This chapter covers the Hospital-at-Home (H@H) programme operating in Catalonia, Spain. The case study includes an assessment of H@H against the five best practice criteria, policy options to enhance performance and an assessment of its transferability to other OECD and EU27 countries.
Integrating Care to Prevent and Manage Chronic Diseases

4. Hospital-at-Home (H@H), Catalonia, Spain
Abstract
Hospital-at-Home (H@H): Case study overview
Description: In 2006, Catalonia, a region in Spain, introduced its first Hospital-at-Home (H@H) programme. H@H offers patients acute, home‑based care that would otherwise be delivered in a hospital setting. This service is designed to improve patient experiences and population health, while reducing the per capita cost of healthcare.
Best practice assessment:
OECD best practice assessment of H@H in Catalonia, Spain
|
Criteria |
Assessment |
|---|---|
|
Effectiveness and efficiency |
Scaling-up H@H across the whole of Spain is estimated to lead to savings equal to EUR 6.03 per person, per year between 2023 and 2050. Average estimated savings across EU27 countries is similar to Spain at EUR 6.75, which equates to 0.004% of total health expenditure. |
|
Equity |
There is a risk H@H excludes those with unstable living environments thereby heightening existing health inequalities. Nevertheless, findings from the literature indicate these types of programmes can promote health equality given health professionals can more readily address a patient’s social determinants of health. |
|
Evidence‑base |
Results from Hernandez et al. (2023[1]) and Herranz et al. (2022[2]) provided inputs to model the health and economic impact of H@H. This study performs well against the Quality Assessment Tool for Quantitative Studies, in particular in terms of data collection methods used and the use of confounders to control for external factors. |
|
Extent of coverage |
There is a high level of acceptance and therefore uptake in Catalonia’s H@H programme – around 82%. However, due to eligibility criteria, approximately 1.3% of patients admitted to hospital are eligible for H@H. |
Enhancement options: Several policy options are available to enhance the performance of H@H against the five best practice criteria. These include, but are not limited to, adjusting reimbursement schemes to better reflect services provided by H@H programmes as well as strengthening community-based care to ensure socially vulnerable patients have access to H@H.
Transferability: Programmes similar to H@H exist in several OECD countries such as Australia, Canada, Germany and the United States. Based on feedback from H@H administrators and a review of the literature, there are several factors that facilitate the transfer of H@H. These include supportive leadership at the hospital level, a sophisticated health information system and a culture of care integration.
Conclusion: The H@H programme is designed to provide care to patients in their own home as opposed to a hospital setting. By doing so, it aims to improve experiences and outcomes, while reducing costs. Findings from this analysis indicate H@H aligns with many best practice criteria and has the potential to be transferred to other OECD and EU27 countries.
Intervention description
This section outlines the Hospital-at-Home (H@H) programme operating in Catalonia, Spain. The H@H programme was developed as part of the Catalan Open Innovation Healthcare Hub, which aims to support integrated care services for patients with chronic conditions. The section first describes the Catalan Open Innovation Healthcare Hub, and second, outlines how H@H fits within this broader initiative.
The Catalan Open Innovation Healthcare Hub
The Catalan Open Innovation Healthcare Hub (hereafter, the Hub) aims to provide all Catalonian residents with high-quality, integrated care services. At a high-level, the Hub combines a population-based approach to health with adaptive case management.
The Hub consists of five key building blocks – 1) health risk assessment, 2) promotion of healthy lifestyles, 3) vertical and horizontal integration, 4) innovative assessment and regulatory issues, and 5) digital support to integrated care services. Box 4.1 provides a high-level description of each block.
Box 4.1. The Catalan Open Innovation Healthcare Hub building blocks
This box outlines the five building blocks that make up the Catalan Open Innovation Healthcare Hub.
Block 1: Health risk assessment
The Hub includes a regional population-based health risk assessment tool, GMA (Adjusted Morbidity Groups). The GMA tool uses patient level data collected from the Catalan Health Surveillance System in order to stratify patients into different risk groups.
There are four risk groups ranging from lowest to highest complexity needs:
GMA‑1: 50% of the population who have the lowest healthcare needs
GMA‑2: 30% of the population
GMA‑3: 15% of the population
GMA‑4: 5% of the population who have the highest healthcare needs.
Block 2: Promotion of healthy lifestyles
Block 2 aims to foster healthy lifestyle behaviours in order to prevent multimorbidity. The key intervention within this block is the “Prehabilitation programme” (hereafter, Prehab). Prehab is a pre‑operative intervention for high-risk patients aged 70 years and above undergoing major elective surgery. It aims to enhance functional capacity in order to reduce postoperative morbidity and accelerate recovery through improving aerobic capacity, nutritional balance, and psychological well-being.
Block 3: Vertical and horizontal integration
Block 3 consists of four evidence‑based integrated care services: 1) programme for chronic and frail patients; 2) support for complex case management including home hospitalisation; 3) healthcare support programmes for nursing homes; 4) integrated are to avoid hospital admissions among subacute and frail patients. Home hospitalisation, also known as Hospital-at-Home (H@H) is the focus of this case study.
Block 4: Innovative assessment and regulatory issues
Block 4 includes three separate items: 1) healthcare planning and health delivery assessment, 2) regulatory issues regarding patients’ self-tracking data, and 3) regulatory aspects regarding data privacy and sharing.
