This chapter covers the integrated care model for multimorbid patients in the Basque Country, Spain. The case study includes an assessment of the model against the five best practice criteria, policy options to enhance performance and an assessment of its transferability to other OECD and EU27 countries.
Integrating Care to Prevent and Manage Chronic Diseases

6. Integrated care model for multimorbid patients, the Basque Country, Spain
Abstract
Integrated care model for multimorbid patients: Case study overview
Description: As part of the strategy for chronicity in the Basque Country, Spain, an integrated care model was implemented. The model includes a comprehensive baseline assessment; individualised care plans; care from a multidisciplinary team; co‑ordinated hospital discharge; patient empowerment programs; and a strong health information system. Eligible patients are identified through a sophisticated risk stratification system, which covers 100% of the population.
Best practice assessment:
OECD Best Practice assessment of the integrated care model in the Basque Country, Spain
|
Criteria |
Assessment |
|---|---|
|
Effectiveness |
The integrated care model increases contacts with the primary care system and reduces hospitalisations, however, its impact on health outcomes is inconclusive |
|
Efficiency |
Mean total healthcare costs are up to 5% lower for those accessing the integrated care model |
|
Equity |
The risk stratification tool used to identify eligible patients covers the entire population, including those in priority population groups Research indicates the integrated care model may reduce health inequalities between the most and least deprived, given more deprived groups experienced a greater fall in hospital use |
|
Evidence‑base |
Studies evaluating the impact of the integrated care model use strong data collection methods and control for relevant confounders. The overall study design however is weakened by the fact that organisations were not randomly allocated into intervention and control groups. |
|
Extent of coverage |
As mentioned, all eligible patients are identified through the risk stratification tool, however, information on uptake among the eligible population is not available |
Enhancement options: the Basque Country’s integrated care model aligns with general recommendations on how to deliver care to chronically ill patients. Therefore, policies to enhance the structure of the intervention are not given. Nevertheless, options to enhance performance against the best practice criteria exist – for example, by improving digital health literacy among the older population and ensuring sufficient resources to cover the additional activities carried out by healthcare professionals. Further, the internal validity of evaluations would be enhanced by randomising participating organisations and using data from all patients, extending the follow-up period and stratifying data by priority population groups.
Transferability: Integrated care models comparable to the Basque Country´s exist in several European regions as part of a European Commission CareWell Project. The intervention will be extended to a further nine regions under the Joint Action on implementation of Digitally Enabled integrated person-centred Care (JADECARE) (2020‑23).
Conclusion: The Basque Country’s integrated care model for multimorbid patients has been shown to have a favourable impact on healthcare utilisation. By using a sophisticated risk stratification tool, all eligible patients, regardless of background, are identified. Nevertheless, data on uptake and impact across different population groups is not available, therefore, it is unclear what impact the model has on health inequalities. Although the model was designed in line with general recommendations for treating chronically ill patients, options are available to enhance the intervention’s performance. Finally, comparable models exist in several European regions highlighting its transfer potential.
Intervention description
Rising rates of multimorbidity in the Basque Country, Spain, prompted the government to implement a strategy for addressing chronicity. An ageing population partnered with poor lifestyle habits have contributed to a rising number of people living with multiple chronic conditions. Multimorbid patients require care from several health professionals working at different levels of care, therefore, it is important patients receive integrated, co‑ordinated care centred around their needs. In response to challenges posed by multimorbidity, in 2010 the Basque Country’s Department of Health launched the “Strategy to tackle the challenges of chronicity” (Ministry of Health and Consumer Affairs, 2010[1]). The aim of the strategy is to “re‑orient the health system toward an integrated care model” that is patient-centred (the CareWell Group, 2018[2]).
The Basque Country has implemented a multi-pronged approach for providing integrated care to multimorbid patients. In line with the strategy for chronicity, the Basque Health Service developed an integrated care model for multimorbid patients.1 The model consists of several key characteristics designed to improve care quality:
Comprehensive baseline assessment performed by a team of health professionals
Development of an individualised therapeutic plan
Care delivered by a multidisciplinary care team
Co‑ordinated hospital discharge
Patient empowerment programs to support self-management
Support from a strong health information system (see Box 6.1 for further details on characteristics 3 to 6).
Box 6.1. Characteristics of Basque’s integrated care model for multimorbidity
This box outlines in further detail characteristics 3 to 6 of the integrated care model for multimorbid patients in the Basque Country, Spain.
Care delivered by a multidisciplinary care team
Several health professionals are involved in caring for multimorbid patients. In addition to a general practitioner (GP), specialists and social workers, the team includes a:
Care manager (usually a primary nurse) who is responsible for case management
Referent internist who supports decisions made at the primary care level as well as co‑ordinating specialists involved in treating the patient in hospital
Hospital liaison nurse (explained below).
Co‑ordinated hospital discharge
The hospital liaison nurse and the primary care nurse work together to co‑ordinate care when the patient is discharged from hospital. This includes following up with the patient 1‑2 days post-discharge as well as monthly telephone calls by the primary care nurse to identify early detection of deterioration.
Patient empowerment programs
The model aims to improve patient self-management by offering KronikOn, a patient empowerment programme. KronikOn provides frail, older patients with 20‑30min educations sessions led by nurses with the aim of helping patients better understand their condition and how to manage it.
Support from a strong health information system
The integrated care model is supported by a strong health information system (HIS). Key features of the HIS include:
Unified electronic health records (EHR) accessible to all health professionals
ePrescription (integrated into the EHR)
Personal health folder where patients can access information on their medical history as well see upcoming appointments; surgery waiting lists; upload information from self-tracking programs; and communicate with health professionals.
Osarean (remote Osakidetza2)
OSAREAN is a multi-channel Health Service Centre that enhances accessibility and continuity by increasing the number of ways in which the public can interact with the health system. It includes the Personal Health Folder, 24x7 eHealth Call Center, patient tele‑monitoring, web appointments, Osakidetza portal and app, online inter-consultations, and telephone visits.
Source: WP5 Jadecare (2020[3]), “Presentation of the original good practice – Basque health strategy in ageing and chronicity: integrated care”.
A sophisticated risk stratification system identifies patients who are eligible to access the integrated care model. Risk stratification is a well-known tool to deploy large‑scale integrated care services. In the Basque Country, a risk stratification tool has been operating since 2012 to assist health professionals identify patients eligible for the multimorbid integrated care model – i.e. Johns Hopkins Adjusted Clinical Groups Predictive Model (ACG-PM). ACG-PM uses patient data to predict utilisation of healthcare services (a proxy measure for patient morbidity) over the next 12 months (see Box 6.2). Each patient receives a Predictive Index (PI) score that reflects their expected use of healthcare services relative to the average citizen in the Basque Country. For example, a PI score of four indicates the patient’s predicted use of healthcare services is four times the average citizen. Health professionals can access a patient’s PI score via the electronic health record (EHR) system – EHRs in the Basque Country cover 100% of the population and are interoperable across different levels of care.
