This chapter covers the TeleHomeCare as implemented in the Italian town of Ceglie Messapica. The case study includes an assessment of TeleHomeCare against the five best practice criteria, policy options to enhance performance and an assessment of its transferability to other OECD and EU27 countries.
Integrating Care to Prevent and Manage Chronic Diseases

9. TeleHomeCare, Ceglie Messapica, Italy
Abstract
TeleHomeCare: Case study overview
Description: TeleHomeCare is a digital intervention designed to support home care through telemonitoring and teleconsultation for patients who suffer from one or more of the following chronic diseases: heart failure, chronic obstructive pulmonary diseases (COPD) and diabetes. TeleHomeCare was initially developed in Ceglie Messapica (a small town near Brindisi, Italy). The intervention involves the patient, caregivers of patients, general practitioners (GP), specialists, and nurses working in the area. The device installed at the patient’s home – called Hospital-at-Home (H@H) – allows the patient to monitor physiological parameters, share measurements with control room operators and care providers. All clinical parameters of the patients based at home are centralised in the hospital, which respect all privacy laws. The device allows doctors to have remote consultations with patients via video.
Best practice assessment:
OECD Best Practice assessment of TeleHomeCare
|
Criteria |
Assessment |
|---|---|
|
Effectiveness |
The effectiveness of TeleHomeCare has not been assessed. The literature shows that telemonitoring can reduce hospitalisations and mortality when monitoring heart failure and COPD; improve mental health quality of life when monitoring COPD; and improve physiological outcomes when monitoring diabetes and COPD. |
|
Efficiency |
TeleHomeCare costs EUR 1 450 per patient per year, while it saves EUR 640 in healthcare services use. |
|
Equity |
Patients who register for TeleHomeCare have to attend a training to acquire sufficient autonomy and use the device safely. The training seeks to overcome cultural limitations and poor aptitude in the use of medical devices and information and communication technologies (ICT). |
|
Evidence‑base |
A set of systematic reviews and meta‑analyses comprised of randomised trials were used to build the knowledge base on the effectiveness of telemedicine. For the cost of TeleHomeCare in Ceglie Messapica, an evaluation carried out by the regional public health agency was used. |
|
Extent of coverage |
TeleHomeCare has been tested in the town of Ceglie Messapica, near Brindisi, Italy. It has not been extended yet to other regions. |
Enhancement options: Monitoring and evaluating clinical outcomes of TeleHomeCare are needed to enhance the effectiveness. While the intervention was evaluated to cost more than it saves, future evaluation of TeleHomeCare should envisage taking a broader perspective, valuing improved quality of life of patients, reduced waiting and travelling times, reduced workload of healthcare workers, and higher work productivity of patients. Efforts should focus on enhancing the internet network to enable access to TeleHomeCare technology and improve access to population groups who are at risk of digital exclusion, in particular older people, disabled people, people in remote locations and those on low incomes.
Transferability: TeleHomeCare is likely to be transferable, since telemonitoring is experimented in many countries, either at the national, regional or local level. In addition, there is political support given most countries have a national eHealth and telehealth policy or strategy. However, population readiness to use telehealth may be a barrier in countries where technology is less advanced.
Conclusion: By favouring continuity of care from hospital to the home setting, TeleHomeCare has the potential to reduce excessive costs due to long hospital stays and emergency services use. Further evaluations on what aspects of the intervention work well and do not work well are needed to improve effectiveness.
Intervention description
With population ageing, more people are affected by multi-morbidity (i.e. having concomitant chronic diseases, either physical or mental). Overall estimates of the prevalence of multi-morbidity across OECD countries are not available. However, country-specific studies suggest that prevalence is high and increasing (OECD, 2019[1]). A recent systematic review and meta‑analysis gathering evidence from 70 community-based studies found that overall pooled prevalence of multi-morbidity was 38% in high-income countries with prevalence increasing with age (Nguyen et al., 2019[2]).
Tertiary prevention helps patient monitor and control their diseases, to reduce symptoms and complications of the disease and hospital stays, improve quality of life, and avoid re‑hospitalisation. New information and communication technologies (ICT) installed at home can support patients and doctors to set up tertiary prevention projects.
TeleHomeCare (sometimes referred to as TeleMedicine) is a digital intervention designed to support home care through telemonitoring and teleconsultation for patients who suffer from chronic diseases, namely heart failure, chronic obstructive pulmonary diseases (COPD) and diabetes. TeleHomeCare was initially a pilot project developed in the hospital in Ceglie Messapica, a small town near Brindisi, the Puglia region of Italy in 2015. The intervention involves the patient, caregivers of patients, general practitioners (GPs), specialists, and nurses working in the area. The objective of TeleHomeCare is to implement an intermediate level of care that improves continuity of care from hospital to a home setting, reducing cost due to prolonged hospital stays and avoiding frequent access to emergency rooms.
The devices of the Hospital-at-Home (H@H) technology are installed at the patient’s home, allowing the patient to self-monitor diseases. These devices are composed of the H@H medical device that allow monitoring physiological parameters (detection of blood pressure, oxygen saturation, heart rate, respiratory rate) and providing oxygen therapy. It comes with the H@H e‑care touchscreen device which provides video consultation, clinical parameter measurements consultation, and remote auscultation (further described in (Bonifazi et al., 2021[3])). Specifically, the devices at home record a patient’s physiological parameters and transmit, in real-time, the information to the control room located in the Community Care Centre in Ceglie Messapica as well as doctors and nurses located at the hospital. Control room operators are responsible for assisting users (i.e. patients, care givers, doctors and nurses) to resolve problems with the H@H system, and alerting GPs in case of anomalies in vital signs. The devices can, if needed, deliver oxygen therapy and endocavitary aspiration. All patient clinical parameters are centralised at the hospital, respecting privacy rules. The technology allows patients and doctors to have remote consultations via video. GPs who voluntary enrol in the programme1 agree to access the H@H system twice a day, 10 times per week, to check patients’ status. The role of specialists is to define the healthcare plan with the GP, and visit patients upon request from the GP. Nurses are in charge for visiting patients at home daily. Patients and care providers are appropriately trained to use the devices.
