This chapter covers the case study of Diabetes in Europe – Prevention using Lifestyle, Physical Activity and Nutrition (DE‑PLAN), a type 2 diabetes mellitus (T2DM) prevention programme for high-risk individuals operating in several European countries. The case study includes an assessment of DE‑PLAN against the five best practice criteria, policy options to enhance performance and an assessment of its transferability to other OECD and EU27 countries.
Healthy Eating and Active Lifestyles

12. Diabetes in Europe: Prevention using lifestyle, physical activity and nutrition
Abstract
DE‑PLAN: Case study overview
Description: the Diabetes in Europe – Prevention using Lifestyle, Physical Activity and Nutritional Intervention in Greece (DE‑PLAN) is a type 2 diabetes mellitus (T2DM) prevention programme for high-risk individuals. The study seeks to prevent T2DM by improving diet and physical activity (PA) levels through a lifestyle, community-based intervention. DE‑PLAN has been implemented in 17 countries across Europe.
Best practice assessment:
Table 12.1. OECD best practice assessment of DE‑PLAN
|
Criteria |
Assessment |
|---|---|
|
Effectiveness |
The intervention successfully impacted participants’ anthropometric and clinical measurements, as well as dietary intake, but had no significant effect on levels of PA |
|
Efficiency |
Economic evaluations of comparable diabetes prevention interventions conclude they are generally cost-effective |
|
Equity |
The intervention does not specifically target vulnerable populations or individuals from lower socio‑economic groups, |
|
Evidence‑base |
The evidence to evaluate outcomes had a “strong” data collection method and also performed well in regards to the study design used |
|
Extent of coverage |
The participation rate was 76% and the dropout rate 35% |
Enhancement options: to enhance effectiveness, further emphasis could be placed on the importance of PA in the bi-monthly meetings, and systematic and brief counselling, as well as information sessions, on smoking cessation and nicotine dependence. Moreover, to enhance effectiveness, the families and broader environments of the participants could also be involved. To enhance equity, the programme could seek to recruit participants from more vulnerable groups, and adapt the intervention to their specific needs. To enhance the evidence‑base, a control group could be included, alternatives to food-frequency questionnaires could be considered, and a wider scope of academic literature could be taken into account. To enhance extent of coverage, less invasive and time‑consuming alternatives to the oral glucose tolerance test (OGTT) could be employed.
Transferability: DE‑PLAN is a broadly transferable intervention as evidenced by its implementation in 17 European countries. Further, it is likely to have political support given it address three high priority issues – diabetes, obesity and physical inactivity.
Conclusion: although data was not available to fully assess the intervention in terms of cost-effectiveness, the DE‑PLAN study in Greece can be considered a best practice in terms of outcomes. To further enhance implementation, programme administrators could take into consideration policy options laid out in this case study, such as including counselling sessions on smoking cessation.
Intervention description
Cardiovascular diseases (CVD) are a leading cause of death, comprising approximately half of all non-communicable disease (NCD) deaths (Benziger, Roth and Moran, 2016[1]). One of the primary determinants of CVD is obesity, as well as its associated comorbidities (diabetes, hypertension) (Rodríguez-Artalejo et al., 2002[2]). In 2017, over 475 million people were affected by diabetes (Institute for Health Metrics and Evaluation, 2019[3]), and in 2018, almost 60% of people in OECD countries were overweight, and 25% were obese (OECD, 2019[4]). However, obesity and diabetes are largely preventable, highlighting the importance of effective health promotion and disease prevention strategies (World Health Organization, 2020[5]).
The DE‑PLAN study is a large‑scale, community-based diabetes prevention programme implemented within a primary care setting. The intervention aligns with the WHO’s Best Buys report, which supports lifestyle programmes seeking to prevent T2DM in the management of diabetes (WHO, 2017[6]). To date, 17 countries have participated in the intervention, each of which have tailored activities to fit local settings and needs. This analysis assesses the impact of the DE‑PLAN study in Greece. This particular intervention was implemented through group-based consultations, but participating countries could also choose to run these as individual sessions. One‑hundred and twenty‑five participants were recruited in primary care (during one of their visits) and occupational settings based on results from a questionnaire seeking to identify high-risk individuals for T2DM. Throughout the intervention, registered dieticians ran six one‑hour sessions across one year at the participants’ place of residence or work in groups of 6‑10. These provided information and a space for discussion on healthy lifestyles, individual and general risk of disease, diet, and exercise. The programme sought to decrease the intake of saturated fat, trans fatty acids, sugars and refined cereals, to promote the intake of at least five portions of fruits and vegetables per day, as well as to increase physical activity (PA) to 30‑40 min of moderate intensity aerobic exercise five times a week. Participants underwent a lipid profile and anthropometric measurements, an oral glucose tolerance test (OGTT) and a clinical evaluation before and after the intervention.
OECD Best Practices Framework assessment
This section analyses DE‑PLAN against the five criteria within OECD’s Best Practice Identification Framework – Effectiveness, Efficiency, Equity, Evidence‑base and Extent of coverage (see Box 12.1 for a high-level assessment of DE‑PLAN). Further details on the OECD Framework can be found in Annex A. Please note, data on the efficiency of DE‑PLAN in Greece was not publically available, therefore, this criterion was assessed according to information from the DE‑PLAN in Catalonia (CAT) and from comparable interventions, and should only be taken as an indicator of the programme’s actual cost-effectiveness.
