure rise. However, transport pipelines do not transport liquified hydrogen. Standard storage system designs usually assume a heat leak equivalent to 0.5 %/d of the liquid contents. Considering liquefied hydrogen as an ideal gas, the pressure resulting from a trapped volume of liquefied hydrogen at one atmosphere vaporising and being heated to 294 K is 85.8 MPa. However, the pressure is 172 MPa when hydrogen compressibility is considered (Rigas and P., 2013[23]).
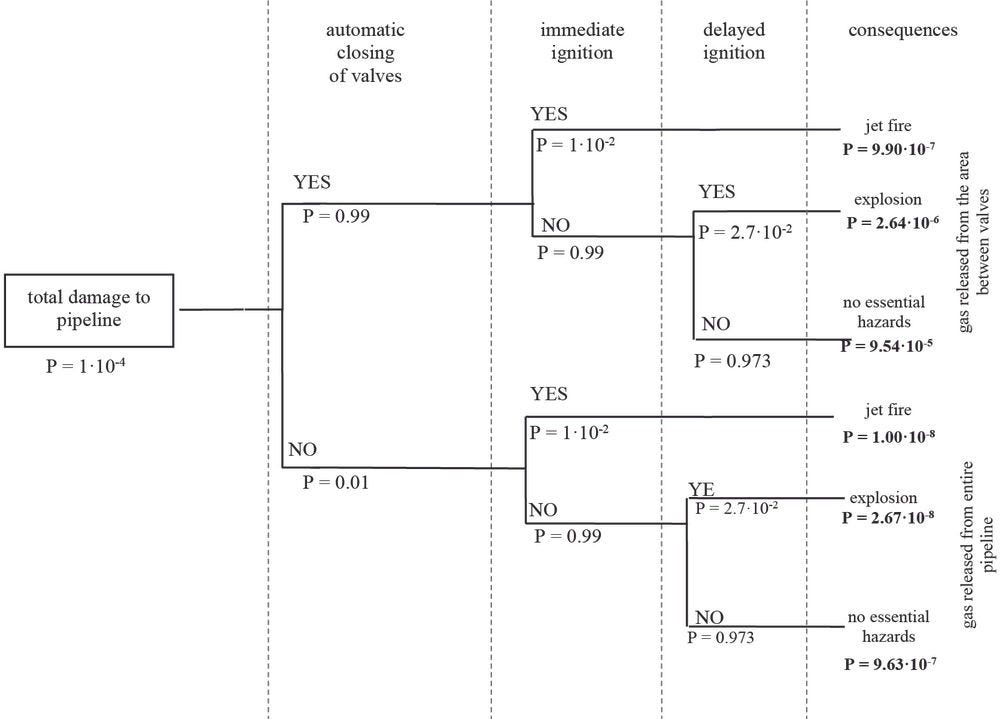
Figure 8.2. Event tree for damage to a hydrogen transport pipeline
An incident consisting in a pipeline failure can lead to several consequences, resulting in serious damage to humans and properties in the surrounding area. Many factors play a role in identifying a hazard area related to the damage, being: the type of failure, hole size, length, and operating pressure of the pipeline, in addition to the time to ignition, meteorological conditions, the ground soil, and the pipeline position. The flow in case of hydrogen leakage through a hole can be characterised to be either choked and or unchoked depending on the release speed (sonic or subsonic flow).
The release rate of high-pressurised hydrogen from a leak in the pipeline depends on the operating pressure, the pipeline diameter, and the length of pipeline from the supply point to the failure point. Due to large differences between the pipeline and its outside ambient, the flow conditions at the release become critical, so that a sonic flow will release from the failure point (Dagdougui et al., 2010[26]).
From the experimental point of view, to estimate the scale of damage to people and buildings caused by high-pressure hydrogen pipeline explosions (Russo, De Marco and Parisi, 2020[27]) conducted a probabilistic risk assessment. The release of hydrogen is simulated using the LimitState:SLAB model. The software tool is a slab analysis tool. To systematically automate the well-known yield line method. First, the size of the hydrogen-air cloud in the flammability range is evaluated and then the overpressure and impulse generated by the blast are evaluated through the Netherland Organisation for Applied Scientific Research (TNO) model. Finally, explosion effects on people and buildings are estimated through probit equations and pressure–impulse diagrams. The study (Russo, De Marco and Parisi, 2020[27]) took into account different relevant effects, from direct and indirect for people to different damages depending on the types of buildings. Proposals for mitigation and prevention systems are featured, alongside distance safety measures, considering both EU guidelines and HSE’s.
The simulations were performed assuming various pipeline geometric characteristics and operating parameters (diameter, temperature, and pressure), various properties of the release source (e.g., hole diameter, distance from the compression station, and distance from the explosion centre), different atmospheric conditions (e.g., wind speed and Pasquill–Gifford atmospheric stability class), and explosive class. The blast probability was calculated using statistical data on the operating properties of pipelines for H2 transmission gathered from the available literature. The information from Air Liquide was used for the failure frequency of hydrogen pipelines per length of pipeline. The value was assumed to be 0.126/year/1 000 km. Finally, the data of the European Gas Pipeline Incident Group (EGIG) were used to determine the frequency of the various sizes of breach. It was defined as follows: a small breach is one with a hole diameter smaller than or equal to 0.02 m; a medium breach is one with the hole diameter larger than 0.02 m and smaller or equal to the diameter of the pipe; and rupture is when the hole diameter is larger than the pipe diameter.
For what concerns blast damage to people, direct and indirect effects are generally distinguished. On the one hand, pressure-sensitive organs (e.g., lungs and ears) can be damaged by a change in pressure. On the other hand, a person can be indirectly involved in the explosion and suffer from indirect damage, such as the impact from flying fragments generated by structure damage or collapse. In addition, people can be thrown away from the overpressure, with a possible subsequent impact.
The European Industrial Gases Association (EIGA) defines harm criteria as being approximately a 1% chance of individual risk of serious injury or fatality and proposes the individual harm exposure threshold for determining safety distances of 3.5∙10-5 /year. The UK Health and Safety Executive (HSE) has specified risk criteria as follows: for workers, maximum tolerable risk is 10-3 per year; for the public, 10-4/year; broadly acceptable risk, 10-6 /year. The Netherlands has its own tolerable risk criteria as detailed in section “Zoning safety measures” below.
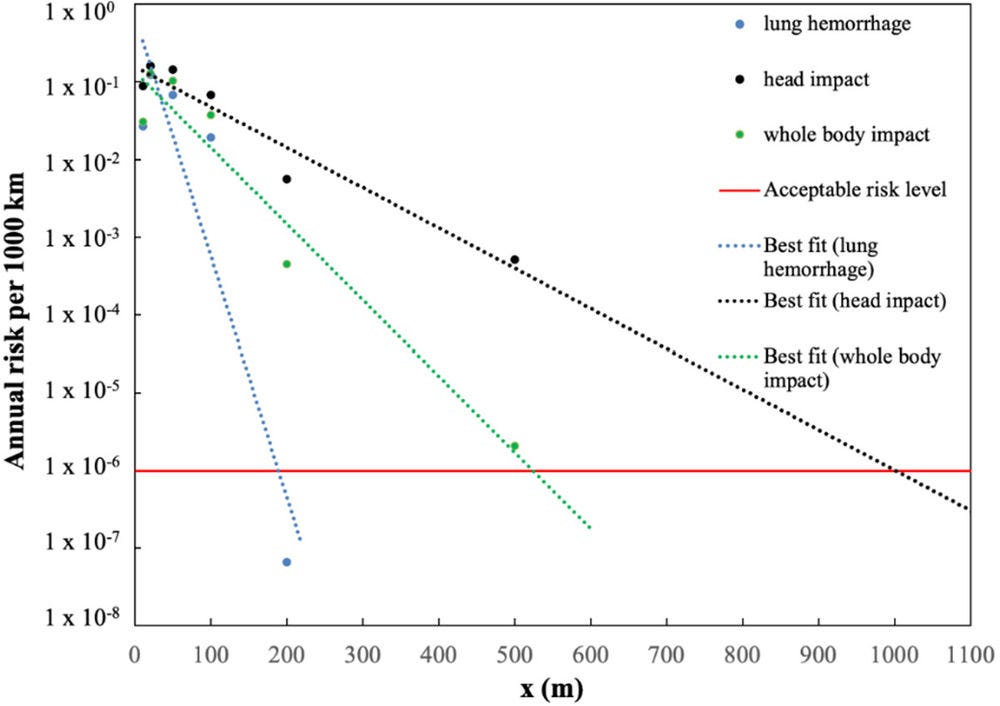
A minimum safe hazard distance between pipelines and populated areas equal to 1 000 m is calculated from comparison with the harm criteria (see Figure 8.3) for certain types of pipeline. It should be pointed here that the safety distance of 1 000 m is prescriptive only.
In the study of (Russo, De Marco and Parisi, 2020[27]) and is calculated based on the particular assumptions made in the QRA. It can be considered as a conservative distance.
Figure 8.3. Annual risk per 1 000 km of damage to people vs. safety distance (m), in the case of blast strength 9 and atmospheric stability class F2
Zoning safety measures
In the Netherlands a zoning policy is in place as a safety measure to prevent major accidents and consequences to people and buildings. This follows an approach aimed at reducing the risk through safety measures at the source of risk. To determine the zoning policy and set the criteria limits for pipelines, the individual risk as a measure of the level of protection to each individual member of the public, and the societal risk as a measure of the disaster potential for the society as a whole. The first is determined with the risk fatality per year, i.e. the probability that an unprotected person residing permanently at a fixed location will be lethally wounded as a result of an accident occurring at a source of risk. The societal risk is the probability of exceeding a certain yearly amount of casualties.
For vulnerable object like schools and hospitals the individual risk limit is 10-6 / year
For less vulnerable objects the above number is a guidance value.
For transport routes the limiting frequency (Flim) per kilometre of pipeline for the occurence of an accident with death (N) casualties is: Flim x N² = 10-² (Laheij and Theune, 2010[28]).
A similar analysis completed by (Witkowski et al., 2017[25]) on hydrogen compression and pipeline transportation processes at the distance of 50 km and the pressure of 10 MPa upstream a pipeline, with safety issues for water electrolysis hydrogen productions, for selected hydrogen flow rates of 0.2, 0.5, 1.0, 2.0, and 2.8 kg/s. These hydrogen mass flow rates were determined by the possible working parameters of different types of compressors and the possible range of safe inner diameter of the pipeline for the transportation process determined similar consequences for human beings. In the case of the hydrogen jet fire, the zones with a fatal effect on humans extend from the location of the pipeline damage over a distance of approximately 120 m for a pipeline diameter of 250 mm. The zones presenting a hazard to human health and life will depend, among others, on the hydrogen pressure and the size of the pipeline damage.
(Houssin-Agbomso, G. and D., 2018[29]) verified the consequences of a specific high pressure gas release on a buried pipeline through a 12 mm diameter breach. The choice of the 12 mm hole size was determined by the fact that it constitutes a representative size of releases in buried pipes resulting from corrosion in the highest number of the 145 000 km long buried pipelines network across Western Europe. The latter element of the pipeline being buried is influenced by environmental factors that contribute in shaping the consequences of leakages occurring on buried high pressure pipes. The experiment therefore determines what were their behaviour and their impact on the soil – i.e., crater formation, or not, according to release parameters – in order to use the appropriate methodology for risk and consequences assessment. Thus, by changing several parameters – like nature of gas, initial gas pressure, type of soil – the threshold between crater formation and gas dispersion in the soil following such leakages was investigated. The crater is influenced by the following specific conditions: high pressure of the releasing gas, vertical upward orientation of the release, and a soil with low plasticity and low cohesiveness like a sandy soil, while it is independent of the nature of the releasing gas. For the other conditions, an uplift of the soil occurs and allows the evacuation of the gas reaching the ground surface with a low velocity and possibly quickly dispersed in the ambient air, for light releasing gases in most atmospheric conditions.
A similar simulation aimed at analysing the impact of different factors in a hydrogen leakage accident evidenced that (1) wind speed, ground roughness, tube pressure and leakage gap area have a great influence on the diffusion distance, wind speed being the most influential; (2) Wind speed, tube pressure and leakage gap area have a great influence on the overpressure distance, gap area being the most influential; (3) Gap area has a significant impact on the combustion distance. The impact of other variables on the combustion distance is very little or negligible; (4) the diffusion distance and overpressure distance reduce as the wind speed and ground roughness increase. In particular, tube pressure and gap area have a great impact on the consequences of the accident; wind speed and ground roughness have a negative correlation to hazardous distance; tube pressure and gap area have positive correlation to hazardous distance; wind speed and ground roughness do not affect combustion distance (Chen and Mao, 2017[30]).
Ignition probability
Since the infrastructural network for hydrogen relies on the same pipeline system of natural gas it might be useful to factor in the ignition probability and safety measures implemented for the transport of other gases.
The ignition probability is described as to be subdivided into direct/immediate and delayed ignition. Two main factors contribute in determining the probability of ignition pressure (p) in the pipeline and the diameter (D) of the pipeline, with a linear relationship existing among them. It has been affirmed that the Pign= pD². Therefore the computed equation states that Pign is 0.80 at the most with a Pdirect:Pdelayed distribution of 0.75:0.25 (Spoelstra and Laheij, 2011[31]).
The effect caused by ignition depends on both the physical state of the transported substance and the type of incident occurring. In the event of rupture of an underground pipeline for a flammable substance such as gaseous hydrogen a jet fire will occur. In case of a delayed ignition a a plume fire for the gaseous hydrogen.
In the same process in case of a leakage the direct ignition will develop a jet fire with a substance in a gaseous physical state and a jet fire combined with a pool fire with a liquid substance. Should the ignition be delayed, both will develop a flammable cloud (National Institute of Public Health and the Environment (RIVM), 2009[32]).
The effects determined by the two accidents we should consider the air entrainment. With gas ruptures the event will form a crate with the mixture with air influencing the velocity of the jet fire. In comparison to vertical jet fires, horizontal jet fires with low momentum can increase ground level heat due to the tilting impact of winds (Spoelstra and Laheij, 2011[31]). However, mixtures of air and hydrogen in low concentrations, up to about 8 -10 vol%, have a lower risk of ignition than natural gas (DNVGL, 2020[33]). The main cause of pipeline rupture are external interferences, safety measures to reduce individual and societal risk are due to focus on reducing the probability of pipeline ruptures as the preemptive way to reduce the adverse effects generated (National Institute of Public Health and the Environment (RIVM), 2009[32]).
Frequency of failures
The frequency of possible failures are determined at 6.1∙ 10-4 /km/year. With a probability of leakage frequency at 0.75 and 0.25 probability for rupture. As rupture is the most probable incident to occur the failure frequency will be 1.5 ∙10-4/km/year mostly caused by external interference (Laheij and Theune, 2010[28]).
By combining historical data of number of incidents in a pipeline depth class and historical damage data and fracture mechanics we derive a function fd = e-2.4 • d-3. (Laheij and Theune, 2010[28]).
To calculate this function, we use specific pipeline parameters: the diameter, pressure, depth of cover, wall thickness, yield strength and Charpy energy. An analysis using this function was conducted on the 12 000 kilometres in length Gasunie network and represented in the calculations by about 1.2 million data points based on the 1977-2005 historical failure data. The final prediction was a 0.7 rupture per year.
The number of leaks and outflow
Natural gas low pressure pipeline leaks mainly occur near the home, precisely in the connecting pipe, the metre connection and the indoor piping through the distribution materials such as iron, asbestos cement and steel. These are commonly the same materials to be used for the hydrogen distribution networks. The main difference with natural gas in terms of leak is related to the outflow volume which is greater with hydrogen. A small leak of around one litre per hour or less, the flow may be laminar and about 30% more hydrogen flows out based on volume. Larger leaks lead to a turbulent flow that releases 190% more hydrogen than natural gas (DNVGL, 2020[33]).
By analysing the H2Incidents database, a total of 53 incidents involving pipe ruptures were found with most of them leading to ignition subsequent to leakage. 35 of them lead to property damage with only 7 involving human life.
Conclusions and knowledge gaps
The main conclusions based on the literature review related to scenario 2 are:
Scenario 2 analyses pipeline transport focusing on the consequences occurring in case of leakage from high and low pressure pipelines. The reports provide an overview of both impacts on the pipeline, buildings and people, and effects leading to the potential hazards. Main conclusions from all reviewed studies so far are:
A maximum value of 1.65∙10-3 death/year/1 000 km was obtained in the case of an explosive class with high ignition power (class 9), stable atmospheric conditions and assuming a failure frequency of hydrogen pipelines equal to 0.126/year/1 000 km.
The individual risk creates a distance between the source of risk and its surroundings. Societal risk limits the population density around the source of risk.
Crater formation from a 12 mm diameter breach in underground pipeline is studied. The 12 mm size was tested as it is most commonly considered as an accidental scenario of the buried pipelines network due to damage by pipe corrosion. It is impacted by high pressure of the releasing gas, vertical upward orientation of the release, and a soil with low plasticity and low cohesiveness like a sandy soil. There is great influence of wind speed, ground roughness, tube pressure and leakage gap area on the diffusion distance and overpressure distance.
Since hydrogen gas is odourless, colourless, and tasteless, leaks are not detected by human senses. Therefore, as a safety measure to counter major consequences from hazards, the use of hydrogen sensors is recommended to successfully detect hydrogen leaks.
Walls collapse at overpressures of 14 kPa,12 and at 42 kPa13 houses are largely destroyed. Due to the higher reactivity of hydrogen, it is expected that a stoichiometric mixture of hydrogen is more likely to cause a detonation.
By reviewing the literature we understand that there is no clear view of the sources that do or do not lead to ignition. Different sources with sufficient energy for ignition were tested but there was discontinuity of igniting a flammable mixture.
Hydrogen leaks are greater in volume flow than those of natural gas. In case of leaks, the risk of hydrogen entering in a home depends on the concentration that can be built up: if the concentration of hydrogen remains below 10 vol%, there is a lower probability of damage because the likelihood of ignition is lower than with natural gas and because no explosion is likely to occur if ignition takes place. For concentrations above 10 vol%, hydrogen presents a greater risk of damage because the chance of deflagration is higher than with natural gas and the pressure builds up much faster.