OECD Best Practices Framework assessment
This section analyses TeleHomeCare against the five criteria within OECD’s Best Practice Identification Framework – Effectiveness, Efficiency, Equity, Evidence‑base and Extent of coverage (see Box 9.1 for a high level assessment of TeleHomeCare). Further details on the OECD Framework can be found in Annex A.
Box 9.1. Assessment of TeleHomeCare
Effectiveness
The evidence of the effect of TeleHomeCare in Ceglie Messapica has not been evaluated yet.
Evidence from the literature show that telemedicine for patients with heart failure (HF) can reduce HF-related mortality, reduce the risk of hospitalisation, and improve quality of life. Regarding telemedicine for patients with diabetes, evidence supports effectiveness on health outcomes, but it does not support an effect on mortality or hospitalisation. Regarding telemedicine for patients with COPD, evidence supports a decrease in hospitalisation and emergency room visits among severe patients, an improvement of mental health quality of life, and a reduction in the number of exacerbations.
Efficiency
The cost of TeleHomeCare in Ceglie Messapica has been evaluated at EUR 1 450 per targeted patient per year, which is greater than estimated savings (EUR 640 per patient per year). However, estimated savings do not take into important factors such as reduced workload for health professionals and travel time for patients.
Equity
There is no evaluation of equity of TeleHomeCare yet,
The evidence on digital health interventions indicates they have the potential to both widen and reduce health inequalities.
The pilot experience in Ceglie Messapica identified technical difficulties in the implementation of TeleHomeCare in areas where there was poor or absence of the internet network coverage.
Evidence‑base
A set of systematic reviews and meta‑analyses were used to build the knowledge base on the effectiveness of telemedicine. For the cost of TeleHomeCare in Ceglie Messapica, an evaluation carried out by the regional public health agency (ARESS) was used.
Extent of coverage
TeleHomeCare has been tested in the town of Ceglie Messapica, near Brindisi, Italy. It has not been extended to other regions.
Effectiveness
The evidence of the effect of TeleHomeCare in Ceglie Messapica has not been evaluated yet. However, systematic reviews and meta‑analyses collected by (Bonifazi et al., 2021[3]) provide evidence of effectiveness for telemedicine (Yun et al., 2018[4]; Faruque et al., 2017[5]; Hong and Lee, 2019[6]). This evidence is presented by type of disease in Table 9.1. Four main outcomes are summarised: mortality, healthcare resources use, quality of life, and other health outcomes. The evidence was complemented with a systematic review on telemonitoring for COPD patients (Cruz, Brooks and Marques, 2014[7]) and for patients with heart failure (Drews, Laukkanen and Nieminen, 2021[8]). Telemedicine for patients with heart failure can reduce all-cause mortality, reduce the risk of hospitalisation, and improve the quality of life of patients. In patients with COPD, telemedicine can reduce the risk of hospitalisation and emergency room (ER) admission, and reduce the number of exacerbations. In patients with diabetes, telemedicine can improve clinical measures.
Table 9.1. Effectiveness of telemedicine for managing heart failure, diabetes and COPD
|
Effect of Telemedicine compared to usual care |
Heart Failure |
Diabetes |
COPD |
|---|---|---|---|
|
Mortality |
Decreasing all-cause mortality (Relative risk (RR) 0.81, 95% CI 0.70‑0.94; I2 = 16%) |
No good evidence found to support a reduction of mortality |
No good evidence to support reduction in mortality rate (RR = 1.43; 95% CI = 0.40‑5.03) (Cruz, Brooks and Marques, 2014[7]) |
|
HF-related mortality (RR 0.68, 95% CI 0.50‑0.91; I2 = 8%) |
|||
|
The all-cause mortality rate significantly lower in studies: published in Europe, involving patients > 65 years, transmitting =3 biologic indicators |
|||
|
Use of healthcare resources |
Reduced risk of HF-related hospitalisation (RR 0.86, 95% CI 0.74‑1.00; I2 = 36%) |
Not available |
Decreased hospitalisation rate of severe patients [RR 0.92, CI 0.31‑1.02]; no difference in moderate patients [RR 1.24, CI 0.57‑2.70] |
|
Decreased emergency room visits in severe patients [RR 0.48, CI 0.31‑0.74]; no difference in moderate patients [RR 1.28, CI 0.61‑2.69] |
|||
|
Quality of life |
Improve quality of life (Drews, Laukkanen and Nieminen, 2021[8]) |
No good evidence found to support an improvement in QoL (quality of life) |
Improved mental health QoL [RR 3.06, CI 2.15‑3.98], failed at improving QoL |
|
Other improvements |
Not available |
Reductions in HbA1C in all 3 follow-up periods (at = 3 mo: ‑0.57%, 95% confidence interval [CI] ‑0.74% to ‑0.40%; at 4‑12 mo: ‑0.28%, 95% CI ‑0.37% to ‑0.20%;at > 12 mo: ‑0.26%, 95% CI ‑0.46% to ‑0.06%. |
Reduced number of exacerbations (Cruz, Brooks and Marques, 2014[7]) |
|
No good evidence found to support a reduced risk of hypoglycaemia |
Efficiency
Looking at telemedicine at large, evidence on cost-effectiveness of care delivered through telemedicine is context-specific and cannot be easily generalised (Oliveira Hashiguchi, 2020[9]).
In the context of TeleHomeCare in Ceglie Messapica, the intervention costs more than it saves money (Bonifazi et al., 2021[3]). The cost of the intervention was estimated at EUR 1 450 per targeted patient per year. This estimate, calculated from a regional healthcare perspective, includes costs related to GPs, nurses, control room operator, and medical device unit. On the other hand, TeleHomeCare significantly reduces the cost of outpatient clinic visits and emergency room visits, while the costs for hospitalisations and pharmaceuticals remain unchanged. The total saving is estimated at EUR 640 per patient per year. However this evaluation does not account for improved quality of life of patients, reduced waiting and travelling times, reduced workload of healthcare workers, and potential indirect cost (e.g. effect on patient’s participation in the labour force and productivity at work).
