This chapter covers the case study of the StopDia Pilot, a lifestyle intervention aiming to prevent type 2 diabetes mellitus among Finland’s Somali population. The case study includes an assessment of the StopDia Pilot against the five best practice criteria, policy options to enhance performance and an assessment of its transferability to other OECD and EU27 countries.
Healthy Eating and Active Lifestyles

10. StopDia pilot
Abstract
StopDia (Somali population) Pilot: Case study overview
Description: StopDia is a lifestyle intervention aiming to prevent type 2 diabetes mellitus (T2DM) by improving diet, encouraging physical activity (PA) and reducing overweight in people at high risk of T2DM. The StopDia pilot was a culturally adapted version of StopDia for adult Somali individuals in the region of Helsinki in Finland, which was delivered in co‑operation with a local mosque to a group of 24 people at high risk based on the Finnish Diabetes Risk Score (FINDRISC) test.
Best practice assessment:
Table 10.1. OECD best practice assessment of StopDia for the Somali population
|
Criteria |
Assessment |
|---|---|
|
Effectiveness |
Evidence from the StopDia pilot indicates it improves diet, however, results for other outcome indicators were not statistically significant More broadly, lifestyle interventions have found to be effective in reducing T2DM risk factors, in particular body weight, and the incidence of T2DM. |
|
Efficiency |
Efficiency studies into the StopDia Pilot are not available. Cost-effectiveness models of lifestyle interventions generally conclude that they are cost-effective. However, some of the assumptions, in particular on how long effects of interventions are sustained, may not be achieved in reality |
|
Equity |
The StopDia pilot improves equity of access through increasing access to prevention for a high-risk and underserved population group. It can also reduce inequality in health outcomes if it is effective |
|
Evidence‑base |
Strong data collection methods were used to evaluate the impact of the StopDia Pilot, however, missing information on withdrawals/dropouts and controlling for confounders limit the overall quality of the study |
|
Extent of coverage |
The proportion of eligible people who participated in the StopDia Pilot was 73%. The intervention has the potential to be scaled-up to a larger population given only a small fraction of the Somali population had the opportunity to participate. |
Enhancement options: many factors associated with effectiveness of T2DM lifestyle interventions are already reflected in the StopDia Pilot. To enhance effectiveness further, the duration of the intervention could be increased. Depending on the cost implications, this may or may not increase efficiency. Participant retention and adherence are important for effectiveness; this could possibly be improved by engaging families of participants. To enhance the evidence‑base, future evaluations of small-scale implementations, such as the StopDia Pilot, could be improved by increasing the sample sizes of studies; increasing the follow-up time; and by evaluating effectiveness in terms of a more complete set of relevant outcome indicators. Finally, to enhance extent of coverage, the StopDia Pilot could be extended to the entire Somali community in in Finland, and to high-risk population groups of other ethnic backgrounds.
Transferability: a high-level comparison of Finland with other OECD countries and non-OECD EU Member States suggests that StopDia can be transferred. For example, it is likely that StopDia would receive political support given it addresses key public health priorities – i.e. T2DM prevention and unhealthy diets. However, more detailed information needs to be analysed to determine transferability for each target country.
Conclusion: initial evidence from the StopDia Pilot found the intervention led to an improvement in outcomes, however, only changes in diet were statistically significant. While effectiveness and cost-effectiveness remains to be convincingly demonstrated, it may constitute an attractive blueprint for interventions to prevent T2DM in ethnic minorities or migrant population groups in various OECD countries and non-OECD EU Member States.
Intervention description
StopDia is a lifestyle intervention aiming to prevent type 2 diabetes mellitus (T2DM) by improving diet, encouraging physical activity (PA) and reducing overweight. Unhealthy diets, sedentary lifestyles and overweight and obesity are major risk factors for T2DM (Uusitupa et al., 2019[1]; Carbone et al., 2019[2]). StopDia was initially designed to reach people at increased risk of T2DM in the general Finnish population. The intervention for the general population is currently being evaluated in a randomised controlled trial (RCT) involving approximately 3 000 participants from the provinces of Northern Savo, Southern Carelia, and Päijät‑Häme, which have a combined population of about 580 000 people (Pihlajamäki et al., 2019[3]).
StopDia has also been adapted for a Somali ethnic minority living in Finland and piloted as a small-scale intervention in this group (hereafter referred to as the “StopDia Pilot”). Among other disadvantaged population groups, immigrants living in Finland have been identified as being at increased risk of T2DM (see Hussein et al. (2020[4]) and Weiste Paakkanen et al. (2013[5])). In addition to different risk profiles across population groups, the effectiveness of lifestyle interventions may depend on cultural factors, requiring adaptations for ethnic minorities.
The StopDia Pilot was aimed at Somali adults living in the region of Helsinki in Finland, and delivered in co‑operation with a local mosque. A group of 24 people participated in the Pilot. The intervention consisted of (Hussein et al., 2020[4]):
Screening for individuals at increased risk, to include people with a risk score of 12 points or more in the Finnish Diabetes Risk Score (FINDRISC) test1 and people with previous gestational diabetes. A researcher as well as volunteers with Somali backgrounds recruited participants at the mosque and were available to assist people take the FINDRISC test.
Group lifestyle counselling through six group meetings spread across 12 weeks. Meetings were led by the Somali researcher who moderated discussions in the group and provided coaching among pairs of participants. Meetings lasted for approximately 1.5 hours each, following a similar structure but revolving around a different theme every time (e.g. “eating well”, “joy of movement”, “active everyday”, etc.). Participants were provided with a workbook, which included a diary of physical activities and fruit and vegetable consumption, to be kept throughout the duration of the intervention and discussed at the group meetings.
Digital support for lifestyle change, using the BitHabit healthy lifestyle mobile application for the 12 weeks. The BitHabit application allows for browsing of behavioural suggestions and selecting those that the users want to perform; daily self-monitoring of selected behaviours; and getting summary feedback for habits. It also provides anonymous information on selections of other users and a self-learning section that provides information on the prevention of T2DM.
OECD Best Practices Framework assessment
This section analyses StopDia against the five criteria within OECD’s Best Practice Identification Framework – Effectiveness, Efficiency, Equity, Evidence‑base and Extent of coverage. Further details on the OECD Framework can be found in Annex A.
Given limited evidence on the StopDia Pilot for certain criteria, the OECD Framework was applied to the StopDia Pilot and more broadly to similar lifestyle interventions that T2DM (see Box 10.1). Results from the assessment should therefore only be taken as an indication of how the StopDia Pilot could perform if implemented widely.
Box 10.1. Assessment of the StopDia pilot and other lifestyle interventions to prevent type 2 diabetes (T2DM)
Effectiveness
There is limited evidence on the effectiveness of StopDia Pilot so far. Findings from the before‑and-after evaluation of the StopDia Pilot suggest that the intervention may have reduced T2DM risk factors, in particular, diet.
Systematic reviews and meta‑analyses show that lifestyle interventions generally reduce incidence T2DM in people at increased risk. However, this evidence is not necessarily applicable to the StopDia Pilot.
Efficiency
No evidence of the cost-effectiveness of the StopDia Pilot is available.
Most simulation models conclude that lifestyle interventions to prevent T2DM are cost-effective. However, estimates of cost per QALY gained range widely, from cost-saving to more than USD 140 000 in the United States. Estimates are sensitive to assumptions on the effectiveness of interventions, participant adherence and therefore the persistence of the intervention effect over time, and to costs.
The context-dependency of cost-effectiveness and the wide range of QALY estimates from prior models leave it uncertain whether existing evidence can be generalised and applied to the StopDia pilot.
Equity
The intervention specifically targeted and successfully reached an ethnic minority in Finland, whose members are at increased risk of T2DM. It increases access in a high-risk and underserved population group, and has a positive effect on equity in terms of access and, possibly, health outcomes.
Evidence‑base
Strong data collection methods were used to evaluate the impact of the StopDia Pilot, however, missing information, for example, on withdrawals/dropouts and controlling for confounders limit the overall quality of the study.
Extent of coverage
Of the 33 people who were identified as eligible for the StopDia Pilot, 24 (73%) agreed to participate in the intervention. Overall, this represents a small fraction of the more than 20 000 people with a Somali background in Finland, indicating the intervention has the potential to be scaled-up to reach a significantly larger population.
Effectiveness
Initial evidence indicates the StopDia pilot is effective, however, it is limited in scope
Initial evidence from a before‑and-after evaluation of the StopDia Pilot indicate the intervention achieved small improvements in lifestyle‑related T2DM risk factors, including increased vegetable consumption, increased physical activity (PA) and weight loss (Hussein et al., 2020[4]).
The pilot was evaluated in terms of overall diet quality (based on using a healthy diet score); intake of fruit, berries, and vegetables; the average number of steps per day; and waist circumference. However, only the difference in vegetable consumption (80% of participants reported eating vegetables at least once a day after the intervention compared to 50%) was statistically significant. The reduction in waist circumference, increase in step count and increase in diet quality were not statistically significant. Consistent with the increased step count, participants reported higher planned and incidental exercise after the intervention, and felt more competent in increasing physical activity (ibid.).