Hydrogen spreads faster in confined spaces than natural gas. Experiments and simulations have shown that in the case of a leak in a non-ventilated space, hydrogen initially accumulates at the top of a confined space due to the difference in density, and then mixes to form a homogeneous blend. With enough ventilation hydrogen escapes to adjacent spaces.
Gaps
From the above findings, most of the experiments conducted out of labs used pre-existing infrastructure developed and built for natural gases that have different properties that might vary depending on the relative specific conditions.
No statistics have yet been compiled for the leakage size distribution and detection in hydrogen distribution networks. The creation of a register of leaks and their extent in future hydrogen distribution networks might help.
Further study is needed to confirm the effects of detonation in confined areas and to further establish the impact compared to natural gas.
Scenario 3 – Road transport: H2 leakage in a confined space/ built environment
The following analysis investigates the properties of hydrogen in confined spaces and built environments. Hydrogen is less likely to cause a fire or explosion hazard in an open or well-ventilated space as it diffuses easily. However, it can cause a safety risk if it accumulates in a confined or poorly ventilated space. Therefore, the safety of FCVs and the related infrastructure, including hydrogen fuelling stations, tunnels, garages, parking, and maintenance workshops is relevant. Accidents in urban built areas also increase the likelihood of hydrogen leakage and the attendant risks. The articles summarised, therefore, describe the potential incidents arising out of hydrogen leakage in parking garages and road accidents involving hydrogen FCVs (HFCV) and some methods to mitigate such incidents.
Sensors in HFCVs
Regulatory attempts have already been proposed for certifying HFCVs to test their crashworthiness. For instance, the new Global Technical Regulation (GTR) proposes the performance-based test methodology for HFCV fuel system integrity certification. If this proposal is accepted, then HFCV’s certification could depend on the system performance during barrier/ rollover crash tests. Under the proposed regulation, an FCV would fail the certification test if the hydrogen leakage rate exceeds 118-L/min or if flammable mixtures develop within the car or the trunk within an hour of the crash.
Within the GTR methodology, certain additional experiments have been performed to analyse the capabilities necessary to detect the presence of flammable mixtures within the car or the trunk (Ekoto et al., 2011[34]). Through in-vehicle leakage tests the importance of sensors, both direct and indirect, were highlighted. Direct sensors measure the hydrogen concentration while indirect sensors or oxygen depletion sensors measure the depletion in oxygen levels. Both the sensors performed equally well once temporal drift corrections were applied. Some other findings from this experiment are as below:
Duplicate in-vehicle (cabin and boot) dispersion characteristics were highly repeatable, with mole fraction profile variations less than 0.01 at most sensor locations.
Releases with high amounts of convective mixing had in-vehicle mixture distributions that were far more homogeneous than distributions from diffusion dominated releases with negligible jet exit velocity.
Porous diffusion boundaries, such as seat cushions, natural ventilation to the ambient environment, present between adjacent compartments within the car slowed the development of elevated H2 concentrations. However, H2-rich mixtures eventually formed in elevated regions for both compartments if the upper edge of the diffusion boundary was located above the ventilation point.
Although increased release rates led to more rapid threshold detection times and higher peak concentrations throughout the vehicle, even small leaks resulted in the rapid development of flammable regions. A simplified analytic analysis indicates these flow rates can easily be exceeded from small ruptures in moderately pressurised storage or delivery components unless appropriate control and mitigation measures are taken.
Ventilation in parking garages
Increase in the demand for H2 fuel in the future will require high investments in the infrastructure sector. In this regard, parking hydrogen vehicles in residential garages pose a potential safety hazard because of the accidents that could arise from hydrogen leaks. The dispersion of hydrogen in a garage: with ventilation and without, has been analysed through numerical and experimental studies (Ehrhart et al., 2020[35]), (Choi et al., 2013[36]). The temporal and spatial evolution of hydrogen concentration as well as flammable regions in a parking garage have been predicted. The volume of the flammable region shows a non-linear growth in time with a latency period. The effects of the leakage flow rate and an additional ventilation fan have also been investigated to evaluate the ventilation performance to relieve accumulation of the hydrogen gas. It is found that expansion of the flammable region is delayed by the presence of a fan via enhanced mixing near the boundary of the flammable region.
(Merilo et al., 2011[37]) performed a series of experiments to investigate hydrogen release accidents in a vehicle garage with both mechanical and natural ventilation. Tests were performed with hydrogen release rates of 1.6 kg/h, 3.3 kg/h, 4.9 kg/h, and 6.7 kg/h and ventilation rates of 0.1 m3/s, 0.2 m3/s, and 0.4 m3/s. The primary hazard was the deflagration of the hydrogen-air mixture and the burning of the hydrogen jet fire inside the garage. The maximum concentration of 17% v/v and overpressure of 0.77 kPa were produced with a 6.7 kg/h release rate and a ventilation rate of 0.1 m3/s. The maximum average peak overpressure was 0.769 kPa. For all mechanical ventilation tests except the 6.7 kg/h release, the overpressures that resulted from the confined deflagrations were all very low and did not represent a risk to people or property.
Very recent experiments performed by USN, (Lach and Gaathaug, 2021[38]) investigate the ventilation efficiency in underground garages. Compressed hydrogen was released from underneath a car inside a semi-closed facility with forced ventilation. Two ventilation rates based on British standards (6 and 10 ACH) and several nozzle sizes were tested. Steady state and blowdown releases were considered. Based on the experiments it was found that:
The peak concentration formed inside the garage is similar for 6 ACH14 and 10 ACH ventilation.
The cloud becomes flammable (reaches the LFL) at different times for each ventilation rate for hydrogen releases with the same mass flow rate.
The residence time of the flammable cloud is halved for a ventilation rate with 10 ACH.
The sufficiency of forced ventilation, used today, on hydrogen concentration was not conclusive in the experimental geometry that was used.
The ventilation rate in underground release should be 10 ACH (or higher) for unignited hydrogen releases, because lower ventilation rates will result in a longer duration of a flammable cloud.
(Hao et al., 2020[39]) studied the impact of no ventilation and mechanical ventilation on dispersion of hydrogen in a confined space using experiments. Two model cars of equal dimensions and having onboard hydrogen storage tanks with working pressure of 70 MPa mounted near the rear seats and the trunk were experimented upon. Vehicle A was equipped with Type IV hydrogen tank15 (non-metallic liner) while Vehicle B was equipped with Type III hydrogen tank (seamless metallic liner). The experiments were performed firstly with an Air Exchange Rate of 0.03 ACH which is considered to be the poorest ventilation and is descriptive of a tight wooden frame structure and sheltered from wind and temperature variations. The second experiments were performed with two fans and vents producing an air exchange rate of 6 ACH. For emergency ventilation 9 ACH is preferred. Fans with dimensions 120x120x38 mm (length, width, thickness), diameter 100 mm, air velocity 86.0 m3/h at the speed of 1600 rpm were placed near the ceiling. Circular vent diameter was 100 mm. The discussions and findings surrounding parking state and idle state as covered in the study by (Hao et al., 2020[39]) are discussed below.
In most scenarios involving parking garages and repair workshops, vehicles are engaged in two states: i) parking state (including start-idle and shutdown process) and ii) idle state. Both these states have different impacts on the hydrogen leakage and volume of flammable concentrations:
Parking state (with a parking time of 8 hours)
Vehicles with Type III hydrogen tanks perform better than vehicles with Type IV hydrogen tanks (Hao et al., 2020[39]). Hydrogen is detected about 20 minutes and 50 minutes after leakage in Type IV and Type III respectively. As hydrogen concentration follows a linear upward trend, after parking for 8 h, the hydrogen leakage of vehicle A and vehicle B resulted in the detection of the highest hydrogen concentration in the poorly ventilated confined space, at 125 ppm and 42 ppm, respectively. However, both the values are much smaller than the safety limit of 10 000 ppm required by GTR standards. Additionally, after hydrogen gas was detected in the sealed chamber, the hydrogen concentration rose at a nearly constant rate. This means that 8 hours is enough to examine the hydrogen leakage of the vehicle without the need to further increase time. 8 hours could simulate the daily use of most vehicles. Interestingly, if the hydrogen concentration is raised at approximately 5.544 ppm/h (as for a car with Type II tank), it would require approximately 1 800 hours to reach the safety limit of 10 000 ppm.
Idle state (with start-up and shutdown purge and idling time of 10 minutes)
When an HFCV is started, it goes through a start-up purge. Before a fuel cell engine starts, in order to supply the hydrogen into the anode rapidly or purge the air in the anode which permeated from the cathode during parking conditions, some hydrogen is supplied into the stack with pressure and then discharged from the tailpipe of the vehicle. This process is named as “start-up purge process.” As a consequence, the hydrogen concentration near the vehicle exhaust outlet rapidly increased to 695 ppm (Type IV) and 232 ppm (Type III). Subsequently, the hydrogen gas can diffuse to other positions in the chamber. Next, the fuel cell engine automatically maintains an idling state for 10 minutes. During idling, hydrogen concentrations do not increase noticeably. Once the vehicle shuts down, the fuel cell engine automatically enters the “shutdown purge process.” In order to decrease the water produced by electrochemical reaction and adjust the humidity inside the fuel cell stack, during the shutdown process of the fuel cell engine, some hydrogen is supplied into the stack with pressure and then discharged from the tailpipe of the vehicle. As a consequence, this process rapidly raises the hydrogen concentration near the tailpipe to 2356 ppm (Type IV) and 130 ppm (Type III; the two types operate at different pressures, hence the resulting difference in concentration) and causes the hydrogen concentration at other positions to increase. However, under the action of mechanical ventilation, the hydrogen concentration in a confined space can be gradually decreased.
It can be conclusively stated that ventilation reduces the hazard associated with hydrogen leakage in confined spaces frequented by HFCVs. This is because firstly, the flow structure and molar fraction of hydrogen is strongly influenced by ventilation parameters. With a ventilation fan, the flammable region decreases as air volume of the fan increases. A fan enables mixing of air near the flammable region and thereby delays expansion of the flammable mixture. Secondly, in the absence of ventilation, flammable regions increase with time. It is important to note that the volume of the flammable region does not increase linearly with time, but increases rapidly after the initial latency period. It can be therefore stated that parking garages need a minimum ventilation requirement for both liquid and gaseous energy carriers, and should be an important consideration in building regulations.
Regulators must note that the extent of risk mitigated depends on several factors such as shape of the vent, type of ventilation (see also Hydrogen Safety Aspects section): i.e. natural or mechanical, and in the case of mechanical ventilation systems such as fans, the size, speed and location of the fan etc. Some findings from the study by (Hajji et al., 2021[40]) which can inform regulators on the parameters of ventilation and their impact on hydrogen stratification are as under:
Hydrogen flows in two directions, parallel to the bottom of the car and to the ceiling. This results in development of flammable regions near the bottom of the car and closer to the top of the ceiling.
Amongst the three generally prevalent configurations of ventilation openings- square, circle and triangle, the square shape generates lower concentration levels and presents the highest extraction efficiency which is equal to 56.06%. This proves that simple geometric shapes (square) are more adaptable to the evacuation of low-fuel gas density as hydrogen.
Different aspect ratios (R, length/height) of the vent have a distinct effect on the hydrogen concentration; when R decreases, there is an increase of fresh air drawn and an increase of the hydrogen evacuation. When combining the two factors: aspect ratio and shape type, hydrogen extraction of the transverse rectangular shape (R = 0.5) is more efficient and it presents better results than the others.
Accident involving HFCVs
A vital issue for HFCVs is the safety concerns when hydrogen is leaking from a damaged vehicle after an accident.
(Sun and Zhiyong, 2018[41]) studied the major hydrogen consequences including impinging jet fires and catastrophic tank ruptures are evaluated separately in terms of accident duration and hazard distances. The hazards associated with hydrogen releases in a 70 MPa fuel cell car involved in an accident caused by collision on a city road (for instance due to tyre burst), would normally last for no more than 1.5 minutes due to the emptying of the tank (although a conservative value could be 3-5 minutes when considering first responder activities). For the probability of a successful fire extinguishment (assuming a fire is caused due to the collision), it is assumed that 50% of the fires will not be suppressed in time. It takes 30 minutes from the fire starting to the triggering of TPRD (4.2 mm diameter). The probability that the TPRD will fail is estimated at 2.22∙10-5. Given the improvement in modern hydrogen tank safety, the likelihood of TPRD failing is low.
First responders would be able to approach the vehicle, conservatively, approximately two minutes after hearing the hissing sound as the hydrogen hazards have been eliminated. For the safety of the general public, a perimeter of 100 metres is suggested to be set in the accident scene if no hissing sound is heard (Sun and Zhiyong, 2018[41]). However, the perimeter can be reduced to 10 metres once the hissing sound of hydrogen release is observed. For the first responders, if there’s no sign of hydrogen release, they should stand at least 10 m away from the burning car, otherwise their risk of fatality would be over 50% in case of catastrophic tank rupture. Blast wave overpressures greater than 1.35 kPa would lead to temporary loss of hearing. Overpressure of 30 kPa is taken as the fatality criterion (50% probability of fatality from missile wounds).
To mitigate the risks of ignition and fire, studies, (Liu and Christopher, 2015[42]) have suggested the use of a portable blower by first responders. Ground effect blowers with a diffuser flush to the floor effectively removes most of the hydrogen to create a safety envelope around the vehicle. In terms of approach direction, first responders should avoid approaching the vehicle from the side opposite the blower. This is because these areas are where the hydrogen concentrations would still be close to the lower flammability limit even despite the presence of a blower. Hydrogen flame lengths can be considered as “fatal distance” and distance to 70℃ temperature boundaries can be considered “no harm distance”. CFD simulations show that flame lengths from hydrogen jet impingement reach 8 metres and the 70℃ envelope is 10 metres. This means that first responders who deal with accidents must stand at least 8 metres away from a car to avoid fatalities and a perimeter of at least 10 metres should be set around the accident scene to protect the public. Some other issues, regulators could consider while drafting regulations related to emergency responses involving HFCV accidents are:
Blowing from the front produces a higher safety margin.
Leak from under the centre of the car is easier to control than leaks from the side.
Forced airflows of 10 m/s can disperse hydrogen from around a car and in its interior to less than the flammability limit of 4 vol% hydrogen (assuming leak rate of 2000 NL/min).
Ground effect blowers with the diffuser flush to the floor removed most of the hydrogen effectively to create a safety envelope around the vehicle.
Regulators can also consider using lessons learnt from CNG vehicles to determine the safety requirements for HFCVs. Comparison studies using CFD (Li and Luo, 2019[43]) between CNG and HFCVs show that the release duration for CNG vehicle is over two times longer than that for hydrogen vehicle, indicating that CNG vehicle jet fire accident is more time-consuming and firefighters have to wait a longer time before they can safely approach the vehicle. In the given experiment, for both hydrogen vehicles and CNG vehicles, the longest hazard distance near the ground occurs about 1 to 4 seconds after the initiation of the thermally-activated pressure relief devices. Afterwards the flames will shrink and the hazard distances will decrease. For firefighters with bunker gear, they must stand 6 m and 14 m away from the hydrogen vehicle and CNG vehicle, respectively. For the general public, a perimeter of 12 m and 29 m should be set around the accident scene for hydrogen vehicles and CNG vehicles, respectively.
Risk assessment on life safety and financial losses in case of FCV accidents
In the study by (Sun and Zhiyong, 2018[41]), the additional risks introduced by the flammable effects of hydrogen are calculated. The study considers “additional” risks rather than “overall” risks because the losses caused by hydrogen powered vehicles and conventional fuel vehicles are similar. As per the study, due to flammable effects of hydrogen, the risk of compensation for fatalities and injuries in the car accident is 8x10-5/year, and compensation costs will be less than 20 million dollars and 2 million dollars for fatalities and injuries, respectively. For repair and replacement loss, the risk of compensation of less than 60 thousand dollars is 8x10-5/year and the risk of compensation less than 7 thousand dollars is 2x10-4/year. The risk of environmental clean-up cost is 2x10-4/year, while the cost is very small (700 dollars). The insurance premium of fatalities and injuries should be higher than that of property loss, to be taken into account in insurance pricing of FCVs.
The Hydrogen Safety Panel prepared a report for the Safety of Mobile Hydrogen and Fuel Cell Technology Applications in October 2019 to suggest future course of action for safer use of inter alia mobile refuelling and high-volume transport applications. These trailers with high pressure (upwards of 19 MPa16) hydrogen cylinders particularly those of composite construction require greater harmonisation of codes and standards (Hydrogen Safety Panel, 2019[44]).
Conclusions and knowledge gaps
The main conclusions based on the literature review related to Scenario 3 are:
Hazards for Fuel Cell Vehicles (FCVs) are generally categorised into two, first being hazards associated with onboard hydrogen and piping systems mostly in the rear of the vehicle and second, hazards related to the onboard battery mostly in the front of the vehicle. The hazards can be associated with each other.
An immediate ignition of continuous release of hydrogen will result in a jet fire, while a delayed ignition could lead to a flash fire or an explosion if in confined space. For an instantaneous release in the case of catastrophic rupture of a hydrogen tank the violent depressurization from the high pressure tank will create an outward blast wave and fragment projectiles.
Hydrogen FCVs could become more publicly acceptable if the general public perceives their safety as comparable to that of the now generally accepted of CNG cars.
Storing a hydrogen fuel cell car in a garage can pose a safety hazard if there is a build-up of flammable mixture within the vehicle and/or the garage structure and an ignition source is present. Ventilation- both natural and mechanical- should be considered for the design of garages, repair workshops etc.
Significant research has also gone into studying dispersion of hydrogen in confined spaces. Hydrogen tends to accumulate below ceilings and roofs where it can reach flammable concentrations. Further, the manner in which hydrogen forms layers: uniform or stratified with varying concentrations, will also impact the safety assessment especially in confined spaces such as residential units.
To calculate the risks posed by hydrogen fuelled systems, probability of ignition is required. Ignition probability is still unknown and more research is required in the field. However, given hydrogen’s low ignition energy, its ignition probability is higher than that of other flammable gases if no additional measures are taken. Current studies reveal a number of mitigating and management measures such as limiting release of hydrogen, preventing leaks from escalating, personal protections and emergency response. The importance of overpressure relief valves and flow restrictors is also stressed.