Block 5: Digital support to integrated care
Block 5 facilitates digital operability across healthcare providers in the region. Several tools are available that promote digital operability – regional information exchange platform, primary care electronic medical record and electronic prescription, personal health folder, ICT tools that support adaptive case management and collaborative work, and cloud-based strategies.
Source: WP6 JADECARE (2020[3]), “Presentation of the original Good Practice: Catalan Open Innovation Hub on ICT-supported Integrated Care Services for Chronic Patients”.
The H@H programme operates within building block three, specifically, as a programme to promote care integration.
Hospital-at-Home (H@H) to promote care integration
Like many developed countries in the world, Spain, including the region of Catalonia, is experiencing an increase in the number of people living with complex health needs due to an ageing population and unhealthy lifestyle behaviours. Health Plans, which are developed in Catalonia every four years, include strategies to address the needs of complex health patients, for example, by prioritising new models of care.
As part of the 2006 Health Plan, the Regional Government of Catalonia prioritised the delivery of integrated care using the conceptual framework outlined within Chronic Care Model (Wagner et al., 1999[4]). One of the strategies to promote integrated care was to implement a Hospital-at-Home (H@H) programme, which was subsequently expanded in the 2011‑15 and 2016‑20 regional health plans (Gonzalez-Colom et al., 2023[5]). H@H is now a mainstream service operating among 27 providers in the region namely eight tertiary hospitals, twelve general hospitals and seven community hospitals.
The remainder of this section provides a description of H@H, as well as information on eligibility and objectives.
What is Hospital-at-Home (H@H)?
H@H is a service offering acute, home‑based care within the comfort of the patient’s home as opposed to in a hospital setting. The service aims to promote vertical care integration by bringing together hospital- and community-based care.
A patient admitted to H@H receives the standard hospital care. The patient is assessed in person daily by the H@H team, which consists of either a nurse or a nurse and a physician (according to the physician’s discretion). Specifically, the registered nurse sees the patient within the first 24 hours of being sent home, with daily visits thereafter lasting around 40 minutes. The registered nurse has access to electronic patient data during their visit, further, they are in contact with the patient’s physician at the hospital, via a dedicated application on a laptop. In addition, the patient’s H@H team1 meet daily to discuss the patient’s progress and to decide when the patient can be discharged.
Interventions available at home include regular tests (e.g. blood and microbiology tests, clinical ultrasound, electrocardiogram), most of the intravenous and nebulised treatments, and oxygen therapy. A pathway for elective transfer back to the hospital (e.g. for additional tests not available at home) and an emergency transfer in case of clinical deterioration is also available.
Despite care being provided in the home, the hospital retains clinical, financial and legal responsibility for the patient.
Who is eligible for H@H?
Patients with acute or exacerbated chronic healthcare needs, as well as surgical patients, who meet the following criteria are eligible for H@H:
Live at home in an area covered by the H@H programme
Have a phone
Have a stable living situation
Have a carer.
Patients are not eligible if they are at high risk of severe clinical deterioration that cannot be treated at home based on medical judgement, are admitted into a short stay unit, and/or have a severe psychiatric disorder.
What are the objectives of H@H?
At a high-level, H@H aims to achieve the “Triple Aim” approach, which is to simultaneously improve patient experiences (quality and satisfaction), improve population health and reduce per capita costs of healthcare.
OECD Best Practices Framework assessment
This section analyses H@H against the five criteria within OECD’s Best Practice Identification Framework – Effectiveness, Efficiency, Equity, Evidence‑base and Extent of coverage (see Box 4.2 for a high-level assessment).
Box 4.2. Assessment of H@H
Effectiveness and efficiency
Scaling-up H@H across the whole of Spain is estimated to lead to savings equal to EUR 6.03 per person, per year between 2023 and 2050.
Average estimated savings across EU27 countries is similar to Spain at EUR 6.75, which equates to 0.004% of total health expenditure.
Equity
There is a risk H@H excludes those with unstable living environments thereby heightening existing health inequalities. Nevertheless, findings from the literature indicate these types of programmes promote health equality given health professionals have better access to information on the patient’s social determinants of health.
Evidence‑base
Results from Hernandez et al. (2023[1]) and Herranz et al. (2022[2]) provided inputs to model the economic impact of H@H. This study performs well against the Quality Assessment Tool for Quantitative Studies, in particular in terms of data collection methods used and the use of confounders to control for external factors.
Extent of coverage
Previous studies have shown that over 80% of patients eligible for Catalonia’s H@H programme agree to participate indicating high levels of acceptance. However, only a small proportion (1.3%) of people who are admitted to hospital are eligible for H@H, which limits the reach of the intervention.
Effectiveness and efficiency
This section highlights key findings from a modelling exercise designed to estimate the economic impact of scaling-up H@H across Spain and transferring it to all other EU27 countries. The estimates were calculated using OECD’s SPHeP-NCD (Strategic Public Health Planning – noncommunicable diseases) model, which relied on real-world evidence on the cost impact of H@H as implemented in Catalonia, Spain. At a high-level, H@H has been shown to reduce the cost per episode of care by over EUR 1 000 when compared to usual care. Savings are generated from lower staffing, catering, infrastructure, and patient testing costs. Further details on modelling assumptions are in Annex 4.A.