Box 12.1. Assessment of DE‑PLAN T2DM prevention programme in Greece
Effectiveness
The intervention has been successful in improving participants’ anthropometric and clinical measurements, as well as dietary intake, but had no significant impact on levels of PA
Efficiency
Cost information is not available for the DE‑PLAN study in Greece
Findings from comparable T2DM prevention interventions and from the DE‑PLAN CAT (Catalonia) indicate that they are generally cost-effective
Equity
The intervention did not specifically target a priority population group
Evidence‑base
DE‑PLAN was evaluated using a non-randomised, open label interventional clinical trial with no control group
The study to evaluate DE‑PLAN had a “strong” data collection method and also performed well in other areas, such as, the study design and reducing selection bias
Extent of coverage
Participation rate was 76% and the dropout rate 35%
Effectiveness
Effect of the intervention on anthropometric and clinical measurements
In Greece, the DE‑PLAN study had significant outcomes in terms of anthropometric and clinical measurements. On average, participants saw a reduction in:
weight of 1kg (p = 0.022)
BMI of 0.5 kg/m2 (p = 0.014)
blood pressure of 6/‑1 mmHg (p < 0.001)
total cholesterol of 0.37 mmol/l (p < 0.0001)
LDL cholesterol of 0.39 (p < 0.0001) (Makrilakis et al., 2010[7]) (see Table 12.2).
Moreover, there was an increase from baseline in the percentage of individuals with normal glucose tolerance one year after the intervention, from 32.0% to 40.8%, as well as a decrease in the percentage of individuals with any type of dysglycaemia, from 68.0% to 53.6%. However, not all results were significant: there was no important change in waist circumference, 2‑h glucose, triglycerides and HDL cholesterol (see Table 12.2).
Table 12.2. Mean anthropometric and clinical data of the participants at baseline and one year after the intervention
|
Characteristic |
Baseline |
1 year |
Difference |
p value |
|---|---|---|---|---|
|
Weight (kg) |
89.0 |
88.0 |
1.0 |
0.022 |
|
BMI (kg/m2) |
32.0 |
31.6 |
0.5 |
0.014 |
|
Waist circumference (cm) |
102.9 |
102.6 |
0.3 |
NS |
|
Blood pressure (mmHg) |
133/79 |
127/80 |
6/‑1 |
< 0.001 (for systolic blood pressure) |
|
Fasting glucose (mmol/l) |
5.8 |
5.7 |
0.15 |
0.017 |
|
2‑h glucose (mmol/l) |
6.6 |
6.6 |
‑0.03 |
NS |
|
Total cholesterol (mmol/l) |
5.9 |
5.5 |
0.37 |
< 0.0001 |
|
Triglycerides (mmol/l) |
1.4 |
1.5 |
‑0.03 |
NS |
|
HDL-C (mmol/l) |
1.3 |
1.3 |
0.00 |
NS |
|
LDL-C (mmol/l) |
4.0 |
3.6 |
0.39 |
< 0.0001 |
Note: BMI refers to body mass index; HDL-C to high-density lipoprotein cholesterol; LDL-C to low-density lipoprotein cholesterol; NS to non significant.
A p value < 0.05 is considered statistically significant.
Source: Makrilakis et al. (2010[7]), “Implementation and effectiveness of the first community lifestyle intervention programme to prevent Type 2 diabetes in Greece. The DE‑PLAN study”, https://doi.org/10.1111/j.1464-5491.2010.02918.x.
Effect of the intervention on lifestyle behaviours
The intervention was relatively successful overall in improving diets, but had no significant effect on levels of PA. Indeed, participants reported fewer weekly servings of whole fat dairy products (p = 0.018), processed meats (p = 0.016), sugars and sweets (p = 0.006) and refined cereals (p = 0.045) (Kontogianni et al., 2012[8]) (see Table 12.3). However, there were no important changes in terms of fruit and vegetable intake or weekly PA levels. The former may be due to the fact that most participants already consumed these foods on a daily basis (three servings per day on average), and did not consider it to be substantially different from the intervention goal (five servings per day) (Kontogianni et al., 2012[8]). Nonetheless, by the end of the study, the diets of 58.7% of the participants had improved, 33.9% had worsened and 7.4% were unchanged (Kontogianni et al., 2012[8]). It is also important to note that these results depend on self-reported food diaries, and thus may not accurately reflect participants’ food intake. The overall results from this study are, nevertheless, significant. Although the intervention did not impact levels of PA, it had positive outcomes both in terms of anthropometric and clinical measurements, as well as in terms of diet, and can thus be deemed effective.
Table 12.3. Mean changes in dietary and PA variables at baseline and 1 year after the intervention
|
Variable |
Baseline |
1 year |
p value |
|---|---|---|---|
|
Whole fat dairy products (servings/week) |
13.4 |
8.9 |
0.018 |
|
Processed meats (servings/week) |
2.30 |
0.56 |
0.016 |
|
Sugars and sweets (servings/week) |
9.6 |
7.8 |
0.006 |
|
Refined cereals (servings/week) |
9.7 |
7.1 |
0.045 |
|
Vegetables (servings/week) |
9.0 |
9.3 |
0.623 |
|
Fruits (servings/week) |
12.1 |
12.6 |
0.447 |
|
Total minutes of exercise/day (during work and leisure time) |
37.2 |
34.0 |
0.311 |
Note: A p value < 0.05 is considered statistically significant.
Source: Kontogianni et al. (2012[8]), “Changes in dietary habits and their association with metabolic markers after a non-intensive, community-based lifestyle intervention to prevent type 2 diabetes, in Greece. The DEPLAN study”, https://doi.org/10.1016/j.diabres.2011.09.010.
