Ruth Lopert
Rohit Malpani
Philip Haywood
David Winickoff
Hermann Garden
Ruth Lopert
Rohit Malpani
Philip Haywood
David Winickoff
Hermann Garden
The COVID-19 pandemic revealed that access to global public goods and other essential technologies was far from adequate. Global public goods are those that are of benefit to all, but have traditionally been underproduced, giving rise to a global policy challenge. This chapter discusses current and proposed models for incentivising the research, development, manufacture and distribution of essential health technologies to enable them to approximate global public goods. The policies outlined also aim to ensure the affordable and equitable global dissemination of these technologies.
The experience of COVID‑19 has highlighted the need for greater focus on the research and development (R&D), manufacturing, distribution and deployment of health technologies to respond to health shocks – notably those technologies (such as vaccines and antimicrobials) related to prevention, control and treatment of communicable diseases. The pandemic demonstrated that the dominant business model for incentivising R&D does not guarantee that these health technologies will be developed, produced and made accessible globally. While massive public sector investment (such as the USD 10 billion investment in Moderna’s COVID‑19 vaccine by the United States Government alone) enabled the successful development of effective COVID‑19 vaccines in record time, access and distribution were – and continue to be – expensive, inconsistent and inequitable.
Furthermore, current frameworks that depend on intellectual property (IP) for innovation and supply of health technologies – such as novel antibiotics, treatments for neglected tropical diseases and vaccines – have failed to deliver solutions for priority public health needs. This is due to a combination of limited market size and financial returns, inadequate infrastructure and co‑ordination, and a paucity of developers and investors committed to R&D for priority health needs. For example, a vaccine that reduces both morbidity from and transmission of a communicable disease may not be developed and distributed because of insufficient market size.
Public goods are, by definition, those that are available and accessible to all, without the possibility of exclusion. Many essential health technologies are not universally available or accessible. Yet there is strong global public interest in producing certain. essential goods and making them available affordably, equitably and expeditiously, so that as far as possible they approximate public goods. This would enable countries to prepare for, and respond to, global health threats such as pandemics and increasing antimicrobial resilience.
Appropriate policy frameworks and incentives are required to ensure that such essential goods approximate global public goods (GPGs), by minimising rivalry and excludability. Where global public health interest is strong, but markets alone fail to deliver the desired outcomes, different models are needed. This chapter analyses a variety of existing and emerging mechanisms for incentivising the development of GPGs and other essential technologies that share their characteristics. From the lessons of the pandemic and this analysis, a number of key policy and funding approaches emerge:
Delinking research, manufacturing and supply from sales revenue: delinkage is critical where essential health technologies are underprovided by competitive, revenue‑driven markets, as in the case of novel antibiotics. Governments could trial novel pull incentives, such as innovation prizes and market entry rewards, to ensure returns independent of market size.
Using blended finance approaches: leveraging public, private and philanthropic funding can facilitate multi-sector engagement, allowing organisations with different objectives to invest alongside each other while achieving their own objectives, and strengthen long-term commitment to developing essential health technologies.
Promoting collective and co‑ordinated management of IP rights and know-how: voluntary licensing, via patent pools or technology access pools – such as the COVID‑19 Technology Access Pool led by the World Health Organization (WHO) – is one mechanism for promoting access. Technology transfer hubs, such as the WHO mRNA Technology Transfer Hub, can also expand use of platform technologies. Sharing of IP and know-how can also help to expand the manufacturing and distribution of essential health technologies. For example, voluntary licences for COVID‑19 antivirals through the Medicines Patent Pool enables their generic production in low- and middle‑income countries.
Preserving a degree of ownership of health technologies developed using public funding, and attaching relevant obligations to public funding or pull incentives. For example, public and philanthropic funders could mandate and enforce clear obligations with respect to access and affordability. Governments could also invest directly in establishing or funding institutions to facilitate the development and production of essential health technologies.
Policy makers could also consider the following:
Mandating or encouraging greater transparency of funding and financing agreements between governments and recipients, and improving overall transparency of financing flows. The costs and outcomes of R&D, production and IP protection, and the prices of products worldwide, should be transparent. Increased transparency reduces uncertainty and allows all parties to make decisions with confidence.
Ensuring that advance purchase agreements are as inclusive as possible of countries and populations worldwide: many high-income countries established these agreements with manufacturers of COVID‑19 vaccines. These agreements should also commit recipient companies to share IP and know-how, in exchange for greater certainty of demand.
Facilitating the alignment of interests and investments between public, philanthropic and private sector funders: developing means of encouraging collaboration between such funders could reduce the rivalry and excludability of essential health technology products.
There is no single best approach to solving the development, manufacturing and distribution challenges of essential health technologies needed for health system resilience in the future. Public support will be required in many policy areas, including financing, regulation, manufacturing and even the direct provision of services. Public support should be accompanied by credible obligations to distribute such technologies equitably, especially in times of crisis.
The COVID‑19 pandemic exposed how unprepared the world was to deal with a crisis of such scale and intensity. This has prompted extensive consideration of how health systems can be made more resilient to future pandemics or other crises. To be resilient, health systems need mechanisms to support their ability to prepare for and respond to future threats, not only to react to existing issues. It is essential to invest in developing health technologies needed to address future threats, and in ensuring health system capacity to effectively deploy them.
The existing health innovation system tends to be most effective at generating new products when they are demanded in sufficient quantities over long periods of time, or at sufficiently high prices to make markets attractive. However, the effectiveness of this model, which favours the efforts of individual firms and relies largely on the granting of intellectual property (IP) rights, has been more limited for other goods, such as novel antibiotics and drugs for neglected tropical diseases.1
Previous OECD research (OECD, 2018[1]) highlighted the paucity of economic incentives for the biopharmaceutical industry to develop and bring to market new antimicrobials, and the gradual drying up of the development pipeline (World Health Organization, 2021[2]). This is because it is not financially viable for pharmaceutical companies to invest in research and development (R&D), given the low return from antibiotics at the point of sale under traditional pharmaceutical funding models. Markets are too small to be commercially attractive. New antibiotics must be used judiciously, in accordance with stewardship principles to prevent the further development of resistant organisms, thus limiting demand. Further, many existing products are old and cheap, which constrains the prices of newer products. In the meantime, resistance to existing antibiotics is a slow-burning crisis whose effects could be devastating in the medium- and long-term (see the chapter on investing in resilience for discussion of the impact of the COVID‑19 pandemic on antimicrobial resistance initiatives).
Similarly, prior to the emergence of SARS‑CoV‑2, there was no market for vaccines against coronaviruses. Vaccines had largely fallen out of favour in the biopharmaceutical industry, with low returns on investment and few big market players. The failure to complete the development of vaccines against earlier coronaviruses (e.g. SARS and MERS) was, in retrospect, a missed opportunity, but these outbreaks were too limited to make the completion of their development viable.
By contrast, the COVID‑19 pandemic has been notable for the unparalleled success and speed of vaccine development. This was facilitated by massive public sector push funding for both R&D and manufacturing capacity; the latter enabling vaccine production prior to the conclusion of phase III trials, and in many cases absorbing the full financial risks of R&D failure (OECD, 2021[3]). In many respects, the scientific response to COVID‑19 has been one of the more positive aspects of the pandemic response. However, it has exposed weaknesses in how policies support and incentivise the development of knowledge needed to produce effective vaccines.
This scientific success sits in stark contrast to the failure to ensure equitable distribution of and affordable access to COVID‑19 vaccines, particularly in developing countries. This challenge has often affected health technologies addressing diseases that are not characterised as pandemics or pandemic threats (UN Secretary General High-Level Panel on Access to Medicines, 2016[4]). As such, the pandemic demonstrated that, absent specific mechanisms for ensuring affordable access and equitable distribution, market forces prevail irrespective of whether global supply is sufficient or not. Supply of COVID‑19 vaccines based on bilateral agreements led to preferential access in high-income countries. This was an outcome that was both inequitable and inefficient. It may have resulted in more than 1 million additional deaths in low- and middle‑income countries in 2021 (Ledford, 2022[5]).
There is also growing recognition that current policy approaches to incentivising pharmaceutical innovation may, in at least some therapeutic areas, need reconsideration. Despite extensive IP protection afforded to biopharmaceuticals, there is evidence of declining R&D productivity over time – see, for example Ringel et al. (2020[6]). There remains a lack of progress in areas of urgent need, such as new antimicrobials. What can be learned from the COVID‑19 experience in terms of potential models? To drive innovation, what kinds of institutional arrangements are needed for agile, equitable, resilient and forward-looking systems? What policy settings and mechanisms will engender strong incentives for innovation and also create effective commitments to equitable, affordable distribution and access? How can we incentivise the development of health technologies to minimise rivalry and excludability? There are strong moral and economic arguments for enhancing the mechanisms and models of health innovation in these areas. These deserve exploration both in theory and practice.
This chapter proceeds as follows. Section 13.2 discusses the concept of global public goods (GPGs) and their characteristics, some of which are common to a broader group of health technologies. It describes the challenges inherent in current models for incentivising the R&D required for the public goods and the broader group of essential health technologies needed for resilient health systems. Section 13.3 describes existing and novel mechanisms for funding R&D, and for ensuring that R&D outputs are deployed affordably and equitably as if they are GPGs, with an emphasis on communicable diseases. Section 13.4 presents the conclusions of the analysis and policy approaches for further consideration.
The concept of GPGs has gained increasing attention as governments recognise that challenges such as climate change and antimicrobial resistance not only require solutions beyond the remit of individual national governments, but cannot be resolved by market forces alone (Kaul and Faust, 2001[7]). Most health products cannot, in the strictest sense, become ”pure” global public goods. Nevertheless, the Secretary General of the United Nations and others have urged that health products like vaccines ought to approximate GPGs as far as possible (UN Secretary-General, 2020[8]). In his view, it is important for public health and equity that this be achieved through appropriate policy and regulation.
This section discusses the nature of GPGs, and by examining the characteristics that affect the extent of a health product’s rivalry and excludability, it then highlights potential targets for policy intervention.
Reisen et al. (2004) define a “public good” as
“… a commodity, measure, fact or service: which can be consumed by one person without diminishing the amount available for consumption by another person (non-rivalry); which is available at zero or negligible marginal cost to a large or unlimited number of consumers (non-exclusiveness); which does not bring about disutility to any consumer now or in the future (sustainability)” Reisen, Soto and Weithöner (2004[9]).
The requirement for non-excludability means that market-based incentives are neither appropriate nor adequate, and governments, often the providers of public goods, are likely to have limited resources to address competing priorities (WHO Commission on Macroeconomics and Health, 2002[10]).
Although many goods and services are not pure public goods, they exhibit some of their characteristics. At one end of the spectrum are private goods, or goods that are both rivalrous and excludable. So-called “club goods” are those that are non-rivalrous but excludable, such as cable television services. “Common goods” are those that are non-excludable but rivalrous, such as fish stocks and highways (Table 13.1). Public goods that confer benefits beyond national borders are referred to as international public goods, and may be regional or global (Moon, Rottingen and Frenk, 2017[11]).GPGs are thus those that are potentially of benefit to all countries, people, or generations.
The willingness of governments to supply GPGs, either through international co‑operation or by their own efforts, can be a response to externalities that cross borders, which no individual country can fully address. GPGs tend to be undersupplied. This may in part reflect concerns about ceding national sovereignty to standard setting bodies or accepting treaty obligations, as well as the difficulties of achieving agreement to collective action by governments with divergent interests and approaches to tackling common challenges, such as climate change. Another factor is the “free‑rider” problem; since GPGs are non-rivalrous and non-excludable, an individual country may choose to wait until another provides a public good that it can consume (Hatefi, Marten and Smith, 2020[12]).
|
|
Rivalry |
||
|---|---|---|---|
|
High |
Low |
||
|
Excludability |
High |
Private goods: food, clothing, automobiles |
Club goods: theatres, private clubs, cable TV |
|
Low |
Common goods: clean air, fish stocks, forests, highways |
Public goods: knowledge, policy, herd immunity, lighthouses |
|
Source: Moon, Rottingen and Frenk (2017[11]), “Global public goods for health: Weaknesses and opportunities in the global health system”, https://doi.org/10.1017/S1744133116000451.
There is no consensus on the classification of GPGs for health. Nonetheless, communicable disease control, pandemic preparedness (Stein and Sridhar, 2017[13]), immunisation, and international disease surveillance are generally considered to be GPGs. Another type of GPG for health encompasses information, standards (such as the International Classification of Diseases), guidelines, and frameworks or treaties intended for the management of cross-border challenges, such as the Framework Convention on Tobacco Control and the International Health Regulations (IHR). A third type of GPG is the production of knowledge through research, potentially including research that ultimately leads to the development of new medicines, vaccines and diagnostics (Love, 2020[14]).
GPGs related to the production of health technologies are rare, for several reasons (Hatefi, Marten and Smith, 2020[12]). First, many products are developed in and by the private sector: for commercial reasons, they are subject to a range of measures that render them excludable. Second, many public goods funded by one or a few countries may be shared with other selected countries in the form of development assistance (Love, 2020[14]),and countries that supply them may choose not to limit excludability and rivalry. Third, most health technology products are inherently rivalrous with respect to consumption. For example, a dose of medication administered to one person is not available to another. It is straightforward to render a patented health product excludable, for example, via its price, and thus many products are excludable due to lack of affordability.
When potential benefits accrue beyond the person to whom a product is administered, for example administration of a vaccine that limits spread of a disease, it may be difficult for a sufficient market to form. Efforts to stimulate and incentivise the R&D of these health technologies are needed that delink the drivers of investment from the size of the market.