Box 6.2. Data used to generate Predictive Index scores
The Predictive Index (PI) score generated by the Johns Hopkins Adjusted Clinical Groups Predictive Model (ACG-PM) relies on several data sources including primary care EHRs, and hospital and specialist outpatient care databases. The types of data collected from these sources include:
Socio-demographic factors including age and gender
Socio‑economic data
Disease diagnoses
Prescriptions
Prior healthcare utilisation.
PI scores are generated every two years, which is one of its limitations – i.e. the patient’s condition in real-time may not accurately reflect the PI generated two years prior.
Source: WP5 Jadecare (2020[3]), “Presentation of the original good practice – Basque health strategy in ageing and chronicity: integrated care”.
OECD Best Practices Framework assessment
This section analyses the Basque Country’s integrated care model for multimorbidity patients against the five criteria within OECD’s Best Practice Identification Framework – Effectiveness, Efficiency, Equity, Evidence‑base and Extent of coverage (see Box 6.3 for a high-level assessment). Further details on the OECD Framework can be found in Annex A.
Box 6.3. Assessment of the Basque Country’s integrated care model for multimorbid patients
Effectiveness
The integrated care model increases patient contacts with the primary care system and reduces hospitalisations
In general, patients, providers and carers are satisfied with the integrated care model and believe it has improved the quality of care delivered
The impact of the integrated care model on health outcomes (e.g. BMI) is inconclusive
Efficiency
Mean total healthcare costs were 5% lower for those who received the integrated care model compared to a control group
A budget impact analysis of the integrated model estimated that the integrated care model would reduce the growth in healthcare costs by 4 percentage points
Equity
The risk stratification tool can identify all patients who are eligible for the integrated care model, including patients in priority population groups
Research indicates the integrated care model may reduce health inequalities between the most and least deprived, given more deprived groups experienced a greater fall in hospital use
Multimorbidity disproportionality affects more deprived patients, therefore, the integrated care model has the potential to reduce health inequalities
Evidence‑base
Studies evaluating the impact of the integrated care model used strong data collection methods and controlled for relevant confounders. Healthcare organisations however weren’t randomly allocated to intervention and control groups, which is a study design weakness.
Extent of coverage
The risk stratification tool used to identify eligible patients covers 100% of the population – data from 2019 identified over 69 000 eligible patients
Information on the proportion of eligible patients who enrolled in the integrated care model is not available
Effectiveness
The Basque Country’s integrated care model increases primary care contacts and reduces hospitalisations. A primary objective of the Basque Country’s integrated care model is to keep patients in a stable condition for longer. This is measured by comparing utilisation of healthcare services between those who have and have not accessed the integrated care model. Results from recent studies show patients who access this model of care are more likely to access primary care and less likely to be hospitalised:
Mateo-Abad et al. (2020[4]) using data from a control and intervention group found those in the latter:
had a higher number of contacts with their GP (via phone) per year (6.7 versus 3.6 in the control group, p=0.002) (results for face‑to-face visits were not statistically significant)
had a lower number of hospitalisations per year (1.6 versus 2.3 in the control group, p=0.008)
had a lower number of emergency visits per year (0.3 versus 1.3 in the control group, p<0.001)
[These results align with a similar study by Mateo-Abad et al. (2020[5]), which included data from five other European regions operating comparable models.]2
Soto-Gordea et al. (2018[2]) also using data from a control and intervention group found those in the latter:
were twice as likely to make contact with primary care
had 7% more contacts with primary care
had a 9% lower probability of being hospitalised
recorded a 4% lower hospitalisation rate.
Patient and provider experiences of the integrated care model are largely positive. Healthcare professionals, patients and carers who participated in the integrated care model provided largely positive feedback – for example, health professionals felt more alert and watchful, while patients felt they received more co‑ordinated care and that management of information improved (Mateo-Abad et al., 2020[4]). Qualitative feedback from patients in a study examining the same model of care across multiple regions in Europe3 however recorded mixed results – for example, patients felt health professionals understood them better, yet they also felt less supported from health and social institutions (Mateo-Abad et al., 2020[5]).
The impact of the integrated care model on clinical outcomes is inconclusive. Impact evaluations undertaken by Mateo-Abad & colleagues in the Basque Country, Spain, (2020[4]) and in multiple European regions (2020[5]) found no statistically significant differences in clinical outcomes between the intervention and control group (e.g. BMI, blood glucose, HbA1c levels).
Efficiency
The integrated care model reduced mean total healthcare costs. A study undertaken by Soto-Gordoa et al. (2018[2]) found mean total healthcare costs for patients in the intervention group was 5% lower than in the control group. This supports a previous budget impact analysis, which predicted that the integrated care model would reduce the rate at which healthcare costs grow (see Box 6.4).
Box 6.4. Results from a budget impact analysis scenario analysis (2013‑20)
Soto-Gordoa et al. (2017[6]) estimated healthcare costs over the period 2013‑20 under two scenarios in the Basque country:
Baseline scenario where there are no changes to the delivery of care for multimorbid patients.
Intervention scenario where the integrated care model for multimorbid patients reduces unstable conditions for eligible patients by an annual rate of 2%.
The analysis estimated that the intervention scenario reduces the rate at which healthcare costs grow by 4 percentage points (i.e. 19% versus 23% or by EUR 684 066) between 2013 and 2020 (Figure 6.1).
Figure 6.1. Estimated impact of the integrated care model on healthcare expenditure – scenario analysis (2013‑20)
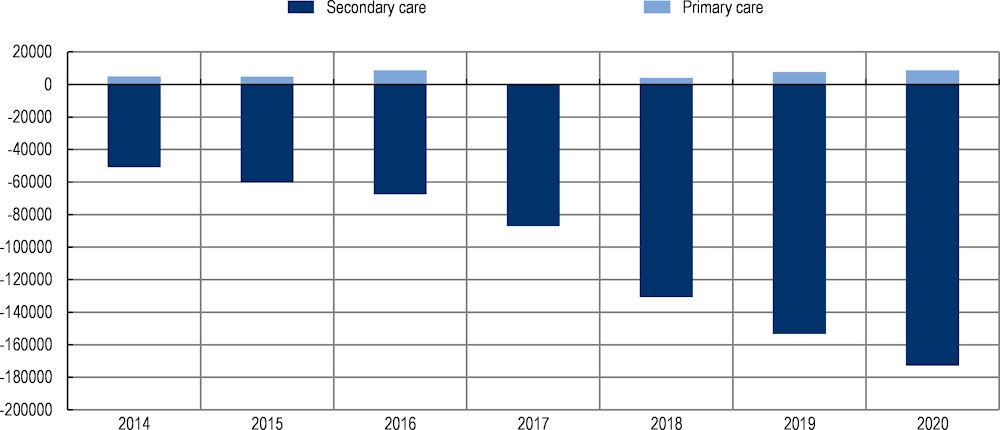
Note: The figures are not cumulative, rather they represent estimated annual changes in expenditure. The baseline year is 2013.