Lifestyle interventions that aim to improve diet and physical activity have so far been found to reduce incidence type 2 diabetes mellitus (T2DM) in people at increased risk
The effectiveness of lifestyle interventions to prevent T2DM has been widely studied. Systematic reviews of the evidence have generally concluded that lifestyle interventions that combine improvements to diet with increases in PA are effective in terms of preventing or delaying the onset of T2DM. It is less clear whether such interventions are also effective in terms of mortality and morbidity, in particular T2DM-related complications. The following recent systematic reviews synthesised evidence of effectiveness:
The Cochrane review by Hemmingsen et al. (2017[6]) concluded that there was evidence of moderate quality2 that interventions that improved diet and increased PA reduced the incidence of T2DM in people at increased risk. However, there is no firm evidence that such interventions reduce the complications associated with T2DM or mortality, nor that changes to diet alone or increased PA alone have an effect on any of these outcomes. The review synthesised results from 12 RCTs of interventions that aimed to improve diet alone, increase PA alone, and interventions that combined changes to diet and PA against standard prevention or no intervention. Primary outcomes were all-cause mortality, incidence of T2DM, and serious adverse events. The review also included a number of secondary outcome measures, including health-related quality of life and measures of blood glucose control. The trials included a total of 5 238 adults between the ages of 45 and 63 years of various ethnic backgrounds, who did not receive glucose‑lowering medicines. Trial follow-up time was from two to six years.
Systematic reviews and meta‑analyses by Haw et al. (2017[7]) and by Uusitupa et al. (2019[1]), which pooled data from 17 and seven RCTs respectively, many of which were also included in the review by Hemmingsen et al. (2017[6]), also found that the combination of improved diet and increased PA reduced the incidence of T2DM in people at increased risk.
Reviews by Haw et al. (2017[7]) and by Uusitupa et al. (2019[1]) also concluded that lifestyle interventions remained effective after they were no longer provided. However, Haw et al. (2017[7]) found that, while interventions had long-term effects, the magnitude of the effects declined over time. Based on four RCTs of lifestyle interventions that followed participants after the end of the active intervention, three of which combined diet and PA, interventions were estimated to lead to a 45% reduction in the risk of developing T2DM at the end of the active intervention period compared to a 28% reduction at the end of the follow-up period. Interventions in these trials lasted from one to six years and follow-up after end of the active intervention for another five to nine years.
In a network meta‑analysis that compared various T2DM prevention interventions against each other, Yamaoka, Nemoto and Tango (2019[8]) found that lifestyle interventions were at least as effective as preventive medication in reducing the incidence of T2DM. Although individual comparisons of the various interventions against control groups suggest that lifestyle interventions may be more effective than medication, differences between lifestyle interventions and medication were not statistically significant. Nevertheless, lifestyle changes may have the advantage that their effects persist after the end of the active intervention while such longer-term effects are not present in medication-based prevention; i.e. medication is only an effective preventive strategy for as long it is administered (Haw et al., 2017[7]).
It is unclear whether evidence from RCTs can be generalised
It is not clear the extent to which conclusions of these reviews can be generalised to all types of interventions that improve diet and increase PA and whether such evidence allows for inferring that the StopDia pilot is likely to be effective. There is some heterogeneity across the studies included in the reviews above, both in terms of the populations targeted and the interventions provided. None of the studies evaluated an intervention identical to StopDia in a population of Somali ethnic background. Study participants comprised people from diverse ethnic backgrounds, mainly Caucasians from Australia, Europe and North America but also African Americans and people from China, India and Pakistan. Studies also used various criteria to identify people at high risk, such as criteria related to impaired glucose tolerance (IGT), impaired fasting glucose (IFG) or dysmetabolism. Interventions varied in terms of the types of diet and PA support provided to participants, the intensity of desired PA, the duration of the interventions and the frequency of contacts with participants. The PA component of interventions was sometimes targeted according to the body mass index (BMI) of participants. In the studies included in the review by Hemmingsen et al. (2017[6]), the number of contacts with participants in the interventions ranged from 3 to 46. Importantly, interventions in the studies included in the reviews by Haw et al. (2017[7]), Hemmingsen et al. (2017[6]) and Uusitupa et al. (2019[1]) lasted from one to six years, which is significantly longer than in the StopDia pilot (12 weeks).
Digitally-delivered diet and physical activity interventions can also be effective
A recent systematic review by Van Rhoon et al. (2020[9]) found some evidence that digitally-delivered diet and PA interventions for adults at high risk of T2DM were effective in terms of achieving short-term (≤6 months) weight loss but remained inconclusive in terms of weight loss in the longer term (≥12 months). Some studies included in the review found reductions in HbA1c3 and fasting glucose levels. However, only one study reported a reduction in incidence of T2DM. The review included 19 studies of 21 interventions, most of which (14/19) were conducted in the United States and the remainder in Australia, Germany, India and Hong Kong. Nine interventions were “stand-alone”, while the others also as included support by a human health coach, delivered either face‑to-face or through digital communication technology.
These conclusions by Van Rhoon et al. (2020[9]) are consistent with a prior review by Bian et al. (2017[10]), who also found that digital lifestyle interventions achieved weight loss and improvements in blood glucose levels. It remains less clear, on the other hand, which characteristics make digital interventions effective and whether they are effective on their own or only in combination with face‑to-face interactions with participants. Based on the review by Van Rhoon et al. (2020[9]), digitally-delivered behaviour change techniques that were associated with effectiveness were encouragement to get social support, goal setting for behaviours and outcomes, feedback on behaviour, self-monitoring of behaviour and outcomes, and problem solving. Self-monitoring and problem solving were also found to be effective behaviour change techniques in a meta-regression analysis by Kebede (2018[11]), who investigated the effectiveness of digitally-delivered interventions in terms of blood glucose levels in patients with poorly controlled T2DM. Van Rhoon et al. (2020[9]) also found that providing health and lifestyle information and advice, diet tracking, and activity tracking were digital features associated with effective interventions.
Efficiency
The efficiency of the StopDia Pilot has not been assessed. However, data on cost of the intervention were collected. These indicate that the intervention had a direct cost of EUR 650 (USD PPP 950) per participant, of which 96% were for human resources.4 Human resource costs were mainly related to upfront investments in adapting the StopDia intervention for the Somali community, including translation and cultural adaptation of materials for participants and the BitHabit application, and recruiting and training of staff for delivery of the intervention (representing 77% of the total direct costs). Direct costs of delivering the intervention, including risk screening, group counselling, and briefing participants on the use of the BitHabit application, represented a smaller share (approximately EUR 120 (USD PPP 175) per participant, equivalent to 18% of the total).
In general, lifestyle interventions generally increase costs in the short-term. They can be expensive as a result of their labour-intensive nature, including, for example, advice and counselling by dieticians, case managers and exercise physiologists, and periodic medical reviews and advice by nurses and physicians. For example, the RCTs included in the systematic review by Hemmingsen at al. (2017[6]), reported mean direct costs per participant in intervention groups ranging from USD 225 to USD 3 625. These costs can be offset, in the medium- to long-term by reductions in medical costs of managing T2DM, in particular from managing T2DM with regular use of medication and from treating the complications that result from T2DM. These cost-offsetting effects are achieved through avoiding or delaying disease onset and disease progression to more severe stages. From a societal perspective, costs can be offset by the benefits of reduced morbidity, such as reduced absenteeism from work and increased productivity.
Three recent systematic reviews of the literature on cost-effectiveness of lifestyle interventions to prevent T2DM in people at increased risk concluded with some confidence that these were cost-effective. Zhou et al. (2020[12]) found a median incremental cost-effectiveness ratio (ICER) of USD 12 510 per quality-adjusted life year (QALY) gained and Roberts et al. (2017[13]) a median of USD 10 980.5
However, these results need to be interpreted with some caution. Limitations of the evidence cast doubts on the validity of a general conclusion that lifestyle interventions are cost-effective and, therefore, also whether it can be assumed that the StopDia Pilot is likely to be cost-effective. First, nearly all cost-effectiveness estimates included in systematic reviews above6 were made using models that generally assume that interventions are as effective as observed in clinical trials. They may all be similarly optimistic in their assumptions on the effects of interventions. Second, the range of base‑case ICERs estimated by the studies included in these reviews is nevertheless wide, varying from being cost-saving (i.e. ICERs < 0) to USD 143 000 per QALY gained.7 This suggests that cost-effectiveness is context dependent and subject to uncertainty. The scope of lifestyle interventions for high‑risk people in systematic reviews includes interventions that differ in terms of their more detailed characteristics and are delivered to different target groups in different settings. These differences affect both, their effectiveness and their costs. Not surprisingly, all reviews find that models are sensitive to assumptions on the effectiveness of interventions, participant adherence and therefore the persistence of the intervention effect over time, and to costs. Also, models generally assume that a favourable effect on T2DM risk factors persists with a diminishing size over time before people return to their baseline trajectories in risk factors and extrapolate effects on outcomes over long time horizons. Because benefits accrue over a long time, models with longer time horizons report more favourable ICERs (NICE Guideline Updates Team, 2017[14]; Roberts et al., 2017[13]). However, it is highly uncertain whether benefits truly last over time horizons of several decades.