Additionally, for reasons of equity and sound public health policy, it may be desirable for some technologies to be made available as if they were GPGs – that is, to minimise excludability and rivalry as far as possible.
There are policies and investment vehicles that can render health products less excludable and less rivalrous, and thus the extent to which a good is excludable and rivalrous is essentially a “social and political choice” (Hatefi, Marten and Smith, 2020[12]) (Box 13.1).For sound public policy reasons, policy making should focus on ensuring that the outputs of publicly-funded investments are as widely accessible as possible (Love, 2020[14]).
There are five characteristics that affect the extent of a product’s rivalry and excludability. These characteristics are all influenced by the choices of private actors, governments and third parties holding rights to underlying knowledge.
Characteristics that affect product rivalry are:
Supply: Increased supply of a health product reduces rivalry. In the early stage of absorbing the COVID‑19 pandemic, many products were in short supply (see the chapter on securing supply chains). Later, when vaccines became available, limited supplies were directed preferentially to (and retained by) high-income countries, even after COVAX (the COVID‑19 vaccine procurement facility) was launched. The result was that fewer than 10% of people on the African continent were fully immunised by the end of 2021 (Schlein, 2021[15])
Appropriate use: Inappropriate use of antimicrobials can accelerate the development of resistant organisms, rendering them ineffective and destroying their utility for future generations (Morel, Edwards and Harbarth, 2017[16]). However, measures to ensure a health product is non-rivalrous (with respect to benefit for future generations) would render the same products excludable due to restrictions on use.
Characteristics that affect product excludability are:
Regulation: Although Governments and international agencies have taken steps to facilitate registration, for example through the WHO Prequalification Program and the Collaborative Registration Procedure, firms that hold rights to health products must also take steps to facilitate this. Many antibiotics are not widely registered; of 17 on-patent products profiled in the Access to Medicines Foundation’s Antibiotic Resistance Benchmark, only six had filings in ten or more low- and middle-income countries (LMICs) (Access to Medicine Foundation, 2021[17]).
Affordability: The average cost per COVID‑19 vaccine dose ranges from USD 2 to USD 40 (UNICEF Supply Division, 2022[18]). This can represent a significant financial burden for low-income countries where average annual per capita health expenditure may be as low as USD 40 (UNDP Data Futures Platform, 2021[19]), and in middle‑income countries that faced potentially unaffordable prices (relative to their governments’ ability to pay) (Robbins, 2021[20]). Market exclusivities (patent protection, data and market exclusivity) enable producers to charge prices significantly above the marginal costs of production. Where a health product remains patented, private actors with exclusive rights can reduce excludability by offering affordable prices, providing voluntary licenses, or not enforcing IP rights. Governments can improve affordability, by regulating prices or by applying IP safeguards that facilitate competition.
Presentation: A health product’s presentation may be inappropriate for a specific population, country or setting. One critical characteristic is thermostability, or the temperature at which a product must be stored, transported, and administered. Several vaccines require cold chains and have very limited shelf-life once removed from storage, but many LMICs have limited or no cold-chain capacity (Das, 2021[21]). Oral medications with once‑daily dosing are simpler for administration and adherence than those requiring injection, intravenous administration, or multiple daily dosing (Nolen and Robbins, 2021[22]). Paediatric formulations of adult products are often an afterthought (Morin et al., 2022[23]), and development may be delayed or may not eventuate. For example, while paediatric formulations of COVID‑19 vaccines followed soon after adult presentations, there has typically been an eight to ten year gap between the development of the adult and paediatric formulations of antiretrovirals (Penazzato et al., 2018[24]).
Note: The Access to Medicines Foundation’s 2021 AMR Benchmark evaluates 17 companies with major stakes in the anti‑infectives space, and compares how they perform across a set of 20 metrics, to track progress and gaps in their efforts to keep medicines and vaccines available, despite the rise of antimicrobial resistance.
It is important for public health and equity that policy makers focus on ensuring publicly funded incentives and investments limit rivalry and excludability as far as possible. This is especially true for technologies, such as vaccines and antibiotics, that are essential for global public health and health system resilience. However, the private sector will necessarily be involved in developing, producing and distributing health technologies. The question, therefore, becomes: what models of innovation can best support a GPG-driven policy approach to delivering those technologies essential for resilient health systems?
These models may be conceptualised in two broad categories: push funding and pull incentives (Cama et al., 2021[25]).2 Push and pull mechanisms can be applied to one or more steps in the drug development process. The extent to which a push or pull mechanism can deliver products that approximate GPGs can be measured directly according to the excludability or rivalry of the immediate output or outcome, or indirectly, by assessing its impact on outcomes and outputs of downstream steps in the drug development process.
Sections 13.3.1‑13.3.5 address how push and pull mechanisms can affect the excludability and rivalry of health products.3 Section 13.3.6 examines end-to‑end push funding programmes that aspire to develop GPGs. Section 13.3.7 describes “enablers” of R&D, or institutions and entities that facilitate, de‑risk, or reduce R&D costs. Section 13.3.8 outlines broader and more inclusive business models before this analysis concludes in Section 13.3.9.
Push funding refers to direct funding for specific stages of R&D projects in the form of grants, investments, tax credits or low-interest loans. Push funding may either support a stage of, or an entire R&D project, or contribute to the costs. Governments contemplating the provision of push funding may be seeking to: provide additional support to the private sector to develop or commercialise new products; address an undersupply of health products for unmet needs that reflect a policy priority, but may not be commercially viable; or enable a government, international agency, or other third party to participate in developing or commercialising a new product and/or prioritise specific needs.
There is substantial push funding by the public sector and philanthropic organisations for all stages of R&D. Røttingen et al. (2013[26]) estimated that of USD 240 billion spent on health R&D in 2009, the public sector and philanthropic sources provided 40% of this funding. Viergever et al. (2016) identified 55 major public and philanthropic funders of health research that collectively spent USD 93 billion (Viergever and Hendriks, 2016[27]). The COVID‑19 pandemic attracted new sources of push funding. According to Policy Cures, over USD 9 billion has been provided by the public sector, government investment vehicles, and philanthropic organisations as push funding for COVID‑19 R&D (Policy Cures Research, 2020[28]). However, such funding likely reflects a temporary increase and may not be sustained as COVID‑19 is increasingly no longer considered a health emergency.
Business models built on novel forms of collaboration can provide push funding for health solutions where markets are insufficient, particularly for vaccines and antibiotics. The Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), the Coalition for Epidemic Preparedness Innovations (CEPI), and Gavi, the Vaccine Alliance, are examples of cross-sectoral collaboration along the trajectory of antimicrobial R&D and delivery (Figure 13.1 and Section 13.3.4).
In addition, the Innovative Health Initiative (IHI, formerly the Innovative Medicines Initiative) facilitates novel collaborations with diverse business models. As the world’s largest public-private partnership (PPP) in life sciences, it is jointly funded by the European Union and the European pharmaceutical industry, with a budget of EUR 5.3 billion over 2008‑20. IHI drives collaboration between key players and stakeholders involved in health research, including universities, research centres, the pharmaceutical and other industries, small and medium-sized enterprises (SMEs), patient organisations, and medicines regulators. A cornerstone of the successful partnership is the commitment of dedicated resources, scientists, and expertise to the projects by the private partners (IMI Innovative Medicines Initiative, 2022[29]).
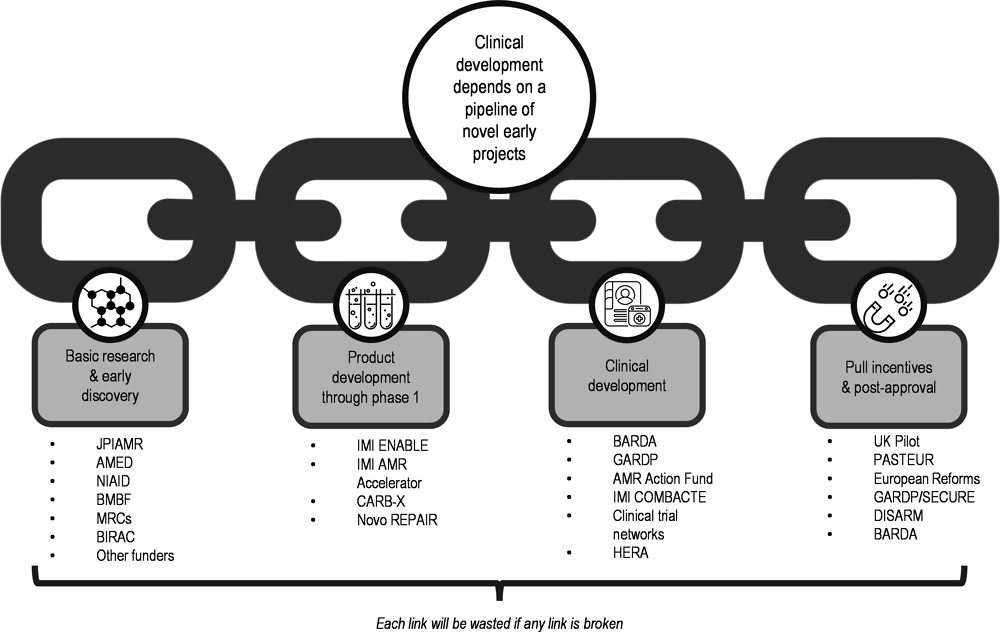
Source: Adapted from CARB-X (2021).
In the area of antibiotics, IHI has invested more than EUR 1 billion of its budget in 14 innovative projects tackling AMR. Several new molecular entities coming out of these projects have entered clinical stages. This interdisciplinary IHI project (COMBACTE‑NET (2022[30])) is working on 11 clinical trials involving six antibacterial molecules, with a dedicated network connecting over 1 000 hospitals and labs in more than 30 European countries. In the area of vaccines, IHI has demonstrated the value of leveraging public and private R&D efforts in the development of an Ebola vaccine (European Commission, 2019[31]) that now has marketing authorisation in Europe and WHO prequalification (Ishola et al., 2021[32]). The large clinical trial infrastructure developed under COMBACTE-NET has contributed to the collective ability to address the COVID‑19 pandemic in Europe and beyond.
The IHI has, however, been subject to some criticism from patients, consumers, providers, payers, public interest organisations and the European Parliament. Criticisms relate to a lack of inclusivity in the choice of research priorities, adequacy of its governance structures, and the dominance of large industry players. Formal evaluations of IHI initiatives have highlighted an imbalance in the representation of stakeholders and poor standards of transparency, with civil society organisations claiming that they failed to meet the goals that justified them, including overcoming market failure and improving the development and availability of health technologies for unmet medical needs (Global Health Advocates, 2021[33]).
Blended finance is a form of push funding that can leverage public, private and philanthropic funding. It has traditionally featured in international development assistance, especially with regard to the construction of infrastructure. However, global health actors are beginning to explore blended finance in the area of health. The OECD (2022[34]) defines blended finance as “…the strategic use of development finance for the mobilisation of additional finance towards sustainable development in developing countries.” The United States Agency for International Development has a six‑step roadmap for blended finance transactions. This framework encourages stakeholders to determine the potential for adopting a blended finance approach by evaluating the sustainability of the underlying programme, the potential for increased efficiency by engaging the private sector, and the presence and interest of private sector players (Lin and Sharma, 2019[35]).
Evidence suggests that blended finance can help organise and facilitate multi-sector engagement and strengthen long-term commitment to fund essential health technologies over short-term financial returns. However, despite a moderate increase in blended finance transactions in the health and education sectors in 2020, the use of blended finance for health-related priorities is low compared to the energy, agriculture and infrastructure sectors (Figure 13.2). Private sector support for Sustainable Development Goal 3‑related priorities has tended to be smaller than government-led initiatives (Apampa, 2022[36])..Further, the scale of health-related funding initiatives is usually smaller than in sectors such as financial services and energy.
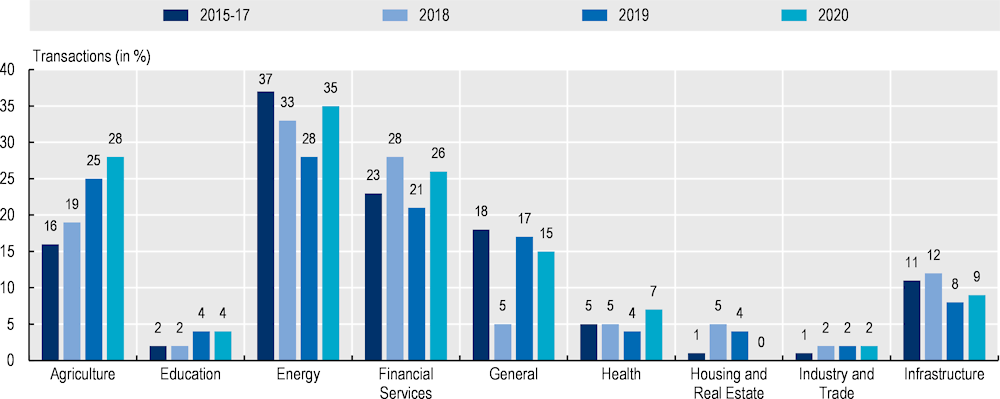
Note: Proportion of closed transactions by sector.
Source: Convergence (2021).
To increase the use of blended finance for health, public funders, philanthropic organisations, and other donors, could combine to provide a catalytic layer of “funds for funds”. Although this approach has been used more to date in the field of international development assistance, the creative pairing of public grants and equity investment has the potential to de‑risk and thus incentivise more private investment. In health, blended finance might be useful in helping overcome “valley of death” problems4 along the innovation chain, supporting market uptake of innovations and increasing deal flows to investors.