Source: Soto-Gordoa (2017[6]), “Incorporating Budget Impact Analysis in the Implementation of Complex Interventions: A Case of an Integrated Intervention for Multimorbid Patients within the CareWell Study”, https://doi.org/10.1016/j.jval.2016.08.002.
Equity
The Basque Country’s risk stratification tool identifies all eligible patients, including those from disadvantaged population groups. The risk stratification tool used to identify patients eligible for the integrated care model covers 100% of the Basque Country population. This ensures all disadvantaged population groups are captured, including those who may have otherwise experienced barriers to access.
Research suggests the integrated care model may reduce health inequalities between men and women and socio-economic groups. Sorto-Gordoa et al. (2019[7]) using an intervention and control group measured inequalities in healthcare access using data on patient participation in the integrated care model and contacts with primary care. Results from the analysis found compared to the control group:
Women and men in the intervention group who are most deprived had a lower probability of hospitalisation (p < 0.05)
Women in the intervention group who are most deprived had a higher probability of a primary care contact, albeit marginally (p < 0.05).
Multimorbidity disproportionally affects patients with a lower socio-economic status, indicating the integrated care model may reduce inequalities. Socio-economic status is a key predictor of health status, for example, analysis of Eurostat data by OECD found men in the most deprived group are 1.5 times more likely to be obese than those in the least deprived group, with this figure increasing to 1.9 for women (OECD, 2019[8]). Poor lifestyle behaviours contribute to higher rates of multimorbidity, which is reflected by data from Basque Country. For example, a study by Orueta et al. (2014[9]) found that in the Basque Country, the least deprived were less likely to have two or more chronic diseases compared to the most deprived (20.4% versus 23.6% of people). By developing a model to improve the level of care delivered to patients with multimorbidity, health inequalities can be reduced, however, a specific analysis examining this topic is not available.
Evidence‑base
Strong data collection methods and controls for confounding factors enhance the quality of studies evaluating the impact of integrated care model in the Basque Country, Spain. This section describes the quality of evidence supporting the effectiveness, efficiency and equity of the integrated care model – i.e. studies undertaken by Mateo-Abad et al. (2020[4]), and Soto-Gordea et al. (2018[2]) and (2019[7]). A summary of the study designs used in both evaluations are below, with further details in Table 6.1:
In their impact evaluation, Mateo-Abad and colleagues (2020[4]) employed a quasi‑experimental study including an intervention and control group (n=101 and n=99, respectively). Generalised regression models, which controlled for relevant confounders, were used to measure differences in healthcare utilisation between the groups based on routine administrative data. Patients weren’t randomly allocated into the intervention or control group, therefore it is unclear if those who agreed to participate accurately represent the target population.
Soto-Gordea and colleagues (2018[2]) relied on retrospective data from routine administrative datasets in their evaluation, which included an intervention and control group. Data for the intervention group was based on 2014 data (n=4 225) while the control group was based on 2012 data (n=3 558). To minimise selection bias between groups, the authors employed propensity score matching4 based on data such as age, gender, morbidity and previous hospitalisations. Differences between the intervention and control group were presented as odds ratios, which controlled for sociodemographic and clinical data.
Soto-Gordea and colleagues (2019[7]) used a retrospective observational study including an intervention and control group to assess the impact of the integrated care model on health inequalities (namely by gender and socio-economic status). The analysis relied on routine administrative datasets from over 16 000 patients (n=8 364 and n=8 239 in the intervention and control group, respectively). Patients weren’t randomly allocated into the intervention or control group, therefore it is unclear if those who agreed to participate accurately represent the target population.
Table 6.1. Evidence‑based assessment – the Basque Country’s integrated care model
|
Assessment category |
Question |
Score for Mateo-Abad et al. (2020[4]) |
Score for Soto-Gordea et al. (2018[2]) |
Score for Soto-Gordoa et al. (2019[7]) |
|---|---|---|---|---|
|
Selection bias |
Are the individuals selected to participate in the study likely to be representative of the target population? |
Can’t tell (a limitation of this study is that it is not randomised and therefore it is unclear if the participants reflect the target population) |
Yes |
Can’t tell (a limitation of this study is that it is not randomised and therefore it is unclear if the participants reflect the target population) |
|
What percentage of selected individuals agreed to participate? |
71% |
N/A |
N/A |
|
|
Selection bias score: |
Weak |
Strong |
Weak |
|
|
Study design |
Indicate the study design |
Quasi‑experimental study design using data from intervention and control group |
Retrospective observational cohort study with an intervention group and a historical control group |
Retrospective observational study using an intervention and control group |
|
Was the study described as randomised? |
No |
No |
No |
|
|
Was the method of randomisation described? |
N/A |
N/A |
N/A |
|
|
Was the method of randomisation appropriate? |
N/A |
N/A |
N/A |
|
|
Study design score: |
Moderate |
Moderate |
Moderate |
|
|
Confounders |
Were there important differences between groups prior to the intervention? |
Yes (the intervention group had a marginally higher starting BMI level) |
Yes (statistically significant difference in the mean age, albeit marginal, and prevalence of different disease combinations) |
Yes (the intervention group was older and utilised a greater level of healthcare services) |
|
What percentage of potential confounders were controlled for? |
80‑100% (age, gender, baseline BMI and comorbidity index) |
80‑100% (gender, age and certain clinical variables) |
80‑100% (gender, age, healthcare service use, socio-economic status) |
|
|
Confounders score: |
Strong |
Strong |
Strong |
|
|
Blinding |
Was the outcome assessor aware of the intervention or exposure status of participants? |
Can’t tell |
N/A (retrospective data) |
N/A (retrospective data) |
|
Were the study participants aware of the research question? |
Can’t tell |
N/A |
N/A |
|
|
Blinding score: |
Weak |
N/A |
N/A |
|
|
Data collection methods |
Were data collection tools shown to be valid? |
Yes (data collected from routine administrative data) |
Yes (data collected from routine administrative data) |
Yes (data collected from routine administrative data) |
|
Were data collection tools shown to be reliable? |
Yes |
Yes |
Yes |
|
|
Data collection methods score: |
Strong |
Strong |
Strong |
|
|
Withdrawals and dropouts |
Were withdrawals and dropouts reported in terms of numbers and/or reasons per group? |
Yes |
N/A (retrospective data) |
N/A (retrospective data) |
|
Indicate the percentage of participants who completed the study? |
87.5% (mainly due to deaths) |
N/A |
N/A |
|
|
Withdrawals and dropouts score: |
Strong |
N/A |
N/A |
Note: N/A = not applicable.