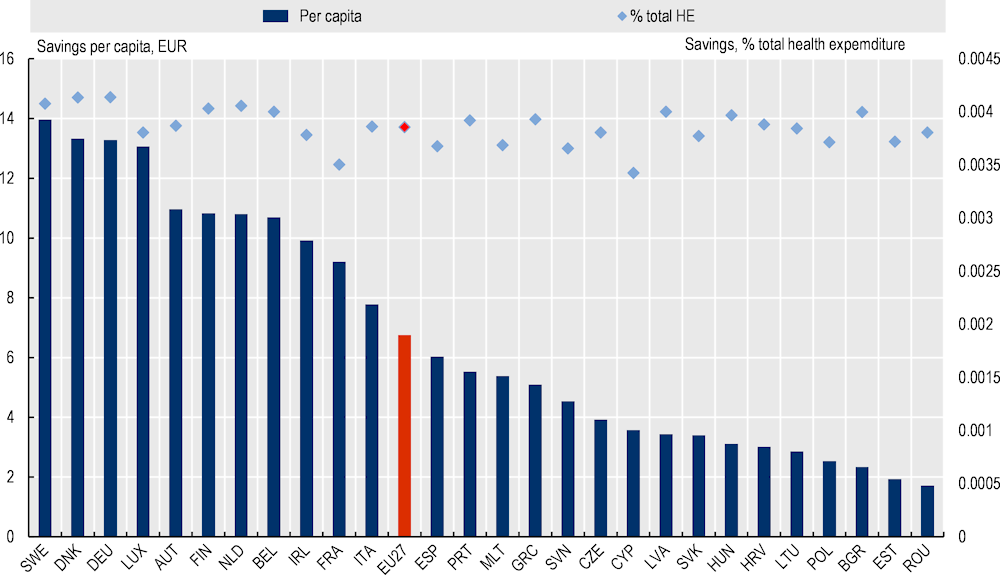
Figure 4.1 outlines average savings, per capita, per year, over the period 2023‑50 for all EU27 countries. On average, EU27 countries are estimated to save EUR 6.75 per person, per year up until 2050 as a result of the hospital-at-home programme. This figure equates to 0.004% of total health expenditure. It is estimated that Sweden would experience the greatest benefit with savings totalling EUR 13.97 per person, compared to EUR 1.72 in Romania.
Scaling-up H@H across the whole of Spain is estimated to lead to similar savings as the EU27 average – i.e. per capita annual savings of EUR 6.03, which equates to 0.004% of total health expenditure.
Figure 4.1. Average savings per capita and as a percentage total health expenditure, 2023‑50 – H@H, EU27 countries
Source: OECD SPHeP-NCD model, 2022.
The analysis outlined above is focused on the economic impact given data availability. However, it is important to note that previous evaluations show H@H does not worsen health outcomes, and, in fact, has been shown to increase patient and caregiver satisfaction. For example, Hernández et al. (2018[6]) in their study evaluating H@H and early discharge (partial substitution of hospital care) in Catalonia, Spain, found the programmes did not lead to an increase in the mortality rate 30 days after discharge. Regarding satisfaction, 98% of patients reported that the treatment they received was “very good”, while 90% and 94% of patients and caregivers stated they would repeat the experience if needed, respectively (Hernández et al., 2018[6]). These findings are supported by the wider literature with a recent systematic review concluding that “HaH generally results in similar or improved clinical outcomes compared with inpatient treatment” (Leong, Lim and Lai, 2021[7]).
Equity
Certain H@H inclusion criteria may exacerbate existing health inequalities. As outlined under the “Intervention description”, to be eligible for H@H, patients must have a stable social situation. This criterion therefore risks excluding socially vulnerable patients, such as those with a low socio-economic background. Further, people from a low socio-economic background may experience barriers to accessing healthcare, therefore it is less likely the healthcare system will identify these patients as eligible for H@H services. For example, across the OECD, 81% of those in the richest income quintile sought care from a doctor in the past three months compared to 75% of those in the poorest quintile, after adjusting for needs (OECD, 2019[8]).
Recent research indicates hospital-at-home programmes might have the potential to reduce health inequalities. As outlined above, research on hospital-at-home programmes and health inequalities is limited. In an effort to address this dearth in the literature, Siu et al. (2022[9]) analysed the impact of hospital-at-home programmes according to socio-economic status in the United States.2 The authors found patients with Medicaid coverage3 who accessed H@H were less likely to revisit the emergency department 30‑days after discharge when compared to usual inpatient care. Findings from the analysis led the authors to conclude that “[H@H] is feasible for economically disadvantaged patients and that these patients may even have greater benefit from [H@H]”. Further, they hypothesis that that patients with a low socio-economic status benefit from such programmes given health professionals can better address the patient’s social determinants of health (e.g. food insecurity).
Evidence‑based
Estimates regarding the effectiveness and efficiency of H@H were calculated using OECD’s SPHeP-NCD microsimulation model. In-depth details of the model are explained elsewhere: http://oecdpublichealthexplorer.org/ncd-doc/.
To estimate the health and economic gains from Catalonia’s Hospital-at-Home programme, the SPHeP-NCD model relied on inputs from Hernandez et al. (2023[1]). This section assesses the quality of this study using the Quality Assessment Tool for Quantitative Studies developed by the Effective Public Health Practice Project (Effective Public Health Practice Project, 1998[10]) (Table 4.1). In summary, the study is considered “strong” in terms of the data collection methods used as well as the use of confounders to control for external factors.