Valves are the best way to limit leaks. Once a leak is detected, ventilation, prevention and management of ignition sources, and implementation of safety distances are some key measures which most studies emphasise.
(Hao et al., 2020[39]) demonstrate that the hydrogen emission for vehicles with Type IV fitted hydrogen tanks fare worse than vehicles with Type III tanks. Regulators can consider incentivising the use of Type III tanks in HFCVs to reduce risks.
For vehicles parked in enclosed spaces, the purge process is a crucial factor because emissions are highest in this state. Parking and idling present less risk from leaking hydrogen as the hydrogen concentration is stable and does not rise with idle time. Car idle times can be determined based on this and the fact that vehicles should avoid multiple purge processes in confined spaces. If the purge process control strategy is not optimised, it could lead to hydrogen concentrations in an enclosed space to exceed the safety limit of 1%.
Gaps
1. Ventilation
In addition to ensuring adequate ventilation in parking garages and enclosed spaces, ventilation should also be considered for tube-trailers transporting hydrogen. More tests need to be performed to verify that vent openings will be adequately sized for credible hydrogen leaks to ensure that hydrogen is not trapped in an enclosure around the cylinders or the pipelines. If there is a roof on the cylinder enclosure of a trailer, the benefits of hydrogen detection sensors should be considered to alert operators and avoid them from opening a door to a flammable mixture.
2. Sensor Location
Although the importance of sensors in HFCVs has already been established, the issue of location of the sensor still requires detailed analysis. Hydrogen distribution strongly depends on release characteristics such as release rate and location. Pinhole leaks from moderate source pressures would produce unacceptably high in-vehicle hydrogen concentrations. Sensors should optimally be located high above the release point. However, much of the sensor’s efficacy would depend on the final vehicle orientation in a crash involving rollovers and therefore further research would be required to take this into account.
3. HFCV Design
i) The main sources of leakage included hydrogen permeation through hydrogen storage vessels, hydrogen leakage in the high-pressure valve, and hydrogen leakage in pipelines and joints. For instance, a concern remains over the robustness of safety valves and the likelihood that they would inadvertently open during impact. However, more research is required on material compatibility, valve performance etc.
ii) Since the highest hydrogen concentration in an enclosed space is noticed to be caused by the purge process, controlling the hydrogen emissions occurring due to the purge process is critical to the improvement of hydrogen safety of vehicles in a garage. This could be done by improving the hydrogen utilisation rate of a fuel cell engine by using components such as a hydrogen circulation pump and optimising air compressor control strategy. Mixing the appropriate amount of air into the FCV exhaust gas to dilute the hydrogen concentration by optimising the pipeline design could also be considered. However, these interventions would require further analysis.
Scenario 4 – Mobility and partially confined spaces: Examples of this scenario include a hydrogen city bus driving in a tunnel involved in a collision accident
This scenario examines the possible hazards from a hydrogen vehicle crash inside a tunnel. A hydrogen vehicle crash can lead to the release of hydrogen and possibly to its ignition. The gas can form flammable clouds and fill the semi-enclosed space of the tunnel. Overpressures can occur as a result of ignition of a cloud of released flammable gas but also as a result of unignited releases of pressurised gas.
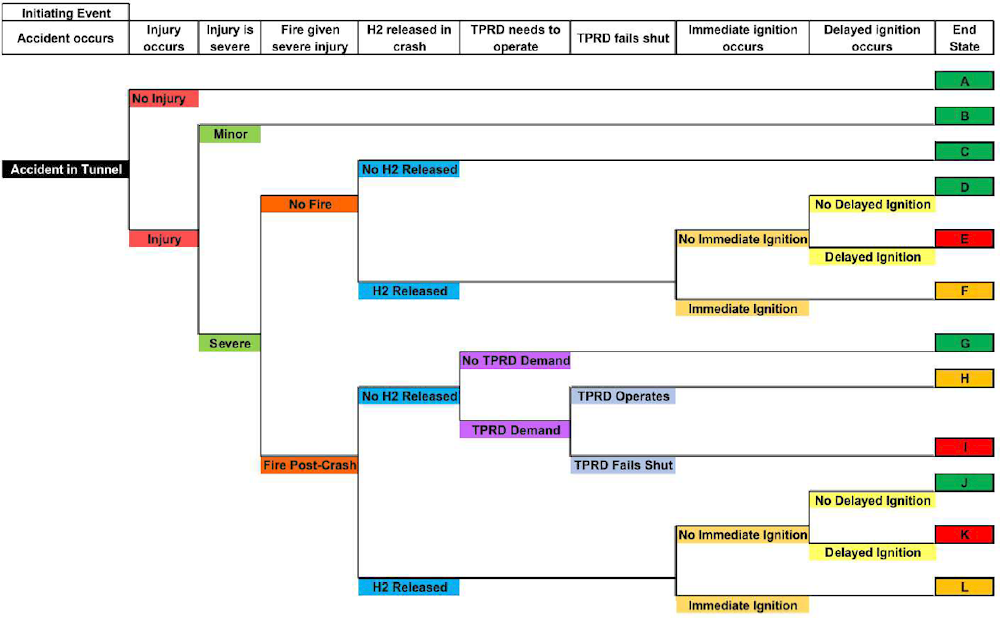
(LaFleur et al., 2017[45]) and (Ehrhart et al., 2019[46]) performed a thorough risk assessment investigating a number of possible scenarios involving a hydrogen vehicle crash inside a tunnel: as can be seen in Figure 8.4, the most likely consequence of a crash is that there will be no additional hazard from the hydrogen fuel (98.1–99.9% probability). If the hydrogen does ignite, it is most likely to result in a jet flame from the pressure relief device released due to a hydrocarbon fire (0.03–1.8% probability).
An older risk study (Middha and Hansen, 2009[47]), examines releases from hydrogen cars (containing 70 MPa17 gas tanks releasing either upwards or downwards or liquid hydrogen tanks releasing only upwards) and buses (containing 35 MPa gas tanks releasing upwards) for two different tunnel layouts and a range of longitudinal ventilation conditions. The worst-case deterministic evaluation of each of the scenarios involved the tunnel filling with stoichiometric hydrogen gas clouds of varying size resulting in very high overpressures (the highest pressure seen was almost 12 barg18 for a 1 000 m3 gas cloud). However, this assumes that the full gas inventory is being mixed homogeneously at stoichiometry, something considered unrealistic by the authors of the study. In fact, very moderate worst-case explosion pressures were predicted when the actual reactivity of the clouds was taken into account, even in cases in which the flammable gas cloud sizes were large. The risk assessment suggested a maximum expected pressure level of 10-20 kPa above ambient.
Figure 8.4. Event sequence diagram for a hydrogen vehicle accident
The shape of the tunnel, the ventilation regime and the different properties of the vehicle thermal pressure relief device (TPRD) are potentially important parameters in determining explosion risks and appropriate mitigation measures. In regard to the tunnel’s shape, larger and ‘taller’ tunnels are considered safer. Findings from HyTunnel , a project established within HySafe, the European Network of Excellence on Hydrogen Safety (Kumar et al., 2009[48]) have shown that the increased ceiling height associated with arched cross-section tunnels reduces the hazard associated with the release of hydrogen, due to increased dilution of the hydrogen stream and a reduction in the momentum of the impinging jet. However, it was noted that the presence of blockage elements, e.g. light armatures or fans, could add some turbulence to flame propagation and make explosions more severe.
For some tunnel tests, obstacles representing vehicles were used to investigate turbulent enhancement. In a series of large-scale hydrogen deflagration and detonation experiments (Groethe et al., 2007[49]) obstacles representing vehicles were used to investigate turbulent enhancement during the release of hydrogen and homogeneous hydrogen mixtures (9.5%, 20% and 30%) inside a 1/5-scale model tunnel. It was found that the presence of vehicle models had no effect in the deflagration, possibly due to the small blockage ratio (cross-area blockage ratio of 0.03).
Tunnel inclination and slope are of interest as well: an older numerical study by (Mukai et al., 2005[50]) found that a 2% slope in a long horseshoe-shaped tunnel resulted in hydrogen collecting near the tunnel ceiling for several dozen minutes, whereas in underwater tunnels with a trough slope, hydrogen is rapidly cleared from the tunnel. In addition, a series of fire experiments and numerical simulations of a carrier loaded with hydrogen FCEVs in a full-scale tunnel (Seike, Ejiri and Kawabata, 2014[51]) showed that even a modest tunnel inclination (2%) hastened the thermal fume propagation of the FCV fires.
An effective tunnel ventilation regime is likely the most important preventive measure against hydrogen hazards. In the study by (Mukai et al., 2005[50]), 60 m3 hydrogen (approximately 5.08 kg) leaked inside a tunnel was immediately carried away from the leaking area under the ventilation velocities of 1 m/s and 2 m/s. A study of a horseshoe-shaped tunnel by (Koutsourakis, Tolias and Giannissi, 2021[52]) showed that for slopes up to 5 % the slope effect on hydrogen dispersion is negligible and no special treatment is required for inclined tunnels. The same study tested also whether the ‘stack-effect’ resulting from inclination inside a tunnel might hazardously cancel out the ventilation. In almost all cases examined the ventilation was proven to be much stronger: ventilation overwhelmed any buoyancy effects. This led to flammable gas concentrations being significantly lower.
There are, however, limits to the positive effects of ventilation. (Wu, 2008[53]) studied the effect of ventilation on the upstream back-layering and the downstream flame from an ignition of hydrogen inside a tunnel. For a smaller hydrogen release rate the tunnel ventilation system could eliminate the upstream back-layering (the smoke flow moving against the ventilation) and control the downstream flame. For a larger rate of hydrogen release (0.25 kg/s and a velocity of 50 m/s) however, the tunnel ventilation system could not provide sufficient air flow. If hydrogen is released at a high enough rate, even in a well-ventilated tunnel, it may produce a near homogeneous mixture at close to stoichiometric conditions, with a corresponding increased explosion hazard (Kumar et al., 2009[48]). Yet, this “worst case scenario” has been considered unrealistic elsewhere (Middha and Hansen, 2009[47]).
(Mukai et al., 2005[50]) also noted that hydrogen with a concentration close to low flammability limit might flow into the power collector portions of electrostatic dust collectors, or at the exhaust fan of the model tunnels for a brief time period. Thus, the distance between the main tunnel and these elements has to be sufficient for the hydrogen to diffuse and mix with the surrounding air. Ventilation can also potentially have negative effects: Simulations performed to test the effect of a ‘push’ or a ‘pull’ fan in underground mines have showed that, especially for the ‘pull’ configuration, in the case of a hydrogen leak, the lower concentration region is being drawn or forced back inside the higher concentration part of the cloud (Angers et al., 2013[54]). This results in higher overpressures in the vicinity of the release point. In experiments testing the effect of different ventilation configurations on unignited horizontal hydrogen jets in the air, (Grune et al., 2021[55]) there were a few cases when low velocity counter-flow ventilation (1.5 m/s) led to a minor increase of the safety distance. The effect was reversed under a stronger flow velocity, which led to a significant reduction of the safety distance. In Grune’s experiments, cross-flow ventilation led to the strongest reduction of the safety distance.
(Giannissi et al., 2021[56]) carried out CFD simulations based on experiments involving hydrogen release inside an enclosure and tested different ventilation configurations based on the experiments conducted by (Grune et al., 2021[55]). The aim was to study the efficiency of mechanical ventilation in case of a high-pressure hydrogen release and provide recommendations on the modelling of ventilated hydrogen dispersion. Simulations agreed with experimental data showing that both co-flow and counter-flow configurations enhanced the mixing and led to a reduction of the longitudinal distance of LFL (compared to the case without ventilation). Attributes of the TPRD, such as its diameter, can also make a difference when it comes to hazard mitigation. (Hussein, Brennan and Molkov, 2020[57]) investigated the release and dispersion of unignited hydrogen in a naturally ventilated covered car park through three different TPRDs with diameters of 3.34, 2.00 and 0.50 mm. A TPRD diameter of 0.5 mm was the safest choice for this particular scenario, since it produced a much more limited flammable cloud than in the other cases. However, the size of the unignited cloud due to the smaller TPRD should be weighed against the potential increase in risk due to longer emptying times in a fire. A risk trade-off needs to be made between the risk of pressure vessel burst and the effect of a smaller flammable cloud.
(Bouix et al., 2021[58]) conducted a set of tests in a real tunnel in France investigating a scenario of a jet fire following the activation of a TPRD. It was found that the temperature of the combustion products of the hydrogen flame, measured near the top of the vault, was much lower with TPRDs with smaller diameter. In a study by (Shentsov, Makarov and Molkov, 2021[59]), releases from TPRDs with diameters of 0.5 and 0.75 mm did not result in a flammable layer formation under the parking ceiling (3.12-3 m height), but releases from TPRDs with a diameter above 0.75 mm did, especially in the absence of mechanical ventilation. In the same study, it was also noted that releases from TPRDs toward obstacles tend to prohibit hydrogen mixing with air and promote the accumulation of a flammable cloud; it was therefore recommended not to park an FCEV with its TPRD directed towards obstructions.
The effect of TPRD orientation on flammable cloud formation inside a naturally ventilated parking area was also studied by (Hussain, Midhat and Balachandran, 2019[60]). It was found that a downward TPRD release at an angle of 30° and 45° directed the hydrogen away from the car, whereas a downward release at 0° briefly surrounded the car doors and passenger escape routes with a flammable cloud. (Shentsov, Makarov and Molkov, 2021[59]) also considered a release angle of 45° to be the overall safest solution. (Bouix D. et al., 2021b[61]) studied upward and downward gas releases following TPRD activation and noted that when the TPRD was directed downwards, the area around the chassis maintained high levels of gas volume. The conclusion was that it is safer not to place the TPRD completely perpendicular to the ground.
Where applicable, it is helpful to perform comparisons between hydrogen fires and hydrocarbon fires. (Seike, Ejiri and Kawabata, 2014[51]) found that the thermal fume from an FCV fire travelled faster than that of a gasoline vehicle fire. (Li, 2019[62]) in a study of fire and explosion hazards of alternative fuel vehicles in tunnels showed that hydrogen jet fires normally are characterised by longer flame lengths and higher heat fluxes compared to fires resulting from the ignition of compressed natural gas. In Li’s numerical study, the flame length increases along with the increasing diameter of the PRDs and can rise up to the height of 40 m. The heat flux can reach 45 kW/m2 for GH2 at 10 m from the fire (compared to 14 kW/m2 for Compressed Natural Gas).The possibility of fire spreading quickly inside a tunnel can therefore be high, as with other vehicle fuels.
Nevertheless, research has shown that a hydrogen fire poses fewer hazards than a hydrogen explosion: a numerical analysis of hydrogen release, dispersion and combustion in a tunnel by (Li et al., 2021[63]) suggested that the deliberate activation of TPRD can mitigate the consequence of a tunnel accident. If hydrogen is ignited right after being injected in the tunnel it forms a jet fire whose Heat Release Rate (HRR) decays with the injection rate. The region of the combustion cloud is limited to the jet fire near the injection and the ceiling. In the case of delayed ignition however, the pressure wave propagates through the detonatable hydrogen cloud. Then, the blast wave decays the unburnable region at a lower speed resulting in a lower overpressure to the surrounding cars. This pressure wave may have severe effects on the human body: for example, in this study it reaches 800 kPa, which can cause lung damage and severe damage to ear drums.
In a combined experimental and modelling study by (Houf et al., 2012[64]) all three of the fuel-cell vehicle’s onboard hydrogen tanks were simultaneously released through three TPRDs toward the road surface. Computational fluid dynamics simulations were used to model the release of hydrogen from the fuel-cell vehicle and to study the behaviour of the ignitable hydrogen cloud inside the tunnel. By increasing the ventilation rate the peak flammable volume, as well as the time required for dilution below the lower flammability limit, were reduced. Simulation results showed that overpressure peaked at an ignition delay of around 5 seconds. Ignition delays of about 4 to 8 seconds resulted in overpressures near or above the fatality threshold level.
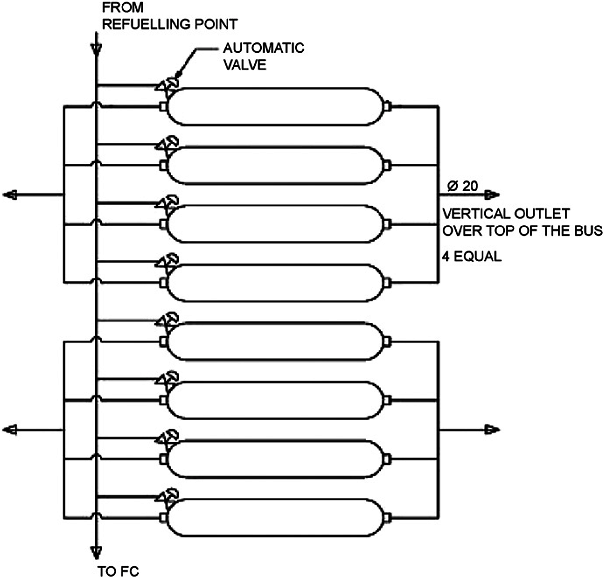
Most studies of hydrogen vehicles inside tunnels are focused either on hydrogen cars or on FCEVs in general without specifying the type of vehicle. It is expected that by the end of July 2022 there will be additional findings that will address hydrogen buses from within the HyTunnel project. It is worth noting however, that there is an older CFD simulation study (Venetsanos et al., 2008[65]) examining hydrogen releases from non-articulated single deck city buses in urban environments and tunnels. Working pressures of 20, 35 and 70 MPa for hydrogen19 and 20 MPa for natural gas were examined. The gas was stored in eight cylinders, each containing either 5 kg hydrogen or 21 kg natural gas (Figure 8.5). Three cases were considered: 1) only one PRD is open and all automatic valves are closed; 2) all automatic valves are open and therefore the gas from all cylinders is released; 3) (worst case scenario), all PRDs and automatic valves are open and the gas from all cylinders is released.