Despite the above studies, evidence on cost-effectiveness of digitally-delivered lifestyle interventions is considered lacking (see, for example, the review by Van Rhoon (2020[9])). Nevertheless, if digital interventions have comparable effectiveness as face‑to-face counselling and if they can be delivered at lower cost through automation and lower human resource needs, it can be assumed they are cost-effective.
Equity
The StopDia Pilot achieved its objective of making the StopDia intervention accessible to the Somali minority in Finland and proved to be well-received (Hussein et al., 2020[4]). The intervention targeted an ethnic minority in Finland, whose members are at increased risk of T2DM and who, for reasons related to culture and language, have lower levels of access to prevention programs compared to the general population (Hussein et al., 2020[4]). The StopDia pilot is therefore a promising concept to increase access in high-risk and underserved population groups, and has a positive effect on equity in terms of access. However, its ultimate effects on equity will depend on whether the intervention can be scaled and achieve broader coverage of high-risk people in the Somali, and other ethnic, minorities (see section on Extent of coverage). While it also has the potential to reduce health inequalities in Finland, further research is needed to better understand the intervention’s impact on health outcomes.
Evidence‑base
Strong data collection methods were used to evaluate the impact of the StopDia Pilot
Evidence evaluating the StopDia Pilot is based on a before‑and -after study (Hussein et al., 2020[4]). Effectiveness of the StopDia pilot has been evaluated by comparing objective lifestyle outcome measures, including height, weight, waist circumference, blood pressure, and nutrition and exercise habits, before and after delivery of the intervention (which are considered “strong” data collection methods, see table below). However, because the evaluation lacked a control group observed differences cannot be attributed to the intervention with confidence (i.e. a “moderate” quality study design was used, see Table 10.2). In addition, differences in measurements of nearly all variables before and after the intervention did not reach statistical significance, which may be related to the fact that 24 people participated in the pilot, providing only a small sample for statistical inference. While effects of the intervention were measured using objective lifestyle‑related T2DM risk factors, in particular BMI, the evaluation did not evaluate effects on T2DM incidence.
Table 10.2. Evidence‑based assessment, StopDia Pilot
|
Assessment category |
Question |
Score |
|---|---|---|
|
Selection bias |
Are the individuals selected to participate in the study likely to be representative of the target population? |
Somewhat likely |
|
What percentage of selected individuals agreed to participate? |
73% |
|
|
Selection bias score: Moderate |
||
|
Study design |
Indicate the study design |
Cohort (one group pre + post) |
|
Was the study described as randomised? |
N/A |
|
|
Was the method of randomisation described? |
N/A |
|
|
Was the method of randomisation appropriate? |
N/A |
|
|
Study design score: Moderate |
||
|
Confounders |
Were there important differences between groups prior to the intervention? |
Can’t tell |
|
What percentage of potential confounders were controlled for? |
N/A |
|
|
Confounders score: Weak |
||
|
Blinding |
Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes |
|
Were the study participants aware of the research question? |
Yes |
|
|
Blinding score: Weak |
||
|
Data collection methods |
Were data collection tools shown to be valid? |
Yes |
|
Were data collection tools shown to be reliable? |
Yes |
|
|
Data collection methods score: Strong |
||
|
Withdrawals and dropouts |
Were withdrawals and dropouts reported in terms of numbers and/or reasons per group? |
Yes |
|
Indicate the percentage of participants who completed the study? |
92% |
|
|
Withdrawals and dropouts score: Strong |
||
Source: Effective Public Health Practice Project (1998[15]), “Quality assessment tool for quantitative studies”, https://www.nccmt.ca/knowledge-repositories/search/14.
Extent of coverage
The participation rate in the StopDia Pilot was relatively high and the intervention reached its objectives in terms of recruitment of participants (Hussein et al., 2020[4]). Of the 33 persons that were found at high risk of T2DM and therefore eligible for the intervention, 24 (73%) agreed to participate in the pilot, of which 22 completed the group counselling session and contributed data to the evaluation after delivery of the intervention (ibid.). This can be considered a high participation rate compared to other studies of lifestyle interventions for T2DM prevention that reported this metric (Aziz et al., 2015[16]; NICE Guideline Updates Team, 2017[14]). Some participants, however, struggled to find time to attend the group sessions and, on average, participants only attended 50% of the sessions that were offered (three of six). All 22 participants registered as users of the BitHabit application (Hussein et al., 2020[4]). The intervention garnered a high level of interest among non-participants.
The high participation rate was attributed to several factors including recruitment and delivery in a culturally familiar and trusted environment. This raises questions about how coverage of the intervention could be extended to the wider Somali community, including people who do not attend religious services at the mosque, or other minority groups who are at high risk of T2DM. According to Statistics Finland, there are approximately 20 000 people of Somali background currently live in Finland (OSF, 2020[17]). This represents some 5% of the 400 000 people with foreign background in the country (ibid.).
It should also be noted that the StopDia Pilot initially offered the BitHabit application as a standalone intervention to those who would not have time to attend group counselling. However, all participants wanted to attend the group counselling sessions and the digital intervention on its own was not taken up (Hussein et al., 2020[4]). Prior OECD research indicates that mobile apps to improve their health have so far achieved only modest uptake (around 2%) in the adult population (15‑64 years) (OECD, 2019[18]).
Policy options to enhance performance
Options to enhance the performance of the StopDia Pilot are based on an analysis of facilitators of and barriers to success of similar interventions elsewhere.
Enhancing effectiveness
Prior reviews of lifestyle interventions to prevent T2DM revealed a number of key success factors for enhancing effectiveness, many of which are already reflected in the design of StopDia (see Box 10.2). However, evidence also suggests that the effectiveness of the StopDia pilot could be improved further by providing the intervention over a longer period of time and possibly also through additional measures to improve adherence to the intervention.
The group counselling sessions in the StopDia pilot could be provided over a longer period of time to enhance effectiveness. Duration of the intervention is a factor likely to be relevant for effectiveness and the three‑month duration of the StopDia pilot, with six group counselling sessions, may be too short. It is not entirely clear from the systematic reviews summarised under “Effectiveness” for how long a lifestyle intervention needs to last to be effective. It is notable, however, that interventions found to be effective generally last for at least one year. Given that effectiveness requires lifestyle changes to be sustained, that effectiveness appears to at least decline after the end of the active intervention (Haw et al., 2017[7]), and that the benefits of T2DM prevention accrue over the long-term, it is reasonable to assume that effectiveness increases with duration of the intervention. A systematic review that focused on implementation-related aspects of T2DM prevention in high-income countries outside of clinical trials found that only 16% of the 38 studies included in the review reported an intervention duration of less than six months (Aziz et al., 2015[16]). Although the frequency of group counselling sessions tends to be lower in interventions of longer duration (e.g. monthly), the absolute number of sessions throughout the duration of the intervention is higher in most interventions than the six groups contacts in the StopDia pilot (ibid.). The review by Aziz et al. (2015[16]) cautiously confirms that studies of interventions that provide a higher degree of contact with participants report a stronger effect, in particular over long durations even if this results in lower frequency of contacts. The authors conclude that the initial 12 months of an intervention can be considered as the intensive phase, in which lifestyle changes are brought about, and that longer interventions then continue with a follow-up and maintenance phase, in which participants receive support to sustain the changes.
Adherence to the intervention could also be improved, possibly by considering whether different approaches to lifestyle changes work for men and women, by engaging entire families, by providing group counselling sessions more flexibly to facilitate attendance, and by considering the balance between intervention intensity and adherence. The latter can be considered if the intervention is scaled to achieve broader coverage (see “Enhancing extent of coverage”). Acceptance and uptake of the intervention are also key prerequisites for lifestyle interventions to achieve their desired effects. The StopDia pilot achieved a high initial uptake, i.e. a high proportion of those identified at high risk agreed to participate in the intervention, compared to other lifestyle interventions that aim to prevent T2DM (see, for example, Aziz et al. (2015[16]) and NICE Guidelines Updates Team (2017[14])). On the other hand, adherence to the intervention in terms of participation in the six group sessions was moderate (Hussein et al., 2020[4]). Available evidence lends strong support to interventions for ethnic minorities that are culturally adapted, delivered in a familiar environment and, in an appropriate language to increase acceptance and uptake, such as the StopDia pilot for the Somali community in Finland.
Evidence cautiously suggests that men and women need to be engaged differently and that entire families of people at high risk may need to be involved in preventive interventions, to increase adherence and thereby the likelihood that lifestyle changes actually occur and can be maintained. While group counselling session in the StopDia pilot were delivered in separate groups for men and women (Hussein et al., 2020[4]), the strategies for lifestyle change were the same. A review of barriers and facilitating factors in interventions to prevent or manage T2DM in vulnerable population groups, including migrants, ethnic minorities and people with low socio‑economic status, found that the most important barriers to uptake were limited knowledge, family and friends, economic factors such as the price of healthy food, cultural and language barriers and work-related commitments (Breuing et al., 2020[19]). Family and friends were also an important facilitator, so their effect depends on whether their behaviour is supportive of the lifestyle change or obstructive (ibid.). For example, it may be difficult to change dietary habits if family members are unwilling to do so. While men saw family as a facilitator, women more often viewed family as a barrier because tasks related to childcare and the household reduced the time available to adhere to preventive behaviours. While knowledge was also reported to be a facilitator, limited knowledge, for instance of how to make sense of dietary advice, was a common barrier. The amount of information on diabetes prevention was sometimes perceived as overwhelming and information about food and cooking was often designed based on western diets, leaving participants from minorities with difficulties in meal preparation and food choices.