Table 4.1. Evidence‑based assessment of Hospital-at-Home
|
Assessment category |
Question |
Score |
|---|---|---|
|
Selection bias |
Are the individuals selected to participate in the study likely to be representative of the target population? |
Somewhat likely |
|
What percentage of selected individuals agreed to participate? |
82% |
|
|
Selection bias score: Moderate |
||
|
Study design |
Indicate the study design |
Prospective cohort study with an intervention and control group (one period only) |
|
Was the study described as randomised? |
No |
|
|
Was the method of randomisation described? |
Not applicable |
|
|
Was the method of randomisation appropriate? |
Not applicable |
|
|
Study design score: Moderate |
||
|
Confounders |
Were there important differences between groups prior to the intervention? |
No |
|
What percentage of potential confounders were controlled for? |
80‑100% |
|
|
Confounders score: Strong |
||
|
Blinding |
Was the outcome assessor aware of the intervention or exposure status of participants? |
Yes |
|
Were the study participants aware of the research question? |
Unknown |
|
|
Blinding score: Weak |
||
|
Data collection methods |
Were data collection tools shown to be valid? |
Yes |
|
Were data collection tools shown to be reliable? |
Yes |
|
|
Data collection methods score: Strong |
||
|
Withdrawals and dropouts |
Were withdrawals and dropouts reported in terms of numbers and/or reasons per group? |
Not applicable (data collected at one point in time) |
|
Indicate the percentage of participants who completed the study? |
Not applicable |
|
|
Withdrawals and dropouts score: Not applicable |
||
Source: Effective Public Health Practice Project (1998[11]), “Quality assessment tool for quantitative studies”, https://www.nccmt.ca/knowledge-repositories/search/14.
Extent of coverage
Studies indicate the participation rate among eligible patients is high, however, eligibility criteria restrict many patients from accessing H@H. A 2018 evaluation of Catalonia’s H@H programme indicates there is a high level of acceptance of H@H among eligible patients (Hernández et al., 2018[6]). Specifically, of all eligible patients, 82% accepted to participate. Nevertheless, based on Hernandez et al. (2023[1]), Herranz et al. (2022[2]) and Gonzalez-Colom et al. (2023[5]), the proportion of the people admitted to hospital who are subsequently admitted to H@H is low at 1.3%. Feedback from programme administrators suggest this is due to the availability of virtual beds. Since the COVID‑19 pandemic, the availability of virtual beds has grown thereby increasing the number of people accessing H@H services. A ratio of H@H admissions to all hospital admissions close to 5% has been suggested as a reasonable goal.
Policy options to enhance performance
This section outlines policy options to enhance the performance of H@H against each of the best practice criteria.
Enhancing effectiveness
Define what constitutes hospital care at home. In Catalonia’s healthcare system, H@H is defined as a care modality in which hospital healthcare professionals provide active treatment to the patient at home, for a condition that would otherwise require the patient to be admitted to a healthcare facility. The 2020 consensus document (Servei Català de la Salut, 2020[12]) on H@H specifically indicates that H@H is not: i) care of patients with a low complexity profile not requiring hospital admission; ii) monitoring and control of palliative patients; iii) urgent home care visits; iv) home‑based primary care support; nor, v) control of major ambulatory surgery or standard post-surgical follow-up without other complications. It is therefore important to clearly define and delineate between H@H and other home‑based services offered in Catalonia.
Explore ways to adjust reimbursement schemes to factor in heterogeneity among H@H programmes. The type of services delivered by H@H programmes in Catalonia differ across the region. For example, some programmes offer basic services to treat older age, multimorbid patients while others offer specialised services such as bone marrow transplants. Despite significant differences in services delivered in the home, H@H reimbursement rates are standardised. To maintain high quality care in the home, reimbursement rates could be revised to take into account service heterogeneity – e.g. by offering different diagnostic-related group (DRG) payments within H@H that align with services provided, or reimbursement based on predictive models (Monterde et al., 2020[13]).
More broadly, policy makers should prioritise training that provides health professionals with the necessary skills to deliver hospital-at-home services, as well as invest in assistance technologies. Such as using electronic health records, uploading patient information, and communicating with patients online (Leong, Lim and Lai, 2021[7]).
Enhancing efficiency
Extending eligibility criteria to capture more patients can reduce the cost per patient. Policy makers could consider expanding the H@H programme to include more patients, for example by covering more types of care provided in the home. However, this should only be done if the quality of care and health outcomes are not adversely affected. Increasing the number of patients accessing H@H can reduce the per patient cost given fixed costs are spread over a large number of people.
A refined DRG system may also improve efficiency. As outlined under “Enhancing effectiveness”, a nuanced DRG system that takes into account different H@H services may improve efficiency given payments will better reflect service provision.
Enhancing equity
Strengthen community-based care to increase uptake among socially vulnerable patients. As outlined under “Equity”, socially vulnerable people may be excluded from accessing H@H given their living situation. For this reason, it is important to strengthen community-based services who can take on these patients (e.g. convalescent centres), as well as promote integration between hospital- and community-based services.