Efficiency
Although no data on the cost-effectiveness of the study in Greece was available, overall analyses of T2DM prevention programmes, as well as a cost analysis of the DE‑PLAN CAT (Catalonia) found these interventions to be generally cost-effective. The former analysis found that the most cost-effective T2DM prevention programmes for high-risk individuals involved a combination of screening for diabetes and impaired glucose tolerance with lifestyle interventions, which amounted to GBP 6 262 (USD PPP 9 204 and EUR PPP 6 296) per QALY gained (Gillies et al., 2008[9]). Moreover, the analysis of the DE‑PLAN CAT found that the incremental cost per participant in a group-based intervention setting was of EUR 10 (USD PPP 14.62) per individual, which represents EUR 108 (USD PPP 157.88) per averted case of diabetes (Sagarra et al., 2014[10]). Additionally, the incremental cost-utility ratio was found to be EUR 3 243 (USD PPP 4 741) per quality-adjusted life‑year (QALY) gained (Sagarra et al., 2014[10]).
Equity
The literature on adult obesity indicates that those from more vulnerable backgrounds, with lower SES groups and/or with a lower level of education are more likely to be overweight or obese. Through designing and implementing an intervention which addresses a health issue that disproportionally affects adults from lower-SES groups, the DE‑PLAN study aims to reduce health inequalities. However, it is unclear whether specific efforts have been made to address other disadvantaged groups, such as children from different ethnic backgrounds and/or who live in remote/regional areas.
It is important to note that obesity interventions delivered in a primary care setting are less likely to reach people with a lower-SES due to access inequalities (OECD, 2019[11]). For example, analysis by OECD estimates that after adjusting for needs, in Greece, 55% of people in the lowest income quintile accessed a GP in the past year compared to 66% in the highest quintile (OECD, 2019[11]).
Evidence‑base
Makrilakis et al. (2010[7]) utilised a non-randomised, open label interventional clinical trial (where information is not withheld from trial participants) with no control group to evaluate DE‑PLAN. In order to evaluate the programme outcomes, anthropometric and clinical measurements were taken, and self-reporting questionnaires focusing on nutritional and PA habits were filled out before and one year after the study (Makrilakis et al., 2010[7]). These were based on the Diabetes Prevention Study (Tuomilehto et al., 2001[12]). The clinical measurements include an OGTT, weight, height, waist circumference and blood pressure measures, and a record of medical histories. Levels of plasma glucose, total and HDL cholesterol as well as triglycerides were assessed at a central accredited university research laboratory, and levels LDL cholesterol were calculated according to the Friedwald formula (Makrilakis et al., 2010[7]).
Using the Quality Assessment Tool for Quantitative Studies (Effective Public Health Practice Project, 1998[13]) the study design scored well in terms of data collection methods, however, several limitations were noted – e.g. confounders were not controlled for and neither researchers not participants were blinded.
Table 12.4 Evidence‑based assessment, DE-PLAN
|
Assessment category |
Question |
Score |
|---|---|---|
|
Selection bias |
Are the individuals selected to participate in the study likely to be representative of the target population? |
Very likely |
|
What percentage of selected individuals agreed to participate? |
76% (the proportion of the eligible population who chose to participate) |
|
|
Selection bias score: Moderate |
||
|
Study design |
Indicate the study design |
Cohort (one group pre and post) |
|
Was the study described as randomised? |
N/A |
|
|
Study design score: Moderate |
||
|
Confounders |
Were there important differences between groups prior to the intervention? |
N/A |
|
What percentage of potential confounders were controlled for? |
Can’t tell |
|
|
Confounders score: Weak |
||
|
Blinding |
Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes |
|
Were the study participants aware of the research question? |
Yes |
|
|
Blinding score: Weak |
||
|
Data collection methods |
Were data collection tools shown to be valid? |
Yes |
|
Were data collection tools shown to be reliable? |
Yes |
|
|
Data collection methods score: Strong |
||
|
Withdrawals and dropouts |
Were withdrawals and dropouts reported in terms of numbers and/or reasons per group? |
Yes |
|
Indicate the percentage of participants who completed the study? |
35% |
|
|
Withdrawals and dropouts score: Moderate |
||
Source: Effective Public Health Practice Project (1998[13]), “Quality assessment tool for quantitative studies”, https://www.nccmt.ca/knowledge-repositories/search/14.
Extent of coverage
Out of the 251 non-diabetic high-risk individuals that were identified from the FINDRISC questionnaires, 191 agreed to participate in the intervention (Makrilakis et al., 2010[7]). However, 66 participants dropped out during the study, leaving only 125 individuals to complete the programme (Makrilakis et al., 2010[7]). The participation rate was therefore 76% and the dropout rate, 35%. The dropout rate may be due to the fact that many participants described the OGTT as unpleasant and time‑consuming, thus making it unlikely for them to return for a second glucose test at the end of the study (Makrilakis et al., 2010[7]). However, participation rates were nonetheless lower than in the DE‑PLAN CAT, where 88.5% of high-risk individuals identified agreed to participate, but whose dropout rates were more comparable, at 41.3% (The DE-PLAN-CAT Research Group, 2012[14]).
Policy options to enhance performance
The DE‑PLAN study includes a range of best practice criteria for community-based T2DM prevention lifestyle programmes. Indeed, the study targeted both diet and PA, as well as involving access to ongoing support within a community setting.