The following are helpful in describing and assessing the contribution of push funding mechanisms to the development of GPGs:
Source of funding: This could include: publicly-funded R&D; R&D funded by philanthropic foundations (including large, multi-purpose philanthropies such as the Gates Foundation or Wellcome Trust, as well as disease‑focused charities that support or invest in R&D); funding provided by a for-profit private sector entity; or mixed R&D funding (which may combine funding from several sources including governments, NGOs and the private sector).
Type of funding: This includes grants, low-interest loans, investments, or tax credits.
Stage of R&D to which funding is applied: This could include one or more of the following: discovery; translation; development; and/or commercialisation.
Direct impact on rivalry and/or excludability: The extent to which the funding facilitates the partial or complete reduction of the rivalry and/or excludability of the expected output or outcome.
Indirect impact on rivalry or excludability: Whether the push funding indirectly reduces the rivalry or excludability of a downstream output or outcome.
Nature and extent of access requirements to reduce rivalry and/or excludability: This refers to the introduction of specific requirements that affect the rivalry and/or excludability of a health product, and include pricing, regulation, overall and allocation of supply, product presentation/ formulation (e.g. for paediatric use)
This section presents an overview of existing push funding mechanisms and considers the extent to which their outputs are able to approximate GPGs, applying the factors that are helpful in describing and assessing these mechanisms (Box 13.2).
CARB-X is a push funding mechanism sponsored by governments and philanthropic entities that provides grants for the early stages of preclinical development and Phase 1 clinical testing for products targeting known drug-resistant bacteria. Two direct outputs are investigational compounds to address a drug-resistant infection, and clinical trial data. CARB-X permits grantees to seek IP rights for compounds developed with its funding (CARB-X, 2022[37]). However, its contracts require grantees to develop stewardship and access plans that include commitments to introduce strategies for responsible stewardship and appropriate access in low- and middle‑income countries (LMICs) within 90 days of a product entering Phase 3 clinical development (CARB-X, 2022[38]). Plans are published if the products gain marketing approval.
Stewardship and access plans can reduce rivalry through manufacturing commitments and support sustainability through stewardship planning. Excludability can also be minimised if developers commit to registering products and setting affordable prices in LMICs. Although each grantee is contractually obliged to develop plans (which remains with the product irrespective of ownership or development rights to it), there is no minimum standard (CARB-X, 2021[39]). Moreover, CARB-X does not require grantees to develop formulations or presentations of products for specific populations, such as children and neonates.
CEPI is a non-profit foundation with donors from the public, private and philanthropic sectors. It was established in the aftermath of the 2014 Ebola outbreak to develop vaccines to prevent and respond to emerging infectious diseases, and to “secure access to such products for the populations who need them” (CEPI, 2022[40]). CEPI is one of the founding members of COVAX, the international partnership to develop, manufacture, procure and distribute COVID‑19 vaccines. Since its establishment, CEPI has focused on three direct outputs: clinical trial data (safety testing in preparation for an outbreak or pandemic, and late‑stage testing in response); knowledge (IP) generated through its investments; and investigational vaccines, including support for manufacturing.
Initially, CEPI’s equitable access policy introduced obligations for grantees that included:
requirements for the production of investigational vaccine stockpiles during an epidemic
requirements for registration, supply, and affordable prices (for example, through prices as low and as close to optimal marginal cost of production as possible for affected populations, with a particular focus on low- and low-middle income countries as well as countries in crisis)
specifying appropriate data sharing practices that included prompt and public disclosure of all clinical trial results, including negative outcomes
retaining “step-in” (march-in) rights for IP, backed by “triggers”,5 to ensure that supply and other equitable access obligations were met (CEPI, 2017[41]).
More recently, however, CEPI modified its equitable access policy in three ways: to limit its step-in rights; to require a less onerous commitment to affordable prices for most countries; and to introduce a degree of opacity to the terms and conditions requiring grantees to ensure equitable access conditions are in place and are respected (MSF Access Campaign, 2019[42]).
Since the COVID‑19 pandemic began, CEPI has invested in multiple vaccine candidates. Some of these candidates have been approved and are in use, several others did not succeed, and a third group is under development for future use as pan-coronavirus vaccines. CEPI investments include a USD 0.9 million investment in the development of Moderna’s mRNA COVID‑19 vaccine in January 2020 (for its Phase I clinical trial). While CEPI’s investment was both modest and upstream (occurring prior to the WHO’s declaration of a public health emergency of international concern), Moderna agreed to CEPI’s equitable access principles. These principles were that appropriate products are first available to populations when and where they are needed at prices that are affordable to the populations at risk, especially low- and middle‑income countries or to public sector entities that procure on their behalf (CEPI, 2021[43]).
Despite this, Moderna’s vaccine has been mainly acquired by high-income countries, and at prices that LMICs may struggle to afford (Rauhala, 2021[44]). CEPI also invested USD 383 million into the development of AstraZeneca’s COVID‑19 vaccine. However, while CEPI was able to secure 300 million doses on behalf of COVAX (CEPI, 2021[43]) through its agreement, the European Union, United States, Canada, and other countries secured 1.9 billion doses in aggregate (Usher, 2021[45]). Licensing of the IP and know-how used to manufacture the vaccine, for example, to additional suppliers in LMICs, could have yielded larger quantities of vaccine than a supply reservation.
CEPI recently finalised a new Strategic Plan, which includes establishing networks and partnerships to address several objectives including the promotion of equitable access (CEPI, 2021[46]). It has identified several effective interventions to reduce rivalry and excludability of funded vaccines – such as decentralised manufacturing, building R&D capacity in LMICs, and supply reservations. However, CEPI may face significant challenges in adequately addressing excludability and rivalry of new pandemic vaccines, either during the early stages of a pandemic when demand may exceed supply (and thus inequities may arise), or where the vaccines have strong commercial potential.
CEPI has also stated that it will pursue “tiered pricing frameworks”, which can deliver affordable prices under specific conditions, including transparency of pricing, price tiers that reflect value for money and capacity to pay, and avoidance of arbitrage. However, CEPI does not mandate technology transfer of vaccine platforms or vaccines from pharmaceutical industry grantees. It will instead seek voluntary solutions that grantees may or may not choose to adopt. Separately, CEPI will seek to engage major R&D funders, such as G20 members, for “adoption of a minimum set of equitable access requirements in all new public funding and procurement arrangements”, to be accompanied by increased government funding commensurate with actual need and not limited by what is available within international development assistance budgets (CEPI, 2021[46]).
The Global Health Innovative Technology Fund (GHIT) is an international public-private partnership fund that provides push funding for medicines, vaccines, and diagnostics for malaria, tuberculosis, and neglected tropical diseases. It is a mixed fund that includes contributions from the Japanese Government, Japanese pharmaceutical companies, and philanthropic organisations (GHIT, 2022[47]). It funds projects at every stage of product development – from preclinical discovery through to product registration – though not all projects are funded on an end-to‑end basis (GHIT, 2022[48]). It also works with other push funders to complete product development (GHIT, 2022[49]). Three outputs across the GHIT investment portfolio are knowledge associated with investigational compounds, vaccines, and diagnostics; data generated at different stages of the drug development process; and health technology products. GHIT has developed product and data access policies that have both direct and indirect impacts on whether the generated data, knowledge and health products can approximate GPGs (GHIT, 2022[50]).
GHIT’s data access policy requires that data should be available transparently and publicly, including in public access repositories or alternatives “that can ensure the transmission of new scientific findings to the larger research and development community globally” (GHIT, 2022[50]). Any data used in a patent application can be disclosed by the GHIT Fund to a third party, although there are restrictions on which data are disclosed, and on the ways in which a third party may share them. Finally, any data generated through funding provided by GHIT can be subject to ownership rights that are negotiated between the different project partners. Thus, while data may be both fully non-excludable (and non-rivalrous), data generated through a partnership or used in a patent application may be excludable.
The GHIT Fund does not prevent project partners from obtaining patents, but they must grant royalty-free licenses to users in least-developed and low-income countries, while licenses for middle‑income countries include royalties. The Fund’s access policy does not specify licensing arrangements for high-income countries (GHIT, 2022[50]). It is not clear whether licensees may sell products to all countries or a subset of countries, or the terms and conditions that would apply. The extent to which health technology products are non-rivalrous and non-excludable may vary on a case‑by-case basis:
GHIT requires that in least-developed countries and LMICs, entities that market health products must set prices based on a no gain/no loss (cost neutral for the manufacturer) policy (GHIT, 2022[50]). However, it is unclear if developers must offer one low price or can apply tiered pricing, which can adversely affect affordability. The policy does not define a high-income country price.
GHIT sets out target product profiles for its priority areas that are intended to “align the unique needs of end-users with desired product attributes and performance criteria” (GHIT Fund, 2018[51]). This may ensure that the end-products do not exclude specific populations, such as children. However, the extent to which such products may be non-excludable will vary on a case‑by-case basis.
GHIT works with the UN Development Program to develop an access and delivery strategy for each product which covers demand forecasting, regulatory strategy, manufacturing, procurement, and supply chain (GHIT Fund, 2018[51]). Such strategies, if adopted, would improve non-rivalry and non-excludability of health products. This should be assessed on a case‑by-case basis.
The AMR Action Fund is a blended financing mechanism that aims to “invest in the clinical development of novel antibiotics to bring them up to commercialisation” (AMR Action Fund, 2022[52]). The Fund comprises 80% industry funding, with further contributions by philanthropic institutions and development banks, including the Wellcome Trust and the European Investment Bank. The Fund assumes 5 years with capital deployment and a subsequent period of 5‑7 years with additional investments as an “engaged owner”. More than 20 pharmaceutical companies are expected to contribute USD 1 billion in additional investments (Garden, forthcoming[53]).
The Fund provides disbursements for clinical development to smaller biopharmaceutical companies that are developing new antibiotics, with an overall objective of the approval of two to four new antibiotics by 2030 (AMR Action Fund, 2022[54]). The primary output of investments provided by the AMR Action Fund is knowledge (IP, clinical trial data, and novel antibiotics). While the Fund has stated that it will select antibacterial treatments that target priority pathogens identified by WHO and the United States Centres for Disease Control and Prevention (CDC), the investments may ultimately focus on antibiotics that can provide a commercial return, even if they do not meet the most urgent global public health needs. This is because decisions on which products to develop are ultimately made by entities that expect a return on investment (ReAct, 2021[55]).
The AMR Action Fund has published several principles and policies that affect sustainability, rivalry, and excludability (AMR Action Fund, 2022[56]). These include:
ensuring companies undertake clinical trials to pursue indications that reflect the greatest unmet needs, generate data that inform appropriate use in vulnerable populations, and develop formulations that facilitate access
supporting portfolio companies to develop regulatory strategies that support broad registration
requiring companies to introduce access and appropriate use plans that are published during Phase 3 clinical trials, and to adhere to principles established through industry declarations, including the Davos Declaration on Antibiotic Resistance and the AMR Industry Alliance.
While the Fund encourages availability and access, it also states that companies can identify countries “where commercialisation is regarded as unfeasible within a reasonable time horizon” and for which new mechanisms, provided by governments, would be required to enable access and appropriate use. This means that for countries that grantees consider commercially non-viable, there may be no pathway for registration, supply, and access for several years. Private companies that provide funding will govern the Fund’s decision making, including with respect to access and appropriate use policies. Except for the European Investment Bank, no governments or government-led institutions participate within or manage the Fund. Even if policies related to access and appropriate use can promote access, such obligations are neither mandatory nor enforceable, and may depend on the sole discretion of the Fund’s investors and recipients.
The Research Investment for Global Health Technology Fund (The RIGHT Fund) in Korea aims to ensure that all knowledge and information gained from grants, projects or other investments are broadly disseminated in terms of price, quantity, quality and timeframe. The RIGHT Fund requires awardees and project participants to sign and adhere to global access agreements. In terms of IP and licensing approaches, guiding principles of The RIGHT Fund global access policy are (RIGHT Foundation, 2020[57]):
Products, data and other innovations resulting from projects should be made available and accessible in terms of price, quantity, quality and timeframe so as to benefit beneficiaries.
Awardees and project participants may apply for and maintain IP rights to developments of projects. The RIGHT Fund will not take ownership of IP rights to funded developments, provided that it is entitled to royalty-free, irrevocable, and worldwide licenses to access and use IP rights to funded developments.
Product access policy: when awardees and project participants are granted a patent deriving from project, awardees and project participants will grant royalty-free, irrevocable, and worldwide licenses to users operating for the benefit of the public market in least-developed countries (LDCs).
Pull incentives encourage private sector engagement by rewarding successful development through creating viable market demand or ensuring future revenue. The following section discusses the advantages and disadvantages of different types of pull incentives.
Pull incentive mechanisms may be classified according to six criteria:
Source of pull incentive: Sources may be public (government), private, philanthropic or mixed. Most pull incentives are sponsored by governments.
Stage of development: This refers to the stage of product development at which the pull incentive is applied. Most pull incentives are applied once a product has been granted marketing authorisation by a regulatory authority.
Direct impact on rivalry and/or excludability: Whether the pull incentive improves the non-rivalry or non-excludability of the anticipated output or outcome.
Indirect impact on rivalry or excludability: Whether the pull incentive indirectly improves the non-rivalry or non-excludability of a downstream output or outcome, such as the affordability of an end product or its availability (production and registration).
Health product access requirements to improve non-rivalry and/or non-excludability: Requirements affecting the non-rivalry or non-excludability of a health product, including its price, registration, overall supply (and allocation), product formulations and presentation.
Indirect impact on rivalry or excludability of unrelated health products: Whether the pull incentive has any impact on the rivalry or excludability of unrelated health products, such as those that allow a recipient to extend market exclusivity for an unrelated health product (which could render the unrelated health product more excludable).