Equity
TeleHomeCare, such as self-monitoring based at home and video consultation for people with chronic diseases, can help address inequalities by reducing barriers to access, including time, distance and limited availability of services. Telemedicine services help provide care to difficult-to-reach patient groups. For instance, in Canada, where Indigenous people tend to have poorer health than non-Indigenous people, Ontario Telemedicine Network included 120 indigenous telemedicine sites and counted 9 628 indigenous patient events (OTN, 2018[10]).
However, there is a risk of digital exclusion, in particular with regards to older people, disabled people, people in remote locations as well as those on low incomes. For instance, older people who do not have knowledge or capacity to learn how to use the new technologies may not be able to use the system, and thus be excluded. To overcome this issues, patients who register for TeleHomeCare must attend training to acquire sufficient autonomy to use the device safely. The training seeks to overcome cultural limitations and poor aptitude in the use of medical devices and ICT.
While there is no study evaluating what impact TeleHomeCare has on health inequalities, the pilot experience in Ceglie Messapica identified technical difficulties implementing TeleHomeCare in areas where there was poor or no internet network coverage.
Evidence‑base
Evidence of effectiveness for the use of tele‑monitoring for heart failure, Diabetes and COPD was gathered from systematic reviews and meta‑analyses as described in the section on “Effectiveness”. An evaluation of the cost associated with TeleHomeCare in Ceglie Messapica was made by the Italian regional public health agency (Bonifazi et al., 2021[3]). Hence, it is not appropriate to assess the evidence‑base of TeleHomeCare using the Quality Assessment Tool for Quantitative Studies from the Effective Public Health Practice Project. Instead, this section summarises the methodology for a selection of articles cited under the section assessing the “Effectiveness” and “Efficiency” of TeleHomeCare Box 9.2.
Box 9.2. Evidence base supporting the effectiveness and efficiency of TeleHomeCare
This box summarises the methodology for the studies outlined in the sections on “Effectiveness” and “Efficiency”.
Effectiveness
Faruque et al. (2017[5]) undertook a systematic review and meta‑analysis to analyse the effectiveness of telemedicine for the management of diabetes compared with usual care, including over 100 randomised control trials (RCTs). The Cochrane Collaboration’s risk of bias (RoB) tool was used. Blinding of participants is generally not feasible for telemedicine interventions. Blinding of outcome assessors was present in 20% of trials. Seventy-eight trials (70%) reported and described an appropriate method of randomisation, but only 30 (27%) reported an adequate allocation concealment process. The intention-to-treat principle was applied in 51 (46%) of the trials. Public funding was exclusively used in 57 trials (51%).
Hong and Lee (2019[6]) conducted a systematic review and meta‑analysis to analyse the effect of telemonitoring on COPD patients using information from 27 RCTs. They used Cochrane risk of bias (RoB) for RCTs and assessed selection bias, allocation bias, performance and detection bias, attrition bias and reporting bias by scoring low, high and unclear risk. Four studies had a high risk of selection bias, and almost all studies reported an unclear allocation concealment. Only two studies reported blindness. Indeed, the blinding of participants was lacking, but treatment for participants cannot be blinded because of intervention characteristics.
Yun et al. (2018[4]) performed a systematic review and meta‑analysis to evaluate the effectiveness of telemonitoring in the management of patients with heart failure. The quality of the 37 selected RCTs was assessed by the Cochrane RoB tool. More than 25% of the studies had a high risk of bias for reporting bias. The risk of device support was designated “uncertain” in the majority of the studies. Because most of the included studies reported objective outcomes, such as death or hospitalisation, the overall risk of detection bias was low.
Cruz et al. (2014[7]) undertook a systematic review to assess the effectiveness of home telemonitoring in patients with COPD. In total, 10 articles (9 studies) met the inclusion criteria, of which: 8 were RCTs (2 high quality, 5 good quality and 1 fair to good quality); 1 was an experimental study with a control group (good quality), and 1 was quasi‑experimental with a control group (good quality).
Drews et al. (2021[8]) undertook a systematic review and meta‑analysis to assess the effect of home telemonitoring in the treatment of patients with decompensated heart failure. In total, 11 articles were included. The Cochrane RoB tool was employed. The intervention was not blinded in any of the primary studies. The overall risk of bias was judged to be high in four primary studies. In three, there was missing patient data. In one study, the study allocation was not adequately randomised or blinded.
Efficiency
Bonifazi et al. (2021[3]) compared costs and savings of patients who received the TeleHomeCare intervention with those of patients with usual care, in Ceglie Messapica, in the period 2015‑19. The control groups were identified ex-post and not through an ad-hoc clinical protocol. Matching each patient in the treatment group with a patient with the same characteristics in the two control group was possible for 179 patients (86.4% of the total patients enrolled). Besides, it was not possible to carry out a cost effectiveness analysis because no clinical data was available on the therapeutic efficacy of telemedicine compared to usual care.
Extent of coverage
The intervention has been initially deployed locally in the hospital of Ceglie Messapica (a small town near Brindisi, Italy), including 207 patients. The intervention has not been extended to other areas of Italy. However, similar telemonitoring programmes are in place in many countries either at the local, regional or national level (Oliveira Hashiguchi, 2020[9]), for instance, Ontario Telemedicine Network in Canada.
Policy options to enhance performance
Policy options available to high-level policy makers (e.g. region / state / national governments) and TeleHomeCare administrators are outlined in this section and refer to each of the five best practice criteria.
Enhancing effectiveness
Monitoring and evaluating clinical outcomes are needed to enhance the effectiveness of TeleHomeCare. Clinical outcomes associated with the use of TeleHomeCare in Ceglie Messapica have not yet been evaluated. An initial evaluation is crucial to define criteria of improvement.