Enhancing effectiveness
Literature on best practices in this field emphasise the importance of healthy diets, weight loss and physical activity in reducing diabetes risk (Galaviz et al., 2015[15]). In upscaling or adapting this intervention, more attention could be granted to the PA and weight loss components of the intervention, to enhance effectiveness. To date, evidence on the impact of the DE‑PLAN study in Greece on levels of PA amongst participants is lacking. The WHO recommends that adults engage in at least 150‑300 minutes of moderate‑intensity, such as brisk walking, or 75‑150 minutes of vigorous-intensity aerobic PA each week (WHO, 2020[16]). This could be emphasised further in the DE‑PLAN information sessions, in order to motivate participants to increase their PA levels. In a randomised clinical trial in the United States focusing on diabetes prevention through lifestyle intervention, for instance, a target of 150 minutes of moderate‑intensity PA was set and promoted throughout information sessions, in addition to weight loss and dietary objectives. By 24 weeks, 74% of participants had met this goal, and 50% had achieved the weight loss target of 7% or more, with average weight loss at 5.6 kg (The Diabetes Prevention Program Research Group, 2002[17]).
To enhance effectiveness, influencing other lifestyle factors such as smoking could also be taken into account in the intervention design and objectives: 30% of the study participants were smokers (Makrilakis et al., 2010[7]), with the Greek national average at 37%, the highest in the EU (Health and Food Safety Directorate General, 2017[18]). Yet actively partaking in this habit increases diabetes risk by 44% (Willi et al., 2007[19]). Systematic and brief motivational counselling, as well as information sessions on smoking cessation and nicotine dependence could be implemented, for example (López Zubizarreta et al., 2017[20]). In addition, intervention administrators could consider involving the participants’ wider families and environments, in order to create a wider support network and to foster health-enhancing behaviour.
Enhancing efficiency
Policy makers and programme administrators should prioritise an efficiency study of DE‑PLAN in Greece given this information isn’t currently available. For example, Sagarra et al. (2014[10]) undertook an efficiency study of DE‑PLAN in Catalonia, Spain, which calculated the cost per quality-adjusted life year.
Enhancing equity
To enhance equity, consideration could be given to widening the recruitment strategy beyond primary care and occupational settings, for example faith-based and other community events. Further, those responsible for recruitment should represent a diverse range of groups in society. This will ensure other population groups who, for example, are less likely to access primary care or be employed are covered by the recruitment strategy (National Diabetes Prevention Program, n.d.[21]). Finally, to understand how DE‑PLAN affects different population groups, data collection efforts should include questions that enable a stratification by vulnerable groups, for example, by family SES and ethnicity. Results from analysis suing stratified data can then be used to adapt DE‑PLAN in order to meet the needs of different vulnerable groups.
Enhancing the evidence‑base
To enhance the evidence‑base, future evaluations would benefit from improving the strength of the study design – for example by randomising patients into an intervention and control group, blinding researchers and participants, and controlling for relevant confounders. In addition, new methods to assess dietary intake, such as mobile technologies, could be considered in order to complement the use of food-frequency questionnaires (Béjar Prado and Vázquez-Limón Ozcorta, 2017[22]). Finally, basing the intervention design in a wider scope of academic literature, such as meta‑analyses or systematic reviews, might allow for a richer foundation.
Enhancing the extent of coverage
To enhance extent of coverage, less invasive and time‑consuming alternatives to the OGTT might be considered to decrease the dropout rate.
Transferability
This section explores the transferability of DE‑PLAN from Greece to other OECD and non-OECD EU countries and is broken into three components: 1) an examination of previous transfers; 2) a transferability assessment using publically available data; and 3) additional considerations for policy makers interested in transferring DE‑PLAN.
Previous transfers
DE‑PLAN has been implemented in 17 countries across Europe demonstrating it is highly transferable intervention (for example, in Greece, Lithuania, Poland and Spain). One factor explaining why DE‑PLAN can be transferred across a range of courtiers is that it utilises existing resources within the country’s primary health care system.
Transferability assessment
The following section outlines the methodological framework to assess transferability and results from the assessment.
Methodological framework
Details on the methodological framework to assess transferability can be found in Annex A.
Indicators from publically available datasets to assess the transferability of DE‑PLAN are listed in Table 12.5. Note, the assessment is intentionally high level given the availability of public data covering OECD and non-OECD European countries.
Table 12.5. Indicators to assess the transferability of DE‑PLAN
|
Indicator |
Reasoning |
Interpretation |
|---|---|---|
|
Population context |
||
|
% of the population with access to recreational green space within 10min walking distance |
DE‑PLAN participants are encouraged to do outdoor activities, therefore DE‑PLAN is more likely to be successful in countries where people have better access to green space |
🡹 = more transferable |
|
Sector specific context (primary care) |
||
|
% of people who visited a GP in the last 12 months at least once |
DE‑PLAN participants are recruited at the primary care level, therefore, DE‑PLAN will have a greater extent of coverage in countries where more people access their GP frequently |
🡹 = more transferable |
|
Health professionals are trained in health-enhancing physical activity |
DE‑PLAN participants are recruited in primary care, therefore GPs who are accustomed to providing healthy lifestyle advice may be more likely to support DE‑PLAN (e.g. encourage patients to access DE‑PLAN) |
“Yes” = more transferable |
|
Political context |
||
|
Operational strategy/action plan/policy to reduce unhealthy eating |
DE‑PLAN will be more successful in countries who prioritise unhealthy eating |
“Yes” = more transferable |
|
Operational strategy/action plan/policy to reduce physical inactivity |
DE‑PLAN will be more successful in countries who prioritise physical inactivity |
“Yes” = more transferable |
|
Operational policy/strategy/action plan for diabetes |
DE‑PLAN will be more successful in countries who prioritise diabetes prevention |
“Yes” = more transferable |
|
Economic context |
||
|
Primary health care expenditure as a percentage of current health expenditure |
DE‑PLAN is a primary care intervention, therefore, it is likely to be more successful in countries that allocate a higher proportion of health spending to primary care |
🡹 = more transferable |
Source: OECD Health Statistics 2021, https://doi.org/10.1787/ae3016b9-en; WHO (n.d.[23]), “Global Health Observatory”, https://www.who.int/data/gho; OECD (2020[24]), How’s Life? 2020: Measuring Well-being, https://dx.doi.org/10.1787/9870c393-en; Eurostat (2017[25]), “Persons visiting a general medical practitioner in the last 12 months by medical speciality, number of visits, educational attainment level, sex and age”, https://ec.europa.eu/eurostat; WHO Regional Office for Europe (2021[26]), “2021 Physical Activity Factsheets for the European Union Member States in the WHO European Region”, https://apps.who.int/iris/bitstream/handle/10665/345335/WHO-EURO-2021-3409-43168-60449-eng.pdf.