Transferable exclusivity rights (TER) provide the recipient with the right to extended exclusivity on another health product, a right which can also be on-sold to a third party.
TERs for antibiotics have been proposed, to be awarded on approval of antibiotics that meet specific criteria. Since a TER provides a recipient (or third party) with additional monopoly rights for another product, it can lead to increased rivalry and excludability, depending on how the additional exclusivity affects price and supply. The costs may be too high for governments. According to Årdal et al., the cost of one new antibiotic to the European Union would be USD 3.2 billion (Årdal, Lacotte and Ploy, 2020[58]). Rome and Kesselheim, through a retrospective analysis of ten antimicrobials that would have secured a TER in the United States between 2007 and 2016, conclude that “while market exclusivity extensions are a politically appealing mechanism to encourage novel antibiotic development, this approach would cost public and private payers billions of dollars” (Rome and Kesselheim, 2020[59]). Moreover, a TER does not guarantee access to an antibiotic; after it is granted, the antibiotic could be removed from the market, for example, as a result of loss of interest by or insolvency of the manufacturer (Årdal, Lacotte and Ploy, 2020[58]). Impediments to improved access may be magnified if a TER does not include other obligations, such as requirements for registration, licensing, production of data to guide use, and affordability.
A patent buyout is a purchase by a government (or a private party acting in the public interest) of patents associated with a health technology product, thereby terminating the period of monopoly conferred by them. Conditions of patent buyouts can also preclude the acquisition of subsequent patents (Kremer, 1998[60]). There are currently no patent buy-out mechanisms in place for health products.
A patent buyout could be triggered at any stage of drug development, with the estimated value likely to increase as a product nears (or achieves) regulatory approval. Thus, estimating the optimal value of a patent buyout may be challenging. A buyout could potentially render both the knowledge and data associated with a health technology fully non-excludable and non-rivalrous. When exercising a patent buyout, a government could maintain ownership of the IP, and require any third party using it (for example, through a voluntary licence) to fulfil designated access conditions. These conditions could relate to supply, pricing, availability of certain presentations or formulations, and product registration. Setting an acceptable price may be difficult, however, given likely uncertainty regarding the size of markets and diverse ways of calculating value.
Monetary prizes are rewards for achieving a specified outcome. Conceptually, patents (Stiglitz, 2007[61]) and patent buyouts are also forms of prizes. Monetary prizes are likely to be funded by governments, though prizes have been funded by the private sector (InnoCentive, 2022[62]) and philanthropic organisations (XPRIZE, 2022[63]).
Several prize funds for health product R&D have emerged. The United States Government has enacted legislation to establish a framework enabling all federal government agencies to run prize competitions (Legal Information Institute, n.d.[64]). In 2017, the European Commission awarded a Horizon Prize for the development of “a rapid test for health care providers to distinguish, at the point of care, between patients with upper respiratory tract infections that require antibiotics and those that can be treated safely without antibiotics” (European Commission, 2017[65]).
Prizes and prize funds can be awarded at different stages of the drug development cycle. Milestone prizes can be awarded for completion of pre‑clinical R&D or completion of any clinical trial phase. End-stage prizes (also known as market entry rewards) can be awarded upon successful regulatory approval (Love and Hubbard, 2009[66]). Since a developer incurs a greater risk of failure and accrues more expenses with each successive stage of drug development, prizes awarded later in the drug development cycle should be larger (Baraldi et al., 2019[67]), although they may be less well aligned with public health needs. Importantly, a milestone or end stage prize could be awarded as an alternative to IP protection. This would mean that the knowledge underlying the health technology product could approximate a GPG if a government chose to introduce appropriate policies and obligations tied to such prize rewards. However, if a prize recipient were to retain some or all its IP rights, then both the knowledge and the health product would remain excludable, and the prize could over-reward the recipient.
There are several potential challenges with prizes. First, governments or other prize sponsors may have difficulty determining the appropriate magnitude of a reward. Second, there can be challenges with allocating prize rewards among beneficiaries, including those entities or individuals that contributed to the development of a health technology product but do not have any formal share or stake in a successful prize submission. Third, the possibility of a prize may discourage potential beneficiaries from sharing of materials, knowledge, or technical know-how (Love and Hubbard, 2009[66]). Several proposals have been developed to address these challenges but to date none have been implemented.
For a health technology product to approximate a GPG, it is not sufficient to simply replace IP rights with a prize. For example, a milestone prize would need to be accompanied by push funding, and there should be one or more developers to develop the product. For an end-stage prize, governments would need to ensure that either the prize recipient or other manufacturers would supply the health product in a manner that would minimise rivalry and excludability.
Governments can provide additional pull incentives via regulatory frameworks. Two categories of regulatory incentives are the priority review voucher (PRV) and market exclusivities.
The PRV programme, first introduced in the United States in 2007, initially awarded a voucher in exchange for a drug or biological product that prevented or treated a tropical disease (US Food and Drug Administration, 2020[68]). This was expanded in 2012 to include rare paediatric conditions, and in 2016 to include emerging infectious diseases. A voucher permits the recipient to obtain a priority review designation for a subsequent application to the US Food and Drug Administration (FDA) that does not itself qualify for priority review (US Government Accountability Office, 2020[69]). This designation can speed regulatory approval, which, if successful, will extend the effective patent life of a product without extending the actual patent term. A PRV can also be sold to a third party, thereby prolonging the period of monopoly of an unrelated product. A PRV can incentivise an area of R&D that is underserved, however it does not require the recipient to forego exclusive rights to the product, thus the knowledge underlying the product may remain excludable. Furthermore, there are no legal requirements to ensure the target product minimises rivalry or excludability (Médecins Sans Frontières, 2017[70]), whether with respect to affordability, registration, supply, product presentation or formulation.
Although the PRV does not extend the legal term of a patent monopoly for a product that is designated for priority review, it does extend the effective patent life. This means that while a product may come to market earlier than it would in the absence of a PRV, it may be offered at prices that maximise the benefits of the PRV. This may be the case if the PRV is used by a third party that purchases it on the open market and needs to recover its investment for a product that may not earn blockbuster revenues. The PRV, while substantial, is inexpensive compared to revenues for best-selling, on-patent medicines in the United States pharmaceutical market (US House of Representatives, 2021[71]).To date, PRVs have been sold to third parties for prices ranging from about USD 67 million to USD 350 million (US Government Accountability Office, 2020[69]).
Several governments have awarded extended periods of market exclusivity to health technology products that have an orphan designation (Gammie, Lu and Ud-Din Babar, 2015[72]), are deemed a “qualifying” antibiotic (Darrow and Kesselheim, 2020[73]), or are the subject of paediatric trials – often neglected in drug development, but now a mandatory requirement in the United States and the European Union (European Medicines Agency, 2015[74]).
Market exclusivity differs from data exclusivity, but neither is an obligation of the Agreement on Trade‑Related Aspects of Intellectual Property Rights. Data exclusivity prohibits reliance on an originator’s pre‑clinical and clinical test data in the evaluation of an application for marketing authorisation of a generic or biosimilar medicine, for a specified period, usually a minimum of five years. Market exclusivity prohibits a regulatory agency from granting marketing approval for a period following the initial marketing authorisation of the originator product, even where the generic does not rely on the originator’s dataset (’t Hoen, Boulet and Baker, 2017[75]). Market exclusivity can extend the duration of market monopoly of a product for which patent protection has expired, and thus the period of time in which the recipient can set prices without the possibility of competition (Institute of Medicine, 2012[76]).
Several studies have shown that the net financial benefits accruing to recipients of paediatric exclusivity generally exceed the costs of conducting the additional studies (Sinha et al., 2018[77]). Medicines gaining additional exclusivity by virtue of orphan drug designation in the United States may already be highly profitable in other indications (PCMA, 2021[78]). Moreover, there are no additional obligations imposed with respect to pricing, registration, supply, or presentation (Technopolis Group, 2019[79]). There are also wider concerns as to whether additional market exclusivity is an effective mechanism for addressing unmet needs, or may be awarded without generating a tangible public health benefit:
Additional market exclusivity may not be the primary driver for developers. Other incentives that accrue from orphan drug designation include substantial R&D tax credits (a form of push funding) (Sarpatwari et al., 2018[80]).
Market exclusivity may not be an adequate incentive for the development of products to address unmet needs. Targeted market exclusivities for antibiotics, such as the Generating Antibiotic Incentives Now (GAIN) Act 2012 in the United States, have not encouraged the development of novel antibiotics (Darrow and Kesselheim, 2020[73]).
Market exclusivity may encourage the gaming of regulatory benefits rather than investment in novel product development. For example, one concern with orphan drug designation is that developers are successfully obtaining orphan designation by defining increasingly narrow indications (so-called “salami-slicing”) within conditions for which drugs may have already been developed, and targeting ever smaller markets (Burns, 2017[81]).
A product developer may be rewarded with additional market exclusivity even if the actual study or trial is unsuccessful or inconclusive. Developers can obtain paediatric exclusivity even if the product fails to show efficacy, or the company does not pursue regulatory approval for use, in paediatric populations (Benjamin et al., 2006[82]; Bostyn, 2021[83]).
An advance market commitment (AMC) is a binding contract that provides a guaranteed market for a product. AMCs are most likely to be funded by governments or philanthropic organisations. An AMC may be negotiated prior to a product’s regulatory approval or following it if focused on reserving or expanding supply for a purchaser (the latter is also known as an advance purchase commitment or APC). AMCs can improve availability and access to health technologies for those on whose behalf they are awarded.
As an example, an AMC for the pneumococcal vaccine negotiated by Gavi, the Vaccine Alliance, increased supply on behalf of Gavi‑eligible countries that might otherwise have been underserved, at a price that was lower than that offered to high-income countries (The World Bank, 2009[84]). Under COVAX, an AMC for COVID‑19 vaccines was established on behalf of 92 least-developed and low-income countries, prior to approval of any of the vaccines (Berkley, 2022[85]).
An AMC can reduce rivalry and excludability over time for those on whose behalf the vaccine is purchased, but can have the contrary effect for those countries (and populations) not included in the mechanism. The AMC for the pneumococcal vaccine used a two‑stage pricing mechanism – a higher price up front to secure supply on behalf of low-income countries, and a subsequent “tail price”, negotiated with the manufacturers, that would be lower. Thus, while Gavi was able to negotiate a low tail price for pneumococcal vaccine on behalf of Gavi‑eligible countries, countries that were ineligible (particularly middle‑income countries) were charged a higher, tiered price. Several were unable to purchase the vaccine (Tricarico et al., 2017[86]; Chen et al., 2019[87]). Furthermore, since the pneumococcal vaccine AMC did not require recipients to either surrender or out-license IP rights, potential additional suppliers that might have competed on price and increased supply were unable to enter the market (Chu, 2017[88]; Liu, 2017[89]). Eventually, the entry of additional suppliers reduced the price of the vaccine to below the tail price negotiated by Gavi (The Pharma Letter, 2019[90]). Thus, a negotiated tail price under an AMC, even if it declines over time, may still be higher than prices achievable through competition (Usher, 2019[91]).
An AMC negotiated by one country or region can also undermine supply to another, and thereby increase rivalry for a vaccine. During the COVID‑19 pandemic, many high-income countries signed AMCs (or APCs) for COVID‑19 vaccines (Usher, 2019[91]). Pharmaceutical manufacturers that prioritised these AMCs had insufficient supply of COVID‑19 vaccines for LMICs, even those where governments (or their donors) had also signed such agreements, either bilaterally or via COVAX or other joint procurement platforms. An AMC for a product required by all countries for which there is limited supply may only avoid worsening rivalry if all countries use such an AMC, and if there is a framework to allocate such supplies equitably, as intended by the establishment of COVAX (Mueller and Robbins, 2021[92]).
A subscription model involves one or more payments to a supplier in exchange for an appropriate supply of a product to treat a defined population for a specified period. This approach has been dubbed the “Netflix model” and differs from payment based on the volume of drugs sold. Subscription-based models can be used both as pull incentives and as a mechanism for managing reimbursement and supply.
Several countries have either launched or are currently piloting subscription-based reimbursement models as pull incentives for new antibiotics (Gotham et al., 2021[93]). Other countries have them under consideration (Vorperian and Quake, 2021[94]). These subscription models treat the provision of a vaccine, antibiotic, or other medicine, as a service, and delink the payment to the manufacturer from the number of units sold. A fixed annual fee (subscription) means that use is not discouraged by high unit prices and ensures that manufacturers are not incentivised to encourage increased use. A subscription model can thus improve the sustainability of an antibiotic for use by future generations (Gotham et al., 2021[93])
Subscription-based models may not be effective for developing new antibiotics unless one country can provide a sufficiently large pull incentive on its own, or several countries act collectively through pooled procurement. The United States Congress is currently considering the Pasteur Act. If enacted, it could provide rewards for individual antibiotics ranging from USD 750 million to USD 3 billion (Gotham et al., 2021[93]).
One possible complication with a subscription model is that, while it aims to pay a fair price, it is not entirely clear how value should be attributed to new antibiotics. This is because the effectiveness of new antibiotics in reducing AMR can only be measured after sustained use (Glover et al., 2019[95]). Nevertheless, these activities show promise as innovative pull mechanisms for de‑risking investment. A number of subscription models have been launched, or are currently being evaluated as proposals or through pilot studies.
The National Health Service (NHS) England and the UK Department of Health and Social Care recently launched a subscription mechanism for antibiotics. This followed the completion of an initial cost-effectiveness review of two antibiotics (ceftazidime‑avibactam and cefiderocol) covered by a pilot antibiotic subscription programme with an annual cap of GBP 10 million and a total of GBP 260 million for two antibiotics over 10 years. Unlike volume‑based payments, under a subscription model, manufacturers are paid upfront fees based on the estimated value of benefits to patients and to the UK National Health Service (Cookson, 2022[96]; Gotham et al., 2021[93]; Plackett, 2020[97]).