If the intervention is scaled to include a larger number of participants, group counselling sessions could be offered more flexibly to make them as accessible as possible and increase uptake. Finding time to attend the group counselling sessions was identified as one reasons for moderate participation rates in the StopDia pilot (Hussein et al., 2020[4]). In broader implementations, counselling could be offered at various times of the day, including during evenings and at weekends, and in various settings to allow participants to choose the most convenient option.
Lastly, the review by Gillett et al. (2012[20]) suggests that there may be a trade‑off between the intensity of the intervention, i.e. the extent of PA and dietary restrictions targeted, and participant uptake and adherence. Discrete choice experiments conducted in the United States found that, while people valued hypothetical interventions with large benefits in terms of weight loss and T2DM risk reduction, they also expressed a high willingness to participate in interventions that involved low lifestyle sacrifices. This implies that the potentially large effects of interventions that aim for high levels of PA and impose significant dietary restrictions can be undermined by poorer uptake and adherence. If, as a corollary, uptake and adherence can be increased by aiming for more moderate lifestyle changes, the overall effect of such “lighter” interventions may ultimately be greater.
Given the StopDia pilot is targeted at a specific population with unique needs, the policy options above should only be considered if deemed appropriate by those with relevant cultural experience. Further, consideration should be given to the trade‑off policies have on different best practice criteria – for example, the StopDia pilot could become more effective with a longer duration, which would increase costs.
Box 10.2. Factors impacting the effectiveness of lifestyle interventions to prevent T2DM
Evidence on other lifestyle interventions to prevent T2DM suggest that interventions that are effective have the following characteristics, most of which are incorporated into the design of the StopDia pilot:
Interventions are targeted to people who are at increased risk of T2DM and whose risk can be reduced through lifestyle changes (NICE, 2017[21]).
Participants are engaged face‑to-face in individual or group sessions (Johnson et al., 2013[22]).
Interventions combine advice on diet with encouraging PA, rather than targeting only one or the other (Hemmingsen et al., 2017[6]).
Support given to participants combines information and education on healthy diets and PA with goal-setting and monitoring of lifestyle versus goals (Hemmingsen et al., 2017[6]; Uusitupa et al., 2019[1]; NICE, 2017[21]). In general, behaviour change techniques such as goal setting, planning, self-monitoring, and feedback are associated with better health outcomes (Browne et al., 2019[23]; Janssen et al., 2013[24]; Forster et al., 2016[25]; Celis-Morales, Lara and Mathers, 2015[26]).
Information given to participants is culturally appropriate and advice can be readily understood and put into practice in daily life (Breuing et al., 2020[19]; NICE, 2017[21]). If possible, interventions foster social support for participants to make lifestyle changes, for example in the family (ibid.).
Contact with participants lasts for a prolonged period of time (e.g. at least 1‑2 years) and participants adhere to advice provided, so that interventions achieve sustained lifestyle changes that result, in particular, in long-term weight loss (Aziz et al., 2015[16]; Haw et al., 2017[7]; Hemmingsen et al., 2017[6]; Uusitupa et al., 2019[1]; NICE, 2017[21]).
Interventions have an initial intensive phase that aims to educate participants and bring about desired lifestyle changes, followed by a less intensive phase that aims to build the independence of participants and sustain changes (Aziz et al., 2015[16]; NICE, 2017[21]).
Enhancing efficiency
Efficiency is calculated by obtaining information on effectiveness and expressing it in relation to inputs used. Therefore policies to boost effectiveness without significant increases in costs will have a positive impact on efficiency.
Enhancing equity
Equity could be further improved by increasing coverage across high-risk population groups, i.e. extending the intervention to the entire Somali community and other minority groups whose members are at high risk of T2DM. This is discussed below in the section on “Enhancing extent of coverage”.
However, transfer of the intervention to other contexts and for other minority groups need to carefully consider their possible effects on equity of access and inequality in health outcomes. T2DM and its risk factors tend to be more prevalent among ethnic minorities in high-income countries.8 These minority groups may be less well served by broad prevention programs for several reasons (e.g. there is some evidence that PA does not have the same protective effect among all ethnic groups (Boyer et al., 2018[27])).
Enhancing the evidence‑base
The evaluation of the StopDia pilot lacked a control group and had a small study size. This is not unusual given the intervention is a pilot that targets a specific group of people. Future evaluation attempts could be more rigorous by:
Increasing sample sizes: to allow of detecting statistically significant differences in relevant indicators.9
Comparing an intervention group against a concurrent control group: to allow for attribution of observed effects to the intervention. Control groups can be created, for instance, through randomisation to the intervention or control or through creation of a non-random control group matched on key personal characteristics of the people in the intervention group. However, it is important to acknowledge this may not be possible due to ethical reasons.
Increasing the follow-up time to at least 1‑2 years: to assess whether effects of lifestyle changes are sustained.
Evaluating effectiveness in terms of a more complete set of relevant outcome indicators: to provide a full evaluation in terms of the intervention logic, from process-related indicators of implementation to effects of implementation on T2DM risk factors and final health outcomes.
By formally estimating cost-effectiveness.
Incidence of T2DM and incidence of T2DM-related complications are particularly relevant indicators of final outcomes for interventions that aim to prevent T2DM. However, because progression to disease and, for incident cases, to disease complications, occur slowly and only in a subset of at-risk people included in studies, evaluating effectiveness in terms of these indicators requires long follow-up time and large samples. The existing evidence from RCTs provide information on final outcome indicators.
Evaluations of broader implementations of interventions outside of clinical trials have tended not to report T2DM incidence, but mainly effects in terms of risk factors, in particular body weight (Aziz et al., 2015[16]; Johnson et al., 2013[22]). Body weight, or BMI, have been established as risk factors for T2DM and weight loss is an attractive intermediate outcome, given that it is easy to measure and has been shown to predict T2DM incidence (Penn et al., 2013[28]). However, studies across different target populations suggest that weight loss is not equally associated with T2DM incidence in all ethnic groups and across all methods of identifying high-risk populations (ibid.). Implementation-focused studies have also reported process-related indicators (ibid. and Ackermann and O’Brien (2020[29])). A list of indicators of interest for lifestyle interventions to prevent T2DM is provided in Annex 10.A.
Enhancing extent of coverage
Coverage of the intervention, and thereby its positive effect on equity, could be enhanced by broadening recruitment for the intervention beyond the narrow target group of the StopDia Pilot. Broadening the coverage of the intervention would obviously require more resources for recruitment of a larger number of participants and for delivery of the intervention.
Risk screening and recruitment for the StopDia intervention for the Somali minority in Finland could initially be expanded beyond the mosque in Helsinki, where only a small fraction of the more than 20 000 people with Somali background in Finland were reached. In England, for example, the evaluation of the early phase of implementation of the NHS Diabetes Prevention Programme (DPP) showed that a broad and community-based recruitment strategy was key to ensuring adequate coverage of the intervention in high-risk populations (Penn et al., 2018[30]).
Coverage could further be expanded by adapting the intervention to other minority groups. Among the more than 400 000 people with foreign backgrounds who live in Finland, other minority groups may also be at increased risk of T2DM. More than 30 000 people are of Iraqi or Turkish background, including a Kurdish community. Kurdish women were identified by Weiste Paakkanen (2013[5]) as one other minority group at particular high risk of T2DM.
Transferability
This section explores the transferability of the StopDia Pilot and is broken into three components: 1) an examination of previous transfers; 2) a transferability assessment using publically available data; and 3) additional considerations for policy makers interested in transferring the intervention.
Previous transfers
The StopDia intervention, which targets all people at high risk of T2DM, has been implemented in three provinces in Finland – Northern Savo, Southern Carelia, and Päijät‑Häme. The StopDia Pilot represents an adapted version of the original intervention to suit the needs of the Somali population. To date, neither StopDia nor the StopDia Pilot have been transferred outside of Finland.
Transferability assessment
The following section outlines the methodological framework to assess transferability and results from the assessment.
Methodological framework
Details on the methodological framework to assess transferability can be found in Annex A.
Indicators from publically available datasets to assess the transferability of the StopDia Pilot are listed in Table 10.3. Please note, the assessment is intentionally high level given the availability of public data covering OECD and non-OECD European countries.