Stratify evaluation indicators by priority population groups. Despite wide‑spread research on hospital-at-home programmes, including several randomised-controlled trials and systematic reviews, there remains little understanding as to whether such programmes affect population groups differently (Leff et al., 2022[14]). To determine whether Catalonia’s H@H programme reduces or widens existing health inequalities, future studies should stratify data according to priority population groups (e.g. by socio-economic status). Findings from such studies will play a key role in adapting H@H to better meet the needs of disadvantaged groups.
Improve access to healthcare services for disadvantaged groups by promoting health literacy. Disadvantaged groups, such as those with a lower socio-economic status, are less likely to access necessary healthcare services (OECD, 2019[8]). Although disadvantaged groups stand to benefit greatly from H@H, for example, due to higher rates of NCDs, they may be less likely to access the programme. Programmes that promote health literacy among disadvantaged groups may increase uptake in H@H among disadvantaged groups (see Box 4.3 for further details).
Box 4.3. Building population health literacy
Recent analysis estimated that more than half of OECD countries with available data had low levels of health literacy (HL). To address low rates of adult health literacy, OECD have outlined a four‑pronged policy approach, which align:
Strengthen the health system role: establish national strategies and framework designed to address HL
Acknowledge the importance of HL through research: measure and monitor the progress of HL interventions to better understand what policies work
Improve data infrastructure: improve international comparisons of HL as well as monitoring HL levels over time
Strengthen international collaboration: share best practice interventions to boost HL across countries.
Source: OECD (2018[15]), “Health literacy for people‑centred care: Where do OECD countries stand?”, https://doi.org/10.1787/d8494d3a-en.
Enhancing the evidence‑base
Listed below are recommendations to enhance the data collected as part of the H@H programme. These suggestions are based on a review of current data collection protocols.
Collect data from a high-quality study that, to the extent possible, replicates a randomised clinical trial to enable the most possible accurate comparisons
Collect data during the intervention (e.g. utilisation of healthcare services, related costs) in addition to before and after the intervention
Collect annual data after the intervention has concluded (e.g. utilisation of healthcare services, related expenditures) rather than on a monthly basis
Collect information about how the intervention is implemented (for example, what are the inclusion criteria; action plan for each health profile) to improve modelling capabilities
Compare the impact of H@H against other initiatives that aim to reduce healthcare costs without negatively affecting health outcomes and patient experiences (e.g. increase use of outpatient and primary care).
Enhancing the extent of coverage
As outlined under “Enhancing efficiency”, consideration could be given to extending eligibility criteria as long as it is safe to do so. This would help increase the proportion of patients admitted to hospital who are eligible for H@H services.
Transferability
This section explores the transferability of H@H and is broken into three components: 1) an examination of previous transfers; 2) a transferability assessment using publicly available data; and 3) additional considerations for policy makers interested in transferring H@H.
Previous transfers
Several OECD and EU countries have implemented hospital-at-home programmes. As outlined in OECD’s recent primary care report, healthcare systems increasingly provide “post-discharge care at home as an alternative to hospital-based care” (OECD, 2020[16]). Example countries include Australia, Canada, Germany, Israel, the United Kingdom, and the United States. This implies home‑based care programmes, such as H@H, are transferable if tailored to the local context.
Several factors facilitate the transfer of hospital-at-home type programmes. Based on feedback from H@H administrators, key factors include:
Strong and supportive leadership at the hospital level
A culture of integrated care
Reimbursement arrangements that incentivise care delivered in a home setting.
Transferability assessment
The following section outlines the methodological framework to assess transferability followed by results from the assessment.
Methodological framework
A few indicators to assess the transferability of H@H were identified (see Table 4.2). Indicators were drawn from international databases and surveys to maximise coverage across OECD and non-OECD European countries. Please note, the assessment is intentionally high level given the availability of public data covering OECD and non-OECD European countries.
Table 4.2. Indicators to assess transferability – Hospital-at-Home
|
Indicator |
Reasoning |
Interpretation |
|---|---|---|
|
Sector specific context (hospital-, primary- and community-based care) |
||
|
Proportion of hospitals using electronic health records (EHRs) for inpatients |
EHRs improve the ability of health professionals to provide integrated patient-centred care. Therefore, the intervention is more transferable in countries that utilise EHRs in secondary facilities. |
🡹 value = more transferable |
|
The extent of task shifting between physicians and nurses in primary care |
H@H is more transferable to settings with a culture of care integration |
The more “extensive” the more transferable |
|
% of tertiary institutions (public and private) that offer ICT for health (eHealth) courses |
H@H is more transferable if health professional students receive eHealth training |
🡹 value = more transferable |
|
% of institutions or associations offering in-service training in the use of ICT for health as part of the continuing education of health professionals |
H@H is more transferable if health professionals have appropriate eHealth training |
🡹 value = more transferable |
|
Economic context |
||
|
Secondary healthcare expenditure as a percentage of current health expenditure |
The intervention is hospital-based therefore, it is likely to be more successful in countries that allocate a higher proportion of health spending to secondary care |
🡹 value = more transferable |
Source: WHO (2015[17]), “Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage”, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage; Odenkirk (2017[18]), “Readiness of electronic health record systems to contribute to national health information and research”, https://dx.doi.org/10.1787/9e296bf3-en; OECD (2021[19]), “OECD Health Statistics: health expenditure and financing”; Eurostat (2022[20]), “Database – Eurostat”, https://ec.europa.eu/eurostat/data/database.