Digital health products, such as TeleHomeCare devices, require patients and health professionals to be digitally health literate. Healthcare systems are growing increasingly digital as evidenced by the growing number of countries with national eHealth strategies (WHO, 2015[11]). Therefore, policy makers should promote digital health literacy so that people can apply their health knowledge/skills to digital products. TeleHomeCare has a training component for the users, however it is important to further develop this component to ensure that people are confident using telemonitoring and teleconsultation. In particular there is a need to focus on the population aged over 50 who are at greater risk of having one or multiple chronic diseases, such as heart failure, diabetes and COPD, and who are less confident using digital tools. Policy efforts should also concentrate on population groups who face barriers to accessing and utilising eHealth products, such as teleconsultation and telemonitoring, given these groups often stand to benefit most (e.g. those with a lower socio-economic status) (Oliveira Hashiguchi, 2020[9]).
Health professionals must also be digitally health literate in order to feel confident using digital products when treating patients. Among OECD countries, one‑third of health workers do not feel accustomed to using digital solutions “due to gaps in knowledge and skills in data analytics” (OECD, 2019[1]). To ensure health professionals can “safely and effectively” adopt digital work tools (e.g. teleconsultation and telemonitoring), it is important they receive adequate support via training and education. For instance, GPs and specialists who are involved in the TeleHomeCare service are trained during the first two weeks by control room operators and telemedicine experts from the H@H system provider.
Enhancing efficiency
Future evaluations of TeleHomeCare should envisage taking a broader perspective, valuing all potentially improved outcomes. In the case of TeleHomeCare in Ceglie Messapica, it is shown that intervention costs exceed money saved (see section on “Efficiency”). However, some outcomes of the intervention could not be valued (e.g. improved quality of life of patients, reduced waiting and travelling times, reduced workload of healthcare workers, and higher work productivity of patients). Including such outcomes to future studies would provide a more holistic and therefore accurate picture of TeleHomeCare’s cost-effectiveness potential. Future studies would also benefit from taking a longitudinal perspective given interventions such as TeleHomeCare often require significant upfront fixed costs.
Enhancing equity
Efforts to enhance internet network quality and coverage can help to increase access to TeleHomeCare and improve access for population groups in remote areas. The pilot experience in Ceglie Messapica identified technical difficulties in the implementation of TeleHomeCare in areas where there was poor or absence of the Internet network coverage. Enhancing Internet network can therefore help people in underserved areas use TeleHomeCare devices.
Policies to increase access and utilisation of TeleHomeCare among disadvantaged population groups can reduce health inequalities. There is a risk of digital exclusion, in particular with regards to older people, disabled people, people in remote locations and those on low incomes. As outlined under “Enhancing effectiveness”, policy efforts should focus on building health literacy and digital health literacy among disadvantaged groups. More direct action that can be implemented by TeleHomeCare administrators include:
Providing training to patients on how to use the TeleHomeCare devices and providing technical support to users, especially older people, disabled people, those in remote locations and on those low incomes.
Collecting data that can be disaggregated by priority population groups (e.g. information on age, disabilities, education, rural location). This information can subsequently be used to amend the implementation of the intervention to suit the needs of priority populations.
Failing to address the needs of disadvantaged population groups risks widening existing health inequalities.
Enhancing the evidence‑base
The impact of TeleHomeCare in Ceglie Messapica on clinical outcomes and final outcomes will be of key interest to policy makers and is therefore encouraged. To date, one study evaluated the impact of TeleHomeCare in Ceglie Messapica on health system costs, however there are no evaluations examining the impact on health outcomes, including final health outcomes (e.g. patient quality of life, work productivity of patients).
Key steps involved in undertaking an evaluation are outlined in OECD’s Guidebook on Best Practices in Public Health (OECD, 2022[12]). These steps are summarised below to assist TeleHomeCare administrators in future evaluation efforts:
Design the evaluation study
Develop a logic model: a logic model summarises the main elements of an intervention and provides a visual overview of the relationship between inputs, activities, outputs and outcomes.
Select evaluation indicators: indicators for each element within the programme logic need to be specified. Example outcome indicators for TeleHomeCare may include EQ‑5D (patient quality of life) and work productivity. Indicators should be SMART (specific, measurable, achievable, relevant and time‑bound) and where possible be stratified to understand the intervention’s impact on inequalities (as discussed under “Enhancing efficiency”).
Choose a study design: process evaluations assess whether an intervention was implemented as intended whereas an outcome evaluation assesses the impact the intervention had on outcomes. Regarding the latter, it is necessary to choose a study design that is appropriate for the intervention.
Choose a data collection method: any evaluation of TeleHomeCare will largely rely on real-world data collected from the control room servers and devices. Additional primary sources of data may also be collected, for example, from user surveys.
Execute the evaluation study
Collect the data: data collection methods should consider logistics, consent, privacy, data security and other ethical considerations, in particular given data from TeleHomeCare contains personal and clinical information.
Analyse the data: it is not possible to detail all the various methods available to analyse data here, however, a first step for any intervention is to analyse descriptive statistics including a look at the pattern of missing data.
Act on evaluation results
Follow-up action: results from the evaluation will provide useful information on how the intervention can be adapted to improve performance.
Disseminate results: evaluation results should be conveyed to the target audience via appropriate channels. In particular, it is important to convey “lessons learnt” and how these will be incorporated into the future design of TeleHomeCare.
Enhancing extent of coverage
To boost the uptake of TeleHomeCare throughout the national territory, it is key to ensure the devices are trusted and non-burdensome. It is also important considering the viewpoints of both patients and healthcare professionals. Patient’s data (both personal and clinical) needs to be secured. Training and technical support provided to both patients and healthcare professionals have to be promoted. The role of advanced practice nurses in remote monitoring has to be considered.
Transferability
This section explores the transferability of TeleHomeCare and is broken into three components: 1) an examination of previous transfers; 2) a transferability assessment using publicly available data; and 3) additional considerations for policy makers interested in transferring TeleHomeCare.
Previous transfers
TeleHomeCare in Ceglie Messapica has not yet been transferred to other areas or regions in Italy. However, similar telemonitoring programmes are in place in many countries either at the local, regional or national level (Oliveira Hashiguchi, 2020[9]), for instance, Ontario Telemedicine Network in Canada.