Source: Effective Public Health Practice Project (1998[10]), “Quality assessment tool for quantitative studies”, https://www.nccmt.ca/knowledge-repositories/search/14.
Extent of coverage
The risk stratification tool used to identify eligible patients covers the entire Basque Country population. The entire population in the Basque Country are stratified into risk groups every two years using ACG-PM (risk stratification model). ACG-PM can therefore identify all patients eligible to receive the integrated care model for multimorbidity (which is offered in all primary care centres and hospitals). For this reason, risk stratification tools are essential for deploying large‑scale integrated care interventions. According to data from 2019, over 69 000 people in the Basque Country were eligible for the integrated care model. Information on the proportion of eligible people who enrolled in the integrated care model is not known.
Policy options to enhance performance
Enhancing effectiveness
Options to change the design of the integrated care model in order to enhance effectiveness are not given as the model aligns with general recommendations on how to deliver care to chronically ill patients. Wagner et al.’s. (1996[11]) Chronic Care Model (CCM) is the most “well-known and widely applied” framework for population-based integrated care models (WHO Europe, 2016[12]). CCM outlines six key elements for delivering care to chronically ill patients, which are known to have a positive impact on outcomes, care quality and cost savings. A summary comparing the key elements of CCM against the Basque Country’s integrated care model – Table 6.2 – reveal the model in the Basque Country aligns with current recommendations. For this reason, there are no recommendations to alter the design of the Basque Country’s integrated care model in order to enhance effectiveness.
Table 6.2. Features of the Basque Country’s integrated care model compared to general recommendations for integrated care models targeting multimorbid patients
|
Recommendation |
Applied in the Basque Country model |
Notes1 |
|---|---|---|
|
Self-management support to ensure the patient and family members have the skills and confidence to manage their condition |
✓ |
The model includes several patient empowerment programs. For example, KronikOn offers patients four 20‑30min education sessions led by nurses. |
|
Strong delivery system involving a multidisciplinary care team, case management and regular follow-up |
✓ |
The model is delivered by a multidisciplinary team involving a GP, specialists, social workers, care manager, a reference internist and a hospital liaison nurse. The hospital liaison nurse is responsible for following-up with patients post hospital discharge. |
|
Decision support based on evidence‑based guidelines |
✓ |
Care for patients is tailored to the specific needs of patients and is based on latest available evidence (i.e. clinical guidelines). However, final decisions on care are made by individual clinicians. . |
|
Clinical information systems that provide care teams with feedback, reminders and individual and population based information for care planning purposes |
✓ |
The model identifies eligible patients via a sophisticated population risk-stratification tool which uses routine healthcare data. Further, the care team have access to a patient’s health information via their EHR, which covers 100% of the population. Patient’s also have access to their EHR as well as a personal health folder to see upcoming appointments and to communicate with their care team, for example, the eHealth Call Center (run by trained nurses) offers 24x7 care) |
Note: The recommendations are based on the Wagner et al.’s (1996[11]) Chronic Care Model. This model includes six key elements for delivering high quality care to chronically ill patients, however, only four are mentioned given two relate to the context or setting in which the model is delivered (and are therefore outside the control of intervention administrators).
1. See “Intervention description” for further information.
Source: Struckmann et al. (2018[13]), “Relevant models and elements of integrated care for multi-morbidity: Results of a scoping review”, https://doi.org/10.1016/j.healthpol.2017.08.008; Wagner et al. (1996[11]), “Organizing Care for Patients with Chronic Illness”, https://doi.org/10.2307/3350391.
Building patient digital health literacy will improve patient-provider communication and collaboration. Mateo-Abad et al. (2020[4]) in their review of the Basque Country’s integrated care model interviewed professionals on their perceptions of this new model of care, including the use of ICT. Overall professionals felt ICT tools were useful but limited when interacting with patients given their level of technical experience. For this reason, it is important to promote policies that build patient digital health literacy, particularly among older populations who are less familiar with digital tools but who stand to benefit most.
Ensure sufficient resources for healthcare professionals in order to avoid burnout. Mateo-Abad et al.’s (2020[4]) review also found primary care professionals felt the integrated care model increased their workload, particularly among nurses who were now responsible for leading weekly education sessions and following-up with patients more regularly. To avoid declining motivation levels among primary care professionals, an increase in the level of human resources may be necessary in order to perform additional activities required under the integrated care model.
Enhancing efficiency
Efficiency is a measure of effectiveness in relation to inputs used. Therefore, interventions that increase effectiveness without significant increases in costs, or reduce costs while keeping effectiveness at least constant, have a positive effect on efficiency.
Enhancing equity
Stratify evaluation indicators by priority population groups. The impact of the Basque Country’s integrated care model is well documented in high-quality research studies using intervention and control groups. Results, however, are not available by all key priority population groups such as ethnic minorities. Future research studies would benefit from stratifying data according to priority population groups, with findings used to adapt the model in order to reduce health inequalities. However, even if such information were available, it would only allow for an assessment of the effect on equity among participating patients. Therefore, it is also important to collect information on the characteristics of eligible patients who do and do not participate in the integrated care model. Given certain priority population groups – such as low SES – typically have less access to care, this information could be used to tailor future recruitment strategies.
Use available data to understand take‑up of the integrated care model among different socio-economic groups. The risk adjustment model (ACG-PM) (see “Intervention description”) used to identify eligible patients include a socio-economic deprivation index. This index can be used to understand take‑up of the integrated care model among eligible participants across different socio-economic groups. Such information can help administrators identify differences in uptake and adapt recruitment strategies accordingly.
Enhancing the evidence‑base
The evidence supporting the Basque Country’s integrated care model is strong in many aspects, nevertheless opportunities for improvement exist. As outlined under “Evidence‑base” the two studies evaluated the impact of the Basque Country’s integrated care model are strong in many areas – for example, both studies have an intervention and control group, controlled for relevant confounders and used high-quality data collection methods (Mateo-Abad et al., 2020[4]; the CareWell Group, 2018[2]). However, the internal validity of future studies could be improved by:
Randomising healthcare organisations into intervention and control groups, to ensure study participants reflect the target population. Typically, randomising occurs at the patient level, however, this is likely to be very difficult in the Basque Country given the health system structure.
Collecting data over a longer follow-up period – at present, data measuring the impact of the integrated care model is based on follow-up data between 9‑12 months.
Using administrative and clinical data base data from all patients who comply with a study’s inclusion criteria.
Enhancing extent of coverage
As outlined under “Enhancing equity”, the characteristics of eligible participants who do and do not participate in the integrated care model is not known. This information is important for tailoring recruitment strategies to maximise the number of eligible patients benefiting from this new model of care.