Results
Data from publically available sources indicate DE‑PLAN is likely to have broad political support in most countries given unhealthy eating, physical inactivity and diabetes prevention is a political priority in nearly all countries (Table 12.6). Further, data on the proportion of people who visit a GP is relatively high in OECD and non-OECD countries indicating DE‑PLAN will likely reach the target population (i.e. 40% in Greece versus 74% average in remaining countries). However, lower levels of spending on primary care highlight potential affordability issues. Data on remaining indicators shows mixed results, further, for these indicators there are high levels of missing data in non-European countries.
Table 12.6. Transferability assessment by country, DE‑PLAN (OECD and non-OECD European countries)
A darker shade indicates DE‑PLAN is more suitable for transferral in that particular country
|
Access to green space (%) |
% people who visited a GP in the last 12 months at least once (2017) |
Inclusion of physical activity and health in curriculum of health professionals |
Unhealthy eating plan |
Physical inactivity plan |
Diabetes plan |
Primary Health Care Expenditure as percentage Current Health Expenditure |
|
|---|---|---|---|---|---|---|---|
|
Greece |
93.85 |
40 |
Not Implemented |
No |
Yes |
No |
45 |
|
Australia |
89.5* |
83 |
n/a |
Yes |
Yes |
Yes |
37 |
|
Austria |
98.41 |
84 |
Implemented |
Yes |
Yes |
No |
37 |
|
Belgium |
94.89 |
87 |
Implemented |
Yes |
Yes |
Yes |
40 |
|
Bulgaria |
n/a |
48 |
Foreseen* |
Yes |
Yes |
Yes |
47 |
|
Canada |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
48 |
|
Chile |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
n/a |
|
Colombia |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
n/a |
|
Costa Rica |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
33 |
|
Croatia |
n/a |
68 |
Not Implemented |
Yes |
Yes |
Yes |
38 |
|
Cyprus |
n/a |
68 |
Not Implemented |
No |
No |
Yes |
41 |
|
Czech Republic |
97.72 |
86 |
Implemented |
Yes |
Yes |
Yes |
33 |
|
Denmark |
89.18 |
86 |
Implemented |
Yes |
Yes |
Yes |
38 |
|
Estonia |
97.25 |
73 |
Implemented |
Yes |
Yes |
No |
44 |
|
Finland |
99.85 |
68 |
Implemented |
Yes |
Yes |
Yes |
46 |
|
France |
93.03 |
85 |
Implemented |
Yes |
Yes |
Yes |
43 |
|
Germany |
95.93 |
89 |
Implemented |
Yes |
Yes |
Yes |
48 |
|
Hungary |
91.49 |
71 |
Implemented |
Yes |
Yes |
No |
40 |
|
Iceland |
61.34 |
n/a |
n/a |
Yes |
Yes |
No |
35 |
|
Ireland |
94.47 |
76 |
Implemented |
Yes |
Yes |
Yes |
47 |
|
Israel |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
n/a |
|
Italy |
88.11 |
71 |
Not Implemented |
Yes |
Yes |
Yes |
n/a |
|
Japan |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
52 |
|
Latvia |
95.23 |
80 |
Implemented |
Yes |
Yes |
Yes |
39 |
|
Lithuania |
94.82 |
76 |
Implemented |
Yes |
Yes |
Yes |
48 |
|
Luxembourg |
98.72 |
89 |
Implemented |
Yes |
Yes |
No |
38 |
|
Malta |
n/a |
83 |
Implemented |
Yes |
Yes |
Yes |
62 |
|
Mexico |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
44 |
|
Netherlands |
97.00 |
71 |
Implemented |
Yes |
Yes |
No |
32 |
|
New Zealand |
n/a |
n/a |
n/a |
No |
Yes |
Yes |
n/a |
|
Norway |
95.40 |
79 |
n/a |
Yes |
Yes |
Yes |
39 |
|
Poland |
92.63 |
64 |
Implemented |
Yes |
Yes |
Yes |
47 |
|
Portugal |
83.33 |
81 |
Implemented |
Yes |
Yes |
Yes |
58 |
|
Republic of Korea |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
57 |
|
Romania |
n/a |
57 |
Implemented |
Yes |
Yes |
Yes |
35 |
|
Slovak Republic |
95.63 |
82 |
Not Implemented |
Yes |
Yes |
Yes |
n/a |
|
Slovenia |
93.50 |
76 |
Implemented |
Yes |
Yes |
Yes |
43 |
|
Spain |
93.26 |
80 |
Not Implemented |
Yes |
Yes |
Yes |
39 |
|
Sweden |
99.14 |
62 |
Implemented |
No |
Yes |
No |
n/a |
|
Switzerland |
97.31 |
n/a |
n/a |
Yes |
Yes |
Yes |
40 |
|
Turkey |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
n/a |
|
United Kingdom |
91.43 |
74 |
Implemented |
Yes |
Yes |
Yes |
53 |
|
United States |
n/a |
n/a |
n/a |
Yes |
Yes |
Yes |
n/a |
* The figure for Australia represent the average cross each major city and refer to access to green space within 400m. The shades of blue represent the distance each country is from the country in which the intervention currently operates, with a darker shade indicating greater transfer potential based on that particular indicator (see Annex A for further methodological details). n/a = data not available.