Table 10.3. Indicators to assess the transferability of the StopDia Pilot
|
Indicator |
Reasoning |
Interpretation |
|---|---|---|
|
Population context |
||
|
% of residents born in a foreign country* |
StopDia (with a focus on a migrant population) will be more applicable in countries with a higher proportion of people born in a foreign country |
🡹 value = more transferable |
|
% of residents born in Somalia* |
As above |
🡹 value = more transferable |
|
% of individuals using the internet for seeking health information in the last 3 months |
StopDia offers a digital service to support healthy lifestyles (BitHabit), therefore, StopDia is more likely to be successful in populations comfortable seeking health information online |
🡹 value = more transferable |
|
ICT Development Index** |
StopDia’s digital support service will be more accessible in more digitally advanced countries |
🡹 value = more transferable |
|
% of the population with access to recreational green space within 10min walking distance |
StopDia participants are encouraged to do outdoor activities, therefore StopDia is more likely to be successful in countries where people have better access to green space |
🡹 value = more transferable |
|
Political context |
||
|
Operational strategy/action plan/policy to prevent T2DM |
StopDia will be more successful in countries which prioritise Type 2 Diabetes prevention |
“Yes” = more transferable |
|
Operational strategy/action plan/policy to reduce unhealthy eating |
StopDia will be more successful in countries which prioritise unhealthy eating |
“Yes” = more transferable |
|
A national eHealth policy or strategy exists |
StopDia includes a digital service therefore it will be more successful in countries that prioritise eHealth |
“Yes” = more transferable |
|
Economic context |
||
|
Prevention expenditure as a percentage of current health expenditure (CHE) |
StopDia is a prevention intervention, therefore, it will be more successful in countries that allocate a higher proportion of health spending to prevention |
🡹 value = more transferable |
* The indicators may understate the proportion of people with foreign ethnic backgrounds because they do not capture second- or third-generation immigrants, i.e. people who have foreign ethnic backgrounds but were born in their host country; the StopDia pilot was designed for people of Somali ethnic background in Finland, including those born in Finland. **The ICT development index represents a country’s information and communication technology.
Source: OECD (2020[31]), “Stock of foreign-born population by country of birth”, https://stats.oecd.org; WHO (n.d.[32]), “Global Health Observatory”, https://www.who.int/data/gho; WHO (2015[33]), “Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage”, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage; ITU (2020[34]), “The ICT Development Index (IDI): conceptual framework and methodology”, https://www.itu.int/en/ITU-D/Statistics/Pages/publications/mis/methodology.aspx; OECD Health Statistics 2021, https://doi.org/10.1787/health-data-en. OECD (2019[35]), “Dataset: ICT Access and Usage by Households and Individuals”, https://stats.oecd.org/Index.aspx?DataSetCode=ICT_HH2.
Results
Findings from the data are in Table 10.4 and show that:
Sweden and Norway are the only countries with a higher proportion of Somali-born people in its population (0.67% and 0.53%, respectively) than in Finland (0.21%). Somali-born people represent more than 0.1% of the total population in Denmark, the Netherlands and the United Kingdom. However, the vast majority of countries for which data are available have a higher proportion of foreign-born people in their populations than Finland.
Digital health literacy (as measured by proportion of people who seek health information online) and ICT development is higher in Finland than all other OECD and non-OECD European countries. For example, 76% of people in Finland are digitally health literate compared to an average of 54% in remaining countries. Therefore, the mHealth component of the StopDia Pilot may be less effective in other countries. Nevertheless, most countries have a standalone eHealth policy indicating there is political support for digital health interventions.
All countries have national action plans or strategies for T2DM prevention and the reduction of unhealthy diets related to NCD, suggesting that T2DM prevention through lifestyle changes may be well aligned with current public health priorities. According to the WHO NCD Country Capacity Survey, Greece and Sweden are the only countries that report neither a stand-alone T2DM prevention strategy nor a strategy to reduce unhealthy diets related to NCDs.
Spending on prevention is higher in Finland than in all countries with the exception of Canada, Italy and the United Kingdom indicating potential funding issues.
In addition, it should be noted that risk screening of potential participants and delivery of the StopDia pilot relied on non-remunerated volunteers, who were from the same ethnic background and who studied nursing or other health care‑related disciplines. Sufficient people with such profiles may not be available in the target setting, especially if the intervention were to be implemented at scale. Delivery might therefore have to rely on health professionals, such as nurses. No data on the number of nurses relative to the population are available for Finland.
Table 10.4. Transferability assessment by country, StopDia Pilot (OECD and non-OECD European countries)
A darker shade indicates that the StopDia Pilot may be more suitable to transfer to that country
|
% residents born in a foreign country |
Residents born in Somalia (%) |
% individuals seeking health information online |
ICT Development Index value |
% population access to green space |
National T2DM plan |
Unhealthy eating action plan |
National eHealth policy |
Prevention expenditure percentage CHE** |
|
|---|---|---|---|---|---|---|---|---|---|
|
Finland |
7 |
0.21 |
76 |
8.10 |
99.85 |
Yes |
Yes |
Yes |
3.98 |
|
Australia |
30 |
0.04 |
42 |
8.20 |
89.5* |
Yes |
Yes |
Yes |
1.93 |
|
Austria |
19 |
0.06 |
53 |
7.50 |
98.41 |
No |
Yes |
No |
2.11 |
|
Belgium |
17 |
0.06 |
49 |
7.70 |
94.89 |
Yes |
Yes |
Yes |
1.65 |
|
Bulgaria |
n/a |
n/a |
34 |
6.40 |
n/a |
Yes |
Yes |
Yes |
2.83 |
|
Canada |
n/a |
n/a |
59 |
7.60 |
n/a |
Yes |
Yes |
Yes |
5.96 |
|
Chile |
n/a |
n/a |
27 |
6.10 |
n/a |
Yes |
Yes |
Yes |
n/a |
|
Colombia |
n/a |
n/a |
41 |
5.00 |
n/a |
Yes |
Yes |
n/a |
2.05 |
|
Costa Rica |
n/a |
n/a |
44 |
6.00 |
n/a |
Yes |
Yes |
Yes |
0.60 |
|
Croatia |
n/a |
n/a |
53 |
6.80 |
n/a |
Yes |
Yes |
Yes |
3.16 |
|
Cyprus |
n/a |
n/a |
58 |
6.30 |
n/a |
Yes |
No |
Yes |
1.26 |
|
Czech Republic |
n/a |
n/a |
56 |
7.20 |
97.72 |
Yes |
Yes |
No |
2.65 |
|
Denmark |
10 |
0.19 |
67 |
8.80 |
89.18 |
Yes |
Yes |
Yes |
2.44 |
|
Estonia |
15 |
0.00 |
60 |
8.00 |
97.25 |
No |
Yes |
Yes |
3.30 |
|
France |
12 |
n/a |
50 |
8.00 |
93.03 |
Yes |
Yes |
n/a |
1.80 |
|
Germany |
16 |
n/a |
66 |
8.10 |
95.93 |
Yes |
Yes |
n/a |
3.20 |
|
Greece |
n/a |
n/a |
50 |
6.90 |
93.85 |
No |
No |
Yes |
1.27 |
|
Hungary |
6 |
0.00 |
60 |
6.60 |
91.49 |
No |
Yes |
No |
3.04 |
|
Iceland |
17 |
0.01 |
65 |
8.70 |
61.34 |
No |
Yes |
Yes |
2.68 |
|
Ireland |
n/a |
n/a |
57 |
7.70 |
94.47 |
Yes |
Yes |
Yes |
2.60 |
|
Israel |
20 |
n/a |
50 |
7.30 |
# n/a |
Yes |
Yes |
No |
0.37 |
|
Italy |
10 |
0.02 |
35 |
6.90 |
88.11 |
Yes |
Yes |
Yes |
4.41 |
|
Japan |
n/a |
n/a |
n/a |
8.30 |
#n/a |
Yes |
Yes |
Yes |
2.86 |
|
Latvia |
12 |
n/a |
48 |
6.90 |
95.23 |
Yes |
Yes |
Yes |
2.58 |
|
Lithuania |
5 |
0.00 |
61 |
7.00 |
94.82 |
Yes |
Yes |
Yes |
2.17 |
|
Luxembourg |
47 |
0.01 |
58 |
8.30 |
98.72 |
No |
Yes |
Yes |
2.18 |
|
Malta |
|
|
59 |
7.50 |
#n/a |
Yes |
Yes |
No |
1.30 |
|
Mexico |
1 |
|
50 |
4.50 |
#N/A |
Yes |
Yes |
No |
2.92 |
|
Netherlands |
13 |
0.15 |
74 |
8.40 |
97.00 |
No |
Yes |
Yes |
3.26 |
|
New Zealand |
26 |
0.02 |
|
8.10 |
#N/A |
Yes |
No |
Yes |
n/a |
|
Norway |
16 |
0.53 |
69 |
8.40 |
95.40 |
Yes |
Yes |
Yes |
2.45 |
|
Poland |
n/a |
n/a |
47 |
6.60 |
92.63 |
Yes |
Yes |
Yes |
2.28 |
|
Portugal |
n/a |
n/a |
49 |
6.60 |
83.33 |
Yes |
Yes |
No |
1.68 |
|
Republic of Korea |
n/a |
n/a |
50 |
8.80 |
n/a |
Yes |
Yes |
n/a |
3.48 |
|
Romania |
n/a |
n/a |
33 |
5.90 |
n/a |
Yes |
Yes |
Yes |
1.42 |
|
Slovak Republic |
4 |
0.00 |
53 |
6.70 |
95.63 |
Yes |
Yes |
n/a |
0.77 |
|
Slovenia |
13 |
0.00 |
48 |
7.10 |
93.50 |
Yes |
Yes |
No |
3.13 |
|
Spain |
14 |
0.00 |
60 |
7.50 |
93.26 |
Yes |
Yes |
No |
2.13 |
|
Sweden |
19 |
0.67 |
62 |
8.50 |
99.14 |
No |
No |
Yes |
3.27 |
|
Switzerland |
30 |
0.09 |
67 |
8.50 |
97.31 |
Yes |
Yes |
Yes |
2.63 |
|
Turkey |
3 |
0.01 |
51 |
5.50 |
n/a |
Yes |
Yes |
No |
n/a |
|
United Kingdom |
14 |
0.17 |
67 |
8.50 |
91.43 |
Yes |
Yes |
Yes |
5.08 |
|
United States |
14 |
0.03 |
38 |
8.10 |
# n/a A |
Yes |
Yes |
Yes |
2.91 |
* The figure for Australia represent the average cross each major city and refer to access to green space within 400m. **CHE = current health expenditure. n/a = no data available. The shades of blue represent the distance each country is from the country in which the intervention currently operates, with a darker shade indicating greater transfer potential based on that particular indicator (see Annex A for further methodological details).