The ability to readily transfer TeleHomeCare, as it is implemented in Ceglie Messapica, heavily depends on whether the service uses proprietary technology.
Transferability assessment
The following section outlines the methodological framework to assess transferability and results from the assessment.
Methodological framework
Details on the methodological framework to assess transferability can be found in Annex A.
Several indicators to assess the transferability of TeleHomeCare were identified (Table 9.2). Indicators were drawn from international databases and surveys to maximise coverage across OECD and non-OECD European countries. Please note, the assessment is intentionally high level given the availability of public data covering OECD and non-OECD European countries.
Table 9.2. Indicators to assess transferability – TeleHomeCare
|
Indicator |
Reasoning |
Interpretation |
|---|---|---|
|
Population context |
||
|
ICT Development Index* |
TeleHomeCare is more likely to be successful in digitally advanced countries |
🡹 value = more transferable |
|
Individuals using the Internet – last 3 m (%) who are aged 55‑74 |
TeleHomeCare is more transferable to a population where elderly people – who are more likely to have chronic diseases- are comfortable using the connected smart devices |
🡹 value = more transferable |
|
Self-reported use of home care services |
TeleHomeCare is more transferrable to a population that already uses home care services |
🡹 value = more transferable |
|
Sector context (digital health sector) |
||
|
Legislation exists to protect the privacy of personally identifiable data of individuals, irrespective of whether it is in paper or digital format |
TeleHomeCare requires to transfer patient data. Therefore, TeleHomeCare is more likely to be successful in countries with legislation to protect patient data. |
‘Yes’ = more transferable |
|
eHealth composite index of adoption amongst GPs** |
TeleHomeCare requires GPs, specialists and nurses to use eHealth technologies. Therefore, TeleHomeCare is more likely to be successful in countries where GPs are comfortable using eHealth technologies |
🡹 value = more transferable |
|
% of tertiary institutions (public and private) that offer ICT for health (eHealth) courses |
TeleHomeCare is more transferable if health professional students receive eHealth training |
🡹 value = more transferable |
|
% of institutions or associations offering in-service training in the use of ICT for health as part of the continuing education of health professionals |
TeleHomeCare is more transferable if health professionals have appropriate eHealth training |
🡹 value = more transferable |
|
Political context |
||
|
A national eHealth policy or strategy exists |
TeleHomeCare is more likely to be successful if national policies support eHealth |
‘Yes’ = more transferable |
|
A dedicated national telehealth policy or strategy exists |
TeleHomeCare is more likely to be successful if the government is supportive of telehealth |
‘Yes’ = more transferable |
|
Economic context |
||
|
% of funding contribution for eHealth programmes provided by public funding sources over the previous two years |
TeleHomeCare is more likely to be successful in a country whose government spends more on eHealth |
🡹 value = more transferable |
|
Special funding is allocated for the implementation of the national eHealth policy or strategy |
TeleHomeCare is more likely to be successful if there already is allocated funding for eHealth |
‘Yes’ = more transferable |
Note: *The ICT development index represents a country’s information and communication technology capability. It is a composite indicator reflecting ICT readiness, intensity and impact (ITU, 2020[13]). **The eHealth composite index of adoption amongst GPs is made up of adoption in regards to electronic health records, telehealth, personal health records and health information exchange (European Commission, 2018[14]).
Source: WHO (2019[15]), “Existence of operational policy/strategy/action plan to reduce unhealthy diet related to NCDs (Noncommunicable diseases)”, https://apps.who.int/gho/data/node.imr.NCD_CCS_DietPlan?lang=en; ITU (2020[13]), “The ICT Development Index (IDI): conceptual framework and methodology”, https://www.itu.int/en/ITU-D/Statistics/Pages/publications/mis/methodology.aspx; European Commission (2018[14]), “Benchmarking Deployment of eHealth among General Practitioners (2018)”, https://op.europa.eu/en/publication-detail/-/publication/d1286ce7-5c05-11e9-9c52-01aa75ed71a1; OECD (2019[16]), “Individuals using the Internet for seeking health information – last 3 m (%) (all individuals aged 16‑74)”; World Bank (2017[17]), “GNI per capita, PPP (constant 2017 international $)”, https://data.worldbank.org/indicator/NY.GNP.PCAP.PP.KD; WHO (2015[11]), “Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage”, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage; Maier and Aiken (2016[18]), “Task shifting from physicians to nurses in primary care in 39 countries: a cross-country comparative study”, https://doi.org/10.1093/EURPUB/CKW098.
Results
The transfer analysis shows the transferability potential of TeleHomeCare in Ceglie Messapica throughout Italy and to other countries. In Italy, there is political drive to deliver eHealth and telehealth with funding allocations, there is also legislation to protect patient data, and health professionals are comfortable using eHealth technologies according to the eHealth composite index (see the table below).
Data from other countries show high transfer potential based on population and digital health sector indicators, for example, other countries exhibit high rates of ICT development and use of home care services, further, health professional have ready access to eHealth training.
Regarding political support, 27 (out of 39 with available data) countries have a national eHealth policy, and 20 have a dedicated national telehealth policy or strategy indicating there is a mix of political will to introduce programs such as TeleHomeCare among countries.
Finally, using data to represent the economic context, most countries (i.e. 29) have special funding allocated for the eHealth policy implementation.
It is important to note though that data from publicly available datasets, alone, is not appropriate to assess the transferability of the TeleHomeCare programme in Ceglie Messapica. Countries interested in setting up a similar programme should do an analysis to identify what the needs and issues are around telemonitoring and teleconsultation, and how a national programme can address these. In addition, since similar telemonitoring programmes are already in place in many countries either at the local, regional or national level (Oliveira Hashiguchi, 2020[9]), countries should consider evaluating how to their programme compares with TeleHomeCare in Ceglie Messapica.