Results
Table 4.3 displays results from the transferability analysis at the country level, with key findings summarised below:
Factors to determine if the sector (i.e. hospital- and community-based care) is ready for H@H show mixed results:
On a positive note, the proportion of hospitals who use electronic health records in hospitals is high (80% on average and in Spain), with many countries reporting 100% use. Nevertheless, this figure is as low as 10% in Poland.
Task shifting, which may reflect whether there is a culture of care integration, shows less positive results with very few countries reporting “extensive” task shifting between health professionals (i.e. 22% of countries with available data compared to the remaining 78% that reported “limited” or no task shifting).
Similarly, indicators measuring ICT training for health professionals show training does not form part of the formal curricula in most countries.
Regarding expenditure, spending on secondary care (which is responsible for funding H@H) is similar in Spain to other countries with available data (around 25%).
Table 4.3. Transferability assessment by country (OECD and non-OECD European countries) – Hospital-at-Home
A darker shade indicates H@H is more suitable for transferral in that particular country
|
Country |
% hospitals using electronic patient records for inpatients |
Extent of task shifting from doctors to nurses in primary care |
% of tertiary institutions that offer ICT for health (eHealth) courses |
% of institutions or associations offering in-service training in the use of ICT for health as part of the continuing education of health professionals |
Spending on secondary healthcare as a percentage of CHE* |
|---|---|---|---|---|---|
|
Spain |
80 |
Limited |
Low |
Medium |
25 |
|
Australia |
20 |
Extensive |
Medium |
High |
31 |
|
Austria |
99 |
None |
Low |
Low |
32 |
|
Belgium |
n/a |
Limited |
Low |
Low |
28 |
|
Bulgaria |
n/a |
None |
Medium |
Medium |
37 |
|
Canada |
69 |
Extensive |
High |
Low |
16 |
|
Chile |
69 |
n/a |
Low |
Low |
n/a |
|
Colombia |
n/a |
n/a |
n/a |
n/a |
11 |
|
Costa Rica |
n/a |
n/a |
Medium |
Medium |
39 |
|
Croatia |
n/a |
Limited |
Low |
Medium |
20 |
|
Cyprus |
n/a |
Limited |
Medium |
Low |
29 |
|
Czech Republic |
100 |
None |
Medium |
n/a |
24 |
|
Denmark |
100 |
Limited |
Medium |
Very High |
25 |
|
Estonia |
100 |
Limited |
Medium |
Low |
22 |
|
Finland |
100 |
Extensive |
Medium |
Medium |
22 |
|
France |
60 |
None |
n/a |
n/a |
25 |
|
Germany |
n/a |
None |
n/a |
n/a |
26 |
|
Greece |
50 |
None |
Medium |
Medium |
42 |
|
Hungary |
n/a |
Limited |
Low |
n/a |
27 |
|
Iceland |
100 |
Limited |
Very High |
Very High |
28 |
|
Ireland |
35 |
Extensive |
n/a |
Low |
25 |
|
Israel |
100 |
n/a |
High |
Low |
26 |
|
Italy |
n/a |
Limited |
Low |
High |
27 |
|
Japan |
34 |
n/a |
n/a |
n/a |
27 |
|
Korea |
n/a |
n/a |
n/a |
n/a |
25 |
|
Latvia |
90 |
Limited |
Low |
Low |
21 |
|
Lithuania |
n/a |
Limited |
Medium |
Low |
27 |
|
Luxembourg |
n/a |
None |
Low |
Low |
25 |
|
Malta |
n/a |
Limited |
Very High |
Very High |
n/a |
|
Mexico |
49 |
n/a |
Medium |
Low |
30 |
|
Netherlands |
n/a |
Extensive |
High |
High |
19 |
|
New Zealand |
100 |
Extensive |
Medium |
Very High |
n/a |
|
Norway |
100 |
None |
Low |
Medium |
26 |
|
Poland |
10 |
None |
High |
Medium |
35 |
|
Portugal |
n/a |
Limited |
Low |
Low |
17 |
|
Romania |
n/a |
None |
n/a |
n/a |
35 |
|
Slovak Republic |
100 |
None |
n/a |
n/a |
30 |
|
Slovenia |
n/a |
Limited |
High |
High |
27 |
|
Sweden |
100 |
Limited |
Very High |
Very High |
20 |
|
Switzerland |
100 |
None |
Low |
Very High |
26 |
|
Türkiye |
n/a |
None |
n/a |
n/a |
n/a |
|
United Kingdom |
100 |
Extensive |
Medium |
High |
23 |
|
United States |
76 |
Extensive |
Low |
Low |
16 |
Note: *CHE = current health expenditure. n/a = no data available.
Source: See Table 4.2.
To help consolidate findings from the transferability assessment above, countries have been clustered into one of three groups, based on indicators reported in Table 14.4. Countries in clusters with more positive values have the greatest transfer potential. For further details on the methodological approach used, please refer to Best Practice case study guide.
Key findings from each of the clusters are below with further details in Figure 4.2 and Table 4.4:
Countries in cluster one have a secondary-care and community-care sector amenable to hospital-at-home programmes. Further these countries typically spend more on secondary care, which is where funding for hospital-at-home programme is sourced.