Source: OECD (2020[31]), “Stock of foreign-born population by country of birth”, https://stats.oecd.org; WHO (n.d.[32]), “Global Health Observatory”, https://www.who.int/data/gho; WHO (2015[33]), “Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage”, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage; ITU (2020[34]), “The ICT Development Index (IDI): conceptual framework and methodology”, https://www.itu.int/en/ITU-D/Statistics/Pages/publications/mis/methodology.aspx; OECD Health Statistics 2021, https://doi.org/10.1787/health-data-en. OECD (2019[35]), “Dataset: ICT Access and Usage by Households and Individuals”, https://stats.oecd.org/Index.aspx?DataSetCode=ICT_HH2.
To help consolidate findings from the transferability assessment above, countries have been clustered into one of four groups, based on indicators reported in Table 10.3. Countries in clusters with more positive values have the greatest transfer potential. For further details on the methodological approach used, please refer to Annex A.
Key findings from each of the clusters are below with further details in Figure 10.1 and Table 10.5:
Countries in cluster one, including Finland, have population, political and economic arrangements in place to transfer the StopDia Pilot. Countries in this cluster are therefore less likely to experience issues associated with implementing and operating the StopDia Pilot in their local context.
Countries in cluster two also have population and economic arrangements to support the StopDia Pilot. Prior to transferring the intervention, however, these countries may wish to consider ensuring the StopDia Pilot aligns with overarching political priorities.
Remaining countries are in clusters three and four, which should consider whether the intervention aligns with political priorities as well as increase funding on preventative care to ensure long-term affordability.
Figure 10.1. Transferability assessment using clustering, the StopDia Pilot
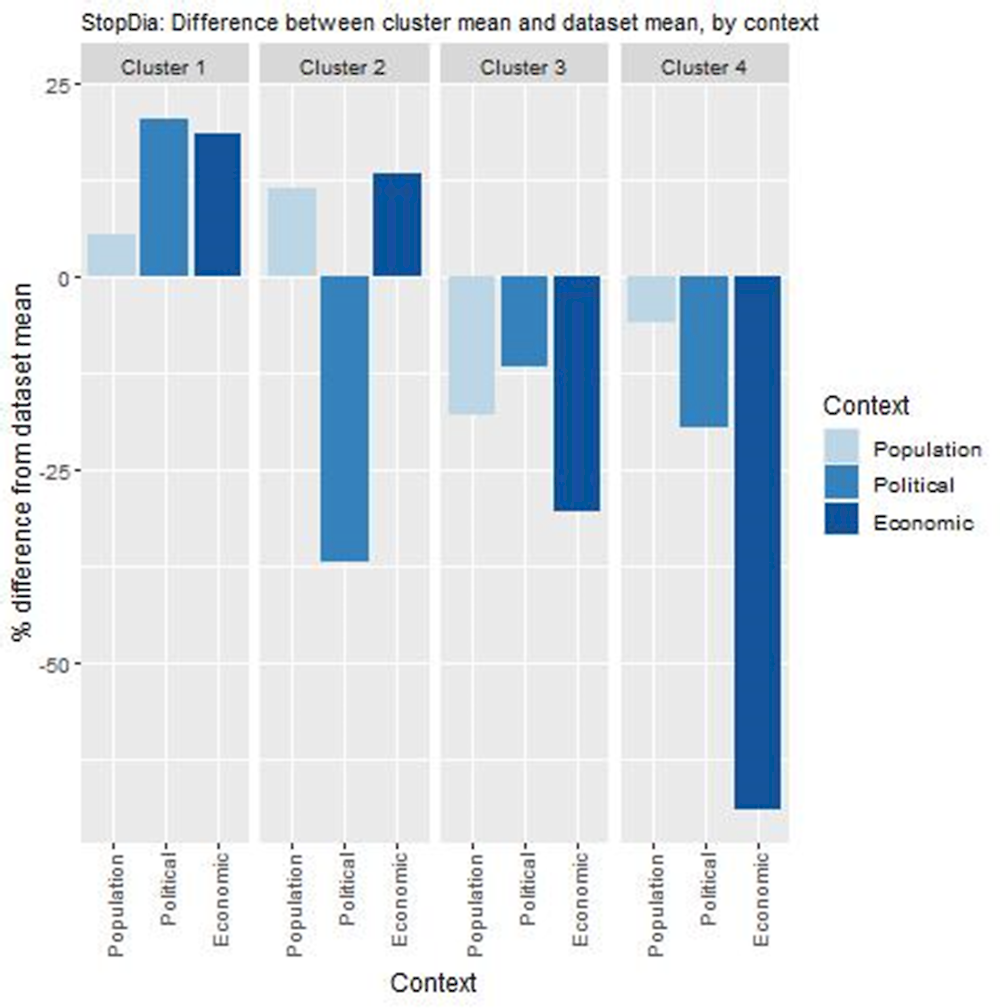
Note: Bar charts show percentage difference between cluster mean and dataset mean, for each indicator.
Source: OECD (2020[31]), “Stock of foreign-born population by country of birth”, https://stats.oecd.org; WHO (n.d.[32]), “Global Health Observatory”, https://www.who.int/data/gho; WHO (2015[33]), “Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage”, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage; ITU (2020[34]), “The ICT Development Index (IDI): conceptual framework and methodology”, https://www.itu.int/en/ITU-D/Statistics/Pages/publications/mis/methodology.aspx; OECD Health Statistics 2021, https://doi.org/10.1787/health-data-en. OECD (2019[35]), “Dataset: ICT Access and Usage by Households and Individuals”, https://stats.oecd.org/Index.aspx?DataSetCode=ICT_HH2.
Table 10.5. Countries by cluster, StopDia Pilot
|
Cluster 1 |
Cluster 2 |
Cluster 3 |
Cluster 4 |
|---|---|---|---|
|
Australia Belgium Bulgaria Canada Chile Croatia Denmark Finland France Germany Ireland Italy Japan Latvia Lithuania Norway Poland Republic of Korea Romania Switzerland United Kingdom United States |
Austria Estonia Hungary Iceland Luxembourg Netherlands Sweden |
Colombia Czech Republic Israel Malta Mexico Portugal Slovak Republic Slovenia Spain Turkey |
Costa Rica Cyprus Greece New Zealand |
New indicators to assess transferability
The transferability assessment based on publically available indicators needs to be interpreted with caution given reliable data are not publicly available for several important indicators and there are gaps in available data for some countries. Additional primary indicators to assess transferability are summarised in Box 10.3. While there appears comparable need for T2DM prevention in the populations of most in countries in the target setting, it is particularly important to assess in more detail which migrant populations/ethnic minorities are priorities for T2DM prevention in each country and how access barriers to T2DM prevention for these groups are best overcome. Also, while in most countries T2DM prevention and reducing unhealthy diets are a priority for public health policy, more detailed analysis is required to identify compatible and synergistic interventions as well as competing interventions that may exist already. Culturally adapted T2DM prevention interventions for minority populations have already been trialled in a number of OECD countries (Lagisetty et al., 2017[36]). Nurses could be one category of health professionals who could support implementation of the intervention in the target settings. However, the most appropriate human resources would need to be assessed in each setting, in particular if people delivering the intervention should have the same ethnic background as participants.
More detailed assessment of transferability should take into account the following information from the owner setting (Finland):
The StopDia intervention was adapted for the Somali community in Finland to overcome language barriers and because of a perception among this, and other migrant communities, that health care services are intended only for people who are ill and not for prevention (Hussein et al., 2020[4]). Both imply access barriers to preventive services delivered to the general population in health care settings.
Direct costs of the StopDia pilot for the Somali minority were estimated at EUR 650 per participant, 96% of which were related to human resources for adapting and delivering the intervention to the Somali community. These estimates do not include costs related to training, which was provided by local universities and research centres. Risk screening and delivery of the intervention was supported by non-remunerated volunteers. Costs of human and other resources may differ in target settings, affecting affordability and cost-effectiveness of the intervention. In particular, human resource costs may be higher if the intervention is delivered over a longer duration and at scale, and can therefore not rely solely on volunteers.
The StopDia Pilot for the Somali minority has not yet been scaled. Implementing the intervention at scale in a target setting would require strong political commitments to garner support in local minority communities and require additional resources in planning and implementation.
Box 10.3. New indicators to assess transferability
In addition to the indicators within the transferability assessment, policy makers are encouraged to collect data for the following indicators:
Population context
Which migrant populations/ethnic minorities are priorities for T2DM prevention in the target context?