Table 9.3. Transferability assessment by country (OECD and non-OECD European countries) – TeleHomeCare
A darker shared indicates TeleHomeCare may be more transferable to that particular country
|
Country |
ICT Development Index (2015) |
Individuals using the Internet who are aged 55‑74 |
Self-reported use of home care services (%) |
Legislation exists to protect the privacy of personally identifiable data of individuals |
eHealth composite index (GPs) |
% of tertiary institutions that offer ICT for health (eHealth) courses |
% institutions or associations offering in-service training in the use of ICT for health as part of the continuing education |
A national eHealth policy or strategy exists |
A dedicated national telehealth policy or strategy exists |
Special funding is allocated for the implementation of the national eHealth policy or strategy |
% funding contribution for eHealth programmes |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Italy |
6.90 |
56.00 |
35.40 |
Yes |
2.19 |
Low |
High |
Yes |
Yes |
Yes |
Very high |
|
Australia |
8.20 |
76.62 |
n/a |
n/a |
n/a |
Medium |
High |
Yes |
No |
n/a |
Very high |
|
Austria |
7.50 |
69.66 |
18.00 |
Yes |
1.91 |
Low |
Low |
No |
No |
Yes |
Very high |
|
Belgium |
7.70 |
77.96 |
45.10 |
Yes |
2.07 |
Low |
Low |
Yes |
Combined* |
Yes |
Very high |
|
Bulgaria |
6.40 |
n/a |
22.30 |
Yes |
1.81 |
Medium |
Medium |
Yes |
Combined |
Yes |
Low |
|
Canada |
7.60 |
88.03 |
n/a |
Yes |
n/a |
High |
Low |
Yes |
No |
n/a |
Very high |
|
Chile |
6.10 |
52.11 |
n/a |
Yes |
n/a |
Low |
Low |
Yes |
No |
n/a |
Very high |
|
Colombia |
5.00 |
n/a |
n/a |
Yes |
n/a |
n/a |
n/a |
n/a |
Yes |
n/a |
n/a |
|
Costa Rica |
6.00 |
64.82 |
n/a |
Yes |
n/a |
Medium |
Medium |
Yes |
Yes |
n/a |
Very high |
|
Croatia |
6.80 |
n/a |
16.40 |
Yes |
2.18 |
Low |
Medium |
Yes |
Yes |
Yes |
Very high |
|
Cyprus |
6.30 |
n/a |
23.50 |
1.93 |
Medium |
Low |
Yes |
Combined |
No |
Very high |
|
|
Czech Republic |
7.20 |
66.85 |
23.00 |
Yes |
2.06 |
Medium |
n/a |
No |
Combined |
No |
Low |
|
Denmark |
8.80 |
93.42 |
51.40 |
Yes |
2.86 |
Medium |
Very high |
Yes |
Yes |
Yes |
Very high |
|
Estonia |
8.00 |
73.25 |
12.70 |
Yes |
2.79 |
Medium |
Low |
Yes |
No |
Yes |
Very high |
|
Finland |
8.10 |
87.83 |
43.60 |
Yes |
2.64 |
Medium |
Medium |
Yes |
Combined |
Yes |
Very high |
|
France |
8.00 |
76.29 |
56.50 |
n/a |
2.05 |
n/a |
n/a |
n/a |
n/a |
n/a |
n/a |
|
Germany |
8.10 |
81.96 |
27.60 |
n/a |
1.94 |
n/a |
n/a |
n/a |
n/a |
n/a |
n/a |
|
Greece |
6.90 |
46.14 |
20.60 |
Yes |
1.79 |
Medium |
Medium |
Yes |
Combined |
Yes |
Very high |
|
Hungary |
6.60 |
54.77 |
24.80 |
Yes |
2.03 |
Low |
n/a |
No |
No |
No |
Very high |
|
Iceland |
8.70 |
97.47 |
34.20 |
Yes |
n/a |
Very high |
Very high |
Yes |
No |
Yes |
Very high |
|
Ireland |
7.70 |
73.99 |
51.90 |
Yes |
2.10 |
n/a |
Low |
Yes |
No |
Yes |
Low |
|
Israel |
7.30 |
73.60 |
n/a |
Yes |
n/a |
High |
Low |
No |
Yes |
Yes |
Very high |
|
Japan |
8.30 |
n/a |
n/a |
Yes |
n/a |
n/a |
n/a |
Yes |
No |
n/a |
n/a |
|
Korea |
8.80 |
87.45 |
n/a |
n/a |
n/a |
n/a |
n/a |
n/a |
n/a |
n/a |
n/a |
|
Latvia |
6.90 |
65.91 |
15.70 |
Yes |
1.83 |
Low |
Low |
Yes |
Combined |
Yes |
Low |
|
Lithuania |
7.00 |
57.68 |
18.30 |
Yes |
1.65 |
Medium |
Low |
Yes |
Yes |
No |
High |
|
Luxembourg |
8.30 |
88.08 |
24.40 |
Yes |
1.78 |
Low |
Low |
Yes |
Combined |
Yes |
Very high |
|
Malta |
7.50 |
n/a |
42.50 |
Yes |
n/a |
Very high |
Very high |
No |
No |
n/a |
Very high |
|
Mexico |
4.50 |
40.49 |
n/a |
Yes |
n/a |
Medium |
Low |
No |
No |
n/a |
High |
|
Netherlands |
8.40 |
92.89 |
59.20 |
Yes |
n/a |
High |
High |
Yes |
Combined |
Yes |
Very high |
|
New Zealand |
8.10 |
n/a |
n/a |
Yes |
n/a |
Medium |
Very high |
Yes |
No |
n/a |
Low |
|
Norway |
8.40 |
95.23 |
27.20 |
Yes |
n/a |
Low |
Medium |
Yes |
Yes |
Yes |
Very high |
|
Poland |
6.60 |
52.08 |
20.80 |
Yes |
1.84 |
High |
Medium |
Yes |
Combined |
Yes |
Very high |
|
Portugal |
6.60 |
45.83 |
17.40 |
Yes |
2.12 |
Low |
Low |
No |
Yes |
Yes |
High |
|
Romania |
5.90 |
n/a |
16.90 |
Yes |
1.79 |
n/a |
n/a |
Yes |
n/a |
n/a |
n/a |
|
Slovak Republic |
6.70 |
54.85 |
18.30 |
n/a |
1.76 |
n/a |
n/a |
n/a |
n/a |
n/a |
n/a |
|
Slovenia |
7.10 |
59.89 |
24.70 |
Yes |
2.00 |
High |
High |
No |
No |
Yes |
Very high |
|
Spain |
7.50 |
76.70 |
39.80 |
Yes |
2.37 |
Low |
Medium |
No |
No |
Yes |
Very high |
|
Sweden |
8.50 |
92.54 |
22.30 |
Yes |
2.52 |
Very high |
Very high |
Yes |
No |
Yes |
Very high |
|
Switzerland |
8.50 |
90.66 |
n/a |
Yes |
n/a |
Low |
Very high |
Yes |
No |
Yes |
Low |
|
Türkiye |
5.50 |
34.13 |
2.90 |
Yes |
n/a |
n/a |
n/a |
No |
Combined |
Yes |
Low |
|
United Kingdom |
8.50 |
87.32 |
27.50 |
Yes |
2.52 |
Medium |
High |
Yes |
Yes |
Yes |
Very high |
|
United States |
8.10 |
78.38 |
n/a |
Yes |
n/a |
Low |
Low |
Yes |
No |
n/a |
n/a |
Note: *Combined with eHealth policy or strategy. n/a = data not available.