Source: OECD Health Statistics 2021, https://doi.org/10.1787/ae3016b9-en; WHO (n.d.[23]), “Global Health Observatory”, https://www.who.int/data/gho; OECD (2020[24]), How’s Life? 2020: Measuring Well-being, https://dx.doi.org/10.1787/9870c393-en; Eurostat (2017[25]), “Persons visiting a general medical practitioner in the last 12 months by medical speciality, number of visits, educational attainment level, sex and age”, https://ec.europa.eu/eurostat; WHO Regional Office for Europe (2021[26]), “2021 Physical Activity Factsheets for the European Union Member States in the WHO European Region”, https://apps.who.int/iris/bitstream/handle/10665/345335/WHO-EURO-2021-3409-43168-60449-eng.pdf.
To help consolidate findings from the transferability assessment above, countries have been clustered into one of three groups, based on indicators reported in Table 12.5. Countries in clusters with more positive values have the greatest transfer potential. For further details on the methodological approach used, please refer to Annex A.
Key findings from each of the clusters are below with further details in Figure 12.1 and Table 12.7:
Countries in cluster one have political and economic arrangements in place to transfer DE‑PLAN. These countries could experience implementation barriers if health professionals feel they do not have the appropriate skills to deliver the intervention. Further, the programme may have limited effect if a relatively low number of eligible patients visit a GP (and are therefore not recruited into the programme).
Countries in cluster two also have political arrangements supportive of DE‑PLAN but would benefit from increasing expenditure on primary care before transferring the intervention to ensure long-term affordability. It is important to note that Greece, which currently operates DE‑PLAN, is in this cluster indicating although ideal, such conditions are not necessarily pre‑requisites for transferring DE‑PLAN.
Countries in cluster three would benefit from ensuring DE‑PLAN aligns with overarching political priorities, as well as ensuring long-term affordability by increasing expenditure on primary care.
Figure 12.1. Transferability assessment using clustering, DE-PLAN
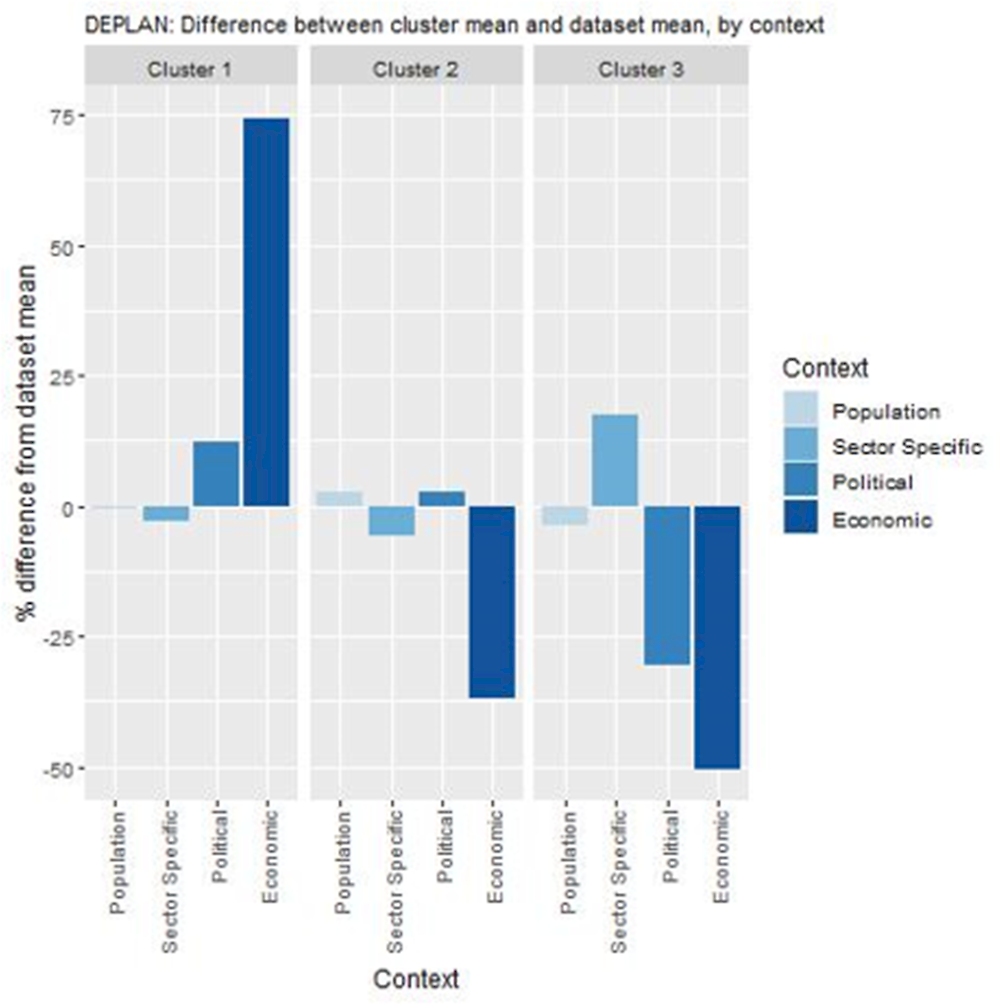
Note: Bar charts show percentage difference between cluster mean and dataset mean, for each indicator.