The European Union Joint Action on Antimicrobial Resistance and Healthcare‑Associated Infections (HCAIs) (EU-JAMRAI) has proposed a multinational initiative to ensure a sustainable supply of antibiotics independent of sales volumes and clinical use (Figure 13.3).
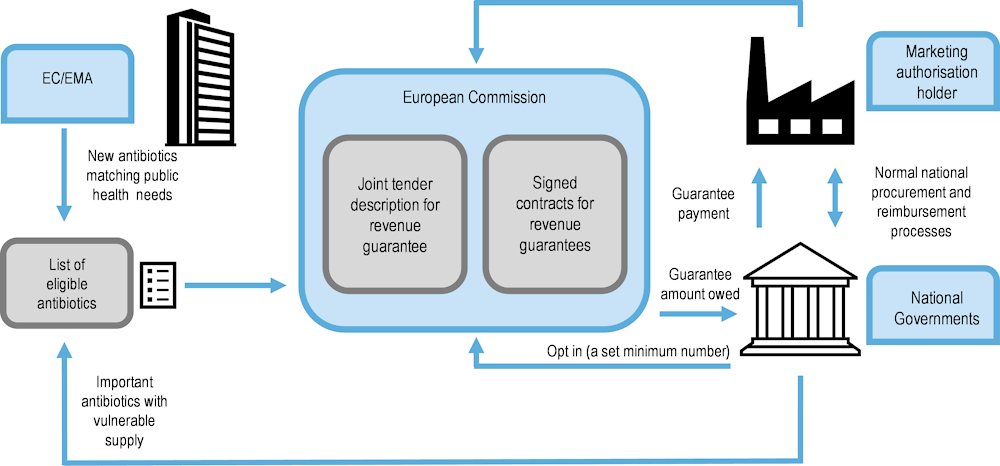
Note: EU-JAMRAI is the European Union Joint Action on Antimicrobial Resistance (AMR) and Healthcare‑Associated Infections (HCAIs).
Source: Adapted from Årdal, Ploy and Lacotte (2021[98]) “D9.2 Strategy for a multi-country incentive in Europe”.
Most R&D is based on providing push and pull incentives to private actors to develop and supply health technology products. There are, however, models of R&D that seek to generate GPGs on an end-to‑end basis through push funding. Three such models are: product development partnerships; public sector development and production of health technology products; and open-source pharmaceutical development.
Product development partnerships (PDPs) are usually not-for-profit entities that enable public, private, academic, and philanthropic entities to partner and aggregate funding and other resources for the development of health technologies. PDPs have been established over the last two decades to target neglected diseases, to develop products on behalf of underserved populations, and more recently to develop products to address drug-resistant infections. These have included treatments for malaria (DNDi, 2019[99]), a vaccine to prevent meningitis in sub-Saharan Africa (PATH, 2018[100]), and a COVID‑19 vaccine (Hotez and Bottazzi, 2021[101]).
However, PDPs have limitations. A PDP may be unable to license an investigational compound for all countries, particularly for high-income countries which represent potential sources of commercial revenues. Further, a PDP may not have the resources to develop a product on behalf of all populations or may not have a manufacturing partner that can satisfy demand.
Governments can undertake R&D through public sector research institutes or university-sponsored research. The outputs are often licensed to private companies or not-for-profit partnerships. Governments can also conduct clinical trials, either to support the efforts of not-for-profit or private entities, or develop products originating in the public sector. Historically, some governments have also manufactured health products.
Governments could develop new products on an end-to‑end basis, and render the knowledge and data GPGs, and introduce policies that enable health technology products to approximate GPGs as far as possible (Sarpatwari, Brown and Kesselheim, 2020[102]). This could be undertaken through one or more government-funded entities or could involve contracting a private sector partner to fulfil certain functions. For example, the United States Army has announced the development of a vaccine against all COVID‑19 and SARS variants that is currently in government-funded clinical trials, and has indicated that it will partner with a private sector entity for the eventual vaccine rollout (Copp, 2021[103]).
Open-source pharmaceutical development is the full end-to‑end development and production of a health technology product in the public domain. It includes transparent working practices, such as the pre‑publication sharing of data and ideas, the possibility of participation of any person in real-time, and a form of shared ownership that ensures all methods and data are GPGs, and that health technology products can approximate GPGs (Balasegaram et al., 2017[104]).
In 2020, the COVID‑19 Moonshot, a collaborative open-science project, was launched with the goal of developing an unpatented oral antiviral drug to treat COVID‑19. It is likely the first open-science community effort to develop an antiviral drug. It endeavours not only to produce a health product as a GPG, but for all data to be published, including negative trial results. The Moonshot project has a “single and shared vision of no IP protection to ensure any resulting therapeutics are accessible and appropriate for people in LMICs” (DNDi, 2021[105]).
Governments can also support drug development by investing in, establishing or funding institutions and entities that can work with private, not-for-profit, or public sector counterparts. Potential enablers include: clinical trial networks, patent pools, regulatory initiatives, data hubs, compound libraries and technology transfer hubs.
Clinical trial networks, often part of PDPs, are collaborations that bring together investigators, ethicists, physicians, and researchers to develop and test the safety and efficacy of health products for one or more diseases. These networks can improve the efficiency and reduce the costs of clinical trials, ensure trial data are publicly disclosed, facilitate R&D in areas of unmet need, and be targeted to optimise or test health products to benefit populations that otherwise are underserved, such as children and pregnant women (Wellcome Trust, 2016[106]).
IP policies, including those concerning the use and licensing of patents, can help create a mix of approaches for leveraging investment for GPGs. As noted above, patent pools are mechanisms for facilitating the sharing of patents and other forms of IP to encourage the development of product combinations and formulations that address unmet needs, or to facilitate competition that can reduce prices of on-patent products.
The Medicines Patent Pool (MPP) signs license agreements with patent holders for both investigational compounds and approved products that can subsequently be out-licensed to third parties for the purposes specified in the in-license agreements (Medicines Patent Pool, 2022[107]; Medicines Patent Pool, n.d.[108]). These patent pools can reduce both rivalry and excludability of knowledge, data, and health products, although the scope of each license agreement depends principally on the preferences of the patent holders, and usually only allows for use of the IP in low- and some middle‑income countries. The MPP has signed voluntary licence agreements with Merck Sharp & Dohme (Merck, 2021[109]) for molnupiravir (an oral COVID‑19 antiviral), with Pfizer (Medicines Patent Pool, 2021[110]) for the antiviral nirmatrelvir, and with Shionogi for the antiviral ensitrelvir fumaric acid (Medicines Patent Pool, 2022[111]).
Regulatory initiatives can enhance access to new health technology products by streamlining registration. The WHO Prequalification Program assesses and prequalifies health products against different diseases to facilitate procurement either by international health agencies or by governments and non-state actors that rely upon WHO assessment (World Health Organization, n.d.[112]). The WHO also hosts the Collaboration Registration Procedure (CRP), which accelerates registration of products with WHO prequalification or approval by a stringent regulator, in up to 58 countries that have joined this Procedure (World Health Organization, 2013[113]).
Data sharing hubs collect data from multiple sources for distribution, sharing, and additional use, including for research. Data sharing hubs can play a role to support health R&D, and include data hubs for compounds, clinical trial data, virus data, and health data that is used to train and validate algorithms. The Global Initiative on Sharing Avian Influenza Data (GISAID) facilitates the sharing of influenza virus data and has also facilitated open sharing of COVID‑19 virus data (Maxmen, 2021[114]). The proposed European Health Data Space will promote exchange and access to different types of health data (electronic health records, genomics data, patient registry data) for health research, health delivery, and policy making (European Commission, n.d.[115]).
Compound libraries enable research entities to employ high-throughput screening to select molecules for further screening and pre‑clinical research (DNDi, n.d.[116]). Several research entities and pharmaceutical companies have made compound libraries fully or partially open for use by third parties. Compound libraries can play an important role in improving drug discovery for areas of need that otherwise are undersupplied and remove barriers to use of such knowledge.
Technology transfer hubs can facilitate the sharing of knowledge, data, IP and know-how for the development and manufacture of health technology products. Since the COVID‑19 pandemic began, the WHO has announced it intends to establish several hubs, including a first hub in South Africa that will expand the capacity of LMICs to develop COVID‑19 mRNA vaccines and scale‑up manufacturing. This would include the transfer of a comprehensive technology package, appropriate training and any licenses required to facilitate production and export of mRNA vaccines to LMICs (World Health Organization, 2021[117]).
Health technology innovation must be embedded in population-based functions and frameworks to realise synergies between national health systems and to support global health co‑operation. Almost a decade apart, Jamison (2013[118]) and Niang et al. (2021[119]) draw similar conclusions from pressing global health challenges: that enhanced investments to scale‑up health technologies and to address socio‑economic determinants of health are both critical to achieving long-term societal gains.
Collaborative platforms that aim to drive innovation in public goods often pursue both economic and social returns according to commercial and welfare logics. In this regard, new business models are springing from broader definitions of value. The Economy for the Common Good (ECG) model (Felber and Hagelber, 2017[120]) and the Human-Centred Business Model (HCBM) (Lessidrenska and Boyer, 2020[121]) are examples of frameworks based on a holistic and integrated set of economic and social priorities (Box 13.4).
Developed by the European Economic and Social Committee (European Economic and Social Committee, 2022[122]) of the European Commission, the ECG model proposes a more inclusive approach to Corporate Social Responsibility (European Commission, 2011[123]). The framework positions companies as drivers of delivering shared value and preventing and mitigating possible adverse outcomes.
The World Bank’s Global Forum on Law, Justice and Development (The World Bank, 2019[124]; Global Forum on Law, 2022[125]) and the OECD Development Centre (OECD, 2022[126]) have developed a framework through which corporate strategies, public policies and regulations incentivise companies to pursue sustainable development (Lessidrenska and Boyer, 2020[121]). The HCBM brings together diverse stakeholders – academia, private sector and professional associations, civil society, and international organisations – based on principles concerning financial mechanisms, fiscal policies, procurement policies, and stakeholder relationships (OECD, 2019[127]).
A recent report by the WHO (2021[128]) highlights the need to promote common goods for health, based on the convergence of health security, non-communicable and communicable disease risks, social determinants and environmental degradation. Further, achieving the Sustainable Development Goals (SDGs) by 2030 will require solutions that are simultaneously technological and societal, especially whole‑of-society approaches that include policy makers, funders, health care providers, researchers and industry as key stakeholders in global health (Lin and Ilona Kickbusch, 2017[129]).
In particular, collaboration and sustainable business models for health innovation must go hand in hand with multi-stakeholder engagement. Otherwise, investment risks and public mistrust of these new technologies could impede research, translation and wide‑scale use. A more systematic path to inclusive engagement and a whole‑of-society approach could help leverage the convergence between health technology innovation, markets and society (OECD, 2020[130]). For this reason, the UN has urged public health actors to:
[a]cknowledge the contribution of and important role played by all relevant stakeholders, including individuals, families and communities, intergovernmental organisations and religious institutions, civil society, academia, the media, voluntary associations and, where and as appropriate, the private sector and industry, in support of national efforts for non-communicable disease prevention and control, and recognise the need to further support the strengthening of co‑ordination among these stakeholders in order to improve the effectiveness of these efforts (United Nations, 2012[131]).
These trends towards a more holistic conception of health innovation have helped give rise to a number of frameworks and approaches to global health and resilience (see the chapter on resilience in other sectors) that explicitly integrate science, technology and society:
One Health: One Health is an integrated, unifying approach that “aims to sustainably balance and optimise the health of people, animals and ecosystems’’ (One Health High-Level Expert Panel, 2022[132]). This framework has been promoted by, for example, the WHO, the World Bank, the United States CDC, and the Food and Agriculture Organization. By recognising the animal-human-environment as a shared source of public health, well-being, and risk, One Health provides a blueprint for joint responses to COVID‑19 (OECD, 2020[130]). It is a core component of the WHO Global Action Plan on Antimicrobial Resistance (World Health Organization, 2015[133]). The One Health approach forms part of the Declaration of G20 Health Ministers (G20 Health Ministers, 2021[134]).
Health in All Policies: The Health in All Policies approach aims to systematically integrate public policies and activities across sectors (Koivusalo, 2010[135]). It takes into account ethical, legal, and social implications, seeks synergies, and avoids harmful impacts in order to improve population health and health equity (Carey, Crammond and Keast, 2014[136]; Pepin et al., 2017[137]).
The extent to which existing push funding mechanisms reduce or limit excludability and rivalry varies, and generally relies on voluntary measures. Many do not require recipients to commit to specific policies or practices to reduce the excludability or rivalry of outputs of the drug development process. Push funding mechanisms, and the obligations they may place on recipients, depend in part on the source of funding, and the nature of governance and decision making. Those mechanisms sponsored in part by governments have introduced specific obligations that ensured affordable prices, encouraged licensing of IP, expanded registration and supply, and required clinical trials supporting use in specific populations, such as children.
Blended finance that brings together the public and private sector may introduce policies to limit excludability or rivalry of a health product, but these policies generally rely on voluntary or contractual obligations that may not address either the underlying causes of excludability or rivalry, or their impacts.
Push funding by commercial entities or private investors may prioritise commercial return on investment, may not extend the benefits of access policies to countries perceived as strong commercial markets, or provide access in countries considered “commercially non-viable”. However, some entities, such as the RIGHT Fund in Korea, use contractual clauses in funding grants and IP approaches to help ensure that the resulting products are made available and accessible in terms of price, quantity, quality and timeliness.