Transferability
This section explores the transferability of Basque Country’s integrated care model and is broken into three components: 1) an examination of previous transfers; 2) a transferability assessment using publicly available data; and 3) additional considerations for policy makers interested in transferring an integrated care model.
Previous transfers
Integrated care models targeting multimorbid patients are common in OECD and EU countries. The rising number of people living with two or more chronic conditions has prompted countries to implement integrated, patient-centred models of care. For example:
OECD’s report on primary care (2020[14]) identified 17 member countries which have developed “new models of primary care”,5 that deliver integrated care to patients.
The European Commission funded ICARE4U project aimed at improving care for multimorbid patients identified 101 models of integrated care across 24 European countries, of which 40% target those aged 65+ (Melchiorre et al., 2020[15]).
The Basque Country’s integrated care model exists in several European countries, and will continue to expand as part of JADECARE. The European Commission co-funded the CareWell project designed to promote the integration of care in several European regions (ended in 2017). As part of the project, the integrated care model outlined in this case study was implemented alongside comparable models in Zagreb (Croatia), Lower Silesia (Poland), Veneto (Italy), Puglia (Italy) and Powys (United Kingdom) (see Box 6.5). As part of the Joint Action on implementation of digitally enabled integrated person-centred care (JADECARE) (2020‑23), nine European regions6 will transfer elements of the Basque Country’s model of integrated care.
Box 6.5. CareWell Project
As part of the CareWell Project, administrators from the Basque Country’s integrated care model worked with partnering regions according to a common framework based on two elements: 1) care co‑ordination and communication between health providers and 2) patient empowerment and home‑based care, all supported by ICT-based platforms.
As part of the Project, eight integrated care related service procedure areas (e.g. self-management, multidisciplinary teams) and 12 ICT tools for integrated care support (e.g. electronic health record, electronic prescription) were identified.
Based on a self-assessment exercise, each region chose which service procedures and ICT tools to implement to improve care integration.
Source: Mateo-Abad et al. (2020[5]), “Impact Assessment of an Innovative Integrated Care Model for Older Complex Patients with Multimorbidity: The CareWell Project”, https://doi.org/10.5334/ijic.4711.
Transferability assessment
The following section outlines the methodological framework to assess transferability and results from the assessment.
Methodological framework
Details on the methodological framework to assess transferability can be found in Annex A.
Several indicators to assess the transferability of the Basque Country’s integrated care model were identified (Table 6.3). Indicators were drawn from international databases and surveys to maximise coverage across OECD and non-OECD European countries. Please note, the assessment is intentionally high level given the availability of public data covering OECD and non-OECD European countries.
Table 6.3. Indicators to assess transferability – the Basque Country’s integrated care model
|
Indicator |
Reasoning |
Interpretation |
|---|---|---|
|
Population context |
||
|
% of older individuals who sought health information online in the past 3 months |
The intervention utilises digital tools to engage with participants, for example, the personal health folder |
🡹 value = more transferable |
|
Sector context (primary and secondary care) |
||
|
Proportion of GPs who work in single‑handed practices |
The intervention is more transferable in countries where GPs feel comfortable working with other health professionals. This indicator is a proxy to measure the willingness of GPs to work in co‑ordinated teams. |
Low = more transferable High = less transferable |
|
Proportion of physicians in primary care facilities using electronic health records |
EHRs improve the ability of health professionals to provide integrated patient-centred care. Therefore, the intervention is more transferable in countries that utilise EHRs in primary care facilities. |
🡹 = more transferable |
|
Proportion of hospitals using electronic patient records for inpatients |
As above |
🡹 = more transferable |
|
The extent of task shifting between physicians and nurses in primary care |
This intervention promotes integrated care provided by multidisciplinary teams. Therefore, the intervention is more transferable in countries where physicians feel comfortable shifting tasks to nurses. |
The more “extensive” the more transferable |
|
The use of financial incentives to promote co‑ordination in primary care |
The intervention is more transferable to countries with financial incentives that promote co‑ordination of care across health professionals. |
Bundled payments or co‑ordinated payment = more transferable |
|
Economic context |
||
|
Primary healthcare expenditure as a percentage of current health expenditure |
The intervention places a stronger emphasis on primary care, therefore, it is likely to be more successful in countries that allocate a higher proportion of health spending to primary care |
🡹 = “more transferable” |
Source: WHO (2018[16]), “Primary Health care (PHC) Expenditure as percentage Current Health Expenditure (CHE)”, https://apps.who.int/nha/database; Oderkirk (2017[17]), “Readiness of electronic health record systems to contribute to national health information and research”, https://dx.doi.org/10.1787/9e296bf3-en; Schäfer et al. (2019[18]), “Are people’s health care needs better met when primary care is strong? A synthesis of the results of the QUALICOPC study in 34 countries”, https://doi.org/10.1017/S1463423619000434; Maier and Aiken (2016[19]), “Task shifting from physicians to nurses in primary care in 39 countries: a cross-country comparative study”, https://doi.org/10.1093/eurpub/ckw098; OECD (2020[14]), Realising the Potential of Primary Health Care, https://doi.org/10.1787/a92adee4-en; OECD (2016[20]), “Health Systems Characteristics Survey”, https://qdd.oecd.org/subject.aspx?Subject=hsc; European Observatory on Health Systems and Policies (2021[21]), “The Health Systems and Policy Monitor”, https://eurohealthobservatory.who.int/countries/overview.
Results
Results from the transferability assessment are summarised below, with country-level details available in Table 6.4. Due to data constraints, the “owner” setting is Spain, as opposed to the Basque Country, which is limitation of the analysis.
The proportion of GPs who work in single practices is mixed among potential transfer countries. These results indicate GPs in some countries would readily accept working in a multidisciplinary team and others not.
Use of EHRs are relatively high in Spain, including the Basque Country, compared to the average of all countries at 99% and 79%, respectively. EHRs are an important for stratifying the population into risk groups in order to identify eligible patients.
The integrated care model is supported by a strong HIS, which includes online support tools for patients. In Spain, approximately 4 in 10 older people use the internet to seek health information, which is marginally higher than the average of countries with available data (37%). Levels of internet use for health related reasons is generally highest in Nordic countries such as Denmark, Finland, Iceland and Norway. In these countries, over half of all adults seek health information online.
Most countries do not employ financing methods that incentivise integrated care, including Spain: among examined countries, 19% and 16% have bundled payments and financial incentives for co‑ordinated care, respectively.