Countries in cluster two also have secondary-care and community-care sectors that are supportive of hospital-at-home programmes. However, on average, they spend less on secondary care indicating potential affordability issues. This cluster includes Spain, indicating high levels of spending on secondary care is not a pre‑requisite for delivering acute, home‑based care.
Countries in cluster three should consider reviewing whether their healthcare sector has the digital capacity to successfully operate hospital-at-home programmes (e.g. use of EHRs to communicate patient data remotely).
Figure 4.2. Transferability assessment using clustering – Hospital-at-Home
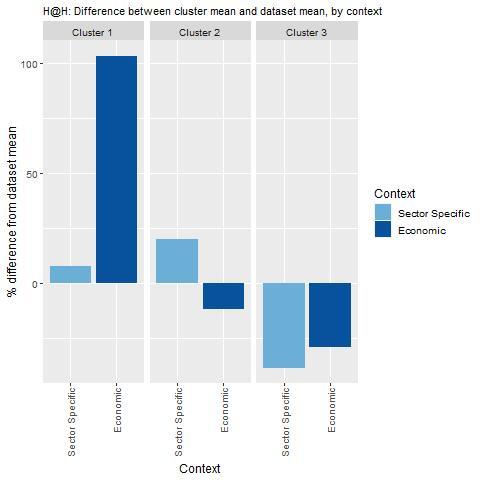
Note: Bar charts show percentage difference between cluster mean and dataset mean, for each indicator.
Source: See Table 14.4.
Table 4.4. Countries by cluster – Hospital-at-Home
|
Cluster 1 |
Cluster 2 |
Cluster 3 |
|---|---|---|
|
Australia Bulgaria Costa Rica Greece New Zealand Poland |
Croatia Cyprus Czech Republic Denmark Estonia Finland Iceland Israel Italy Lithuania Mexico Netherlands Norway Slovak Republic Slovenia Spain Sweden Switzerland United Kingdom |
Austria Belgium Canada Chile France Hungary Ireland Latvia Luxembourg Portugal United States |
Note: The following countries were omitted from the analysis due to high levels of missing data: Colombia, Germany, Japan, Malta, Korea, Romania and Türkiye.
New indicators to assess transferability
Data from publically available datasets alone is not ideal to assess the transferability of public health interventions. Box 4.4 outlines several new indicators policy makers could consider before transferring H@H.
Box 4.4. New indicators, or factors, to consider when assessing transferability – Hospital-at-Home
In addition to the indicators within the transferability assessment, policy makers are encouraged to collect information for the following indicators:
Population context
Are patients accepting of receiving care in their homes?
Sector specific context (hospital-, primary- and community-based care)
Do reimbursement models in primary and secondary care promote hospital-at-home type services?
Do health professionals (e.g. registered nurses) have the skills and feel comfortable providing care in a patient’s home?
Do electronic health records have the necessary functions to provide hospital care at home?
Do payment and billing mechanisms support hospital care at home?
Do healthcare professionals have the skills as well as the motivation to work as a team to deliver patient care?
Are hospital- and community-based services well integrated?
Are leadership groups within hospitals supportive of hospital-at-home type programmes?
What proportion of the population live near a hospital?
How suitable are patients’ homes for hospital-at-home type care?
Political context
Has the intervention received political support from key decision-makers? (E.g. a national strategy to address ageing and chronicity)
Has the intervention received commitment from key decision-makers?
Economic context
What is the cost of implementing and operating the intervention in the target setting and to whom?
Conclusion and next steps
A rise in the number of people living with complex health needs led to the implementation of Catalonia’s H@H programme. H@H is a service offering patients acute, home‑based care within the comfort of the patient’s home as opposed to a hospital setting. H@H aims to improve patient experiences and population health, while reducing the per capita cost of healthcare.
H@H is estimated to lead to cost savings when scaled-up across Spain and transferred to other EU27 countries. On average, EU27 countries are estimated to save EUR 6.75 per person, per year up until 2050 as a result of the hospital-at-home programme. This figure equates to 0.004% of total health expenditure. These savings are similar to those estimated in Spain.
Several policy options are available to enhance the performance of H@H against the five best practice criteria. These include, but are not limited to, adjusting reimbursement schemes to better reflect services provided by H@H programmes and strengthening community-based care to ensure socially vulnerable patients can access H@H.
Hospital-at-home type programmes are more transferable to countries with certain characteristics. Feedback from H@H administrators and a review of the literature identified several factors that facilitate the transfer of hospital-at-home type programmes. These include having strong leadership at the hospital level, a culture of integrated care, reimbursement schemes that incentivise care delivery in the home, as well as a sophisticated health information system.
Box 4.5 summarises next steps for policy makers and funding agencies interested in H@H.