Source: OECD Health Statistics 2021, https://doi.org/10.1787/ae3016b9-en; WHO (n.d.[23]), “Global Health Observatory”, https://www.who.int/data/gho; OECD (2020[24]), How’s Life? 2020: Measuring Well-being, https://dx.doi.org/10.1787/9870c393-en; Eurostat (2017[25]), “Persons visiting a general medical practitioner in the last 12 months by medical speciality, number of visits, educational attainment level, sex and age”, https://ec.europa.eu/eurostat; WHO Regional Office for Europe (2021[26]), “2021 Physical Activity Factsheets for the European Union Member States in the WHO European Region”, https://apps.who.int/iris/bitstream/handle/10665/345335/WHO-EURO-2021-3409-43168-60449-eng.pdf.
Table 12.7. Countries by cluster, DE‑PLAN
|
Cluster 1 |
Cluster 2 |
Cluster 3 |
|---|---|---|
|
Bulgaria Canada Finland Germany Ireland Italy Japan Lithuania Malta Poland Portugal Republic of Korea Slovak Republic United Kingdom |
Australia Belgium Costa Rica Croatia Cyprus Czech Republic Denmark France Greece Latvia Mexico Norway Romania Slovenia Spain Switzerland |
Austria Estonia Hungary Iceland Luxembourg Netherlands Sweden |
Note: Due to high levels of missing data, the following countries were omitted from the analysis: Chile, Colombia, Israel, New Zealand, Turkey, and the United States.
New indicators to assess transferability
Data from publically available datasets is not ideal to assess the transferability of DE‑PLAN. For example, information on existing diabetes prevention interventions in primary care. Therefore, Box 12.2 outlines several new indicators policy makers should consider before transferring DE‑PLAN.
Box 12.2. New indicators to assess transferability
In addition to the indicators within the transferability assessment, policy makers are encouraged to collect data for the following indicators:
Population context
What is the ethnicity and cultural diversity of the target population?
What is the level of acceptability amongst potential patients: a) of lifestyle interventions; and b) collection of patient data, such as weight?
What is the level of health literacy amongst the population? (e.g. on the impact of diet and exercise)
How easily can potential participant’s access group sessions? (e.g. urban versus regional area, public transport links etc.)
Sector specific context (primary care)
What, if any, lifestyle interventions exist for patients at risk of diabetes?
What is the level of self-efficacy amongst health professionals to deliver lifestyle interventions?
What is the level of acceptability amongst health professionals of lifestyle interventions?
What availability do health professionals have to deliver a lifestyle intervention?
What proportion of the population access primary care as the entry point to receiving health care?
Is there a culture of health promotion and disease prevention within the health care system?
Political context
Has the intervention received political support from key decision-makers?
Has the intervention received commitment from key decision-makers?
Economic context
What is the cost of implementing and operating the intervention in the target setting?
Conclusion and next steps
The prevalence of T2DM is growing to epidemic proportions throughout the population (Makrilakis et al., 2010[7]). Diabetes is one of the primary determinants of CVD, a leading cause of death (Rodríguez-Artalejo et al., 2002[2]). The DE‑PLAN programme seeks to prevent the onset of diabetes through a screening and lifestyle intervention.
The results from the study show that the intervention has been successful in positively impacting participants’ anthropometric and clinical measurements, as well as their dietary intake, but did not have any significant impact on levels of PA. Although data on costs was not available for the DE‑PLAN in Greece, comparable programmes have been shown to be cost-effective. Furthermore, evidence used to develop the programme is of medium- to high-quality, and to evaluate the intervention can be deemed medium quality. The extent of coverage of the study in terms of participation and dropout rates was similar to other implementations of the DE‑PLAN study in Europe. In terms of equity, the programme did not target a priority population group and thus did not seek to advance equality for a particular priority population group. Finally, the study did include best practice criteria overall for community-based T2DM prevention lifestyle programmes, such as targeting both diet and PA. However, further changes, such as additional emphasis on PA and smoking, could be considered to achieve the intervention’s primary outcome: to prevent the development of type 2 diabetes in Greece.
Based on the available information, DE‑PLAN is a broadly transferable intervention as evidenced by its implementation in 17 European countries. Further, it is likely to have political support given it address three high priority issues – diabetes, unhealthy eating and physical inactivity. Nevertheless, prior to transferral, policy makers must consider other indicators such as acceptability among health care professionals.
Next steps for policy makers and funding agencies regarding the DE‑PLAN intervention are outlined in Box 12.3.
Box 12.3. Next steps for policy makers and funding agencies
Next steps for policy makers and funding agencies to enhance DE‑PLAN are listed below:
Providing support to DE‑PLAN administrators to implement policies outlined in this case study, for example, funding more in-depth evaluations of DE‑PLAN which would allow researchers to assess the impact of DE‑PLAN across different priority population groups
Ensure funding for future scale‑up and transfer efforts
Promote findings from the DE‑PLAN case study to better understand what countries/regions are interested in transferring the intervention
Promote “lessons learnt” from regions that have transferred DE‑PLAN to their local setting
References
[22] Béjar Prado, L. and E. Vázquez-Limón Ozcorta (2017), “Is there any alternative to traditional food frequency questionnaire for evaluating habitual dietary intake?”, Nutrición Hospitalaria, Vol. 34/4, https://doi.org/10.20960/nh.650.