For the tunnel scenario only Case 1 and Case 3 were examined, as consequences of Case 2 were expected to lie somewhere in between. In both Case 1 and Case 3 the flammable cloud shape was similar for all hydrogen storage pressures but the shape of the natural gas cloud was significantly different. In Case 3, hydrogen reached the tunnel ceiling and dispersed along the ceiling towards both tunnel openings. The flammable volume was 1.34 times larger for Case 3 and 73 times larger than that of methane. For Case 3, the methane flammable cloud, also ascended towards the ceiling, but it extended much further transversely and less so longitudinally, surrounding the bus. The authors note that critical cases in tunnels may lead to a fast deflagration. For methane, for Case1, the predicted flammable mass is much lower compared to hydrogen, whereas for Case 3, the predicted methane flammable mass is much higher compared to hydrogen. Additionally, it was noted that with turbulence generating features, e.g. obstacles, there is the possibility of a detonation. The authors’ conclusion is that hydrogen storage systems should be designed to avoid simultaneous opening of all PRDs. They also recommended that, in order to mitigate the consequences from the hydrogen release, either the number of PRDs opening should be limited or their vents to the atmosphere should be restricted.
Figure 8.5. Assumed storage system arrangement for hydrogen bus
Another accident scenario revolved around the possibility of TPRD failure, something that would lead to a tank explosion. (Bouix et al., 2021[58]) performed tank explosion experiments to determine the size and progression of the blast wave and the propagation velocity of the reactive wave. To examine the reactive wave, a line of thermocouples was placed along the axis of the tunnel near the ceiling. The thermocouples’ response allowed the identification of two regimes: the first one was probably reactive with an average velocity of about 25 m/s and the second one corresponded to the convection of the burnt gas cloud by the flow in the tunnel and had an average velocity of 3.5 m/s.
Traditional models for blast wave decay inside tunnels are derived from studies involving high explosives. (Bouix et al., 2021[58]) used an older model derived from the study of TNT explosions (Silvestrini, Genova and Leon Trujillo, 2009[66]) to determine the extent of the contribution of chemical energy to the blast wave from the explosion of a hydrogen-filled tank: it was estimated at 12%. (Molkov et al., 2020[67]) finding them to be non-appropriate to describe blast wave decay after hydrogen tank rupture presented a universal correlation for blast wave decay after hydrogen tank rupture in a tunnel fire. The validated CFD model was then applied to perform numerical experiments. This model however, has not been used in other studies.
A numerical study by (Shentsov, D. and W., n.d.[68]) made a preliminary exploration of the possible consequences from a blast wave following a tank explosion inside a tunnel. The article attempted to quantify risk by determining ‘no-harm’, ‘injury’ and ‘fatality’ zones and scenarios within different types of 150 m long tunnels according to maximum overpressures predicted: these were to 1.34 kPa, 16.5 kPa and 100 kPa, respectively and described as temporal loss of hearing, 1% eardrum rupture probability and 1% fatality probability respectively. The conclusion was that people in the tunnel would encounter fatality in the field that is nearer to the explosion. Further from the ‘fatality’ zone threshold (40 m from the point of the explosion), all cases of tunnel area and mass combinations examined in the simulations fall into the ‘injury’ zone but in most cases examined in which tank mass is above 0.58 kg (regardless of the tunnel cross-section) are above the ‘injury’ threshold for the whole length of the tunnel. All cases were well below the “fatality” threshold of 100 kPa but the “no-harm” limit was not obtained at 140 m (10 m away from the tunnel exit) in any tunnel type examined and for all hydrogen mass inventories down to 0.58 kg. There is therefore no “no-harm” zone.
A solution to problems posed by the possibility of tank rupture could be found in the leak-no-burst tank, which is developed as part of the HyTunnel project (Kashkarov, Makarov and Molkov, 2021[69]). In case of a fire, heat is transferred through the composite overwrap of the tank, melting a polymer liner. This initiates controlled hydrogen microleaks, keeping pressures in check. With this technology a tank rupture will not occur.
Conclusions and knowledge gaps
The main conclusions based on the literature review related to Scenario 4 are:
Scenario 4 examined the scenario of a traffic accident involving a hydrogen city bus or car inside a tunnel.
A risk analysis conducted by (LaFleur et al., 2017[45]) showed that a hydrogen accident within a tunnel is most likely to be a minor crash, which has no additional consequence due to no hydrogen release (probability of 94.1%).
Of the scenarios in which hydrogen does ignite, by far the most likely consequence is a jet flame resulting from the release of hydrogen through the TPRD due to the heat from a typical accident-related hydrocarbon fire. The possibility of fire propagating inside a tunnel is high.
Suitable ventilation of a tunnel can significantly reduce the probability of an explosion. However, there may be the possibility that even in a well-ventilated tunnel, a high release rate of hydrogen could produce a near homogeneous mixture at close to stoichiometric conditions, with a corresponding increased explosion hazard. Similarly, a large fire may reach the tunnel ceiling and spread under it, which could result in serious damage to the tunnel equipment and structures along the ceiling. Ceiling design and mitigation measures are important.
The ventilation regime should be planned with great care since, under certain circumstances, ventilation can have adverse effects, as it has been shown to happen with low velocity counter-flow ventilation (Grune et al., 2021[55]) and with ‘push’ or ‘pull’ fans (Angers et al., 2013[54]).
In a study by (Mukai et al., 2005[50]) it was found that there is a possibility that there is a brief time in which hydrogen with a concentration at about low flammability limit flows into the power collector portions of electrostatic dust collectors, or at the exhaust fan of the model tunnels. The distance between the main tunnel and these elements has to be sufficient for the hydrogen to diffuse and mix with the surrounding air.
Obstructions inside the tunnel and particularly at the level of the tunnel pose a potential risk in respect to possible fast deflagration or transition to detonation.
In a scenario involving TPRD activation, flammable gas venting to the environment must be considered and the time delay prior to ignition becomes a parameter. Ignition delays can result in dangerously high overpressures. An immediate ignition poses fewer hazards compared to a delayed ignition. Therefore, the deliberate activation of TPRD can mitigate the consequences of a tunnel accident and also reduce the risk of tank rupture.
Storage systems involving more than one TPRDs should be designed to avoid simultaneous opening of all TPRDs. In addition, either the number of TPRDs openings should be limited or their vents to the atmosphere restricted.
TPRD size and orientation are important factors that can limit the formation of flammable clouds under the ceiling of a tunnel or a closed parking lot. Small TPRD sizes (< 1 mm) are generally recommended. Vertically downwards release direction should be avoided to reduce the flammable cloud under the car and around the car doors. Release direction backward at 45o angle is recommended.
The possibility of a TPRD failing with an explosion ensuing can cause severe consequences: there is currently ongoing interest on the matter with numerous studies published.
Gaps
Although lots of research has been performed investigating the safe use of hydrogen vehicles inside tunnels and other confined spaces, some gaps have been identified. A relatively recent review article by Sandia (Glover, Baird and LaFleur, 2020[70]) identified the following gaps in research:
Temperature and thermal effects to structures, or, in other words, how a hydrogen fire or explosion can damage the tunnel. In particular, the risk study by Sandia (LaFleur et al., 2017[45]) mentioned the potential degradation of structural epoxy at 90°C, or its melting at 140°C.
It also has to be noted that hydrogen explosions are more likely to produce an oscillatory pressure-time profile than hydrocarbon explosions, which may have implications for the structures subjected to a hydrogen explosion (Kumar et al., 2009[48]).
Experiments or numerical studies involving vehicles of different size or class can be performed, since, as the vehicular class increases so does the amount of stored fuel. Several different classes of vehicles were evaluated in the studies, including hydrogen cars and buses, liquid hydrogen cars, and multiple hydrogen cars on a cargo truck. The possible effects of deflagration or detonation on structural components of a tunnel can also be different for each of the different hydrogen vehicle classes.
A series of experiments were performed to show that the spontaneous ignition of released hydrogen is caused by transient shock formation and mixing associated with rupture of a burst disk between compressed hydrogen and air (Dryer et al., 2007[71]). However, the study was conducted in ambient conditions outdoors. Further research can evaluate the effect that ventilation inside a tunnel has on the results.
Closer collaboration between the hydrogen industry and research organisations is needed for knowledge transfer, e.g., maximum allowable TPRD diameter, TPRD orientation, tank heat resistance, etc.
However, dedicated research projects on hydrogen safety inside tunnels, like the HyTunnel-CS EU-funded project (https://hytunnel.net/), are still ongoing. Gaps like hydrogen dispersion and combustion inside tunnels resulting from leakage from bus and train along with thermal effects on tunnel structure are expected to be closed within this project.
Scenario 5 – Mobility and partially confined spaces: accidents at a hydrogen refuelling stations
This particular scenario looks into accidents and safety concerns emerging from hydrogen fuel stations, alongside with risk assessments and case studies which help identify safety aspects and input for hydrogen related standards and regulations.
Based on studies by (Sakamoto et al., 2016[72]) it has been possible to understand the nature of accidents and incidents at hydrogen fuelling stations in Japan and the USA within the time period 2004-2014. The collected data included the incidents and accidents involving several types of hydrogen fuelling stations.20 Most types of accidents and incidents were small leakages of hydrogen, but some had led to serious consequences, such as fire. Most of the leakages occurred at the joint parts due to inadequate torque and inadequate sealing. Other causes included design error of the main bodies of apparatuses and human error. One of the characteristics of HRS accidents in Japan was the high percentage of leak accidents occurring at pipe joint sections (whereas accidents due to design error, that is, poorly planned fatigue, were common in the United States).
Several experiments aimed at defining safety measures to strengthen levels of safety at hydrogen refuelling stations (Nilsen and Rikheim, 2003[73]), (Kikukawa, Mitsuhashi and Miyake, 2009[74]), (Hecht and Ehrhart, 2021[75]). Several authors (Nilsen et al., 2003; Kikukawa et al., 2008) mentioned the necessity to install a fire protection wall along station boundaries and that, whenever possible, hydrogen processing systems or storage at high pressures should be placed outdoors in well ventilated areas. (Hecht and Ehrhart, 2021[75]) further highlighted that, via his work on simulations of liquid hydrogen dispersion and flame behaviour to study distances of separation from bulk liquid hydrogen storage, the exposure distances are meant to prevent fire spread, so firewalls can be used to mitigate this hazard and reduce the necessary distance (exposures: the furthest distance to a heat flux of 20 kW/m2 (6 340 BTU/hr-ft2), the visible flame length, or an overpressure of 70 kPa ).
(Nilsen and Rikheim, 2003[73]) further remarks that if, for some reason, hydrogen systems have to be located indoors, it is very important that the risk of leaks and gas accumulation is assessed. In addition, (Kikukawa, Mitsuhashi and Miyake, 2009[74]) highlights how, in densely populated areas, where large safety distances may be impossible to achieve, stricter requirements to quality, inspection and protection of refuelling stations against impact should be implemented. Moreover, (Nilsen and Rikheim, 2003[73]) argues that fences around the units may lead to reduced safety distance requirements if they are designed so that flammable concentrations will not reach outside these barriers.
(Gye et al., 2019[76]) performed a quantitative risk assessment (QRA) of a high-pressure hydrogen refuelling station in an urban area with a large population and high congestion between the instruments and equipment considering the following main accident scenarios 1) catastrophic rupture of the tube-trailer with release pressure equal to 100 bar, 2) leakage from the dispenser with release pressure 70 Mpa21 and leak size 0.11, 1.11 and 11.11 mm). They concluded that leakage from dispensers and rupture from tube-trailers are the main contributions to the hydrogen refuelling station risks.
This conclusion is further confirmed by (Yoo et al., 2021[77]). Their study showed that the catastrophic rupture of a tube trailer and a liquefied hydrogen tank are the worst accidents because they induce fires and explosions. (Gye et al., 2019[76]) argue that to decrease the risk, mitigation, and safety barrier system with certain detectors, such as Emergency Detection System (EDS), also confirmed by (Yoo et al., 2021[77]), which will cause an immediate shutdown in an emergency situation deemed necessary. In agreement with the previous authors is (Khalil, 2017[78]) who underlines the need for high sensitivity detection devices that can detect leaked flammable as well as the use of pressure and temperature sensors in all confined spaces containing flammable gas systems.
Latest research in the field derives from (Yoo et al., 2021[77]), which aimed to perform a quantitative risk assessment (QRA) of GHRSs and LHRSs. A comparative study was performed to enhance the decision-making of engineers in setting safety goals and defining design options. The effect of vapour cloud fire classified by the level of heat radiation (4 kW/m2) resulted in first-degree burns to people remaining in the area indicated by the blue circle or within 210 and 182 m downwind from the centre of the accident for the GHRS and LHRS, respectively (Figure 8.6) Severe damage with high heat radiation (37.5 kW/m2) occurred near the station within 70 m (for GHRS) and 62 m (for LHRS) from the centre of the accident, which can damage equipment and reach 100% lethality within 1 min inside the area of the red circle Figure 8.6. In addition, sufficient energy to induce ignition on wood and plastic classified by a heat radiation level of 12.5 kW/m2 was present within 120 m and 110 m for the GHRS and LHRS, on the basis of the experiments’ conditions.
Considering both the worst-case scenarios, fire occurring in the GHRS had a greater effect on the surrounding people and buildings than the LHRS, whereas a greater explosion effect was observed for the LHRS owing to the formation of a LH2 pool on the ground.
The results of the risk assessment indicated that the LHRS had a lower risk than the GHRS. The following supplemental safety measures are proposed to risk the risk level at GHRS & LHRS: detachable coupling, hydrogen detachment sensor, and automatic as well as manual ESD buttons.
Figure 8.6. Worst-case scenario for the GHRS (top) and LHRS (bottom)
When it comes specifically to safety distances of hydrogen refuelling stations, (Kim et al., 2013[79]), examined a simulation of hydrogen leak and explosion given conditions of a set of pressures, 10, 20, 30, 40 MPa22 and a set of hydrogen ejecting hole sizes, 0.5, 0.7, 1.0 mm, using a commercial CFD tool, FLACS (Figure 8.7). The simulations are based on real 3D geometrical configuration of a hydrogen fuelling station that is being commercially operated in Korea (Figure 8.8).
Figure 8.7. Simulation of experiment scenarios
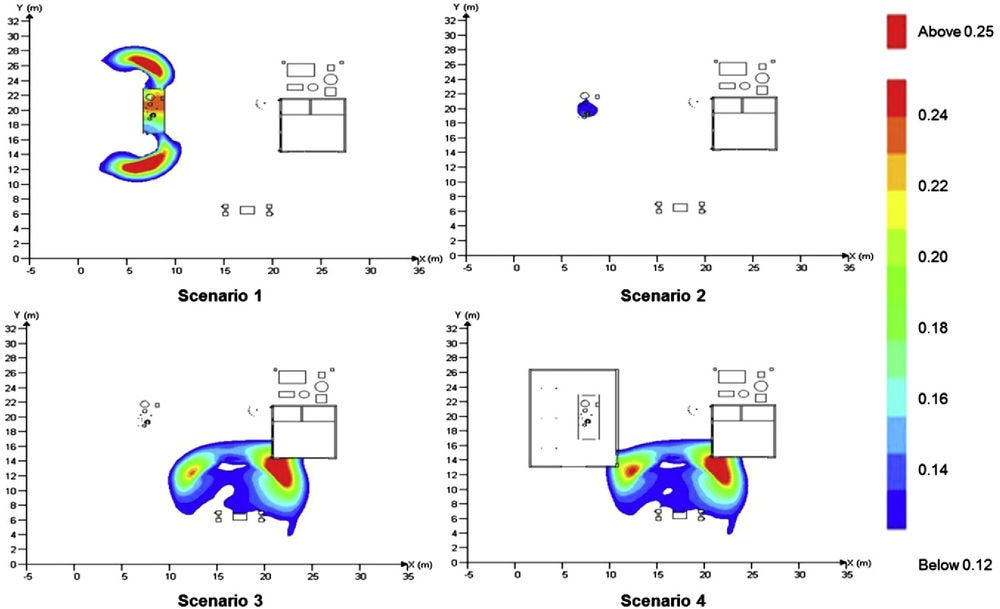
Source: Simulation of hydrogen leak and explosion for the safety design of a hydrogen fuelling station in Korea (Kim et al., 2013[80])
It was found that under scenario 1 (explosion taking place in storage tank) hydrogen storage tank should be configured 5 m further away from the current location of the hydrogen production facility. Next, in scenario 2, an explosion which takes place in a production facility, the explosion does not affect other facilities (placed at a distance of approximately 10 m). In scenario 3, an explosion takes place in the dispenser, it does not reach the hydrogen storage tank nor the hydrogen production facility. In Scenario 4, dispenser with protective wall, it was found that the maximum pressures at the protective wall and operation control room are 0.23 and 0.27 bar. The protective wall and operation room should remain at an additional distance of 2 m away from the dispenser (the difference between scenario 3 and 4 is whether a protective wall is installed or not).
Also, (Russo et al., 2018[81]) presented a study on a hydrogen station to be installed with the aim of determining safety distances. Results of calculations of safety distances for dispenser and compressor show that the most severe scenario, corresponding to a leak size equal to 100% of pipe diameter has higher frequency compared to other leak sizes due to higher probability of ignition, although the exact value of ignition probability remains uncertain. Moreover, the study also found out that safety distances are reduced when safety systems are effective and activated within a short notice, by employing for instance a dispenser which operates in parallel with an emergency shutdown function that interrupts the flow of hydrogen gas. Pressure indicator or switch shall monitor the compressor to initiate its shutdown whenever necessary.