Box 4.5. Next steps for policy makers and funding agencies – Hospital-at-Home
Next steps for policy makers and funding agencies to enhance H@H are listed below:
Review policy options in this case study to identify and prioritise health policy, for example, strengthening horizontal care integration
Promote findings from this case study to countries who may be interested in transferring H@H to their local context
Annex 4.A. H@H: Modelling assumptions
Annex Table 4.A.1. Modelling assumptions
|
Description |
|
|---|---|
|
Target group |
Everyone aged 18 years and over who has a modelled disease. |
|
Exposure |
It is assumed that 1.30% of the target group accesses the H@H programme. This assumption is based on Hernandez et al. (2023[1]) and Herranz et al. (2022[2]) hereby 441 of all 33 859 patients who were admitted to hospital accessed H@H |
|
Effectiveness |
None. |
|
Costs |
For each year the patient has the disease, costs should be decreased to 53.2% of the original cost. This is based on an upcoming paper by Hernandez et al.:
Further, it is assumed that 75% of yearly cost in the incidence year are due to the first 30 days. Therefore, the cost decrease is 53.2% * 75% = 40% |
|
Timeframe |
Years 2023‑50. |
References
[10] Effective Public Health Practice Project (1998), Quality Assessment Tool for Quantitative Studies, https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/ (accessed on 28 July 2021).
[11] Effective Public Health Pratice Project (1998), Quality assessment tool for quantitative studies, https://www.nccmt.ca/knowledge-repositories/search/14.
[20] Eurostat (2022), Database - Eurostat, https://ec.europa.eu/eurostat/data/database (accessed on 15 March 2022).
[5] Gonzalez-Colom, R. et al. (2023), Five years of Hospital at Home adoption in Catalonia: impact and challenges, Cold Spring Harbor Laboratory, https://doi.org/10.1101/2023.01.25.23284997.
[6] Hernández, C. et al. (2018), “Implementation of Home Hospitalization and Early Discharge as an Integrated Care Service: A Ten Years Pragmatic Assessment”, International Journal of Integrated Care, Vol. 18/0, p. 12, https://doi.org/10.5334/ijic.3431.
[1] Hernandez, C. et al. (2023), The Value of Admission Avoidance: Cost-Consequence Analysis of One-Year Activity in a Consolidated Service, Cold Spring Harbor Laboratory, https://doi.org/10.1101/2023.01.05.23284217.
[2] Herranz, C. et al. (2022), “Prospective cohort study for assessment of integrated care with a triple aim approach: hospital at home as use case”, BMC Health Services Research, Vol. 22/1, https://doi.org/10.1186/s12913-022-08496-z.
[14] Leff, B. et al. (2022), “A research agenda for hospital at home”, Journal of the American Geriatrics Society, Vol. 70/4, pp. 1060-1069, https://doi.org/10.1111/jgs.17715.
[7] Leong, M., C. Lim and Y. Lai (2021), “Comparison of Hospital-at-Home models: a systematic review of reviews”, BMJ Open, Vol. 11/1, p. e043285, https://doi.org/10.1136/bmjopen-2020-043285.
[13] Monterde, D. et al. (2020), “<p>Performance of Comprehensive Risk Adjustment for the Prediction of In-Hospital Events Using Administrative Healthcare Data: The Queralt Indices</p>”, Risk Management and Healthcare Policy, Vol. Volume 13, pp. 271-283, https://doi.org/10.2147/rmhp.s228415.
[18] Oderkirk, J. (2017), “Readiness of electronic health record systems to contribute to national health information and research”, OECD Health Working Papers, No. 99, OECD Publishing, Paris, https://doi.org/10.1787/9e296bf3-en.
[19] OECD (2021), OECD Health Statistics: health expenditure and financing.
[16] OECD (2020), Realising the Potential of Primary Health Care, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/a92adee4-en.
[8] OECD (2019), Health for Everyone?: Social Inequalities in Health and Health Systems, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/3c8385d0-en.
[15] OECD (2018), “Health literacy for people-centred care: Where do OECD countries stand?”, OECD Health Working Papers, No. 107, OECD Publishing, Paris, https://doi.org/10.1787/d8494d3a-en.
[12] Servei Català de la Salut (2020), Model organitzatiu d’hospitalització a domicili de Catalunya: Alternativa a l’hospitalització convencional, Servei Català de la Salut, https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/normatives_instruccions/2020/model-organitzatiu-hospitalitzacio-domicili.pdf.
[9] Siu, A. et al. (2022), “Health equity in Hospital at Home: Outcomes for economically disadvantaged and non‐disadvantaged patients”, Journal of the American Geriatrics Society, https://doi.org/10.1111/jgs.17759.
[4] Wagner, E. et al. (1999), “A survey of leading chronic disease management programs: are they consistent with the literature?”, Manag Care Q, Vol. 7/3, pp. 56-66.
[17] WHO (2015), Atlas of eHealth country profiles: The use of eHealth in support of universal health coverage, Global Observatory for eHealth, https://www.afro.who.int/publications/atlas-ehealth-country-profiles-use-ehealth-support-universal-health-coverage.
[3] WP6 (2020), WP6 (2020), Presentation of the original Good Practice: Catalan Open Innovation Hub on ICT-supported Integrated Care Servies for Chronic Patients.
Notes
← 1. A typical H@H team includes one co‑ordinator (i.e. physician or nurse), several advanced practice nurses and physicians.
← 2. The study population were aged 18 years and over with fee‑for-service Medicare or had coverage from a private insurer that contracted for hospital-at-home services. Patients with Medicaid were dually eligible or had Medicaid Managed Care. Patients were eligible if they were admitted into one of four New York city hospitals with a medical diagnosis.
← 3. Medicaid provides health coverage to millions of Americans, including eligible low-income adults, children, pregnant women, elderly adults, and people with disabilities.