[1] Benziger, C., G. Roth and A. Moran (2016), The Global Burden of Disease Study and the Preventable Burden of NCD, Elsevier B.V., https://doi.org/10.1016/j.gheart.2016.10.024.
[13] Effective Public Health Practice Project (1998), Quality assessment tool for quantitative studies, https://www.nccmt.ca/knowledge-repositories/search/14.
[25] Eurostat (2017), Persons visiting a general medical practitioner in the last 12 months by medical speciality, number of visits, educational attainment level, sex and age.
[15] Galaviz, K. et al. (2015), “Lifestyle and the Prevention of Type 2 Diabetes: A Status Report”, American Journal of Lifestyle Medicine, Vol. 12/1, pp. 4-20, https://doi.org/10.1177/1559827615619159.
[9] Gillies, C. et al. (2008), “Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis”, BMJ, Vol. 336/7654, pp. 1180-1185, https://doi.org/10.1136/bmj.39545.585289.25.
[18] Health and Food Safety Directorate General (2017), Public Health, https://ec.europa.eu/newsroom/sante/newsletter-specific-archive-issue.cfm?newsletter_service_id=327&newsletter_issue_id=3764&pdf=true&fullDate=&lang=default.
[3] Institute for Health Metrics and Evaluation (2019), GBD Results Tool | GHDx, http://ghdx.healthdata.org/gbd-results-tool (accessed on 19 September 2019).
[8] Kontogianni, M. et al. (2012), “Changes in dietary habits and their association with metabolic markers after a non-intensive, community-based lifestyle intervention to prevent type 2 diabetes, in Greece. The DEPLAN study”, Diabetes Research and Clinical Practice, Vol. 95/2, pp. 207-214, https://doi.org/10.1016/j.diabres.2011.09.010.
[20] López Zubizarreta, M. et al. (2017), “Tabaco y diabetes: relevancia clínica y abordaje de la deshabituación tabáquica en pacientes con diabetes”, Endocrinología, Diabetes y Nutrición, Vol. 64/4, pp. 221-231, https://doi.org/10.1016/j.endinu.2017.02.010.
[7] Makrilakis, K. et al. (2010), “Implementation and effectiveness of the first community lifestyle intervention programme to prevent Type 2 diabetes in Greece. The DE-PLAN study”, Diabetic Medicine, Vol. 27/4, pp. 459-465, https://doi.org/10.1111/j.1464-5491.2010.02918.x.
[21] National Diabetes Prevention Program (n.d.), Recruiting participants for your type 2 diabetes prevention lifestyle change program, https://coveragetoolkit.org/wp-content/uploads/2018/03/NDPP_Recruiting_Participants_Tipsheet.pdf (accessed on 11 December 2020).
[24] OECD (2020), How’s Life? 2020: Measuring Well-being, OECD Publishing, Paris, https://doi.org/10.1787/9870c393-en.
[11] OECD (2019), Health for Everyone?: Social Inequalities in Health and Health Systems, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/3c8385d0-en.
[4] OECD (2019), The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/67450d67-en.
[2] Rodríguez-Artalejo, F. et al. (2002), “Dietary patterns among children aged 6-7 y in four Spanish cities with widely differing cardiovascular mortality”, European Journal of Clinical Nutrition, Vol. 56/2, pp. 141-148, https://doi.org/10.1038/sj.ejcn.1601296.
[10] Sagarra, R. et al. (2014), “Coste-efectividad de la intervención sobre el estilo de vida para prevenir la diabetes tipo 2”, Revista Clínica Española, Vol. 214/2, pp. 59-68, https://doi.org/10.1016/j.rce.2013.10.005.
[14] The DE-PLAN-CAT Research Group (2012), “Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention”, Diabetologia, Vol. 55/5, pp. 1319-1328, https://doi.org/10.1007/s00125-012-2492-6.
[17] The Diabetes Prevention Program Research Group (2002), “The Diabetes Prevention Program (DPP): Description of lifestyle intervention”, Diabetes Care, Vol. 25/12, pp. 2165-2171, https://doi.org/10.2337/diacare.25.12.2165.
[12] Tuomilehto, J. et al. (2001), “Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance”, New England Journal of Medicine, Vol. 344/18, pp. 1343-1350, https://doi.org/10.1056/nejm200105033441801.
[16] WHO (2020), WHO Guidelines on Physical Activity and Sedentary Behaviour, https://www.who.int/publications/i/item/9789240015128.
[6] WHO (2017), Best buys and other recommended interventions for the prevention and control of noncommunicable diseases, WHO, Geneva, https://apps.who.int/iris/bitstream/handle/10665/259232/WHO-NMH-NVI-17.9-eng.pdf;jsessionid=3E76AC01272E9377F8382B8BC19545AF?sequence=1 (accessed on 10 September 2019).
[23] WHO (n.d.), Global Health Observatory, https://www.who.int/data/gho (accessed on 25 August 2021).
[26] WHO Regional Office for Europe (2021), 2021 Physical Activity Factsheets for the European Union Member States in the WHO European Region, https://apps.who.int/iris/bitstream/handle/10665/345335/WHO-EURO-2021-3409-43168-60449-eng.pdf.
[19] Willi, C. et al. (2007), “Active Smoking and the Risk of Type 2 Diabetes”, JAMA, Vol. 298/22, p. 2654, https://doi.org/10.1001/jama.298.22.2654.
[5] World Health Organization (2020), Obesity and overweight, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 June 2020).