Source: See Table 9.2.
To help consolidate findings from the transferability assessment above, countries have been clustered into one of three groups, based on indicators reported in the table above.
Countries in clusters with more positive values have the greatest transfer potential. For further details on the methodological approach used, please refer to Annex A.
Key findings from each of the clusters are below with further details in Figure 9.1 and Table 9.4:
Countries in cluster one have political, economic and sector specific arrangements in place to transfer TeleHomeCare. However, population uptake may be low given digital health literacy is typically below average for these countries. Italy, where TeleHomeCare currently operates, falls under this cluster indicating digital health literacy, although ideal, is not a prerequisite for this intervention.
Countries in cluster two have a population considered digitally health literate, in addition, these countries have political arrangements in place to support TeleHomeCare. However, prior to transferring this intervention, countries in cluster two may wish to consider introducing policies to ensure the digital health sector is ready to deliver this intervention (e.g. staff have the appropriate skills). Further, it will be important to ensure the intervention is affordable in the long run.
Countries in cluster three should undertake further analysis to ensure TeleHomeCare aligns with political priorities, and that the population and a digital health sector are ready to maximise TeleHomeCare’s potential.
Figure 9.1. Transferability assessment using clustering – TeleHomeCare
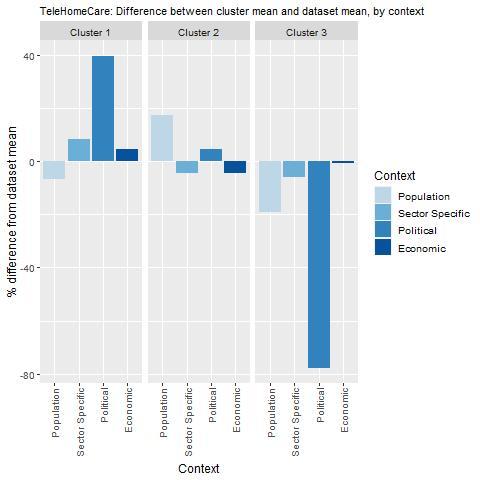
Note: Bar charts show percentage difference between cluster mean and dataset mean, for each indicator.
Source: See Table 9.2.
Table 9.4. Countries by cluster – TeleHomeCare
|
Cluster 1 |
Cluster 2 |
Cluster 3 |
|---|---|---|
|
Bulgaria Costa Rica Croatia Cyprus Czech Republic Denmark Finland Greece Italy Netherlands Norway Poland Türkiye United Kingdom |
Australia Belgium Canada Chile Estonia Iceland Ireland Latvia Lithuania Luxembourg New Zealand Sweden Switzerland United States |
Austria Hungary Israel Malta Mexico Portugal Slovenia Spain |
Note: Due to high levels of missing data, the following countries were omitted from the analysis: Colombia, France, Germany, Japan, Korea, Romania, the Slovak Republic.
New indicators to assess transferability
Data from publicly available datasets is not ideal to assess the transferability of TeleHomeCare. For example, there is no publicly available information the level of public acceptability of telemonitoring and teleconsultation interventions. Therefore, Box 9.3 outlines several new indicators policy makers should consider before transferring TeleHomeCare.
Box 9.3. New indicators, or factors, to consider when assessing transferability – TeleHomeCare
In addition to the indicators within the transferability assessment, policy makers are encouraged to collect data for the following indicators:
Population context
How acceptable are telemonitoring and teleconsultation interventions amongst the public?
Does the population trust their personal health information will be used, stored and managed appropriately?
What proportion of the population is able to use a telemonitoring device?
Sector specific context (digital health)
What, if any, compatible interventions exist?
What, if any, competing interventions exist? (e.g. other telemonitoring and teleconsultation tools)?
How acceptable are digital products to treat patients with diabetes, COPD and heart failure amongst the health profession?
Do regulations support integration of telemonitoring and teleconsultation into the healthcare guidelines? (relevant for countries who do not fall under GDPR rules (Genders Data Protection Regulation))
Do healthcare clinics and hospitals have the appropriate technical equipment to provide TeleHomeCare?
What healthcare facilities operate in the target setting? (e.g. number of hospital, outpatient centres)
Political context
Has the intervention received political support from key decision-makers?
Has the intervention received commitment from key decision-makers?
Economic context
Are there additional cost of implemented the intervention in the target setting beyond those estimated by TeleHomeCare administrators?
Conclusion and next steps
The TeleHomeCare intervention assessed here, is a telemonitoring and teleconsultation programme for patients with heart failure, diabetes and COPD, implemented in Ceglie Messapica, a town near Brindisi in Italy. With TeleHomeCare, physiological parameters of the patient are recorded at home and transmitted in real-time to the control room located in the Community Care Centre in Ceglie Messapica, and to doctors and nurses located at the hospital. This programme creates an intermediate level of care that improves continuity of care from hospital to the home setting, and has the potential to reduce excessive costs caused by prolonged hospital stays and frequent access to emergency rooms.