Table 6.4. Transferability assessment by country (OECD and non-OECD European countries) – the Basque Country’s integrated care model
A darker shade indicates that the Basque country’s integrated care model is more suitable for transferral in that particular country
|
Country |
% older people using the internet for health information |
% GPs in single practices |
% PC* using EHRs |
% hospitals using EHRs |
Task shifting in PC* |
Financial incentives |
Primary expenditure percentage CHE** |
|---|---|---|---|---|---|---|---|
|
Spain |
41 |
Low |
99 |
80 |
Limited |
No incentive |
39 |
|
Australia |
n/a |
Low |
96 |
20 |
Extensive |
Bundled |
37 |
|
Austria |
32 |
High |
80 |
99 |
None |
Co‑ordinated payment |
37 |
|
Belgium |
37 |
High |
n/a |
n/a |
Limited |
Bundled |
40 |
|
Bulgaria |
12 |
High |
n/a |
n/a |
None |
Bundled |
47 |
|
Canada |
n/a |
Low |
77 |
69 |
Extensive |
Bundled |
48 |
|
Chile |
n/a |
n/a |
65 |
69 |
n/a |
No incentive |
n/a |
|
Colombia |
n/a |
n/a |
n/a |
n/a |
n/a |
No incentive |
n/a |
|
Costa Rica |
n/a |
n/a |
n/a |
n/a |
n/a |
No incentive |
33 |
|
Croatia |
18 |
n/a |
3 |
n/a |
Limited |
No incentive |
38 |
|
Cyprus |
36 |
Low |
n/a |
n/a |
Limited |
No incentive |
41 |
|
Czech Republic |
41 |
High |
n/a |
100 |
None |
No incentive |
33 |
|
Denmark |
56 |
Medium |
100 |
100 |
Limited |
Co‑ordinated payment |
38 |
|
Estonia |
32 |
High |
99 |
100 |
Limited |
No incentive |
44 |
|
Finland |
60 |
Medium |
100 |
100 |
Extensive |
No incentive |
46 |
|
France |
39 |
n/a |
80 |
60 |
None |
Bundled |
43 |
|
Germany |
55 |
High |
n/a |
n/a |
None |
Co‑ordinated payment |
48 |
|
Greece |
20 |
High |
100 |
50 |
None |
No incentive |
45 |
|
Hungary |
42 |
High |
n/a |
n/a |
Limited |
No incentive |
40 |
|
Iceland |
56 |
Low |
100 |
100 |
Limited |
Co‑ordinated payment |
35 |
|
Ireland |
40 |
Low |
95 |
35 |
Extensive |
No incentive |
47 |
|
Israel |
n/a |
n/a |
100 |
100 |
n/a |
Co‑ordinated payment |
n/a |
|
Italy |
27 |
Medium |
n/a |
n/a |
Limited |
Bundled |
n/a |
|
Japan |
n/a |
n/a |
36 |
34 |
n/a |
No incentive |
52 |
|
Korea |
n/a |
n/a |
n/a |
n/a |
n/a |
No incentive |
57 |
|
Latvia |
28 |
High |
70 |
90 |
Limited |
Bundled |
39 |
|
Lithuania |
31 |
Medium |
n/a |
n/a |
Limited |
No incentive |
48 |
|
Luxembourg |
46 |
Medium |
n/a |
n/a |
None |
No incentive |
38 |
|
Malta |
34 |
Medium |
n/a |
n/a |
Limited |
No incentive |
62 |
|
Mexico |
n/a |
n/a |
30 |
49 |
n/a |
Co‑ordinated payment |
44 |
|
Netherlands |
67 |
Medium |
n/a |
n/a |
Extensive |
Bundled |
32 |
|
New Zealand |
n/a |
Low |
95 |
100 |
Extensive |
No incentive |
n/a |
|
Norway |
52 |
Low |
100 |
100 |
None |
No incentive |
39 |
|
Poland |
25 |
Medium |
30 |
10 |
None |
No incentive |
47 |
|
Portugal |
19 |
Low |
n/a |
n/a |
Limited |
No incentive |
58 |
|
Romania |
17 |
Medium |
n/a |
n/a |
None |
No incentive |
35 |
|
Slovak Republic |
39 |
High |
89 |
100 |
None |
No incentive |
n/a |
|
Slovenia |
30 |
High |
n/a |
n/a |
Limited |
No incentive |
43 |
|
Sweden |
44 |
Low |
100 |
100 |
Limited |
Co‑ordinated payment |
n/a |
|
Switzerland |
57 |
Medium |
40 |
100 |
None |
No incentive |
40 |
|
Türkiye |
11 |
Low |
n/a |
n/a |
None |
No incentive |
n/a |
|
United Kingdom |
45 |
Low |
99 |
100 |
Extensive |
No incentive |
53 |
|
United States |
n/a |
n/a |
83 |
76 |
Extensive |
No incentive |
n/a |
Note: *PC = primary care. **CHE = current health expenditure. n/a = no data available.
Source: See Table 6.3.
To help consolidate findings from the transferability assessment above, countries have been clustered into one of three groups, based on indicators reported in Table 6.3. Countries in clusters with more positive values have the greatest transfer potential. For further details on the methodological approach used, please refer to Annex A.
Key findings from each of the clusters are below with further details in Figure 6.2 and Table 6.5:
Countries in cluster one typically have populations where internet use for healthcare purposes is high. Given the integrated care model incorporates various digital tools, this may indicate higher levels of engagement from the population. However, expenditure on primary care is relatively low in these countries indicating potential long-term affordability issues. Spain, where this model of care operates, is in this cluster, meaning conditions in which these clusters could improve on, although ideal, are not pre‑requisites.
Similar to cluster one, countries in cluster two have populations who are digitally health literate, however, unlike cluster one, they spend relatively more on primary care indicating long-term affordability. Before transferring this model of care, countries in cluster two should consider whether their healthcare system is prepared, for example, by ensuring electronic sharing of patient data and acceptance of multidisciplinary care teams.
Countries in cluster three operate healthcare systems that would support this model of care and often spend relatively more on primary care. Nevertheless, the overall success of the intervention may be hampered by the population’s low level of digital health literacy.