Figure 8.8. Facility-distance layout of a hydrogen fuelling station as considered in the experiment
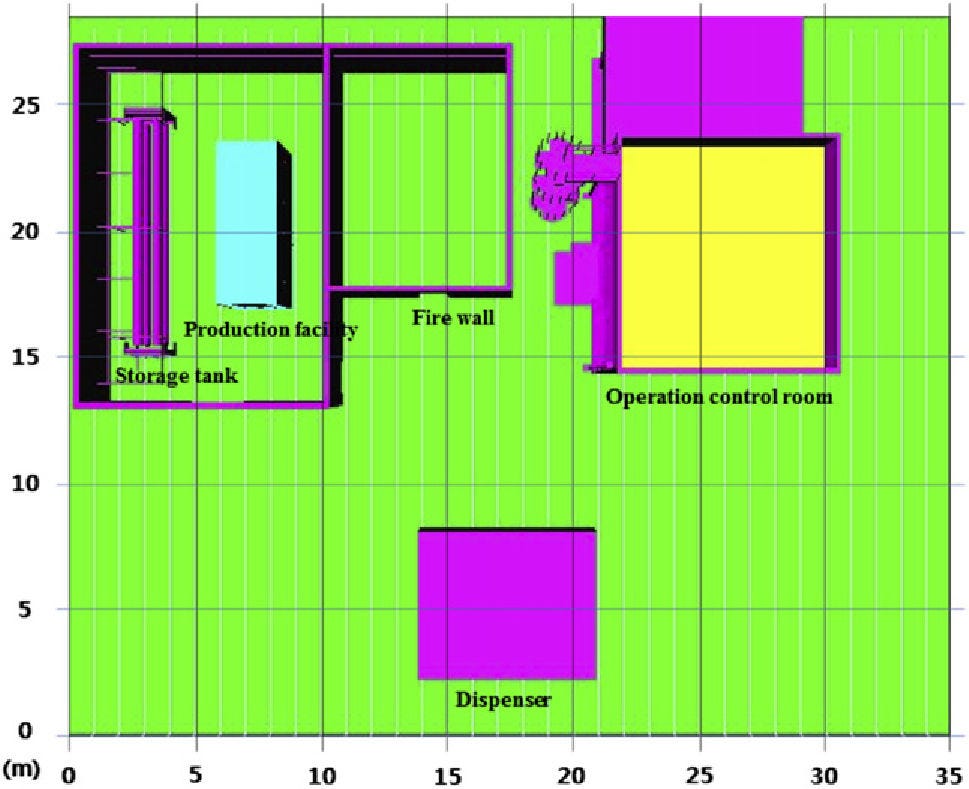
Source: Simulation of hydrogen leak and explosion for the safety design of a hydrogen fuelling station in Korea (Kim et al., 2013[80]).
(Takeno et al., 2007[82]) performed an experimental investigation on the hypothetical scenario of dispersion and explosion of high-pressurised hydrogen gas (40 MPa) which leaks through a large-scale break in piping and blows down to the atmosphere. In the worst-case scenario case (pipe diameter = 10mm, H2 pressure = 40MPa, time = 0.85 s), an overpressure greater than 50 kPa was detected at 10 m away from the ignition point. Through these experiments, it was clarified that the explosion power depends not only on the concentration and volume of hydrogen / air pre-mixture, but also on the turbulence characteristics before ignition.
(LaChance et al., 2009[83]) conducted an analysis to support development of risk-informed separation distances for hydrogen codes and standards, the minimum separation distances between a bulk gaseous hydrogen storage facility and other facilities that help reduce the potential for injury and facility damage. The hydrogen-specific data was utilised to generate system leakage estimates for a 20.7 MPa and 103.4 MPa facility. For a 0.1% leak size, the system leakage frequency is 3 ∙ 10-2/year and 6 ∙ 10-2/year for the 20.7 MPa and 103.4 MPa systems, respectively. What emerges from these values is that a 0.1% leak would be anticipated during the lifetime of these facilities. Larger and less frequent leak sizes of at least 1% should be used as the basis for separation distances to reduce the likelihood of accidents involving humans. If evaluated on a cumulative distribution basis, leaks equal to or less than 0.1% of the component flow area were estimated to represent 95% of the system leakage frequency. This allows us to conclude that separation distances based on the size of leak would guarantee that they cover the majority of possible leakage events.
(Khalil, 2017[78]) used a visual flowcharting methodology to develop a probabilistic model to quantify occupational risks of fire and explosion events initiated by leaks that ignite within enclosed spaces. The case study applied to HRS served as an example for demonstrating functionality of the proposed probabilistic model. The proposed probabilistic model is a solid simulation tool for training relevant stakeholders to better understand potential occupational risks associated with ignition of leaked flammable gases within confined spaces in a wide variety of industrial settings. The research showed that, for these analyses scenarios, small leakage23 from the compressors is associated with an intolerable occupational risk frequencies, which exceed both the acceptance criterion at 1.0 ∙ 10-4 /year and NFPA’s guideline at 2.0 ∙ 10-5/year.
(Honselaar, Pasaoglu and Martens, 2018[84]) conducted an inter-comparison among the QRAs of permitted HRSs in the Netherlands revealing major inconsistencies on different areas of the QRA including for instance the application of failure scenarios. Their conclusion was that it is recommended to develop specific QRA guidelines for HRSs. It should be clear for permitting authorities what the HRS consists of and how it operates. A checklist of HRS sub-systems and components and an extensive description of sub-systems, components, preventive and mitigation measures, configurations (including piping and instrumentation diagrams) and input parameters is recommended. Establishing a national, independent review function for QRAs of HRSs is also advisable. Such an entity would have the potential to become a centre of expertise that could collect existing and future QRAs of HRSs to monitor the latest developments and progress towards the consistent application of the approach as well as provide guidance to permitting authorities on how to apply the approach for HRSs.
(Kodoth et al., 2019[85]) described the importance and need of verification of life parameters in QRA to reduce uncertainty linked with the risk calculation. Failure frequency estimation is one of the important measures of risk quantification. In traditional reliability assessment, mean time to failure (MTTF) is one of the most used life parameters in QRA. It is observed that two stations can have similar survival time but small to large differences in the usage (i.e., number of fillings). If the failure rate is estimated as a function of time, the mean failure rate will be approximately the same for both stations. On the other hand, if the failure rate is estimated by the number of fillings, the failure rate will vary depending on the actual usage of the station. The actual usage conditions are discarded when using the survival time and this may lead to uncertainty in the failure estimation. This leads to another conclusion that the failure rate estimated as a function of number of fillings is more reliable and realistic than the estimation based on survival time. Moreover, the number of fillings is more representative of the true failure rate. This means that the survival time does not always represent the actual usage of the HRSs.
(Kodoth et al., 2020[86]) further conducted leak frequency analysis for hydrogen-based technology using Bayesian and frequentist methods. The leak rate is estimated to be 0.16/year, 0.20/year and 0.42/year based on the time-based,24 leak-hole-size, and non-parametric methods, respectively. This paper proposed leak rate estimation using time-based evaluation methods that utilise historical HRS accident information. In addition, leak frequency estimates from another two methods (non-parametric and leak-hole-size) were examined. In the non-parametric approach, the leak frequency was estimated based on a Bayesian update. It can be observed that there is no major margin between the results resulting from the time-based and leak-hole-size methods. The asset manager can pick the most appropriate leak rate data based on the data on accidents and method availability. One of the possible solutions is to consider a conservative value for the design, in which case, the non-parametric model leak rate of 0.24/year can be used. The base value selected can be used in design to set performance standards for the availability and reliability in the operation maintenance of HRSs. If the leak rate is estimated to be high, inspections activities shall be more frequent to limit the unrevealed leak time (evaluated from the estimated leak frequency) and increase the process of safety. Moreover, the unrevealed leak time can be used to the specification of hydrogen sensors to detect leaks of hydrogen. This will ensure the component and process serve requirements in the performance standard, leading to increased process safety in HRSs.
The National Institute for Public Health and the Environment in the Netherlands performed a QRA in 2016 to assess the risk and identify the impact distances in hydrogen refuelling stations (National Institute for Public Health and the Environment, 2016[87]). The calculations were conducted with the tool, SAFETI-NL 6.7. The direct ignition probability of gaseous hydrogen was assumed equal to 1 and of liquid hydrogen equal to 0.9. It is also assumed that hydrogen will ignite in the first 20 seconds after the release of the gas. The main assumption for the modelling were:
0.001 probability of failure of the emergency shut-off device. A higher value of 0.01 was also examined.
Same failure frequencies as for LPG discharge hose were used.
1 000 kg of hydrogen per day were assumed.
Compressor works for 10 hr/d.
Both 35 and 70 MPa can be refuelled at the delivery column and there were also two buffer storages of 44 and 95 MPa.
The estimated distances to 10-6 risk contours were 30 m for the LH2 delivering via tank and for the gaseous hydrogen dispensing system supplied by pipeline or local production, while they were 35 m for gaseous hydrogen delivering via tube or cylinder trailer. For gaseous hydrogen with delivery via pipeline or tube trailer, the risk after about 50 metres was 10-9, whereas for LH2 supplied by a tanker a risk of 10-9 was reached at 270 m. The proposed QRA distances can be further reduced with the use of proper safety measures. Moreover, an ignition probability equal to 1 that is used is overly conservative. QRA with lower ignition probability and taking also into account certain preventive and mitigation measures are recommended to be carried out to re-evaluate the risk.
(Hecht and Ehrhart, 2021[75]) calculated minimum distance (given by a safety factor of 2) from outdoor LH2 refuelling stations to exposures, like compressor, buildings, human. The estimated distances were lower than 30 m for all group of exposures based on NFPA-2 (including overpressure criteria too), i.e. group 1 – the furthest distance to an average mole fraction of 8%, a heat flux of 4 732 kW/m2 or an overpressure of 5 kPa, group 2–- the furthest distance to a heat flux of 4 732 kW/m2 or an overpressure of 16 kPa, and group 3 – furthest distance to a heat flux of 20 kW/m2 , visible flame length or an overpressure of 70 kPa. These distances should not be confused and compared directly with the values presented in (National Institute for Public Health and the Environment, 2016[87]), because different assumptions were made in the two studies, e.g. in (Hecht and Ehrhart, 2021[75]) a leak diameter of 1% of the flow area and maximum operation pressure of 1.2 MPa were assumed. Moreover, (Hecht and Ehrhart, 2021[75]) haven't calculated the distances based on risk contours, but based on the furthest distance to selective hazardous criteria of the abovementioned exposure groups.
Conclusions and knowledge gaps
The main conclusions based on the literature review related to scenario 5 are:
Scenario 5 examined the scenario of accidents at hydrogen fuel stations. The main findings are:
Most of the leakages in accidents involving hydrogen fuelling stations in Japan and USA up to 2016 occurred at the joint parts due to inadequate torque and inadequate sealing, which therefore needs to be carefully designed and supervised.
The catastrophic rupture of a tube trailer and a liquefied hydrogen tank are the worst accidents of hydrogen refuelling stations, because they induce fires and explosions.
In terms of safety measures it is recommended to maintain risk within accepted levels for liquid hydrogen fuelling stations, the research pointed out that hydrogen storage tanks should be configured at least 5 m further away (based on the conditions outlined in the experiment) from the current location of the hydrogen production facility and a protective wall surrounding the dispenser shall be implemented as a physical barrier protecting from the expansion of a potential explosion. Walls/fences around the units may lead to reduced safety distance requirements if they are designed so that flammable concentrations will not reach outside these fences. However, careful design is required, because obstructions and confinements may lead to more severe explosions in case of ignition. If the leakage frequency is estimated to be high, the inspection interval should be more frequent to reduce the unrevealed leak time.25
Fire occurring in the GHRS had a greater effect on the surrounding people and buildings than the LHRS, whereas a greater explosion effect was observed for the LHRS owing to the formation of a pool of LH2 on the ground: the results of the risk assessment indicated that the LHRS had a lower risk than the GHRS.
It is to notice how the proper design of a gas and flame detection system would increase the chance to detect the leaks at an early stage. This is especially true if in a compressed hydrogen gas fuelling station the ultrasonic technology is used properly to detect leakage at an early stage even with small hole diameter. The complete gas and flame detection system could activate immediately the safety solution in order to avoid the formation of hazardous conditions.
In terms of life parameters in QRA to reduce uncertainty associated with the risk calculation, (Kodoth et al., 2019[85]) argues that the failure rate estimated as a function of the number of fillings is more reliable and realistic than the estimation based on survival time. The number of fillings is more illustrative of the true failure rate as it takes into account the station’s usage and loading.
To reduce the potential for significant consequences to a person at the site boundary due to expected accidents, (LaChance et al., 2009[83]) demonstrates that larger and less frequent leak sizes of at least 1% should be used as the basis for distances between a bulk gaseous hydrogen storage facility and other facilities that help reduce the potential for injury and facility damage.
(Khalil, 2017[78])’s research points out how the probabilistic visual flowcharting based model for consequence tool can simulate what-if accident scenarios and quantify sensitivities of the predicted frequencies of occupational risks to different values of inputs to this model. The HRS case study showed that those accidents involving H2 small leaks (SL) in the compressor's room could lead to undesirable occupational risk frequencies that exceed the 1.0 ∙ 10-4//year acceptance criterion and in excess of the 2.0 ∙ 10-5/year risk value proposed by NFPA as a guideline driven by the comparative risk to gasoline refueling stations. The predicted frequencies of risks associated with the base case SL scenario can be summarised as follows:
Fire-related injuries: 6.62 ∙ 10-4/year (Best Estimate) and 4.26 ∙ 10-3/year (Upper Bound)
Explosion-related injuries: 1.12 ∙ 10-3/year (Best Estimate) and 3.85 ∙ 10-3/year (Upper Bound)
The proposed model could find application as a training tool for first responders to fire and explosion events which are subsequent to leaks of flammable gases.
Gaps
Based on the mapping exercise the following gaps are identified:
It would be helpful to further analyse qualitatively the most recent accidents and incidents that took place at hydrogen refuelling stations involving small leakages of hydrogen to provide an update vision on leakage-type-based analysis at hydrogen fuelling stations using natural gas and other resources and offsite-type hydrogen fuelling stations.
The need for establishment of a national, independent review function for QRAs of HRSs in the Netherlands along with developing specific QRA guidelines for HRSs is revealed. Further analysis into more advanced countries on this end such as Japan could be useful to facilitate the successful implementation of such recommendations.
Closer collaboration between the hydrogen industry, standardisation institutes eg: NEN/PGS and research organisations is needed for knowledge transfer, e.g. separation distances via optimisation of piping diameters.
Scenario 6 – Domestic use: Safety of hydrogen in buildings with focus on hydrogen based residential heating
This scenario relates to safety issues that arise from the local production and/or storage of hydrogen, followed by its distribution for domestic use. The following aspects of this scenario were considered, a) risks that arise due to hydrogen gas leaks during the distribution of hydrogen by low pressure distribution networks into the houses for heating and cooking and b) the accumulation of hydrogen in a house in the case of a leak and possible prevention and mitigation measures.
Hydrogen leaks from a low-pressure distribution network
An investigation into past gas leak incidents from natural gas distribution networks in the UK determined that most leaks occur in the connecting pipe, followed by the gas meter connection and the indoor piping, i.e. in and near the house. Most reported leaks occur from network components made of materials such as grey and ductile iron, asbestos cement and steel. In the case of the UK, these materials, besides steel, are already being removed from the gas distribution network as part of the ongoing Iron Mains Risk Reduction Replacement Programme, so they should not be an issue in the future (V. D. Noort et al., 2020[88]).These findings are supported by (Mouli-Castillo et al., 2021[89]), who investigated gas leak incidents in the UK and who report that the majority of public reports of gas escapes are related to faulty metal joints in piping. They suggest that replacing the currently used UK gas network piping, which is composed of approximately 74% polyethylene and 26% metal parts, with a 100% polyethylene network would amount to a 2.5 factor reduction in reported flammable gas escapes and a 3.5 factor reduction in “gas in building” events for both natural gas and hydrogen. This benefit is only applicable to releases upstream of the gas metre, which compose 85% of the currently reported natural gas releases, as the replacement of the metallic components of domestic pipework was not considered within the scope of this risk assessment (Mouli-Castillo et al., 2021[89]). Tests are ongoing to understand the implication of long-term use of hydrogen in polyethylene (PE) pipelines (ERM and HSL, 2019[90]), although past research on the long-term exposure of polyethylene to a hydrogen atmosphere suggests that the tensile behaviour and the microstructure of the polymer are not significantly affected even in the long term (Castagnet et al., 2012[91]).
Due to the nature of hydrogen, the outflow volume of hydrogen from a pipeline leak will be greater than in the case of natural gas for the same mass flow. When the gas leak is small (around 1 L/h or less), the gas outflow may be laminar and, as a result, about 30% more hydrogen than natural gas will flow out based on volume. However, for larger leaks, the gas flow becomes turbulent and 190% more hydrogen than natural gas is released based on volume (V. D. Noort et al., 2020[88]).
Risk modelling conducted by DNV GL on behalf of Netbeheer Nederland, was used to study and compare the release and dispersion of natural gas and hydrogen from a low-pressure distribution pipeline in the open air and underground (V. D. Noort et al., 2020[88]). Experiments are being conducted as part of the H21 research programme, taking place in the UK, to support and validate the results of this risk analysis model.
The risk modelling concluded that due to its lower density, hydrogen will rise faster when blown off or leaked into the open air than natural gas. This does not lead to higher risks, as the amount of energy released is approximately the same and initial calculations of the safety contours around a leak in a distribution pipe show that they are lower than for natural gas. The contour of the gas cloud is similar to that of natural gas (V. D. Noort et al., 2020[88]).
In the case of hydrogen ignition in an open space and at low concentrations (<10 % v/v hydrogen in air), a fire will break out, but no overpressures will occur at concentrations below 10% hydrogen (V. D. Noort et al., 2020[88]). Any leak of pure hydrogen will of course result in concentration over 10% in a certain volume immediately neighbouring the leak, but for small leaks these volumes will be small. Further modelling work was conducted by DNV GL on the heat radiation – and the lethality – of a flare fire that could occur in the event of a rupture in an underground low-pressure distribution pipeline that is then exposed to the open air. In all four standard operation scenarios examined in this work, the heat radiation of hydrogen, and therefore the lethality and risk resulting from this heat radiation, is lower than that of natural gas under identical conditions (Table 8.3) (Coster, Triezenberg and Beks, 2018[92]).