Procurement-based pull incentives can promote access in included countries but can exacerbate excludability and/or rivalry for omitted countries and populations. Others, such as transferable exclusivity rights and extended market exclusivity, can either exacerbate the rivalry or excludability of one health technology product, or adversely affect the rivalry or excludability of another. These pull incentives may prove significantly more costly to public health budgets than the direct funding of R&D. They have the potential to be “gamed” by recipients for commercial benefit while not necessarily encouraging innovation. Further, they need not include additional obligations on recipients to reduce excludability or rivalry of resulting health products.
A priority review voucher may extend the effective patent life of another health product, without imposing any obligations to reduce the excludability or rivalry of the index product. Advanced market commitments and subscription-based reimbursement models rely on procurement as a means of incentivising the development or ensuring the supply of a health technology product. Furthermore, procurement-based pull incentives do not require recipients to share IP and know-how. This means knowledge and data associated with such health products remain excludable, limiting the entry of additional suppliers that could expand supply and reduce prices through competition. Other pull incentive mechanisms, such as innovation prizes and patent buyouts, are yet to be scaled up for use.
The pandemic has underlined the importance of developing health technologies to prepare for and respond to future crises, and ensuring that the resulting products are as widely accessible as possible. Many options for financing and incentivising the development of these products exist, and no single approach will be appropriate in every circumstance. However, it is clear that market forces will prevail, absent specific provisions or mechanisms for ensuring affordable access and equitable distribution. Whichever incentives or financing approaches funders elect to employ, they should evaluate the implications for pricing, supply and access, and consider defining and enforcing clear obligations to minimise excludability and rivalry. These considerations, which are relevant to essential health products for health emergencies, also have salience outside of shocks, and contribute to resilient and equitable health systems.
Appropriate policy and regulation should ensure that certain essential health technologies are able to approximate GPGs as far as possible. Most health products cannot become “pure” GPGs. Nevertheless, it is important for public health and equity that publicly funded incentives and investments are framed to limit rivalry and excludability. Policy makers can address at least five characteristics of health technology products to positively affect a product’s rivalry and excludability: supply; exclusive ownership of IP rights; registration; affordability; and presentation. Push and pull mechanisms that address any of these characteristics can reduce rivalry and/or excludability.
Collaboration, shared financing and IP rights arrangements lie at the heart of models that can provide health solutions where there is market failure. Where health technologies are underprovided by competitive, revenue‑driven markets, delinking research, manufacturing and supply from sales revenues is critical.
Collaboration is important to balance investment and rewards with societal priorities. Governments, non-profit partnerships, funders, public researchers and the pharmaceutical industry should consider collaborating more to drive sustainable innovation and market development for health technologies. The concept of aspiring for essential health technologies to become GPGs helps to align stakeholder priorities better for shared investment, value creation and appropriation.
Blended financing could facilitate multi-sector engagement and strengthen long-term commitment to developing essential health technologies. Policy makers could support blended finance mechanisms to attract and better integrate private resources, de‑risk investment and enable more sustainable innovation where there is market failure. Some companies earned record profits from COVID‑19 vaccine sales, even as supply and affordability were critical problem for many countries. Blended finance mechanisms should ensure that future collaborations benefit public and private sectors equitably.
Collective and co‑ordinated management of IP rights and know-how could help expand access to essential health technologies. Voluntary licensing via patent pools or technology access pools (such as the WHO-led COVID‑19 Technology Access Pool) is one mechanism for promoting access. Technology transfer hubs, such as the WHO mRNA Technology Transfer Hub, can also reduce the excludability of platform technologies. This is particularly important in the context of novel and existing platform technologies, which are increasingly central to the development of health technologies. Even though they are often constructed over decades of publicly funded research, many platform technologies are excludable because IP rights are held by disparate entities.
Policy makers could facilitate expanding manufacturing and supply of health technology products through IP arrangements and decentralised manufacturing. Exclusive manufacturing by a patent holder of a health technology product may not be adequate to meet overall demand, thereby exacerbating rivalry. Sharing IP and know-how can be one means of expanding manufacturing and supply. Decentralised manufacturing of health technology products – especially in regions or continents that may otherwise face supply shortages but are able or willing to procure relevant health technologies – can reduce rivalry (see the chapter on securing supply chains). Decentralised or open manufacturing can also foster competition and reduce prices, thereby improving affordability and reducing excludability.
Public funders of health technology products could define and mandate clear obligations to reduce excludability and rivalry. Push funding mechanisms that aggregate or mix public and private sector funding often rely too heavily on voluntary measures that do not address the causes of excludability or rivalry, or neglect such issues entirely. Furthermore, push funding mechanisms governed only by companies or private investors may prioritise commercial markets, or may not address access barriers in markets viewed as commercially non-viable. Where private sector funding either precedes or follows public sector investment, recipients are likely to prioritise commercial returns over minimising rivalry and excludability of a product.
Governments should consider maintaining a degree of ownership of, or interest in, the development or manufacture of a health product when providing public funding or pull incentives for it. Governments could use this interest to require affordable pricing, encourage licensing of IP, mandate expanded registration and/or supply, and facilitate the conduct of clinical trials to ensure that products can be used by specific populations, such as children. While voluntary measures designed with or by recipients can reduce excludability and rivalry on a case‑by-case basis, they may not be as far-reaching or rigorous as those mandated by funders.
Governments could trial novel pull incentives, such as innovation prizes and patent buyouts, to assess the extent to which these can minimise excludability and rivalry of health products. Pilot projects with prizes or patent buyouts could be used to determine whether prizes are suited to stimulating development of health products to address unmet health needs; whether outputs can approximate GPGs; and whether the technical challenges of determining and administering prizes can be overcome in practice.
Even though push and pull mechanisms are both needed to incentivise the development of novel health technologies, policy makers may wish to reconsider the value of some pull incentives. Transferable exclusivity rights and extended market exclusivity can exacerbate rivalry of the index product or another product to which exclusivity has been transferred. These pull incentives may also prove significantly more costly to public health budgets than the actual R&D expenses incurred, and they have the potential to be “gamed” for commercial benefit. In addition, they may not be well suited to supporting commercial operations for smaller firms that require near-term revenues, and they do not impose additional obligations on recipients to minimise excludability or rivalry.
Advance purchase agreements should be as inclusive as possible of countries and populations worldwide, and should require recipients to share IP and know-how. They should not preclude longer-term efforts to reduce excludability or rivalry through other means, including competition. Pull incentives such as advance market commitments, advance purchase agreements and buy-down agreements – which can partially de‑risk private sector R&D investments – may benefit countries that are included in such mechanisms, but they may worsen the situation for excluded countries or populations. They may also limit the ability of governments to reduce the rivalry or excludability of a health technology product over the long-term. Where appropriate, these instruments should also integrate obligations to avoid misuse or overuse of health technology products that should be conserved for use by future generations.
Governments (and, where relevant, not-for-profit entities) could explore novel R&D models that are designed to prioritise the public interest. Models such as product development partnerships, public sector development and production, and open-source pharmaceutical development may be particularly relevant. Not-for-profit entities have played a valuable role in working with companies to reduce rivalry and excludability of new medicines, including ensuring that such medicines gain marketing authorisation widely, accelerating paediatric drug development to minimise the lag between availability of treatments for adults and children, and improving affordability.
Governments could invest in, establish or fund institutions to improve the supply of GPGs, including patent pools, clinical trial networks, regulatory initiatives, data hubs, compound libraries and technology transfer hubs. Efforts by governments (and companies) to use donations to reduce rivalry or excludability of a health technology product, while well intentioned, may not always be readily usable by recipient countries. As such, donations – in accordance with interagency guidelines for medicine donations issued by WHO and other international health agencies (WHO et al., 2011[138]) – should be used only in exceptional circumstances, be time limited and comport with internationally mandated guidelines and the preferences of recipient countries.
Policy makers could mandate or encourage greater transparency of funding and financing agreements between governments and recipients, and improve overall transparency of total GPG-related financing flows. Such transparency could improve the ability of all governments and non-state actors to assess whether health technology products, as well as underlying data and knowledge, will be non-excludable and non-rivalrous. Furthermore, funding and financing mechanisms should require greater transparency with respect to the product being funded, the costs of research and production, IP protections, and the prices of the product worldwide. Finally, governments should report on funding and financing of relevant push and pull mechanisms, using the framework of Total Official Support for Sustainable Development, to improve transparency of such funding flows and to increase financing.
Policy makers could facilitate the alignment of interests and investments between public and philanthropic funders. There is currently no permanent governance mechanism by which to ensure that the divergent interests and investments of multiple public and philanthropic funders can be reconciled. For example, the Access to COVID‑19 Tools Accelerator, a governance mechanism established to accelerate the introduction of COVID‑19 technologies after the pandemic began, was not fully able to encourage such co‑ordination and has been disbanded (WHO, 2022[139]). Policy makers could consider encouraging collaboration between such funders to reduce the rivalry and excludability of health technology products, and ensure appropriate dialogue and engagement with private sector funders.
No one policy will resolve the challenges that are the subject of this chapter. The policy options suggested need to be considered alongside measures to estimate the benefits associated with each. The sharing of this information will allow the goal of universal access to essential health technologies to become closer in reality.
[17] Access to Medicine Foundation (2021), 2021 Antimicrobial Resistance Benchmark, https://accesstomedicinefoundation.org/publications/2021-antimicrobial-resistance-benchmark (accessed on 31 May 2022).
[52] AMR Action Fund (2022), Antimicrobial Resistance Research & Development - AMR Action Fund, AMR Action Fund, https://www.amractionfund.com/ (accessed on 9 December 2022).
[54] AMR Action Fund (2022), Our Investments, https://www.amractionfund.com/our-investment#page-section-1 (accessed on 23 June 2022).
[56] AMR Action Fund (2022), Stewardship and Access Principles, https://www.amractionfund.com/our-investments#page-section-2 (accessed on 31 May 2022).
[36] Apampa, A. (2022), Can blended finance help fill the financing gap in health and education?, Convergence, https://www.convergence.finance/news-and-events/news/3sJLhFFmJi5M60tQ7zut69/view (accessed on 9 December 2022).
[58] Årdal, C., Y. Lacotte and M. Ploy (2020), “Financing Pull Mechanisms for Antibiotic-Related Innovation: Opportunities for Europe”, Clinical Infectious Diseases, Vol. 71/8, pp. 1994-1999, https://doi.org/10.1093/CID/CIAA153.
[98] Årdal, C., M. Ploy and Y. Lacotte (2021), D9.2 Strategy for a multi-country incentive in Europe, EU-JAMRAI, https://eu-jamrai.eu/wp-content/uploads/2021/03/EUjamrai_D9.2_Strategy-for-a-multi-country-incentive-in-Europe_INSERM-FHI.pdf (accessed on 8 February 2023).
[104] Balasegaram, M. et al. (2017), “An open source pharma roadmap”, PLOS Medicine, Vol. 14/4, p. e1002276, https://doi.org/10.1371/JOURNAL.PMED.1002276.
[67] Baraldi, E. et al. (2019), “Economic incentives for the development of new antibiotics Report commissioned by the Public Health Agency of Sweden”.
[82] Benjamin, D. et al. (2006), “Peer-Reviewed Publication of Clinical Trials Completed for Pediatric Exclusivity”, JAMA, Vol. 296/10, pp. 1266-1273, https://doi.org/10.1001/JAMA.296.10.1266.
[85] Berkley, S. (2022), The Gavi COVAX AMC Explained, Gavi, the Vaccines Alliance, https://www.gavi.org/vaccineswork/gavi-covax-amc-explained (accessed on 31 May 2022).
[83] Bostyn, S. (2021), Tackling Salami Slicing and Indication Stacking in Orphan Drug Innovation Incentives, Bill of Health, https://blog.petrieflom.law.harvard.edu/2021/09/15/orphan-drug-innovation-incentives/ (accessed on 31 May 2022).
[81] Burns, J. (2017), “Orphan Drugs: Way Too Many, Way Too Expensive”, Managed Care (Langhorne, Pa.), Vol. 26/6, pp. 12-17.
[25] Cama, J. et al. (2021), “To Push or to Pull? in a Post-COVID World, Supporting and Incentivizing Antimicrobial Drug Development Must Become a Governmental Priority”, ACS Infectious Diseases, Vol. 7/8, pp. 2029-2042, https://doi.org/10.1021/ACSINFECDIS.0C00681.
[37] CARB-X (2022), CARB-X: Combating Antibiotic-Resistant Bacteria, https://carb-x.org/about/overview/ (accessed on 31 May 2022).
[38] CARB-X (2022), Stewardship and Access - CARB-X, https://carb-x.org/about/stewardship-and-access/ (accessed on 31 May 2022).
[39] CARB-X (2021), Stewardship & Access Plan (SAP) Development Guide, https://cms.wellcome.org/sites/default/files/2021-03/stewardship-access-plan-2021.pdf (accessed on 31 May 2022).
[136] Carey, G., B. Crammond and R. Keast (2014), “Creating change in government to address the social determinants of health: How can efforts be improved?”, BMC Public Health, Vol. 14/1, https://doi.org/10.1186/1471-2458-14-1087.
[40] CEPI (2022), Why we exist – CEPI, https://cepi.net/about/whyweexist/ (accessed on 31 May 2022).
[46] CEPI (2021), “2022-2026 Strategy”, https://cepi.net/wp-content/uploads/2021/03/20211201-CEPI-2022-2026-Strategy.pdf (accessed on 31 May 2022).