Figure 6.2. Transferability assessment using clustering – the Basque Country’s integrated care model
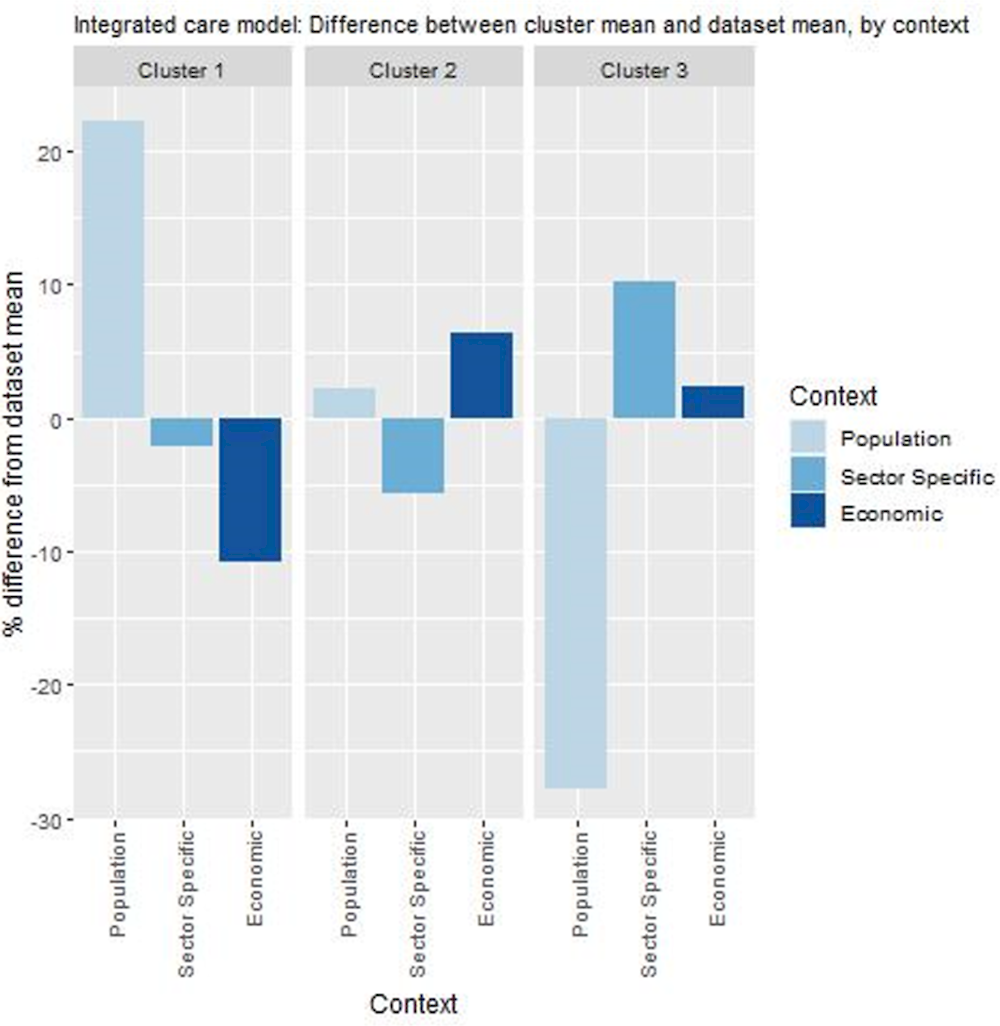
Note: Bar charts show percentage difference between cluster mean and dataset mean, for each indicator.
Source: See Table 6.3.
Table 6.5. Countries by cluster – the Basque Country’s integrated care model
|
Cluster 1 |
Cluster 2 |
Cluster 3 |
|---|---|---|
|
Cyprus Denmark Germany Iceland Malta New Zealand Norway Portugal Spain Sweden United Kingdom |
Australia Bulgaria Canada Czech Republic Finland France Greece Ireland Luxembourg Netherlands Poland Romania Slovak Republic Switzerland |
Austria Belgium Croatia Estonia Hungary Italy Latvia Lithuania Mexico Slovenia |
Note: Due to high levels of missing data, the following countries were omitted from the analysis: Chile, Colombia, Costa Rica, Israel, Japan, Korea, Türkiye and the United States.
New indicators to assess transferability
Data from publicly available datasets is not ideal to assess the transferability of the Basque Country’s integrated care model. For example, there is no international data measuring the level of trust between health professionals, which is necessary for multidisciplinary care teams. Therefore, Box 6.6 outlines several new indicators policy makers could consider before transferring this integrated care model.
Policy makers and relevant stakeholders could also assess their readiness to implement integrated care models using the SCIROCCO (Scaling Integrated Care in Context) Maturity Assessment Model (an EU funded project). The model includes 12 domains for assessing readiness such as, “structure and governance” and “information and eHealth services” (SCIROCCO, n.d.[22]). The Basque Country has completed this assessment with results available using the following link: https://www.scirocco-project.eu/regions-self-assessment/experience-basque-country/.
Box 6.6. New indicators, or factors to consider, when assessing transferability – the Basque Country’s integrated care model
In addition to the indicators within the transferability assessment, policy makers are encouraged to review and/or collect data for the following indicators:
Population context
What is the population’s attitude towards receiving care from health professionals who are not doctors?
What is the level of health literacy among patients? (i.e. are patients likely to engage in shared decision-making?)
Sector specific context (primary and secondary care)
What integrated care models currently exist?
What is the level of acceptability (trust) among health professionals to work together as a co‑ordinated team?
Does the clinical information system support: a) sharing of patient data across health professionals? b) Sharing of patient data across healthcare facilities?
Do health provider reimbursement schemes support co‑ordinated care? (E.g. bundled payments, add-on payments that incentivise co‑ordinated care)
Do regulations support integrated care models? (i.e. professional competencies and practice scope)
Is there an acceptance of evidence‑based care guidelines among health professionals?
Access to population data including patient level information on demographics, diseases, and healthcare use? (Necessary for developing the risk stratification tool)
What is the level of patient data operability?
Political context
Has the intervention received political support from key decision-makers? (E.g. a national strategy to address ageing and chronicity)
Has the intervention received commitment from key decision-makers?
Economic context
What is the cost of implementing and operating the intervention in the target setting and to whom?
Conclusion and next steps
In response to rising rates of multimorbidity, the government in the Basque Country, Spain, implemented a new integrated care model. The new integrated care model is defined by six key characteristics: comprehensive baseline assessments; individualised care plans; multidisciplinary teams; co‑ordinated hospital discharge; patient empower programs; and a strong health information system. Patients eligible for this model of care are identified through a sophisticated risk stratification system.
The Basque Country’s integrated care model reduces demand for hospital services resulting in lower costs. Studies using data from an intervention and control group found patients who participate in the integrated care model are more likely to see primary care thereby reducing hospital admissions and emergency visits. By reducing demand for secondary care services, it is estimated that the integrated care model reduces costs by 5%.
The uptake and impact of the integrated care model across different population groups is not known. The Johns Hopkins Adjusted Clinical Groups Predictive Model (ACG-PM) risk stratification tool is used to identify patients eligible for the integrated care model, which covers 100% of the population. Nevertheless, data on uptake among different population groups – e.g. low SES – is not available. Similarly, it is not clear what impact the integrated care model has on patients with different characteristics and therefore its impact on health equity.
The Basque Country’s integrated care model aligns with the Chronic Care Model, which is considered “gold standard”, nevertheless, policies to enhance performance are available. For example, by building digital health literacy among the older population who are less familiar with digital tools and who stand to benefit most; ensuring sufficient resources to compensate for an increase in responsibilities among healthcare professionals, in particular nurses; and enhancing the quality of future evaluations by stratifying data by different populations groups, increasing the follow-up time and randomising patients into intervention and control groups.