Table 8.3. The results of calculations of the heat radiation and the site-specific risk (SSR)
Results cover both hydrogen and natural gas leaks from an exposed pipeline of four different configurations for a pipeline failure rate of 3.74⋅10-5 km-1 year-1
|
|
Hydrogen |
Natural gas |
|||
|---|---|---|---|---|---|
|
Gas pressure (bar) |
Pipeline diameter (mm) |
Peak heat (kW/m2) |
Distance SSR (m) |
Peak heat (kW/m2) |
Distance SSR (m) |
|
0.1 |
63 |
83 |
10-6: n/a 10-7: n/a 10-8: 3.3 |
60 |
10-6: n/a 10-7: n/a 10-8: 4.5 |
|
0.1 |
110 |
101 |
10-6: n/a 10-7: 0.1 |
105 |
10-6: n/a 10-7: 2.4 |
|
8 |
110 |
38 |
10-6: n/a 10-7: n/a 10-8: 6 |
57 |
10-6: n/a 10-7: 10 10-8: 17 |
|
8 |
114 |
37 |
10-6: n/a 10-7: 3.6 |
56 |
10-6: n/a 10-7: 10.6 |
For larger hydrogen gas leaks, the overpressure increases on ignition from hydrogen concentrations above 10% v/v and with a stoichiometric mixture (around 30% v/v) overpressures can occur that exceed 10 kPa.26 Due to the high reactivity of hydrogen, it is expected that a stoichiometric mixture of hydrogen is more likely to cause a detonation than natural gas. Further study is needed to confirm this and to further establish the possible impact compared to natural gas.
Overall, based on the results of this study, the risks of natural gas and hydrogen are expected to be comparable in the case of free flow in the open air (V. D. Noort et al., 2020[88]).
For underground releases, the outflow of hydrogen through the soil can be accurately described with models, provided that the soil composition and its permeability are known. Besides soil composition, permeability is also influenced by the weather conditions (rain, freezing weather). At equal pressures, hydrogen is more likely to cause crater formation than natural gas. This can occur mainly at higher pressures in the gas distribution system (>200 kPa) and not at low pressures (<20 kPa). Crater formation could be favourable, as it ensures that the hydrogen is released into the atmosphere faster and does not diffuse underground into confined spaces. Overall, in the case of underground leaks, the chance of an unsafe situation is expected to be lower if a permeable top layer is present, but if the top layer is impermeable (e.g. due to the nature of the soil or due to freezing weather), the likelihood of hydrogen migrating into buildings increases (V. D. Noort et al., 2020[88]).
A more in-depth investigation of gas leaks from pipes under open and covered surfaces was conducted as part of the H100 project (ERM and HSL, 2019[90]). A series of eight generic flow regimes were analysed, focusing on the distance to which hydrogen gas can travel to a minimum hazardous flux level and how this distance changes if methane gas is used instead of hydrogen. The switch from methane to hydrogen makes minimal difference to the range at which significant gas dispersion will occur in the case of leaks from uncovered pipes. In cases where the leak is covered, however, the release range of hydrogen may be significantly larger. Horizontal distances travelled below ground from the point of release were found to be typically 6%-25% further for hydrogen compared to natural gas across the range of conditions tested. The most serious potential consequences (large hydrogen flow rates) are associated with (very rare) hydrogen gas releases into large open channels that lead directly into vulnerable buildings. Such an unlikely scenario might occur due the presence of a service duct that is not properly sealed where it enters a property. When such an easy route is present, a 25% increase in hydrogen travel distance compared to methane may occur.
An experiment conducted by the HyDelta consortium studied the extent of the entry of air to a hydrogen distribution pipeline in the event of a pipe fracture (Lueb, 2021[93]). During this experiment, pipes with diameters of DN100 (114.3 mm outer diameter) and DN200 (219.1 mm outer diameter) were filled with hydrogen and then their ends were opened to the air to simulate a pipe rupture. The hydrogen/air ratios in the pipes were measured before and after the opening of the pipe ends. As expected, after the leakage occurred, the hydrogen contained in the pipes flowed out immediately. Explosive hydrogen/air mixtures were then observed in both the DN100 and the DN200 pipes as the air entered the pipes. The explosive concentrations persisted for the entire duration of the experiment (90 minutes). The air inflow was faster in the case of the DN 200 pipe than in the DN 100 pipe. As a result, the explosive mixture formation was slightly faster in the case of the DN 200 pipe. The effect of wind was negligible during the experiment. Further experiments should be conducted in the future to investigate the effect of the entry of air in a natural gas pipeline and compare the risk to the risk from hydrogen.
In the Netherlands, the HyDelta consortium is currently filling in the knowledge gaps that inhibit the use of hydrogen in the existing Dutch natural gas infrastructure. As part of the project, a variety of factors that might affect the use of hydrogen in the infrastructure were studied. One such factor was the leak tightness of the pipeline connections during hydrogen transport and whether the same requirements that are currently applied to natural gas can also be applied to hydrogen (Lueb and Kooiman, 2022[94]). The leakage rates of three gases, natural gas, hydrogen and nitrogen, were measured as they were flowing through the service lines at pressures of 3, 10 and 20 kPa. The measurements were subsequently combined with a theoretical assessment of the risk arising from small hydrogen leaks, such as those that might occur under the currently used leak tightness guidelines. The study concluded that it is not necessary to have stricter tightness requirements for hydrogen than for natural gas when it comes to new service lines. However, in the case of the currently used connecting pipes, the leakage rate of hydrogen was 1.83 times higher than the leakage rate of natural gas. As such, the suggestion was made that the tightness requirements should be stricter, ensuring that the maximum permissible leakage rate for hydrogen is 74% of that of natural gas.
In another study conducted by the HyDelta consortium (Lueb and Kooiman, 2022[95]), the compatibility of currently used pressure regulators with hydrogen was tested. 40 pressure regulators, that had been previously removed from the natural gas distribution network, were tested with hydrogen and 10 of these regulators were further tested with natural gas. Based on the results of these tests, it was concluded that the existing domestic pressure regulators can be safely used with hydrogen, and it is therefore unnecessary to replace the regulators as part of the conversion to hydrogen. It was however observed that the under-pressure shut-off valve in several of the tested regulators closed prematurely when hydrogen was used, increasing the likelihood of more failures occurring. Additionally, an increase in the valve shut off pressure was observed when hydrogen was used. As all hydrogen appliances will be equipped with a flame protection device, that should be sufficient to mitigate the safety risk.
A study was carried out to determine the risks that might arise during the purging of the Dutch natural gas pipelines with hydrogen and to determine the appropriate purging speed to be used to safely displace the natural gas (Lueb, 2021[96]). Pipes of diameters DN100 and DN200 that were 200 metre long, were initially filled with 100% natural gas. Hydrogen was then introduced into the pipes at different flow rates. It was determined that the purging of the natural gas in the pipes with hydrogen, including flaring, could be carried out safely. A purging speed of 0.2 m/s was sufficient to safely purge the natural gas with hydrogen in both types of pipes. However, as in practice purging the network pipes might be more difficult than purging the test pipes, a minimum purging speed of 0.4 m/s is suggested, to ensure that the pipes are completely purged of natural gas. For a shorter purging process, a purging speed of 1.0 m/s is suggested as optimal.
Hydrogen dispersion and accumulation in a house following a leak
Experimental testing of the leak rates of methane and hydrogen from various gas joints and fittings currently used in domestic gas installations in the UK was conducted as part of the Hy4heat project (Ryan and Roberts, 2020[97]). The tests showed that hydrogen was compatible with all of the fittings and pipes tested. Components that displayed no leaks when methane flowed through would also not display any leaks when hydrogen flowed through and components that displayed a leak with methane would also cause a hydrogen leak. It can therefore be considered safe to use the same materials and fittings for internal pipework for hydrogen as is currently used for methane, at least in the short term, in the context of a community trial. The hydrogen that leaked from damaged components was larger in volume than methane under the same conditions (1.2:1 volumetric leak ratio between hydrogen and methane for small leaks along threads and 2.8:1 from large leaks from drilled holes), however, as hydrogen has less than one-third of the energy of methane on a volumetric basis, the amount of energy outflow is, in every case, less for hydrogen than for methane. Furthermore, the measured concentration of hydrogen within flammability limits resulting from a large leak in a domestic room, was only 1.3 -1.8 times higher that of natural gas, evidence that hydrogen dissipates more quickly than methane under the same leak conditions.
In another set of experiments conducted as part of the Hy4heat project, the dispersion and accumulation of hydrogen and methane when they are released within confined spaces in residential buildings was examined (Simpson, Allason and Johnson, 2020[98]). The confined spaces that were considered were kitchen cupboards and an inset metre box. Gas releases from holes ranging from 0.6 mm to 7.2 mm diameter with a pressure of 10 kPa were examined. Based on these tests it was determined that releases of both methane and hydrogen generally formed layers of nominally uniform concentration above the point of their release. In all releases of hydrogen and methane into the metre box, flammable concentrations were only observed in the wall and floor cavities. No flammable concentrations of either gas were observed in the rooms of the house. For hydrogen, the highest release rate tested (18.6 m3/h through a 7.2 mm hole) produced highly reactive hydrogen concentrations above 30% v/v within a high-level layer in the kitchen. Hydrogen concentrations of 30% v/v have a burning velocity about a factor of 5 higher than the worst case for methane. This can have a significant effect on the severity of any subsequent explosion, even where some venting is available through weak parts of the structure such as windows.
Further tests conducted using different combinations of vent openings in the cupboard and kitchen wall, showed that the addition of a ceiling vent, ducted to the external wall, was very beneficial in reducing the maximum concentration of hydrogen seen within the kitchen and the presence of cupboard vents helped reduce the concentration of hydrogen in the cupboards. The resulting recommendation from these tests was, therefore, that for community trials, venting in any cavity should be made mandatory, as specified by Building Regulations ADJ (i.e. an exemption should not be granted for hydrogen appliances).
The dispersion of hydrogen in a gas metre box was also studied by DNV GL on behalf of Alliander N.V. (Bierling, Vlap and Bahlmann, 2020[99]). In a series of measurements, 100% natural gas and 100% hydrogen were released into a metre box at flow rates ranging from 1 to 25 lt/hr to simulate a flammable gas leak from the piping connected to the gas metre. The tests were performed initially with the ventilation grilles of the box open and then they were repeated with taped grilles, so as to simulate metre boxes that do not have ventilation openings. Most tests with open ventilation grilles were carried out once, so the measurements that were obtained only give an indication of the concentrations of natural gas and hydrogen in the metre cupboard. Duplicate or triplicate measurements must be made to ensure that the measured gas concentrations are statistically substantiated.
The tests of natural gas and hydrogen leakage, with open ventilation grilles, showed that, with increasing leakage rates, the gas concentration in the cabinet increased proportionally. The gas concentration eventually levelled off at approximately 2 % v/v, for both natural gas and hydrogen. The measured gas concentration of hydrogen was greater at the same leakage rates than the measured gas concentration of natural gas, but only by a factor of approximately 1.02. The measured gas concentrations were comparable except in the case of the 3 L/h leakage rate where the measured concentration of hydrogen was 3.7 times higher than that of methane. Because hydrogen is much lighter than natural gas, it was expected in advance that hydrogen would rise faster, so that it would disperse more quickly through the top ventilation grid. However, the tests showed that this was not the case. The hydrogen concentration in the box was comparable to the natural gas concentration, at the same leakage rates. The researchers could not give an explanation to this and suggested that further tests will need to be conducted. Furthermore, for some tests for both natural gas and hydrogen, a sinusoidal trend was seen in the measured gas concentrations. This could be a side-effect of chimney effect taking place in the box or could be caused by vortex formation in the box. Additional tests would have to be performed to determine the actual cause. In the tests with taped grates, and therefore no ventilation, higher gas concentrations were detected than in the tests with open grilles, but the measured gas concentrations remained below 4% v/v for both types of gas.
At the domestic property level, gas dispersion and accumulation tests have also been conducted, to determine the risks that arise by hydrogen leaks in various locations within a house. Such tests have been conducted both as part of the Hy4heat project (Simpson, Allason and Johnson, 2020[100]) and as part of the HyHouse study (Crowther et al., 2015[101]). During the Hy4heat experiments, hydrogen and methane were released within a two-story domestic property. The release sites were the basement of the house and the kitchen boiler cupboard. Based on the results of these tests, it was determined that having the kitchen door open, in experiments where methane or hydrogen were released in the boiler cupboard, resulted in higher explosive gas concentrations in the rooms outside the kitchen whilst not having much effect on the concentrations measured at the high point in the kitchen. For hydrogen gas releases in the basement, a minimum gas flow rate of 25.5 m3/h through a 10 mm diameter release orifice was required to generate significant flammable concentrations. It should be noted that while the inclusion of furniture and other obstacles is unlikely to have any effect on the accumulation of gas within the property, it is known to have a significant effect on the potential explosion severity in natural gas explosions. Given the increased reactivity of hydrogen mixtures, it is important to take this effect into account when assessing the experimental results and the potential risk.
A mitigation measure that was tested was the addition of a ceiling vent27 which had the effect of reducing the maximum concentration of hydrogen seen within the kitchen. Other mitigation measures, such as air bricks added to the basement, showed less conclusive results, with some smaller vent tests recording an increase in the maximum hydrogen concentration. The tests undertaken with the larger vent size in the basement did demonstrate a reduction in maximum hydrogen concentration, however, as the results were inconclusive, this could be an area that requires further investigation. The potential effect of atmospheric wind conditions on the results of the venting experiments was not considered and future experiments should study the impact of wind speed and direction on the air flow through the house to better quantify the effects of the mitigation measures tested (Simpson, Allason and Johnson, 2020[100]).
In the HyHouse project (Crowther et al., 2015[101]), test gases were injected into a two-storey farmhouse at different flow rates and the concentration and distribution of those gases throughout the house was measured. The following test gases were used: 100% Natural Gas, 100% Hydrogen, 3% v/v Hydrogen (97% Natural gas), 10% v/v Hydrogen (90% Natural gas), Town gas (50% Hydrogen, 25% CO2 and 25% Natural gas). A range of leaks were simulated from locations in the living room, the kitchen and the cupboard under the stairs. This was complemented by several high-rate gas releases to simulate a leaking hydrogen vehicle or gas main, using 100% hydrogen and 100% natural gas.
Significant flammable gas stratification was observed in the downstairs rooms of the property, with increasing definition at higher injection rates and thus higher gas concentrations. Flammable gas stratification was evident at different levels of house air tightness. At hydrogen injection rates under 39.5 lt/min, as would be expected from a minor gas leak, hydrogen gas concentrations within the property did not exceed the lower flammability limit (LFL) for hydrogen. For 79 lt/min 100% hydrogen injections, hydrogen concentrations did reach the LFL in the room of injection, but concentrations throughout the rest of the house did not reach the LFL until the very end of the injection period. This suggests that flammable concentrations are unlikely to be achieved during short term, low-rate releases, even in properties with low air permeability rates (e.g. ~3 m3 h-1 m-2).
Overall, the flammable gas concentrations resulting from the release of hydrogen in the HyHouse project were found to be, on average, about 1.6 times greater than the concentrations resulting from methane releases. Based on these concentrations from HyHouse, the ignition likelihood of hydrogen (for the same energy release rate) was estimated to be greater than that of methane by a factor of 4. When only the large gas releases were considered, this factor was reduced to 2. This difference in ignition probability is primarily due to the ignition energy being approximately one order of magnitude lower for hydrogen than for methane (Mouli-Castillo et al., 2021[89]).
The concentrations observed in HyHouse would not lead to flammable gas concentrations that would result in severe structural damage if ignited. The large releases of hydrogen and methane were shown to result in flammable gas concentrations that are likely to induce a similar overpressure if ignited. In such a case, the associated potential to cause severe structural damage was comparable for hydrogen and methane.
Experiments conducted as part of the Naturalhy project (Lowesmith et al., 2009[24]), simulated the release of methane / hydrogen mixtures in a test rig designed to represent a typical domestic room of dimensions 3 m (length) by 3 m (width) by 2.3 m (height). Four different gases were used in the experiments: 100% methane and mixtures of hydrogen and methane which contained 10%, 20% and 50% v/v hydrogen. In all cases, the gas concentrations (of either methane or hydrogen) measured at different locations within the enclosure but at the same height, were the same. The gas accumulation was uniform in the horizontal plane but only varied with height above the floor. The recorded gas concentrations increased with time until a steady state concentration was reached which prevailed until the gas release was terminated.
Gas concentrations were very low (less than 1% v/v) for all sensors located at low heights within the enclosure (1.1 m or lower). All sensors located 1.6 m or higher gave similar results, indicating uniform gas accumulation between 1.6 m and the ceiling. Between these two regions, the gas concentration varied significantly with height. The formation of a uniform gas layer was observed at an early stage after gas release, and the gas concentration in the layer increased until a steady state concentration was reached. After the gas release was terminated, high gas concentrations persisted for some time close to the ceiling but at lower heights, the concentrations were significantly lower and gas accumulation was quickly dispersed.
A mathematical model was developed to compare the absolute gas concentration levels achieved during the experiments while taking into account the effect of different wind conditions and the different heights of ventilation openings. The predictions of the model showed good agreement with the experimental data, demonstrating that the model performs well for upward directed, relatively low momentum, releases of buoyant gas. Consequently, the model was used to investigate the influence of changes in the hydrogen content in the released methane/hydrogen mixture on the gas accumulation. Raising the percentage of hydrogen resulted in an increase in the volume flow rate of the gas released into the enclosure, leading to a rise in the gas concentration and an increase in the volume of the region in which the gas accumulates. However, the rise in hydrogen content also led to enhanced gas buoyancy which in turn led to an increase in the ventilation air flow. Consequently, the rise in concentration was not as great as might otherwise have been expected.
While hydrogen dispersion tests have been successfully carried out in two-storey houses, the applicability of the test results to other types of accommodation, such as flats or bungalows, might require further investigation (Mouli-Castillo et al., 2021[89]).
The hydrogen dispersion tests have been further supplemented by gas ignition potential tests and consequence assessments. Experiments have been conducted to assess and compare the potential for household electrical items to ignite hydrogen or methane mixtures with air (Crewe, Johnson and Allason, 2020[102]). The items used in these experiments included white goods in new and used condition, plugs and switches, light fittings and extractor fans. In the majority of the tests, no ignition occurred with either hydrogen or methane or ignition occurred with both hydrogen and methane. Very few domestic appliances caused hydrogen to ignite, but not methane. These included hair dryers, toasters, vacuum cleaners, tumble dryers and irons. Nearly all of these appliances can only be used with a human operator present, who would most likely be able to smell a gas release. For this reason, the odourisation of hydrogen to lower the detection threshold is the priority of various currently ongoing projects (Mouli-Castillo et al., 2021[103]).