[43] CEPI (2021), Enabling Equitable Access to COVID-19 Vaccines: Summary of equitable access provisions in CEPI’s COVID-19 vaccine development agreements, https://cepi.net/wp-content/uploads/2020/12/Enabling-equitable-access-to-COVID19-vaccines-v4-18Mar2021.pdf (accessed on 31 May 2022).
[41] CEPI (2017), “CEPI Policy Documentation”, https://msfaccess.org/sites/default/files/2018-09/CEPIoriginalPolicy_2017.pdf (accessed on 31 May 2022).
[87] Chen, C. et al. (2019), “Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis”, The Lancet Global Health, Vol. 7/1, pp. e58-e67, https://doi.org/10.1016/S2214-109X(18)30422-4.
[88] Chu, M. (2017), Pfizer Korea wins pneumonia vaccine patent dispute with SK Chemicals, Korea Biomedical Review, http://www.koreabiomed.com/news/articleView.html?idxno=2061 (accessed on 31 May 2022).
[30] COMBACTE (2022), COMBACTE: Combatting Bacterial Resistance in Europe, COMBACTE, https://www.combacte.com/ (accessed on 9 December 2022).
[96] Cookson, C. (2022), “UK launches world-first ‘subscription’ model for antibiotic supply”, Financial Times.
[103] Copp, T. (2021), US Army Creates Single Vaccine Against All COVID & SARS Variants, Researchers Say, Defense One, https://www.defenseone.com/technology/2021/12/us-army-creates-single-vaccine-effective-against-all-covid-sars-variants/360089/ (accessed on 31 May 2022).
[73] Darrow, J. and A. Kesselheim (2020), “Incentivizing antibiotic development: Why isn’t the generating antibiotic incentives now (gain) act working?”, Open Forum Infectious Diseases, Vol. 7/1, https://doi.org/10.1093/OFID/OFAA001.
[21] Das, M. (2021), “COVID-19 vaccine and the cold chain implications for global adoption”, Indian journal of public health, Vol. 65/3, pp. 307-310, https://doi.org/10.4103/IJPH.IJPH_1353_20.
[105] DNDi (2021), “Another Triumph of Science, But Defeat for Access?”, https://dndi.org/wp-content/uploads/2021/08/DNDi-COVID-19-Policy-Report-2021.pdf (accessed on 31 May 2022).
[99] DNDi (2019), Malaria ASAQ, https://dndi.org/research-development/portfolio/asaq/ (accessed on 31 May 2022).
[116] DNDi (n.d.), Drug discovery: It all starts in the compound library, https://dndi.org/research-development/drug-discovery/ (accessed on 31 May 2022).
[31] European Commission (2019), Vaccine against Ebola: Commission grants market authorisation, European Commission, https://ec.europa.eu/commission/presscorner/detail/en/ip_19_6246 (accessed on 9 December 2022).
[65] European Commission (2017), Horizon prize for better use of antibiotics, https://ec.europa.eu/info/research-and-innovation/funding/funding-opportunities/prizes/horizon-prizes/better-use-antibiotics_en (accessed on 31 May 2022).
[123] European Commission (2011), EU strategy 2011-14 for Corporate Social Responsibility, EC, https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2011:0681:FIN:EN:PDF (accessed on 21 September 2021).
[115] European Commission (n.d.), European Health Data Space, https://ec.europa.eu/health/ehealth-digital-health-and-care/european-health-data-space_en (accessed on 31 May 2022).
[122] European Economic and Social Committee (2022), European Economic and Social Committee - Homepage, European Economic and Social Committee, https://www.eesc.europa.eu/en (accessed on 9 December 2022).
[74] European Medicines Agency (2015), “The EU Paediatric Regulation in 5 minutes”, https://www.ema.europa.eu/en/documents/presentation/presentation-european-union-paediatric-regulation-5-minutes-paolo-tomasi_en.pdf (accessed on 31 May 2022).
[120] Felber, C. and G. Hagelber (2017), The Economy for Common Good, The Next System Project.
[134] G20 Health Ministers (2021), Declaration of the G20 Health Ministers, G20, Rome, https://www.g20.org/ (accessed on 9 September 2021).
[72] Gammie, T., C. Lu and Z. Ud-Din Babar (2015), “Access to Orphan Drugs: A Comprehensive Review of Legislations, Regulations and Policies in 35 Countries”, PLOS ONE, Vol. 10/10, p. e0140002, https://doi.org/10.1371/JOURNAL.PONE.0140002.
[53] Garden, H. (forthcoming), “Collaborative action where markets fail: Strengthening innovation and access to health technologies”, OECD Publishing, Paris.
[50] GHIT (2022), Access Policies | Global Health Innovative Technology Fund, https://www.ghitfund.org/applyforfunding/accesspolicy/en (accessed on 23 June 2022).
[49] GHIT (2022), Co-investment | Global Health Innovative Technology Fund, https://www.ghitfund.org/investment/coinvestment/ (accessed on 23 June 2022).
[47] GHIT (2022), GHIT Fund | Global Health Innovative Technology Fund, https://www.ghitfund.org/en (accessed on 23 June 2022).
[48] GHIT (2022), Investment Overview | Global Health Innovative Technology Fund, https://www.ghitfund.org/investment/overview/en (accessed on 23 June 2022).
[51] GHIT Fund (2018), “GHIT Fund 2.0 Strategic Plan: FY2018 – FY2022”, https://www.ghitfund.org/assets/othermedia/StrategicPlan_2018-2022_eng.pdf (accessed on 31 May 2022).
[125] Global Forum on Law, J. (2022), Global Forum on Law, Justice and Development | Generating Innovative Legal Solutions to Development Challenges, Global Forum LJD, https://www.globalforumljd.com/ (accessed on 9 December 2022).
[33] Global Health Advocates (2021), The European Parliament calls for openness, transparency and inclusiveness in Innovative Health Initiative, Global Health Advocates, https://www.ghadvocates.eu/the-european-parliament-calls-for-openness-transparency-and-inclusiveness-in-innovative-health-initiative/ (accessed on 9 December 2022).
[95] Glover, R. et al. (2019), “Subscription model for antibiotic development”, BMJ, p. l5364, https://doi.org/10.1136/bmj.l5364.
[93] Gotham, D. et al. (2021), “Reimbursement models to tackle market failures for antimicrobials: Approaches taken in France, Germany, Sweden, the United Kingdom, and the United States”, Health Policy, Vol. 125/3, pp. 296-306, https://doi.org/10.1016/J.HEALTHPOL.2020.11.015.
[12] Hatefi, A., R. Marten and R. Smith (2020), “Global-scale action in health: a common language is a critical starting point to bolster global health financing”, Health Policy and Planning, Vol. 35/8, pp. 1133-1136, https://doi.org/10.1093/HEAPOL/CZAA090.
[101] Hotez, P. and M. Bottazzi (2021), A COVID Vaccine for All, Scientific American, https://www.scientificamerican.com/article/a-covid-vaccine-for-all/ (accessed on 31 May 2022).
[29] IMI Innovative Medicines Initiative (2022), IMI - Europe’s partnership for health, IMI Innovative Medicines Initiative, https://www.imi.europa.eu/ (accessed on 9 December 2022).
[62] InnoCentive (2022), About - InnoCentive, https://www.innocentive.com/about-us/about/ (accessed on 31 May 2022).
[76] Institute of Medicine (2012), Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act, The National Academies Press, Washington, DC, https://doi.org/10.17226/13311 (accessed on 31 May 2022).
[32] Ishola, D. et al. (2021), “Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial”, The Lancet Infectious Diseases, https://doi.org/10.1016/S1473-3099(21)00125-0.
[118] Jamison, D. et al. (2013), “Global health 2035: a world converging within a generation”, The Lancet, Vol. 382, pp. 1898-1955, https://doi.org/10.1016/S0140-6736(13)62105-4.
[7] Kaul, I. and M. Faust (2001), Global public goods and health: Taking the agenda forward.
[135] Koivusalo, M. (2010), “The state of Health in All policies (HiAP) in the European Union: potential and pitfalls”, Journal of Epidemiology & Community Health, Vol. 64/6, pp. 500-503, https://doi.org/10.1136/JECH.2009.102020.
[60] Kremer, M. (1998), “Patent buyouts: A mechanism for encouraging innovation”, The Quarterly Journal of Economics, Vol. 113/4, pp. 1137-1167, https://doi.org/10.1162/003355398555865.
[5] Ledford, H. (2022), “COVID vaccine hoarding might have cost more than a million lives”, Nature, https://doi.org/10.1038/D41586-022-03529-3.
[64] Legal Information Institute (n.d.), 15 U.S. Code § 3719 - Prize competitions, Legal Information Institute, https://www.law.cornell.edu/uscode/text/15/3719 (accessed on 31 May 2022).
[121] Lessidrenska, T. and D. Boyer (2020), “Human-Centered Business Model - Social and Environmental Principles”, https://www.globalforumljd.org/ (accessed on 9 July 2021).
[35] Lin, A. and P. Sharma (2019), Greater than the sum of its parts: blended finance roadmap for global health, USAID , Washington, D.C.
[129] Lin, V. and P. Ilona Kickbusch (2017), Progressing the Sustainable Development Goals through Health in All Policies: Case studies from around the world, WHO, Government of South Australia, https://www.who.int/publications/m/item/progressing-the-sustainable-development-goals-through-health-in-all-policies (accessed on 26 August 2021).
[89] Liu, A. (2017), Pfizer wins Indian patent to Prevnar 13, draws cries of ‘monopoly’ from MSF, Fierce Pharma, https://www.fiercepharma.com/vaccines/pfizer-gains-indian-patent-to-prevnar-13-draws-monopoly-criticism-from-msf (accessed on 31 May 2022).
[14] Love, J. (2020), The Use and Abuse of the Phrase “Global Public Good” – Developing Economics, Developing Economics, https://developingeconomics.org/2020/07/16/the-use-and-abuse-of-the-phrase-global-public-good/ (accessed on 19 April 2021).
[66] Love, J. and T. Hubbard (2009), “Prizes for innovation of new medicines and vaccines.”, Annals of health law / Loyola University Chicago, Vol. 18/2, pp. 155-186, https://www.mendeley.com/catalogue/4176a5c8-4151-3009-9f1a-1638d21e8272/ (accessed on 31 May 2022).
[114] Maxmen, A. (2021), “One million coronavirus sequences: popular genome site hits mega milestone”, Nature, Vol. 593/7857, p. 21, https://doi.org/10.1038/D41586-021-01069-W.
[70] Médecins Sans Frontières (2017), Spotlight on: USFDA neglected disease PRV, Access Campaign, https://msfaccess.org/spotlight-usfda-neglected-disease-prv (accessed on 31 May 2022).
[107] Medicines Patent Pool (2022), Home - MPP, Medicines Patent Pool, https://medicinespatentpool.org/ (accessed on 9 December 2022).
[111] Medicines Patent Pool (2022), Shionogi and the Medicines Patent Pool (MPP) sign licence agreement for COVID-19 oral antiviral treatment candidate to increase access in low- and middle-income countries, MPP, https://medicinespatentpool.org/news-publications-post/shionogi-and-the-medicines-patent-pool-mpp-sign-licence-agreement-for-covid-19-oral-antiviral-treatment-candidate-to-increase-access-in-low-and-middle-income-countries (accessed on 9 December 2022).
[110] Medicines Patent Pool (2021), Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries, MPP, https://medicinespatentpool.org/news-publications-post/pfizer-and-the-medicines-patent-pool-mpp-sign-licensing-agreement-for-covid-19-oral-antiviral-treatment-candidate-to-expand-access-in-low-and-middle-income-countries (accessed on 9 December 2022).
[108] Medicines Patent Pool (n.d.), About Us - MPP, https://medicinespatentpool.org/who-we-are/about-us (accessed on 31 May 2022).
[109] Merck (2021), The Medicines Patent Pool (MPP) and Merck Enter Into License Agreement for Molnupiravir, an Investigational Oral Antiviral COVID-19 Medicine, to Increase Broad Access in Low- and Middle-Income Countries, Merck Media, https://www.merck.com/news/the-medicines-patent-pool-mpp-and-merck-enter-into-license-agreement-for-molnupiravir-an-investigational-oral-antiviral-covid-19-medicine-to-increase-broad-access-in-low-and-middle-income-countri/ (accessed on 9 December 2022).
[11] Moon, S., J. Rottingen and J. Frenk (2017), “Global public goods for health: Weaknesses and opportunities in the global health system”, Health Economics, Policy and Law, Vol. 12/2, pp. 195-205, https://doi.org/10.1017/S1744133116000451.
[16] Morel, C., S. Edwards and S. Harbarth (2017), “Preserving the ’commons’: addressing the sustainable use of antibiotics through an economic lens”, Clin Microbiol Infect, Vol. 23, p. 718, https://doi.org/10.1016/j.cmi.2017.08.002.
[23] Morin, S. et al. (2022), “Pediatric COVID-19 Therapeutics: Seizing the Right Research and Development Opportunities to Accelerate Access for Children”, Pediatric Infectious Disease Journal, Vol. 41/1, pp. E1-E5, https://doi.org/10.1097/INF.0000000000003331.
[42] MSF Access Campaign (2019), “THE WRONG PRESCRIPTION FOR VACCINE ACCESS”, https://msfaccess.org/open-letter-cepi-board-members-revise-cepis- (accessed on 31 May 2022).
[92] Mueller, B. and R. Robbins (2021), Where a Vast Global Vaccination Program Went Wrong, The New York Times, https://www.nytimes.com/2021/08/02/world/europe/covax-covid-vaccine-problems-africa.html (accessed on 31 May 2022).