Monitoring and evaluating clinical outcomes arising from TeleHomeCare are needed to enhance what aspects of the intervention work well and do not work well – findings from the analysis can subsequently be used to improve overall effectiveness. While the intervention was evaluated to cost more than it saves, future evaluations of TeleHomeCare should envisage taking a broader perspective by incorporating improved patient quality of life, reduced waiting and travelling times, reduced workload of healthcare workers, and higher work productivity of patients. Policy efforts should also focus on enhancing internet network coverage to enable access to the TeleHomeCare technology and improving reach to population groups with a risk of digital exclusion, in particular older people, disabled people, people in remote locations and those on low incomes.
TeleHomeCare is likely to be transferable since telemonitoring operates in many countries, either at national, regional or local level. In addition, there is political support given most countries have a national eHealth and telehealth policy or strategy. However, population readiness to use telehealth may act as a barrier for countries that are less digitally advanced.
Next steps for policy makers and funding agencies regarding TeleHomeCare are summarised in Box 9.4.
Box 9.4. Next steps for policy makers and funding agencies – TeleHomeCare
Next steps for policy makers and funding agencies are listed below:
Monitor and evaluate clinical outcomes of TeleHomeCare
Evaluate the improved quality of life of patients, reduced waiting and travelling times, reduced workload of healthcare workers, and higher work productivity of patients, resulting from TeleHomeCare
Ensure that TeleHomeCare addresses digital inclusion, to reduce rather than exacerbate health inequalities
Identify needs and issues around telemonitoring, and how a national programme can address these
For countries which already have in place similar programmes or pilot at the national, regional or local level, compare them with TeleHomeCare in Ceglie Messapica.
References
[3] Bonifazi, F. et al. (2021), Telemonitoring of chronic diseases: The Experience of Azienda Sanitaria Locale BR, Brindisi (IT) with heart failure, diabetes and chronic obstructive pulmonary disease, AReSS Puglia, Agenzia Regionale Strategica per la Salute ed il Sociale, https://www.itemoxygen.com.
[7] Cruz, J., D. Brooks and A. Marques (2014), “Home telemonitoring effectiveness in COPD: a systematic review”, International Journal of Clinical Practice, Vol. 68/3, pp. 369-378, https://doi.org/10.1111/IJCP.12345.
[8] Drews, T., J. Laukkanen and T. Nieminen (2021), “Non-invasive home telemonitoring in patients with decompensated heart failure: a systematic review and meta-analysis”, ESC Heart Failure, https://doi.org/10.1002/EHF2.13475.
[14] European Commission (2018), Benchmarking Deployment of eHealth among General Practitioners (2018), https://op.europa.eu/en/publication-detail/-/publication/d1286ce7-5c05-11e9-9c52-01aa75ed71a1.
[5] Faruque, L. et al. (2017), “Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials”, CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne, Vol. 189/9, pp. E341-E364, https://doi.org/10.1503/CMAJ.150885.
[6] Hong, Y. and S. Lee (2019), “Effectiveness of tele-monitoring by patient severity and intervention type in chronic obstructive pulmonary disease patients: A systematic review and meta-analysis”, International journal of nursing studies, Vol. 92, pp. 1-15, https://doi.org/10.1016/J.IJNURSTU.2018.12.006.
[13] ITU (2020), The ICT Development Index (IDI): conceptual framework and methodology, https://www.itu.int/en/ITU-D/Statistics/Pages/publications/mis/methodology.aspx (accessed on 26 February 2021).
[18] Maier, C. and L. Aiken (2016), “Task shifting from physicians to nurses in primary care in 39 countries: a cross-country comparative study”, The European Journal of Public Health, Vol. 26/6, pp. 927-934, https://doi.org/10.1093/eurpub/ckw098.
[2] Nguyen, H. et al. (2019), “Prevalence of multimorbidity in community settings: A systematic review and meta-analysis of observational studies”, Journal of Comorbidity, Vol. 9, p. 2235042X1987093, https://doi.org/10.1177/2235042X19870934.
[12] OECD (2022), Guidebook on Best Practices in Public Health, OECD Publishing, Paris, https://doi.org/10.1787/4f4913dd-en.
[1] OECD (2019), Health in the 21st Century: Putting Data to Work for Stronger Health Systems, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/e3b23f8e-en.
[16] OECD (2019), Individuals using the Internet for seeking health information - last 3 m (%) (all individuals aged 16-74), Dataset: ICT Access and Usage by Households and Individuals.
[9] Oliveira Hashiguchi, T. (2020), “Bringing health care to the patient: An overview of the use of telemedicine in OECD countries”, OECD Health Working Papers, No. 116, OECD Publishing, Paris, https://doi.org/10.1787/8e56ede7-en.
[10] OTN (2018), OTN Annual Report, https://otn.ca/wp-content/uploads/2017/11/otn-annual-report.pdf (accessed on 12 November 2021).
[15] WHO (2019), Existence of operational policy/strategy/action plan to reduce unhealthy diet related to NCDs (Noncommunicable diseases), https://apps.who.int/gho/data/node.imr.NCD_CCS_DietPlan?lang=en.
[11] WHO (2015), Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage, Global Observatory for eHealth, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage.
[17] World Bank (2017), GNI per capita, PPP (constant 2017 international $), https://data.worldbank.org/indicator/NY.GNP.PCAP.PP.KD.
[4] Yun, J. et al. (2018), “Comparative Effectiveness of Telemonitoring Versus Usual Care for Heart Failure: A Systematic Review and Meta-analysis”, Journal of cardiac failure, Vol. 24/1, pp. 19-28, https://doi.org/10.1016/J.CARDFAIL.2017.09.006.
Note
← 1. Voluntary enrolment by GPs may have an impact on which patients are selected to receive TeleHomeCare, thereby influencing generalisability of evaluation findings.