The consequence assessment of potential hydrogen gas ignitions within structures constructed from varying types of material such as glass, wood, concrete and metal, concluded that for concentrations of around 15-20% v/v hydrogen, the consequences of an ignition would be roughly comparable to those of a 10% v/v methane ignition (Hardy et al., 2021[104]). Towards the higher end of this concentration band, the hydrogen ignition starts to become more severe than methane. Beyond 20% v/v (up to around 40% v/v) the consequence of a hydrogen ignition gets progressively more severe. The presence of obstructions within the combustion zone could cause turbulence of flammable gas mixtures leading to increased peak overpressure for both hydrogen and methane. Peak overpressures for hydrogen can be higher due to the faster flame speed. There was no evidence of hydrogen exhibiting a general transition from deflagration to detonation in a pseudo domestic environment. A general detonation was only achieved using chemical detonators.
As part of the Naturalhy project, a series of large-scale explosion experiments involving methane/hydrogen mixtures was conducted in a 69.3 m3 enclosure to assess the effect of different hydrogen concentrations on the resulting explosion overpressures (Lowesmith et al., 2011[105]). The tests studied methane, 80:20 methane: hydrogen and 50:50 methane: hydrogen mixtures. The results showed explosion severity (overpressure) increased with increasing hydrogen fraction. This increase was small when adding up to 20% v/v hydrogen to the methane, however the increase became significant when 50% v/v hydrogen was added. For the vented confined explosions studied, it was also observed that the addition of obstacles within the enclosure, to simulate the congestion caused by furniture, equipment and pipework, resulted in increased flame speeds and overpressures above the levels measured in an empty enclosure. Predictions of the explosion overpressure and flame speed were made using a modified version of the Shell Global Solutions model, SCOPE. Comparisons of the model predictions with the experimental data showed generally good agreement.
Hydrogen odourisation
One of the main mitigation measures suggested for the early detection of hydrogen gas leaks is the odourisation of hydrogen. Research has been conducted to identify odorants that are effective when added to hydrogen and are also compatible with hydrogen appliances (Mouli-Castillo et al., 2021[103]), (Murugan et al., 2019[106]). Tests conducted in the UK, determined that Odorant NB,28 which is a blend of 78% t-butyl mercaptan and 22% dimethyl sulphide and which is currently used for natural gas, is also effective for hydrogen. Other odorants, such as THT,29 have also been tested and were found to be effective and compatible with network components and hydrogen appliances (Murugan et al., 2019[106]), (Top and Teunissen, 2020[107]) However, both NB and THT were shown to be incompatible with fuel cells due to the sulphur that they contain, which causes significant degradation to the fuel cell components (Murugan et al., 2019[106]).
Conclusions and knowledge gaps
The main findings based on the literature review related to scenario 6 are:
Due to increased interest in the residential use of hydrogen for heating, large research projects and demonstrations are currently underway, mainly in the UK and the Netherlands, which aim to investigate the possibility of substituting natural gas with hydrogen for heating. Experiments conducted as part of these projects are currently filling in the knowledge gaps when it comes to the safe delivery and use of hydrogen in residential buildings. Below are summarised the main findings of these projects, along with the recommendations they make to minimise the risk from the use of hydrogen in houses.
Hydrogen distribution network
Based on findings from the H100 project, it has been concluded that switching to hydrogen without any changes to the current UK gas network and infrastructure would lead to a doubling in the risk of fatalities resulting from severe structural damage (relative to the risk from the use of methane in the current gas network). As most observed flammable gas leaks are caused by metallic network components, by switching to a 100% polyethylene network and by implementing additional mitigation measures downstream of the gas meter, a 100% hydrogen network could be as safe as the currently used natural gas network (Mouli-Castillo et al., 2021[89]) Figure 8.9.
Hydrogen usage in buildings
Based on the results of hydrogen dispersion experiments, small hydrogen leaks (97% of currently reported natural gas leaks in the UK are from holes no larger than two millimetres) should not create sufficiently large flammable clouds to produce injuries. Medium sized leaks (from holes between three and seven millimetres in size) could produce flammable gas clouds in small rooms, notably those with the door closed and / or rooms with poor ventilation. However, as noted by the experts, these leaks are most often caused by third party damage and rarely occur spontaneously, so generally the appropriate steps are readily taken to stop the development of the leak, i.e. opening windows, closing the emergency control valve (ECV) and alerting the gas company. Large leaks (from holes greater than seven millimetres) can produce high gas concentrations in large areas of a house. A significant percentage of these leaks arise from third party damage, including malicious intent. The likelihood of such leaks could be reduced by implementing the appropriate mitigation measures, such as the introduction of excess flow valves to the household piping and the installation of flame failure devices to all hydrogen appliances (Brown et al., 2021[108]).
Recommendations from pilot studies
The introduction of two excess flow valves (EFVs). This would help reduce the likelihood of large hydrogen leaks developing into a hazardous scenario by a factor of 4 (i.e. the flow of gas will be stopped before a flammable atmosphere can develop). This reduced likelihood will then be similar to the likelihood of hazardous scenarios that can ensue in the current UK natural gas network (Mouli-Castillo et al., 2021[89]).
The first EFV should be either in the service pipe or immediately after the emergency control valve. The second EFV should be either integrated in the hydrogen gas metre (Brown et al., 2021[108]) or added upstream of the metre (Mouli-Castillo et al., 2021[89]). The gas metre should be installed outside of the property, where possible, and comply with current best practice and BS6400-1:2016 (Brown et al., 2021[108]). Since in the Netherlands the gas metres are inside the house, some initial research has been conducted on the effectiveness of ventilation for the gas metre cabinets (Bierling, Vlap and Bahlmann, 2020[99]), but further information is needed to better understand potential risks.
The installation of Flame Failure Devices (FFDs) in all hydrogen appliances to reduce the likelihood that appliances will be, unwittingly, left on whilst unlit. A significant cause of current fires and explosions (about 40% of all of those occurring downstream of the emergency control valve) is the absence of FFDs, particularly on hobs (Brown et al., 2021[108]), (Mouli-Castillo et al., 2021[89]).
There should be non-closable vents with an equivalent area of 10,000 mm², located as close to the ceiling level as possible and no more than 0.5 m below the ceiling level in all rooms with gas appliances or hydrogen-carrying pipes installed (Brown et al., 2021[108]).
All the cupboards and other appliance compartments (e.g. boilers) where hydrogen appliances are present should have vents (Brown et al., 2021[108]).
An odorant of the same effectiveness should be added to hydrogen as is currently used for natural gas (Brown et al., 2021[108]).
Hydrogen detection alarms should be installed where residents are unable to smell the gas odorant (Brown et al., 2021[108]).
Mechanical crimp fittings should be used in pipework instead of soldered joints, which are weaker and more prone to leakages (Mouli-Castillo et al., 2021[89]).
Concerning the potential use of hydrogen for cooking, a stronger flexible pipe could be installed at the rear of the cooker to limit the likelihood of damage when the cooker is displaced. Additionally, the cooker should be fixed to the wall using a chain and Rawl bolts to limit the loading on the flexible cooker connection (Mouli-Castillo et al., 2021[89]).
As a lot of the required infrastructure for the use of 100% hydrogen for domestic heating is not yet installed and would require years of preparation, it has been suggested that, in the short term, a 20% blend of hydrogen with natural gas could be used for heating and would still be compatible with the existing infrastructure and heating appliances (Castek and Harkin, 2021[109]).
Figure 8.9. Risk factor calculations based on the likelihoods of events determined in our assessment
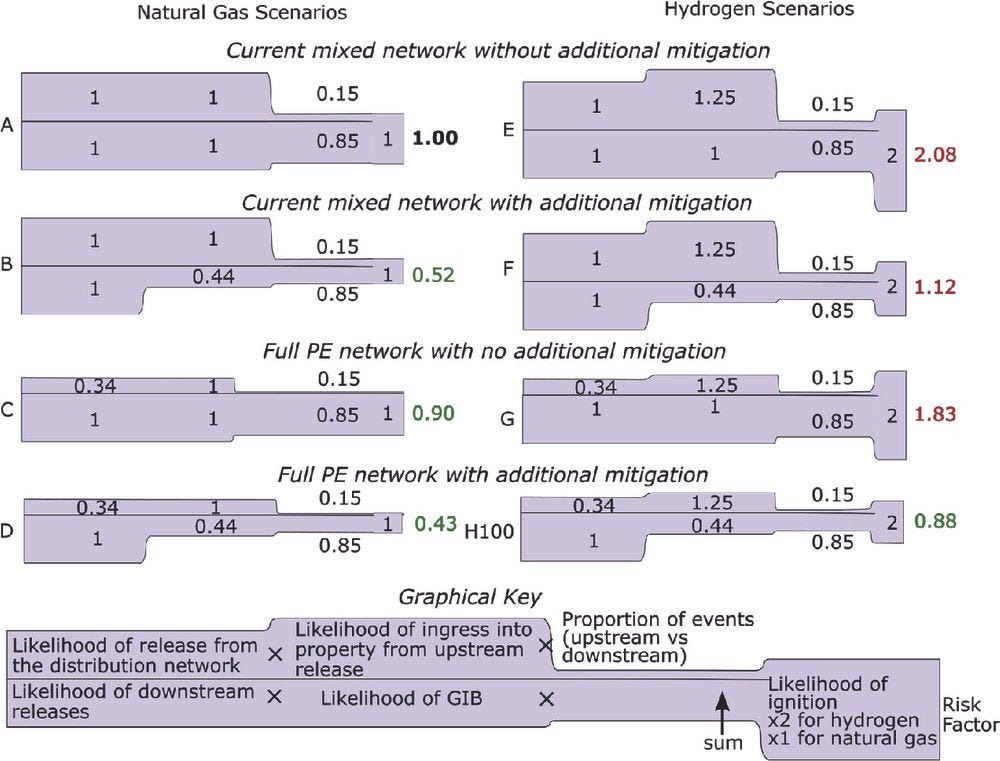
Note: The scenarios A, B, C and D correspond to the risks that arise by the use of natural gas, and scenarios E, F, G and H100 are scenarios corresponding to the use of hydrogen in the gas network. Scenario A is the current use of natural gas in the UK network. Scenario H100 is representative of the H100 100% hydrogen network.
Source: (Mouli-Castillo et al., 2021[89]).
Gaps
As most of the studies on the residential use of hydrogen that were covered in this report are still on-going, it is likely that some of the current gaps in our knowledge concerning hydrogen safety will be addressed in the near future.
The projects covered in this report focused on the use of hydrogen in properties that are masonry-built and that contain a standard range of ignition sources. This is suitable for the UK needs, as it corresponds to the majority of domestic settings in the UK. However, the applicability of the results to other types of accommodation might require further investigation. Such kinds of accommodation are blocks of flats, high-rise buildings, houses in multiple occupation, mechanically ventilated buildings and buildings that contain an atypical number of ignition sources (Brown et al., 2021[108]), (Mouli-Castillo et al., 2021[89]).
There is not currently sufficient information on the safety concerns associated with the use of 100% hydrogen in the internal pipework of houses. Additionally, as there is currently a limited number of heating appliances available that run on 100% hydrogen (and these appliances are still largely part of various demonstration projects and have only been in operation for the past 2-3 years), it is unknown what the impact from the use of hydrogen will be on the maintenance requirements of the heating systems. As the current demonstration projects continue and more information on the use of hydrogen is compiled, evidence on this impact should be obtained over the next 5 years (Castek and Harkin, 2021[109]).
References
[54] Angers, B. et al. (2013), CFD Simulations of the effect of ventilation on hydrogen release behavior and combustion in an underground mining environment,.
[99] Bierling, B., H. Vlap and R. Bahlmann (2020), Proefopstelling verspreiding waterstof in de meterkast., DNV GL.
[7] BloombergNEF (2022), Hydrogen-10 predictions for 2022, https://about.bnef.com/blog/hydrogen-10-predictions-for-2022/.
[110] Bossel, U. (2006), “Does a hydrogen economy make sense?”, Proceedings of the IEEE, Vol. 94/10, pp. 1826-37.
[61] Bouix D., S. et al. (2021b), Full-scale tunnel experiments for fuel cell hydrogen vehicles: gas dispersion., Proceedings of 9th International Conference on on Hydrogen Safety (ICHS 2021), 21-24 September 2021, ID43,.
[58] Bouix, D. et al. (2021), Full-scale tunnel experiments for fuel cell hydrogen vehicles: jet fire and explosions, 9th International Conference on Hydrogen Safety (ICHS 2021), 21-24 September 2021, ID42 1197-1210.
[108] Brown, S. et al. (2021), Hy4Heat Safety Assessment Conclusions Report incorporating Quantitative Risk Assessment., ARUP.
[20] Campbell, J. (2005), Questions and issues on hydrogen pipelines. In Proceedings of the DOE Hydrogen Pipeline Working Group workshop., https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/hpwgw_questissues_campbell.pdf (accessed on 24 February 2022).
[91] Castagnet, S. et al. (2012), “Effect of long-term hydrogen exposure on the mechanical properties of polymers used for pipes and tested in pressurized hydrogen”, International Journal of Pressure Vessels and Piping, Vol. 89, pp. 203-209, https://doi.org/10.1016/j.ijpvp.2011.11.008.
[109] Castek, R. and S. Harkin (2021), Evidence review for hydrogen for heat in buildings.
[30] Chen, H. and Z. Mao (2017), The study on the results of hydrogen pipeline leakage accident of different factors, IOP Conference Series: Earth and Environmental Science.
[36] Choi, J. et al. (2013), “A CFD simulation of hydrogen dispersion for the hydrogen leakage from a fuel cell vehicle in an underground parking garage”, International Journal of Hydrogen Energy, Vol. 38, pp. 8084-8091.
[92] Coster, R., D. Triezenberg and R. Beks (2018), Risk Analysis for Hydrogen Distribution Systems, DNV GL.
[102] Crewe, R., M. Johnson and D. Allason (2020), Ignition Potential Testing with Hydrogen and Methane, DNV GL.
[101] Crowther, M. et al. (2015), Energy storage component research & feasibility study scheme: HyHouse safety issues surrounding hydrogen as an energy storage vector., Kiwa Gastec.
[26] Dagdougui, H. et al. (2010), “Hazard and risk evaluation in hydrogen pipelines”, Management of Environmental Quality: An International Journal, Vol. 21/5, pp. 712 – 725.
[33] DNVGL (2020), Behavior of hydrogen leaks in the gas distribution network, report OGNL.184991.
[71] Dryer, F. et al. (2007), “Spontaneous ignition of pressurised releases of hydrogen and natural gas into air”, Combustion Science and Technology, Vol. 179/4, pp. 663-694.
[46] Ehrhart, B. et al. (2019), “Ehrhart, B., Brooks, D., & Muna, A., Lafleur, C. (2019). Risk assessment of hydrogen fuel cell electric vehicles in tunnels.”, Fire Technology, Vol. 56.
[35] Ehrhart, B. et al. (2020), “isk assessment and ventilation modeling for hydrogen releases in vehicle repair garages”, International Journal of Hydrogen Energy, Vol. 46/23.
[34] Ekoto, I. et al. (2011), “Performance-based testing for hydrogen leakage into passenger vehicle compartments”, International Journal of Hydrogen Energy, Vol. 36, pp. 10169-10178.
[90] ERM and HSL (2019), H100 Hydrogen Characterisation Final Report, SGN.
[19] FCH2JU (2021), Statistics, lessons and recommendations from the analysis of the hydrogen incidents and accidents database (HIAD 2.0), Fuel Cells and Hydrogen joint Undertaking.
[14] FCH2JU (2020), Minutes of the FCH2JU workshop of electroysis on 18 November 2020, Fuel Cells and Hydrogen 2 joint Undertaking.
[8] Gallandat, N., K. Romanowicz and A. Zuettel (2017), “An Analytical Model for the Electrolyser Performance Derived from Materials Parameters”, Journal of Power and Energy Engineering, Vol. 5/10, pp. 34-49.
[56] Giannissi, S. et al. (2021), “On the CFD modelling of hydrogen dispersion at low-Reynolds number release in closed facility”, International Journal of Hydrogen Energy, Vol. 46/57, pp. 4060-4071.
[16] Glover, A., A. Baird and D. Brooks (2020), Final Report on Hydrogen Plant Hazards and Risk Analysis Supporting Hydrogen Plant Siting near Nuclear Power Plants (No. SAND2020-7946)., Sandia National Lab.(SNL-NM),.
[70] Glover, A., A. Baird and C. LaFleur (2020), Hydrogen fuel cell vehicles in tunnels.
[49] Groethe, M. et al. (2007), “Large-scale hydrogen deflagrations and detonations,”, International Journal of Hydrogen Energy, Vol. 32/13, pp. 2125–2133.
[55] Grune, J. et al. (2021), Hydrogen jet structure in presence of forced co-, counter- and cross-flow ventilation, Proceedings of 9th International Conference on Hydrogen Safety (ICHS 2021), 21-24 September 2021, ID41.
[76] Gye, H. et al. (2019), “Quantitative risk assessment of an urban hydrogen refuelling station”, International Journal of Hydrogen Energy, Vol. 44/2, pp. 1288-1298, https://doi.org/10.1016/j.ijhydene.2018.11.035.
[40] Hajji, Y. et al. (2021), “Green hydrogen leaking accidentally from a motor vehicle in confined space: A study on the effectiveness of a ventilation system.”, International Journal of Energy Research, Vol. 45/13, p. 18935.
[39] Hao, D. et al. (2020), “Experimental Study on Hydrogen Leakage and Emission of Fuel Cell Vehicles in Confined Spaces”, Automotive Innovation, Vol. 3, pp. 111-122.
[104] Hardy, N. et al. (2021), Hy4Heat Gas Ignition and Explosion Data Analysis, Kiwa Gastec.