What are the main barriers for these priority populations that keep them from accessing preventive services and health care? (E.g. Is health literacy a problem?)
In which setting are the access barriers for these priority populations best overcome?
How acceptable are lifestyle interventions to these priority populations?
Sector specific context (community and migrant health)
What, if any, compatible and synergistic interventions exist? (E.g. Other health interventions for migrant populations and/or ethnic minorities and T2DM prevention interventions for the general population that could support the intervention to prevent T2DM.)
What, if any, competing interventions exist? (E.g. Other interventions that aim to prevent T2DM in migrant populations and/or ethnic minorities.)
Are necessary human resources and work force skills available to adapt and deliver the intervention to priority migrant populations and/or ethnic minorities?
Political context
Will the intervention receive political support from key decision-makers in the target setting?
Will the intervention receive commitments from key decision-makers in the target setting?
Economic context
What is the cost of implementing the intervention in the target setting? (E.g. How do infrastructure and human resource needs, and the respective costs of these resources, differ between the owner and target settings?)
How do costs in the target setting affect anticipated cost-effectiveness of the intervention?
Conclusion and next steps
The StopDia Pilot was a culturally adapted version of StopDia for Somali adults in the region of Helsinki, Finland. The Pilot was delivered to a group of 24 people in co‑operation with a local mosque. Findings from the before‑and-after evaluation of the Pilot found the intervention achieved small improvements in lifestyle‑related T2DM risk factors, including increased vegetable consumption, increased physical activity (PA) and weight loss. Outcomes were measured using strong data collection methods, for example, volunteers from the local community were provided with comprehensive training to take objective participant measurements (e.g. height and weight). However, similar to most public health interventions, the quality of evidence was weaker in other areas (e.g. RCTs are considered to be of higher quality than a cohort pre / post study with only an intervention group, which was used to evaluate the StopDia Pilot). Future evaluations of StopDia for the Somali population would be improved by increasing the sample sizes of studies; increasing the follow-up time; and by evaluating effectiveness in terms of a more complete set of outcome indicators.
While lifestyle interventions can be effective and cost-effective in high-risk population groups, it should also be noted that they are costly to implement and that their success crucially depends on participant adherence and on sustaining lifestyle changes in the longer term. At the same time, the literature suggests that participant uptake and retention in interventions are frequent challenges in broad implementations, as is adherence by participants to diet and PA recommendations. While interventions can be designed to improve adherence, this also implies that lifestyle interventions can be a part of broader T2DM prevention strategies but are alone not sufficient. They need to be integrated with other prevention policies, such as building a healthy environment that encourages people to undertake physical activity as part of their everyday life and policies that encourage healthy food choices, such as regulation and taxation.
A transferability assessment of the StopDia Pilot to other OECD and non-OECD EU countries broadly indicates the intervention would have political support given most countries have national plans in place to address T2DM and unhealthy eating. Further, the digital component of the intervention will likely be encouraged by most governments given the increasing focus on eHealth. However, limitations on available data to assess transferability mean further analysis is needed before choosing to transfer the intervention (see Box 10.4).
Box 10.4. Next steps for policy makers and funding agencies
Next steps for policy makers and funding agencies to enhance the StopDia Pilot are listed below:
Support policy efforts outlined in this case study, for example, funding to expand recruitment, which is necessary to increase the sample size and therefore validity of future evaluation results
Support research into whether the StopDia Pilot can be adapted to suit other ethnic minority groups who are also at higher risk of T2DM
Promote findings from the StopDia Pilot case study to understand what countries/regions are interested in transferring the intervention
References
[41] Abate, N. and M. Chandalia (2003), “The impact of ethnicity on type 2 diabetes”, Journal of Diabetes and its Complications, Vol. 17/1, pp. 39-58, https://doi.org/10.1016/s1056-8727(02)00190-3.
[29] Ackermann, R. and M. O’Brien (2020), Evidence and Challenges for Translation and Population Impact of the Diabetes Prevention Program, Springer, https://doi.org/10.1007/s11892-020-1293-4.
[16] Aziz, Z. et al. (2015), A systematic review of real-world diabetes prevention programs: Learnings from the last 15 years, BioMed Central Ltd., https://doi.org/10.1186/s13012-015-0354-6.
[10] Bian, R. et al. (2017), “The Effect of Technology-Mediated Diabetes Prevention Interventions on Weight: A Meta-Analysis”, Journal of Medical Internet Research, Vol. 19/3, p. e76, https://doi.org/10.2196/jmir.4709.
[27] Boyer, W. et al. (2018), “Protective role of physical activity on type 2 diabetes: Analysis of effect modification by race-ethnicity”, Journal of Diabetes, Vol. 10/2, pp. 166-178, https://doi.org/10.1111/1753-0407.12574.
[19] Breuing, J. et al. (2020), “Barriers and facilitating factors in the prevention of diabetes type 2 and gestational diabetes in vulnerable groups: A scoping review”, PLOS ONE, Vol. 15/5, p. e0232250, https://doi.org/10.1371/journal.pone.0232250.
[23] Browne, S. et al. (2019), “Effectiveness of interventions aimed at improving dietary behaviours among people at higher risk of or with chronic non-communicable diseases: an overview of systematic reviews”, Eur J Clin Nutr, Vol. 73, pp. 9-23, https://doi.org/10.1038/s41430-018-0327-3.
[2] Carbone, S. et al. (2019), Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness, W.B. Saunders, https://doi.org/10.1016/j.pcad.2019.08.004.
[28] Casarini, D. (ed.) (2013), “Importance of Weight Loss Maintenance and Risk Prediction in the Prevention of Type 2 Diabetes: Analysis of European Diabetes Prevention Study RCT”, PLoS ONE, Vol. 8/2, p. e57143, https://doi.org/10.1371/journal.pone.0057143.
[46] CDC (2020), National Diabetes Statistics Report 2020 - Estimates of Diabetes and Its Burden in the United States, https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
[26] Celis-Morales, C., J. Lara and J. Mathers (2015), Personalising nutritional guidance for more effective behaviour change, Cambridge University Press, https://doi.org/10.1017/S0029665114001633.
[47] Eddy, D. and L. Schlessinger (2003), “Archimedes”, Diabetes Care, Vol. 26/11, pp. 3093-3101, https://doi.org/10.2337/diacare.26.11.3093.
[40] Eddy, D., L. Schlessinger and R. Kahn (2005), “Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes”, Annals of Internal Medicine, Vol. 143/4, https://doi.org/10.7326/0003-4819-143-4-200508160-00006.
[15] Effective Public Health Practice Project (1998), Quality assessment tool for quantitative studies, https://www.nccmt.ca/knowledge-repositories/search/14.
[25] Forster, H. et al. (2016), Personalised nutrition: The role of new dietary assessment methods, Cambridge University Press, https://doi.org/10.1017/S0029665115002086.
[20] Gillett, M. et al. (2012), “Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: A systematic review and economic evaluation”, Health Technology Assessment, Vol. 16/33, pp. 1-235, https://doi.org/10.3310/hta16330.
[7] Haw, J. et al. (2017), “Long-term sustainability of diabetes prevention approaches: A systematic review and meta-analysis of randomized clinical trials”, JAMA Internal Medicine, Vol. 177/12, pp. 1808-1817, https://doi.org/10.1001/jamainternmed.2017.6040.
[6] Hemmingsen, B. et al. (2017), “Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus”, Cochrane Database of Systematic Reviews 12, https://doi.org/10.1002/14651858.CD003054.pub4.
[4] Hussein, I. et al. (2020), WP7 - Finland Individual pilot action report.
[34] ITU (2020), The ICT Development Index (IDI): conceptual framework and methodology, https://www.itu.int/en/ITU-D/Statistics/Pages/publications/mis/methodology.aspx (accessed on 26 February 2021).
[24] Janssen, V. et al. (2013), “Lifestyle modification programmes for patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials”, European Journal of Preventive Cardiology, Vol. 20/4, pp. 620-640, https://doi.org/10.1177/2047487312462824.
[22] Johnson, M. et al. (2013), “Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence”, Diabetic Medicine, Vol. 30/1, pp. 3-15, https://doi.org/10.1111/dme.12018.
[11] Kebede, M. et al. (2018), “Effectiveness of Digital Interventions for Improving Glycemic Control in Persons with Poorly Controlled Type 2 Diabetes: A Systematic Review, Meta-analysis, and Meta-regression Analysis”, Diabetes Technology & Therapeutics, Vol. 20/11, pp. 767-782, https://doi.org/10.1089/dia.2018.0216.
[36] Lagisetty, P. et al. (2017), “Culturally Targeted Strategies for Diabetes Prevention in Minority Population: A Systematic Review and Framework”, Diabetes Educator, Vol. 43/1, pp. 54-77, https://doi.org/10.1177/0145721716683811.
[38] Lindström, J. and J. Tuomilehto (2003), “The diabetes risk score: A practical tool to predict type 2 diabetes risk”, Diabetes Care, Vol. 26/3, pp. 725-731, https://doi.org/10.2337/diacare.26.3.725.
[43] Meeks, K. et al. (2016), Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: a systematic review and meta-analysis, Springer-Verlag Italia s.r.l., https://doi.org/10.1007/s11739-015-1302-9.