The integrated care model in the Basque Country, Spain, has been transferred to several regions indicating transferability potential. As part a European Commission funded project, CareWell, the Basque Country’s integrated care model was further developed together with the models in regions in Croatia, Poland, Italy and the United Kingdom. A further nine regions across Europe will implement elements of the model as part of the Joint Action on implementation of digitally enabled integrated person-centred care (JADECARE) (2020‑23).
Next steps for policy makers and funding agencies in regards to the Basque Country’s integrated care model are summarised in Box 6.7.
Box 6.7. Next steps for policy makers and funding agencies – the Basque Country’s integrated care model
Next steps for policy makers and funding agencies to enhance the Basque Country’s integrated care model are listed below:
Support researchers undertake more rigorous evaluations to increase the internal validity of studies
Support policies to build digital health literacy, particularly a mong older populations to maximise the intervention’s potential
Promote findings from this case study to better understand what countries/regions are interested in transferring this intervention
Promote “lessons learnt” from countries that have transferred the Basque Country’s integrated care model to their local setting.
References
[10] Effective Public Health Pratice Project (1998), Quality assessment tool for quantitative studies, https://www.nccmt.ca/knowledge-repositories/search/14.
[21] European Observatory on Health Systems and Policies (2021), The Health Systems and Policy Monitor, https://eurohealthobservatory.who.int/countries/overview (accessed on 9 June 2021).
[19] Maier, C. and L. Aiken (2016), “Task shifting from physicians to nurses in primary care in 39 countries: a cross-country comparative study”, The European Journal of Public Health, Vol. 26/6, pp. 927-934, https://doi.org/10.1093/eurpub/ckw098.
[5] Mateo-Abad, M. et al. (2020), “Impact Assessment of an Innovative Integrated Care Model for Older Complex Patients with Multimorbidity: The CareWell Project”, International Journal of Integrated Care, Vol. 20/2, p. 8, https://doi.org/10.5334/ijic.4711.
[4] Mateo-Abad, M. et al. (2020), “Impact of the CareWell integrated care model for older patients with multimorbidity: a quasi-experimental controlled study in the Basque Country”, BMC Health Services Research, Vol. 20/1, https://doi.org/10.1186/s12913-020-05473-2.
[15] Melchiorre, M. et al. (2020), “Integrated Care Programs for People with Multimorbidity in European Countries: eHealth Adoption in Health Systems”, BioMed Research International, Vol. 2020, pp. 1-23, https://doi.org/10.1155/2020/9025326.
[1] Ministry of Health and Consumer Affairs (2010), A Strategy to Tackle the Challenge of Chronicity in the Basque Country, https://ec.europa.eu/eip/ageing/sites/eipaha/files/practices/chronicitybasquecountry.pdf.
[17] Oderkirk, J. (2017), “Readiness of electronic health record systems to contribute to national health information and research”, OECD Health Working Papers, No. 99, OECD Publishing, Paris, https://doi.org/10.1787/9e296bf3-en.
[14] OECD (2020), Realising the Potential of Primary Health Care, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/a92adee4-en.
[8] OECD (2019), The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/67450d67-en.
[20] OECD (2016), Health Systems Characteristics Survey 2016, https://qdd.oecd.org/subject.aspx?Subject=hsc.
[18] Schäfer, W. et al. (2019), “Are people’s health care needs better met when primary care is strong? A synthesis of the results of the QUALICOPC study in 34 countries”, Primary Health Care Research & Development, Vol. 20, https://doi.org/10.1017/s1463423619000434.
[9] Schooling, C. (ed.) (2014), “Prevalence and Costs of Multimorbidity by Deprivation Levels in the Basque Country: A Population Based Study Using Health Administrative Databases”, PLoS ONE, Vol. 9/2, p. e89787, https://doi.org/10.1371/journal.pone.0089787.
[22] SCIROCCO (n.d.), Maturity Model, https://www.scirocco-project.eu/maturitymodel/ (accessed on 9 December 2020).
[6] Soto-Gordoa, M. et al. (2017), “Incorporating Budget Impact Analysis in the Implementation of Complex Interventions: A Case of an Integrated Intervention for Multimorbid Patients within the CareWell Study”, Value in Health, Vol. 20/1, pp. 100-106, https://doi.org/10.1016/j.jval.2016.08.002.
[7] Soto-Gordoa, M. et al. (2019), “Gender and socioeconomic inequalities in the implementation of the Basque programme for multimorbid patients”, European Journal of Public Health, Vol. 29/4, pp. 681-686, https://doi.org/10.1093/eurpub/ckz071.
[13] Struckmann, V. et al. (2018), “Relevant models and elements of integrated care for multi-morbidity: Results of a scoping review”, Health Policy, Vol. 122/1, pp. 23-35, https://doi.org/10.1016/j.healthpol.2017.08.008.
[2] the CareWell Group (2018), “Impact of stratification on the effectiveness of a comprehensive patient-centered strategy for multimorbid patients”, Health Services Research, Vol. 54/2, pp. 466-473, https://doi.org/10.1111/1475-6773.13094.
[11] Wagner, E., B. Austin and M. Korff (1996), “Organizing Care for Patients with Chronic Illness”, The Milbank Quarterly, Vol. 74/4, p. 511, https://doi.org/10.2307/3350391.
[16] WHO (2018), Primary Health Care (PHC) Expenditure as % Current Health Expenditure (CHE).
[12] WHO Europe (2016), Integrated care models: an overview, https://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf.
[3] WP5 Jadecare (2020), Presentation of the original good practice - Basque health strategy in ageing and chronicity: integrated care.
Notes
← 1. Eligible patients are aged 65+, have two of the following three chronic conditions (diabetes, heart failure and chronic obstructive pulmonary disease), have been hospitalised in the past year, and have a Predictive Index score in the 95th percentile (based on Johns Hopkins Adjusted Clinical Groups Predictive Model). Recent updates have extended the target group and include all patients above 13 years of age.
← 2. Other regions included in the study include Zagreb (Croatia), Lower Silesia (Poland), Veneto (Italy), Puglia (Italy) and Powys (United Kingdom).
← 3. Ibid.
← 4. Propensity scores reflect the probability of patient being in the intervention group based on observable characteristic (i.e. allows researchers to construct an artificial control group that is, to the extent possible, the same as the intervention group).
← 5. A “new model of primary care” meeting the following four characteristics: 1) multidisciplinary practices or inter-professional practices; 2) comprehensive health services in the community; 3) population health management (generally based on risk stratification using sophisticated IT systems); and 4) engagement of patients in shared decision-making (OECD, 2020[14]).
← 6. Regions in Bosnia and Herzegovina, Croatia, the Czech Republic, Denmark, Greece, Italy (two regions) Portugal and Serbia.