[75] Hecht, E. and B. Ehrhart (2021), Analysis to support revised distances between bulk liquid hydrogen systems and exposures, ICHS 202, September 21-24.
[84] Honselaar, M., G. Pasaoglu and A. Martens (2018), “Hydrogen refuelling stations in the Netherlands: An intercomparison of quantitative risk assessments used for permitting”, International Journal of Hydrogen Energy, Vol. 43/27, pp. 12278-12294, https://doi.org/10.10.
[64] Houf, G. et al. (2012), “Releases from hydrogen fuel-cell vehicles in tunnels”, International Journal of Hydrogen Energy, Vol. 37, pp. 715-719.
[29] Houssin-Agbomso, D., B. G. and M. D. (2018), “Consequences of a 12-mm diameter high pressure gas release on a buried pipeline. Experimental setup and results”, Journal of Loss Prevention in the Process Industries, Vol. 54, pp. 183-189.
[60] Hussain, T., T. Midhat and R. Balachandran (2019), “Investigating the effect of local addition of hydrogen to acoustically excited ethylene and methane flames”, International Journal of Hydrogen Energy, pp. 11168-11184.
[57] Hussein, H., S. Brennan and V. Molkov (2020), “Dispersion of hydrogen release in a naturally ventilated covered car park”, International Journal of Hydrogen Energy, Vol. 45/43, pp. 23882–23897.
[44] Hydrogen Safety Panel (2019), “Safety of Mobile Hydrogen and Fuel Cell Technology Applications: An Investigation by the Hydrogen Safety Panel”, https://h2tools.org/sites/default/files/Safety_of_Mobile_Hydrogen_and_Fuel_Cell_Technology_Applications-Oct_2019.pdf (accessed on 5 June 2023).
[18] Ichard, M. et al. (2012), “CFD computations of liquid hydrogen releases”, International Journal of Hydrogen Energy, Vol. 37, pp. 17380-17389.
[4] IEA (2021), Global hydrogen review, International Energy Agency.
[6] IRENA (2018), Hydrogen from renewable power: Technology outlook for the energy transition, International Renewable Energy Agency.
[21] Jang, C. and S. Jung (2016), “Numerical computation of a large‐scale jet fire of high‐pressure hydrogen in process plant”, Energy Science & Engineering, Vol. 4/6, pp. 406-417.
[3] Kalamaras, C. and A. Efstathiou (2013), “Hydrogen production technologies: current state and future developments”, Conference papers in science, Hindawi, Vol. 2013.
[10] Kasai, N. et al. (2016), “The qualitative risk assessment of an electrolytic hydrogen generation system”, International Journal of Hydrogen Energy, Vol. 41/30, pp. 13308-13314.
[69] Kashkarov, S., D. Makarov and V. Molkov (2021), “Performance of hydrogen storage tanks of type IV in a fire: effect of the state of charge”, Hydrogen, Vol. 2, pp. 386–398, https://doi.org/10.3390/hydrogen2040021.
[78] Khalil, Y. (2017), “A probabilistic visual-flowcharting-based model for consequence assessment of fire and explosion events involving leaks of flammable gases”, Journal of Loss Prevention in the Process Industries, Vol. 50(A), pp. 190-204.
[74] Kikukawa, S., H. Mitsuhashi and A. Miyake (2009), “Risk assessment for liquid hydrogen fueling stations”, International Journal of Hydrogen Energy, Vol. 34/2, pp. 1135-1141, https://doi.org/10.1016/j.ijhydene.2008.10.093.
[80] Kim, E. et al. (2013), “Simulation of hydrogen leak and explosion for the safety design of hydrogen fueling station in Korea”, International Journal of Hydrogen Energy, Vol. 38/3, pp. 1737-1743, https://doi.org/10.1016/j.ijhydene.2012.08.079.
[79] Kim, E. et al. (2013), “Simulation of hydrogen leak and explosion for the safety design of hydrogen fueling stations in Korea”, International Journal of Hydrogen Energy, Vol. 38/3, pp. 1737–1743.
[86] Kodoth, M. et al. (2020), “Leak frequency analysis for hydrogen-based technology using bayesian and frequentist methods”, Process Safety and Environmental Protection, Vol. 136, pp. 148-156.
[85] Kodoth, M. et al. (2019), “Verification of appropriate life parameters in risk and reliability quantifications of process hazards”, Process Safety and Environmental Protection, Vol. 127, pp. 314–320.
[52] Koutsourakis, N., I. Tolias and S. Giannissi (2021), Numerical study of the effects of tunnel inclination and ventilation on the dispersion of hydrogen released from a car, Proceedings of 9th International Conference on Hydrogen Safety, September 2021, ID105.
[5] Krishnan, S. et al. (2020), “Power to gas (H2): alkaline electrolysis. In Technological learning in the transition to a low-carbon energy system”, Academic Press, pp. 165-187.
[48] Kumar, S. et al. (2009), HyTunnel Final Report, HySafe Deliverable 111..
[111] Kunihiro, T. (2017), Centre for Supply Control and Disaster Management, Tokyo Gas Co., Ltd.
[38] Lach, A. and A. Gaathaug (2021), “Effect of Mechanical Ventilation on Accidental Hydrogen Releases—Large-Scale Experiments”, Energies, Vol. 14, p. 3008.
[83] LaChance, J. et al. (2009), Analyses to support development of risk-informed separation distances for hydrogen codes and standards, SANDIA REPORT SAND2009-0874.
[45] LaFleur, C. et al. (2017), Hydrogen fuel cell electric vehicle tunnel safety study.
[28] Laheij, G. and C. Theune (2010), Consequences of a risk based approach for natural gas pipelines, Pipeline Technology Conference.
[42] Liu, W. and D. Christopher (2015), “Dispersion of hydrogen leaking from a hydrogen fuel cell vehicle”, International Journal of Hydrogen Energy, Vol. 40, pp. 16673-16682.
[62] Li, Y. (2019), “Study of fire and explosion hazards of alternative fuel vehicles in tunnels”, Fire Safety Journal, Vol. 110, p. 102871.
[63] Li, Y. et al. (2021), “Numerical analysis of hydrogen release, dispersion and combustion in a tunnel with fuel cell vehicles using all-speed CFD code GASFLOW-MPI”, International Journal of Hydrogen Energy, Vol. 46, pp. 12474-12486.
[43] Li, Z. and Y. Luo (2019), “Comparisons of Hazard distances and accident durations between hydrogen vehicles and CNG Vehicles”, International Journal of Hydrogen Energy, Vol. 44/17, pp. 8954-8959.
[24] Lowesmith, B. et al. (2009), “Gas build-up in a domestic property following releases of methane/hydrogen mixtures.”, International Journal of Hydrogen Energy, Vol. 34/14, pp. 5932-5939.
[105] Lowesmith, B. et al. (2011), “Vented confined explosions involving methane/hydrogen mixtures”, Int. J. Hydrogen Energy, Vol. 36, pp. 2337–2343.
[93] Lueb, S. (2021), HyDelta: WP 1C Pipes and indoor installations (components): D1C.1a – Entry of air into a hydrogen pipeline in case of a pipe rupture, HyDelta.
[96] Lueb, S. (2021), HyDelta: WP 1C Pipes and indoor installations (components); D1C.1 Research question 187 – Purging of natural gas pipelines with hydrogen., HyDelta.
[95] Lueb, S. and A. Kooiman (2022), HyDelta: WP 1C – Pipes and indoor installations (components); D1C.4 – question 185 – domestic pressure regulators., HyDelta.
[94] Lueb, S. and A. Kooiman (2022), HyDelta: WP 1C Pipes and indoor installations (components): D1C.2 question 124 – Tightness of distribution pipes., HyDelta.
[22] Matthijsen, A. and E. Kooi (2006), “Safety distances for hydrogen filling stations”, Journal of Loss Prevention in the Process Industries, Vol. 19/6, pp. 719-723.
[37] Merilo, E. et al. (2011), “Experimental study of hydrogen release accidents in a vehicle garage”, International Journal of Hydrogen Energy, Vol. 36/3, pp. 2436-2444.
[47] Middha, P. and O. Hansen (2009), “CFD simulation study to investigate the risk from hydrogen vehicles in tunnels”, International Journal of Hydrogen Energy, Vol. 34/14, pp. 5875–5886.
[67] Molkov, V. et al. (2020), Dynamics of blast wave and fireball after hydrogen tank rupture in a fire in the open atmosphere.
[89] Mouli-Castillo, J. et al. (2021), “A quantitative risk assessment of a domestic property connected to a hydrogen distribution network”, International Journal of Hydrogen Energy, Vol. 46/29, pp. 16217–16231, https://doi.org/10.1016/j.ijhydene.2021.02.114.
[103] Mouli-Castillo, J. et al. (2021), “A comparative study of odorants for gas escape detection of natural gas and hydrogen”, International Journal of Hydrogen Energy, Vol. 46/27, pp. 14881-14893, https://doi.org/10.1016/j.ijhydene.2021.01.211.
[50] Mukai, S. et al. (2005), CFD Simulation on Diffusion of Leaked Hydrogen Caused by Vehicle Accident in Tunnels, 1st International Conference on Hydrogen Safety, 8-10 September 2015.
[106] Murugan, A. et al. (2019), Hydrogen Odorant and Leak Detection Part 1, Hydrogen Odorant, SGN.
[87] National Institute for Public Health and the Environment, T. (2016), Risk and impact distances of hydrogen refuelling stations.
[32] National Institute of Public Health and the Environment (RIVM) (2009), Reference Manual Bevi Risk Assessments version 3.2, https://www.rivm.nl/sites/default/files/2018-11/Reference-Manual-Bevi-Risk-Assessments-version-3-2.pdf (accessed on 5 June 2023).
[73] Nilsen, S. and H. Rikheim (2003), Risk assessments of Hydrogen Refuelling station concepts based on onsite production..
[23] Rigas, F. and A. P. (2013), Myths and facts about hydrogen hazards, 13th international symposium on loss prevention and safety promotion in the process industries.
[81] Russo, P. et al. (2018), “Quantitative Risk Assessment on a Hydrogen Refuelling Station”, Chemical Engineering Transactions, Vol. 67, pp. 739-744, https://doi.org/10.3303/CET1867124.
[27] Russo, P., A. De Marco and F. Parisi (2020), “Assessment of the Damage from Hydrogen Pipeline Explosions on People and Buildings”, Energies, p. 13.
[97] Ryan, N. and S. Roberts (2020), Hy4Heat Work Package 7 – Lot 1: Safety Assessments for the Suitability of Hydrogen in Existing Buildings., Steer Energy.
[72] Sakamoto, J. et al. (2016), “Leakage-type-based analysis of accidents involving hydrogen fueling stations in Japan and USA.”, International Journal of Hydrogen Energy, Vol. 41/46.
[12] Schefer, R. et al. (2009), “Experimental evaluation of barrier walls for risk reduction of unintended hydrogen release”, International Journal of Hydrogen Energy, Vol. 34, pp. 1590-1606.
[51] Seike, M., Y. Ejiri and N. Kawabata (2014), “Fire experiments of carrier loaded FCV in fullscale model tunnel”.
[68] Shentsov, V., M. D. and D. W. (n.d.), Stand-alone hemisphere-tank rupture in tunnel fire: effect of hydrogen inventory on blast wave strength in far field, Proceedings of the Ninth International Seminar on Fire and Explosion Hazards.
[59] Shentsov, V., D. Makarov and V. Molkov (2021), Effect of TPRD diameter and direction of release on hydrogen dispersion in underground parking, Proceedings of 9th International Conference on Hydrogen Safety (ICHS 2021), 21-24 September 2021, ID88.
[66] Silvestrini, M., B. Genova and F. Leon Trujillo (2009), “Energy concentration factor. A simple concept for the prediction of blast propagation in partially confined geometries”, Journal of Loss Prevention in the Process Industries, Vol. 22, pp. 449–454.
[98] Simpson, G., D. Allason and M. Johnson (2020), Cupboard Level Leakage and Accumulation Data Report., DNV GL.
[100] Simpson, G., D. Allason and M. Johnson (2020), Property Level Leakage and Accumulation Data Report, DNV GL.
[15] Skjold, T. et al. (2017), “3D risk management for hydrogen installations.”, International Journal of Hydrogen Energy, Vol. 42/11, pp. 7721-7730.
[13] Spada, M., P. Burgherr and P. Rouelle (2018), “Comparative risk assessment with focus on hydrogen and selected fuel cells: Application to Europe”, International Journal of Hydrogen Energy, Vol. 43/19, pp. 9470-9481.
[31] Spoelstra, M. and G. Laheij (2011), Towards a method to calculate risks of underground pipelines transporting hazardous substances, https://www.icheme.org/media/9217/xxii-paper-39.pdf.
[41] Sun, K. and L. Zhiyong (2018), “Risk Assessment on Life Safety and Financial Loss for Road Accident of Fuel Cell Vehicle”, International Journal of Hydrogen Energy, Vol. 44, p. 17.
[82] Takeno, K. et al. (2007), “Dispersion and explosion field tests for 40 MPa pressurised hydrogen”, International Journal of Hydrogen Energy, Vol. 32, pp. 2144-2153.
[2] Tchouvelev, A. et al. (2006), Quantitative Risk Comparison of Hydrogen and CNG Refuelling Option, Final Technical Report to Natural Resources Canada for the Codes and Standards Workshop of the CTFCA.
[11] Tchouvelev, A. et al. (2021), “Development of risk mitigation guidance for sensor placement inside mechanically ventilated enclosures–Phase 1”, International Journal of Hydrogen Energy, Vol. 46/23, pp. 12439-12454.
[1] Tian, Z. et al. (2021), “Review on equipment configuration and operation process optimization of hydrogen refueling station”, International Journal of Hydrogen Energy..
[107] Top, H. and C. Teunissen (2020), Odor assessment of selected odorants in hydrogen and natural gas-hydrogen mixtures, DVN GL.
[17] Uijt de Haag, P. and B. Ale (2005), Guidelines for quantitative risk assessment, Publication Series on Dangerous Substances (PGS 3), VROM,.
[88] V. D. Noort, A. et al. (2020), Gedrag van waterstof bij lekkages in het gasdistributienet, DVN GL.
[65] Venetsanos, A. et al. (2008), “CFD modelling of hydrogen release, dispersion and combustion for automotive scenarios”, Journal of Loss Preventation in the Process Industries, Vol. 21/2, pp. 162-184.
[25] Witkowski, A. et al. (2017), “Comprehensive analysis of hydrogen compression and pipeline transportation from thermodynamics and safety aspects”, Energy, Vol. 141, pp. 2508-2518.
[53] Wu, Y. (2008), “Assessment of the impact of jet flame hazard from hydrogen cars in road tunnels.”, Transportation Research Part C: Emerging Technologies, Vol. 16/2, pp. 246-254.
[77] Yoo, B. et al. (2021), “Comparative risk assessment of liquefied and gaseous hydrogen refuelling stations”, International Journal of Hydrogen Energy, Vol. 46/71, pp. 35511-35524.
[9] Zarei, E., F. Khan and M. Yazdi (2021), “A dynamic risk model to analyze hydrogen infrastructure”, International Journal of Hydrogen Energy, Vol. 46/5, pp. 4626-4643.
Notes
← 1. However, one should note that each (hydrogen) production route depends also on the geographical region, process configuration (IEA, 2021[4]).
← 2. In the 1940s, the world’s largest water electrolysis plant was built in Rjukan, Norway (Hydrohub, 2020).
← 3. This includes: Canadian Hydrogen Installation Code - BNQ, design code for hydrogen station GB50177-2005 (China), hydrogen technologies code - NFPA2 (USA) etc.
← 4. That is, pipeworks that transports hydrogen at a pressure between atmospheric pressure to 3.4 MPa (Table 3).
← 5. A measure of risk created by mathematically adjusting a value in order to permit comparisons.
← 6. HIAD: Hydrogen incident reporting database.
← 7. Fuel cells and hydrogen joint undertaking, https://www.fch.europa.eu.
← 8. Nonetheless, compressors at hydrogen production plants operate at a lower pressure as hydrogen is prepared for transportation either by pipelines (usually between 2-5 MPa) or road tube trailers (legal limit at 20 MPa in China and 25 MPa in the United States).
← 9. This is related to Scenario 1 as electrolyser units (alkaline, PEM) are located indoors.
← 10. More applicable to pipework connected to a hydrogen vessel where a large inventory is available.
← 11. Nonetheless, the software Safeti (Det Norske Veritas) is applicable to for hazardous substances in general, not specifically validated for hydrogen.
← 12. 14 kPa = 0.14 bar = 140 mbar.
← 13. 42 kPa = 0.42 bar = 420 mbar.
← 14. Air changes per hour.
← 15. For details on different tank types please check section 2.4.
← 16. 1MPa = 10 bar.
← 17. 70MPa = 700 bar.
← 18. 12 bar above atmospheric pressure, 12 bar = 1200 kPa.
← 19. It is worth noting that currently, heavy duty hydrogen vehicles, such as buses and trucks in Europe typically use gaseous hydrogen compressed to 35 MPa, while a pressure of 70 MPa is the norm for hydrogen cars.
← 20. In Japan, onsite-type hydrogen fuelling stations using natural gas and other resources and offsite-type hydrogen fuelling stations, in the USA, some hydrogen fuelling stations considered in this study were of the offsite type.
← 21. 70MPa = 70,000 kPa = 700 bar.
← 22. 1 Mpa = 10 bar.
← 23. Nonetheless, risk for medium and large leakage meet the acceptance criterion at 1.0 ∙ 10-4 /year.
← 24. A method which uses information on HRS accidents over time.
← 25. Unrevealed leak time is calculated as a function of leak rate and inspection interval. Unrevealed leak time is one area within safety and risk management of hydrogen stations that has not yet been addressed in any other research paper. The authors believe that in addition to process safety time, unrevealed leak time is an equally critical parameter that needs to be considered in the engineering safety designs. It determines the time period when the leak exists at the installation due to an unrevealed leak failure.
← 26. 10kPa = 100 mbar.
← 27. Ducted to the external wall of the kitchen.
← 28. NB: a blend of two chemicals, t-butyl mercaptan (TBM) and dimethyl sulphide.
← 29. THT: a chemical, Tetrahydrothiophene.