[42] Montesi, L., M. Caletti and G. Marchesini (2016), “Diabetes in migrants and ethnic minorities in a changing World”, World Journal of Diabetes, Vol. 7/3, p. 34, https://doi.org/10.4239/wjd.v7.i3.34.
[21] NICE (2017), Type 2 diabetes: prevention in people at high risk, https://www.nice.org.uk/guidance/ph38/resources/type-2-diabetes-prevention-in-people-at-high-risk-pdf-1996304192197 (accessed on 22 September 2020).
[14] NICE Guideline Updates Team (2017), Type 2 diabetes: prevention in people at high risk [A] Evidence reviews for interventions for people at high risk of type 2 diabetes, https://www.nice.org.uk/guidance/ph38/evidence/evidence-reviews-pdf-4600705357.
[37] OECD (2022), Guidebook on Best Practices in Public Health, OECD Publishing, Paris, https://doi.org/10.1787/4f4913dd-en.
[31] OECD (2020), Stock of foreign-born population by country of birth, https://stats.oecd.org/Index.aspx?QueryId=48877.
[35] OECD (2019), Individuals using the Internet for seeking health information - last 3 m (%) (all individuals aged 16-74), OECD, Paris.
[18] OECD (2019), The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/67450d67-en.
[17] OSF (2020), Population Structure, https://www.stat.fi/til/vaerak/2019/02/vaerak_2019_02_2020-05-29_tie_001_en.html.
[30] Penn, L. et al. (2018), “NHS Diabetes Prevention Programme in England: Formative evaluation of the programme in early phase implementation”, BMJ Open, Vol. 8/2, p. 19467, https://doi.org/10.1136/bmjopen-2017-019467.
[3] Pihlajamäki, J. et al. (2019), “Digitally supported program for type 2 diabetes risk identification and risk reduction in real-world setting: Protocol for the StopDia model and randomized controlled trial”, BMC Public Health, Vol. 19/1, pp. 1-13, https://doi.org/10.1186/s12889-019-6574-y.
[13] Roberts, S. et al. (2017), Preventing type 2 diabetes: Systematic review of studies of cost-effectiveness of lifestyle programmes and metformin, with and without screening, for pre-diabetes, BMJ Publishing Group, https://doi.org/10.1136/bmjopen-2017-017184.
[39] Schünemann, H. et al. (2020), “Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence”, in Higgins, J. and J. Thomas (eds.), Cochrane Handbook for Systematic Reviews of Interventions, https://training.cochrane.org/handbook/current/chapter-14 (accessed on 9 November 2020).
[44] Ujcic-Voortman, J. et al. (2009), “Diabetes prevalence and risk factors among ethnic minorities”, The European Journal of Public Health, Vol. 19/5, pp. 511-515, https://doi.org/10.1093/eurpub/ckp096.
[1] Uusitupa, M. et al. (2019), Prevention of type 2 diabetes by lifestyle changes: A systematic review and meta-analysis, MDPI AG, https://doi.org/10.3390/nu11112611.
[9] Van Rhoon, L. et al. (2020), “A systematic review of the behaviour change techniques and digital features in technology-driven type 2 diabetes prevention interventions”, DIGITAL HEALTH, Vol. 6, p. 205520762091442, https://doi.org/10.1177/2055207620914427.
[5] Weiste Paakkanen, A. et al. (2013), “Migrant Health and Wellbeing Study (Maamu)”, European Journal of Public Health, Vol. 23/suppl_1, https://doi.org/10.1093/eurpub/ckt124.116.
[33] WHO (2015), Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage, Global Observatory for eHealth, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage.
[32] WHO (n.d.), Global Health Observatory, https://www.who.int/data/gho (accessed on 25 August 2021).
[45] WHO Regional Office for Europe (2020), Diabetes Data and Satistics, https://www.euro.who.int/en/health-topics/noncommunicable-diseases/diabetes/data-and-statistics (accessed on 9 November 2020).
[8] Yamaoka, K., A. Nemoto and T. Tango (2019), “Comparison of the Effectiveness of Lifestyle Modification with Other Treatments on the Incidence of Type 2 Diabetes in People at High Risk: A Network Meta-Analysis”, Nutrients, Vol. 11/6, p. 1373, https://doi.org/10.3390/nu11061373.
[12] Zhou, X. et al. (2020), “Cost-effectiveness of diabetes prevention interventions targeting high-risk individuals and whole populations: A systematic review”, Diabetes Care, Vol. 43/7, pp. 1593-1616, https://doi.org/10.2337/dci20-0018.
Annex 10.A. StopDia pilot indicators
A full range of potential indicators to measure the impact of StopDia, or other lifestyle interventions that aim to prevent T2DM, are listed below. This includes process-related indicators to evaluate implementation and indicators of intermediate and final outcomes. Data on outcome indicators need to be collected at different points in time, in particularly at baseline before delivery of the intervention and at different follow-up periods after delivery of the intervention. Indicators considered widely accepted and therefore high-priority have been highlighted.
Annex Table 10.A.1. Indicators to evaluate lifestyle interventions to prevent type 2 diabetes mellitus (T2DM)
Indicators in bold and italics are considered to be of high priority
|
Indicator category |
Indicators |
|---|---|
|
Final outcomes (effects on T2DM-related and general health indicators) |
Incidence of type 2 diabetes mellitus (T2DM) Time to progression to T2DM Incidence of T2DM-related adverse events and complications Change in fasting glucose or HbA1c levels Change in perceived health status (% of participants in good or very good health) or health-related quality of life Number of quality-adjusted life years (QALYs) gained Number of disability-adjusted life years (DALYs) averted |
|
Intermediate outcomes (effects on T2DM risk factors) |
Change in body-mass index Change in waist circumference Change in index scores of diet quality, focussing on intake of fat, saturated fat and fibre Self-efficacy for healthy eating and physical activity Change in consumption of fruits and vegetables Change in consumption of processed and unprocessed red meat, white rice, and sugar-sweetened beverages Change in consumption of tobacco and alcohol Change in percentage of participants who eat or skip breakfast Change in percentage of participants who eat snacks in between meals Percentage of population whose free sugar intake is less than 10% of total calorie intake Percentage of the population who consume less than 5 grammes of salt per day Percentage of the population whose saturated fatty acid intake is less than 10% of total calorie intake (less than 1% for trans fatty acids) Number of steps taken per day Changes in the amount of moderate to vigorous physical activity (PA) each week percentage adults (18+) reporting doing at 150min of moderate‑intensity physical activity in a week OR 75min or vigorous-intensity (or a combination of the two) Percentage population who engage in performance enhancing physical activity at least once a week (aerobic and/or muscle strengthening) |
|
Process-related indicators |
Percentage of high-risk population targeted by the intervention Percentage of high-risk population who agree to participate in the intervention (penetration) Percentage of participants who attend a minimum number of face‑to-face counselling sessions (participation) Content actually delivered in face‑to-face sessions vs. content planned Percentage of participants who actively use the digital lifestyle application Participant satisfaction |
Source: Further details can be found within OECD (2022[37]), Guidebook on Best Practices in Public Health, https://doi.org/10.1787/4f4913dd-en.
Notes
← 1. See Lindström and Tuomilehto (2003[38]) and https://www.mdcalc.com/findrisc-finnish-diabetes-risk-score.
← 2. Based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, applied in Cochrane reviews for assessing certainty (or quality) of a body of evidence (Schünemann et al., 2020[39]).
← 3. HbA1c – glycated haemoglobin – is a form of hemoglobin that is chemically linked to a sugar and is used as an indicator of how well blood sugar levels are controlled
← 4. Cost data provided by administrators of the StopDia pilot in Finland.
← 5. Roberts (2017[13]) reported a median of GBP 7 490, converted at 2017 purchasing power parities. The review by the NICE Guideline Updates Team (2017[14]) reported no median cost per QALY. It included 9 studies, all of which were also included by Zhou et al. (2020[12]) or Roberts et al. (2017[13]). Zhou et al. (2020[12]) included 28 studies and Roberts et al. (2017[13]) 27 studies; 12 studies were included in both reviews.
← 6. With the notable exception of the model described in Chapter 6 note 7.
← 7. The latter estimate can be considered an outlier, and was produced by the Archimedes model (Eddy and Schlessinger, 2003[47]) used to estimate cost-effectiveness of the Diabetes Prevention Program (DPP) in the United States from the perspective of a health care payer (Eddy, Schlessinger and Kahn, 2005[40]). The reasons for the difference in findings between the Archimedes model and the Markov-type models used in the majority of studies are not entirely clear. They are discussed at length in the earlier review by Gillett et al. (2012[20]), which concludes that the differences are likely driven by, among other factors, more conservative, and possibly more realistic, assumptions on baseline disease progression and occurrence of complications, and on the effectiveness of the intervention.
← 8. See, for example, Abate and Chandalia (2003[41]), CDC (2020[46]), Montesi, Caletti and Marchesini (2016[42]), (Meeks et al. (2016[43]), Ujcic-Voortman et al. (2009[44]), and WHO Regional Office for Europe (2020[45]).
← 9. The 24 participants in the StopDia pilot for the Somali community is a small sample to detect statistically significant differences.