[119] Niang, M. et al. (2021), “Why is repositioning public health innovation towards a social paradigm necessary? A reflection on the field of public health through the examples of Ebola and Covid-19”, Globalization and Health 2021 17:1, Vol. 17/1, pp. 1-11, https://doi.org/10.1186/S12992-021-00695-3.
[22] Nolen, S. and R. Robbins (2021), Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries, The New York Times, https://www.nytimes.com/2021/11/16/health/covid-pill-pfizer.html (accessed on 31 May 2022).
[34] OECD (2022), Blended Finance, OECD, Paris, https://www.oecd.org/dac/financing-sustainable-development/blended-finance-principles/ (accessed on 9 December 2022).
[126] OECD (2022), Development Centre, OECD, Paris, https://www.oecd.org/dev/ (accessed on 9 December 2022).
[3] OECD (2021), “Access to COVID-19 vaccines: Global approaches in a global crisis”, OECD Policy Responses to Coronavirus (COVID-19), OECD Publishing, Paris, https://doi.org/10.1787/c6a18370-en.
[130] OECD (2020), Development Co-operation Report 2020: Learning from Crises, Building Resilience, OECD Publishing, Paris, https://doi.org/10.1787/f6d42aa5-en.
[127] OECD (2019), The Human-Centred Business Model (HCBM): A Holistic Approach to a New Model for Sustainable Business, OECD, Paris, https://www.oecd.org/dev/human-centred-business-model-hcbm.htm (accessed on 9 December 2022).
[1] OECD (2018), Pharmaceutical Innovation and Access to Medicines, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/9789264307391-en.
[132] One Health High-Level Expert Panel (2022), “One Health Theory of Change”, One Health High-Level Expert Panel.
[100] PATH (2018), Lining up for hope—and a meningitis vaccine, https://www.path.org/articles/lining-up-for-hopeand-a-meningitis-vaccine/ (accessed on 31 May 2022).
[78] PCMA (2021), Orphan Drugs Sound Small but Can Lead to Big Drug Spending, https://www.pcmanet.org/big-drug-spending/ (accessed on 31 May 2022).
[24] Penazzato, M. et al. (2018), “Shortening the decade-long gap between adult and paediatric drug formulations: A new framework based on the HIV experience in low- and middle-income countries: A”, Journal of the International AIDS Society, Vol. 21, https://doi.org/10.1002/JIA2.25049.
[137] Pepin, D. et al. (2017), “Collaborating for health: Health in all policies and the law”, Journal of Law, Medicine and Ethics, Vol. 45/1_suppl, pp. 60-64, https://doi.org/10.1177/1073110517703327.
[97] Plackett, B. (2020), “Why big pharma has abandoned antibiotics”, Nature, Vol. 586/7830, pp. S50-S52, https://doi.org/10.1038/D41586-020-02884-3.
[28] Policy Cures Research (2020), COVID-19 R&D tracker, https://www.policycuresresearch.org/covid-19-r-d-tracker (accessed on 8 November 2020).
[44] Rauhala, E. (2021), Moderna agreed to ’equitable access’ for its coronavirus vaccine, but most of its doses are going to wealthy countries, The Washington Post, https://www.washingtonpost.com/world/coronavirus-vaccine-access-poor-countries-moderna/2021/02/12/0586e532-6712-11eb-bf81-c618c88ed605_story.html (accessed on 31 May 2022).
[55] ReAct (2021), Ensuring sustainable access to antibiotics for everyone, everywhere, https://www.reactgroup.org/wp-content/uploads/2021/09/ReAct-Report-Ensuring-sustainable-access-to-effective-antibiotics-for-everyone-everywhere-How-to-address-the-global-crisis-in-antibiotic-Research-and-Development-March-2021.pdf (accessed on 31 May 2022).
[9] Reisen, H., M. Soto and T. Weithöner (2004), Financing global and regional public goods through ODA: analysis and evidence from the OECD Creditor Reporting System, OECD, Paris, https://www.oecd.org/development/pgd/24482500.pdf (accessed on 17 May 2021).
[57] RIGHT Foundation (2020), Global Access Policy, RIGHT Foundation, https://rightfund.org/en/access-policy/ (accessed on 9 December 2022).
[6] Ringel, M. et al. (2020), “Breaking Eroom’s Law”, Nature Reviews Drug Discovery, https://doi.org/10.1038/d41573-020-00059-3.
[20] Robbins, R. (2021), Moderna, Racing for Profits, Keeps Covid Vaccine Out of Reach of Poor, The New York Times, https://www.nytimes.com/2021/10/09/business/moderna-covid-vaccine.html (accessed on 31 May 2022).
[59] Rome, B. and A. Kesselheim (2020), “Transferrable market exclusivity extensions to promote antibiotic development: An economic analysis”, Clinical Infectious Diseases, Vol. 71/7, pp. 1671-1675, https://doi.org/10.1093/CID/CIZ1039.
[26] Røttingen, J. et al. (2013), “Mapping of available health research and development data: what’s there, what’s missing, and what role is there for a global observatory?”, The Lancet, Vol. 382/9900, pp. 1286-1307, https://doi.org/10.1016/s0140-6736(13)61046-6.
[80] Sarpatwari, A. et al. (2018), “Evaluating the impact of the Orphan Drug Act’s seven-year market exclusivity period”, Health Affairs, Vol. 37/5, pp. 732-737, https://doi.org/10.1377/HLTHAFF.2017.1179.
[102] Sarpatwari, A., D. Brown and A. Kesselheim (2020), “Development of a National Public Pharmaceutical Research and Development Institute”, Journal of Law, Medicine and Ethics, Vol. 48/1, pp. 225-227, https://doi.org/10.1177/1073110520917023.
[15] Schlein, L. (2021), Pandemic Will End When Vaccine Inequity Ends, WHO Chief Says , https://www.voanews.com/a/pandemic-will-end-when-vaccine-inequity-ends-who-chief-says-teaser-who-says-boosters-should-be-given-to-the-elderly-and-people-who-are-immunocompromised-and-not-to-those-at-low-risk-such-as-children-/6362485.html (accessed on 31 May 2022).
[77] Sinha, M. et al. (2018), “Labeling Changes and Costs for Clinical Trials Performed under the US Food and Drug Administration Pediatric Exclusivity Extension, 2007 to 2012”, JAMA Internal Medicine, Vol. 178/11, pp. 1458-1466, https://doi.org/10.1001/jamainternmed2018.3933.
[13] Stein, F. and D. Sridhar (2017), “Health as a “global public good”: Creating a market for pandemic risk”, BMJ (Online), Vol. 358, https://doi.org/10.1136/bmj.j3397.
[61] Stiglitz, J. (2007), Prizes, Not Patents, Project Syndicate, https://www.project-syndicate.org/commentary/prizes--not-patents (accessed on 31 May 2022).
[75] ’t Hoen, E., P. Boulet and B. Baker (2017), “Data exclusivity exceptions and compulsory licensing to promote generic medicines in the European Union: A proposal for greater coherence in European pharmaceutical legislation”, Journal of Pharmaceutical Policy and Practice, Vol. 10/1, p. 19, https://doi.org/10.1186/s40545-017-0107-9.
[79] Technopolis Group (2019), “Study to support the evaluation of the EU Orphan Regulation”, European Commission.
[90] The Pharma Letter (2019), First alternative pneumonia vaccine breaks Pfizer and GSK’s decades-long stranglehold, says MSF, https://www.thepharmaletter.com/article/first-alternative-pneumonia-vaccine-breaks-pfizer-and-gsk-s-decades-long-stranglehold-says-msf (accessed on 31 May 2022).
[124] The World Bank (2019), Human-Centred Business Model: Sustainable Business Practices for Sustainable Development Outcomes, The World Bank, https://www.worldbank.org/en/events/2019/02/21/human-centered-business-model (accessed on 9 December 2022).
[84] The World Bank (2009), Pilot Advance Market Commitment For Vaccines against Pneumococcal Diseases, The World Bank, Washington.
[86] Tricarico, S. et al. (2017), “Pneumococcal conjugate vaccine implementation in middle-income countries”, Pneumonia, Vol. 9/1, https://doi.org/10.1186/S41479-017-0030-5.
[4] UN Secretary General High-Level Panel on Access to Medicines (2016), Report of the UN Secretary General’s High-Level Panel on Access to Medicines, High-Level Panel on Access to Medicines, http://www.unsgaccessmeds.org/final-report (accessed on 9 December 2022).
[8] UN Secretary-General (2020), Quick, Equal, Affordable Access to COVID-19 Vaccine Must Be Considered Global Public Good, Secretary-General Says in Remarks to Africa Dialogue Series, UN Press, https://press.un.org/en/2020/sgsm20089.doc.htm (accessed on 9 December 2022).
[19] UNDP Data Futures Platform (2021), Vaccine affordability: Global Dashboard for Vaccine Equity, https://data.undp.org/vaccine-equity/affordability/ (accessed on 31 May 2022).
[18] UNICEF Supply Division (2022), COVID-19 Market Dashboard, UNICEF, https://www.unicef.org/supply/covid-19-market-dashboard (accessed on 9 December 2022).
[131] United Nations, G. (2012), Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases Annex Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases, UN, http://www.who.int/publications/en/. (accessed on 8 November 2021).
[68] US Food and Drug Administration (2020), Tropical Disease Priority Review Voucher Program, https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/tropical-disease-priority-review-voucher-program (accessed on 31 May 2022).
[69] US Government Accountability Office (2020), “Drug Development: FDA’s Priority Review Voucher Program”, GAO-20-251.
[71] US House of Representatives (2021), Drug Pricing Investigation: Majority Staff Report.
[45] Usher, A. (2021), “CEPI criticised for lack of transparency”, Lancet (London, England), Vol. 397/10271, pp. 265-266, https://doi.org/10.1016/S0140-6736(21)00143-4.
[91] Usher, A. (2019), “Low-cost pneumonia vaccine breaks into global market”, Lancet (London, England), Vol. 393/10185, pp. 2025-2026, https://doi.org/10.1016/S0140-6736(19)31084-0.
[27] Viergever, R. and T. Hendriks (2016), “The 10 largest public and philanthropic funders of health research in the world: What they fund and how they distribute their funds”, Health Research Policy and Systems, Vol. 14/1, p. 12, https://doi.org/10.1186/s12961-015-0074-z.
[94] Vorperian, S. and S. Quake (2021), The PASTEUR Act can help win the war against superbugs, STAT News, https://www.statnews.com/2021/06/25/pasteur-act-help-fight-superbugs-antimicrobial-resistance/ (accessed on 31 May 2022).
[106] Wellcome Trust (2016), “Clinical Trial Networks for Antibiotic Development: Why they’re important and how they should be developed.”.
[139] WHO (2022), External Evaluation of the Access To COVID-19 Tools Accelerator (ACT-A), World Health Organization, https://www.who.int/publications/m/item/external-evaluation-of-the-access-to-covid-19-tools-accelerator-(act-a) (accessed on 9 December 2022).
[10] WHO Commission on Macroeconomics and Health (2002), Global public goods for health : the report of Working Group 2 of the Commission on Macroeconomics and Health, World Health Organization, https://apps.who.int/iris/handle/10665/42518.
[138] WHO et al. (2011), “Guidelines for Medicine Donations, revised 2010”, World Health Organization, https://apps.who.int/iris/handle/10665/44647.
[2] World Health Organization (2021), 2020 Antibacterial agents in clinical and preclinical development: an overview and analysis, World Health Organization, https://apps.who.int/iris/handle/10665/340694.
[117] World Health Organization (2021), Establishment of a COVID-19 mRNA vaccine technology transfer hub to scale up global manufacturing, https://www.who.int/news-room/articles-detail/establishment-of-a-covid-19-mrna-vaccine-technology-transfer-hub-to-scale-up-global-manufacturing (accessed on 31 May 2022).
[128] World Health Organization (2021), Financing common goods for health, World Health Organization, https://apps.who.int/iris/handle/10665/345090.
[133] World Health Organization (2015), Global action plan on antimicrobial resistance, World Health Organization, https://www.who.int/publications/i/item/9789241509763.
[113] World Health Organization (2013), “WHO Prequalification of Medicines Programme”, WHO Drug Information, No. 27, World Health Organization, https://apps.who.int/iris/handle/10665/331154.
[112] World Health Organization (n.d.), History and Mission of WHO Prequalification, https://extranet.who.int/pqweb/about (accessed on 31 May 2022).
[63] XPRIZE (2022), XPRIZE Foundation, https://www.xprize.org/ (accessed on 23 June 2022).
← 1. “Neglected Tropical Diseases” is an umbrella term for several parasitic, viral, and bacterial diseases that cause substantial illness for more than 1 billion people globally, and for which research and development of effective treatments is considered commercially unattractive. Examples include diseases such as Chagas disease, Human African trypanosomiasis, and dengue fever. More than 70% of countries and territories that report the presence of neglected tropical diseases are low-income or lower middle‑income economies.
← 2. IP rights are a form of pull incentive, intended to encourage R&D by protecting future revenues once R&D milestones are reached.
← 3. A full examination of the role of push and pull funding should also examine their impact on the rivalry and excludability of the knowledge resulting from early-stage discovery and development; data (e.g. clinical data) used to inform the development of health technology products; and platform technologies on which health products are increasingly being developed.
← 4. The “valley of death” is often used to describe the gap between proof of concept of a potential novel therapeutic and its translation (clinical development, commercialisation). The valley of death is characterised by high risk of failure, significant resource needs, and limited capital (i.e. funding running out prior to financial returns).
← 5. With respect to step-in rights, a trigger is one or more defined events or conditions, which, if realised, should lead to the presumptive or automatic exercise of march-in rights by a government or third party related to IP held by an assignee.